Abstract
This prospective study determined the use of intracranially recorded spectral responses during naming tasks in predicting neuropsychological performance following epilepsy surgery.
We recruited 65 patients with drug-resistant focal epilepsy who underwent preoperative neuropsychological assessment and intracranial EEG recording. The Clinical Evaluation of Language Fundamentals evaluated the baseline and postoperative language function. During extra-operative intracranial EEG recording, we assigned patients to undergo auditory and picture naming tasks. Time-frequency analysis determined the spatiotemporal characteristics of naming-related amplitude modulations, including high gamma augmentation at 70–110 Hz. We surgically removed the presumed epileptogenic zone based on the intracranial EEG and MRI abnormalities while maximally preserving the eloquent areas defined by electrical stimulation mapping. The multivariate regression model incorporating auditory naming-related high gamma augmentation predicted the postoperative changes in Core Language Score with r2 of 0.37 and in Expressive Language Index with r2 of 0.32. Independently of the effects of epilepsy and neuroimaging profiles, higher high gamma augmentation at the resected language-dominant hemispheric area predicted a more severe postoperative decline in Core Language Score and Expressive Language Index. Conversely, the model incorporating picture naming-related high gamma augmentation predicted the change in Receptive Language Index with an r2 of 0.50. Higher high gamma augmentation independently predicted a more severe postoperative decline in Receptive Language Index. Ancillary regression analysis indicated that naming-related low gamma augmentation and alpha/beta attenuation likewise independently predicted a more severe Core Language Score decline. The machine learning-based prediction model suggested that naming-related high gamma augmentation, among all spectral responses used as predictors, most strongly contributed to the improved prediction of patients showing a >5-point Core Language Score decline (reflecting the lower 25th percentile among patients). We generated the model-based atlas visualizing sites, which, if resected, would lead to such a language decline. With a 5-fold cross-validation procedure, the auditory naming-based model predicted patients who had such a postoperative language decline with an accuracy of 0.80. The model indicated that virtual resection of an electrical stimulation mapping-defined language site would have increased the relative risk of the Core Language Score decline by 5.28 (95% confidence interval: 3.47–8.02). Especially, that of an electrical stimulation mapping-defined receptive language site would have maximized it to 15.90 (95% confidence interval: 9.59–26.33).
In summary, naming-related spectral responses predict neuropsychological outcomes after epilepsy surgery. We have provided our prediction model as an open-source material, which will indicate the postoperative language function of future patients and facilitate external validation at tertiary epilepsy centres.
Keywords: paediatric epilepsy surgery, intracranial electroencephalography (iEEG) recording, event-related high gamma augmentation, high-frequency oscillations (HFOs), ripples
Sonoda et al. report that naming-related spectral responses on intracranial EEG can predict neuropsychological outcomes after epilepsy surgery. They provide the machine learning-based prediction model as open-source material, so that investigators can use it to predict the p language function of their own patients.
Introduction
Invasive recording using intracranial electrodes aims to localize the seizure onset zone and functionally important areas for resective epilepsy surgery.1 Electrical stimulation mapping (ESM) remains the gold standard for defining the language areas.2-5 Investigators have implemented an alternative method for language mapping partly because ESM has limitations. Stimulation of non-epileptic areas may induce non-habitual seizures,6 which may increase the risk of complications and reduce the reliability of the ESM sessions. A study of 122 patients reported that ESM-induced seizures in 36% of patients.7 Some patients may not be able to tolerate hourly ESM sessions. A tertiary epilepsy centre reported that ESM failed to localize the language areas in the presumed dominant hemisphere in four-fifths of children at the age of 10 years or younger.8
Measurement of task-related spectral responses on intracranial EEG (iEEG) is a method that complements the ESM.9–12 Task-related augmentation of high-frequency broadband activity, including high gamma (70–110 Hz), is suggested to reflect cortical activation.13,14 Such amplitude augmentation was reported to be associated with increased neural firing,15,16 haemodynamic activation on functional MRI,17 and increased metabolism on glucose PET.18 A meta-analysis of 15 studies reported that sites showing naming-related high gamma augmentation had 6.44 times increased odds to be classified as ESM-defined language areas.19 Our preliminary study reported that resectioning sites showing naming-related high gamma augmentation increased the risk of new deficits requiring speech therapy, independent of objective neuropsychological assessment.20 Two iEEG studies of 11 and 17 patients reported that resection of high gamma sites was marginally associated with a postoperative decline of neuropsychological function.21,22 A prospective study of a larger cohort of patients with neuropsychological data is necessary to provide definitive evidence supporting the utility of high gamma-based language mapping.
The present study intended to achieve the following aims.
Aim 1: We aimed to clarify the relationship between cortical resection involving naming-related high gamma activation sites and neuropsychological performance following surgery. We determined whether high gamma-based mapping would predict postoperative language performance independently of epilepsy and neuroimaging profiles available preoperatively.
Aim 2: We determined whether naming-related modulations of iEEG frequency bands other than high gamma would likewise predict postoperative language performance. Previous iEEG studies reported that sites showing high gamma augmentation frequently exhibit low gamma augmentation and alpha/beta suppression simultaneously.23–25 While high gamma augmentation is suggested to be better time-locked to a given task than alpha/beta suppression,13,26 it remains to be determined whether high gamma-based mapping would play the most critical role in predicting postoperative language function.
Aim 3: We generated a machine learning-based prediction model identifying electrode sites, which, if resected, would lead to a postoperative decline in language function. We designed the model based on iEEG spectral responses but not on ESM findings and assessed its prediction accuracy using a cross-validation procedure. Furthermore, we performed a simulation study and determined whether this prediction model revealed an increased relative risk of a postoperative language decline related to virtual resection of an ESM-defined language site. We have provided our prediction model as open-source material. Investigators can use this model to predict a given patient’s postoperative language outcome at their own epilepsy centres.
Materials and methods
Patients
We prospectively recruited and studied a consecutive series of patients satisfying the following criteria. The inclusion criteria consisted of: (i) drug-resistant focal epilepsy; (ii) age 4 years and above; (iii) neuropsychological evaluation including the baseline language function27; (iv) extra-operative iEEG recording as part of our presurgical evaluation at Detroit Medical Center in Detroit between January 2009 and February 2019; and (v) measurement of naming-related spectral responses on iEEG. The exclusion criteria consisted of: (i) history of previous epilepsy surgery; and (ii) massive structural lesions (such as megalencephaly or perisylvian polymicrogyria), which would make the Sylvian or central sulcus unidentifiable. The Wayne State University Institutional Review Board approved the study. We obtained informed consent/assent in writing from the patients or the guardians of patients.
Language-dominant hemisphere
We previously discussed the rationale of our approach to estimate the language-dominant hemisphere.20,28,29 It is infeasible to expect all surgical candidates, especially young children, to successfully undergo a Wada test or functional MRI for lateralizing the dominant hemisphere.30,31 We aimed to generate a model that can predict postoperative language function without relying on the Wada test, functional MRI or ESM.20,32 Thus, we estimated the dominant hemisphere based on the handedness and anatomical MRI findings.33–35 We treated the left hemisphere as dominant if the patient was right- or left-handed but free of a developmental cortical lesion (such as dysplasia) in the left neocortical area. Conversely, we treated the right hemisphere as dominant if the patient was left-handed and had a developmental cortical lesion in the left neocortex.20,29
Intracranial EEG
We surgically implanted platinum disc electrodes on the pial surface to determine the boundary between the seizure onset zone and functionally important areas.28,36 We recorded iEEG with a sampling rate of 1000 Hz and a band-pass of 0.016–300 Hz for 3–7 days.37 We discontinued anti-epileptic drugs to capture habitual spells and localize the seizure onset zone responsible for habitual seizures.36 We performed the following iEEG analysis using common average reference (i.e. an average of iEEG voltages at all channels excluding those affected by the seizure onset zone, interictal spikes, MRI lesions or artefacts).
MRI
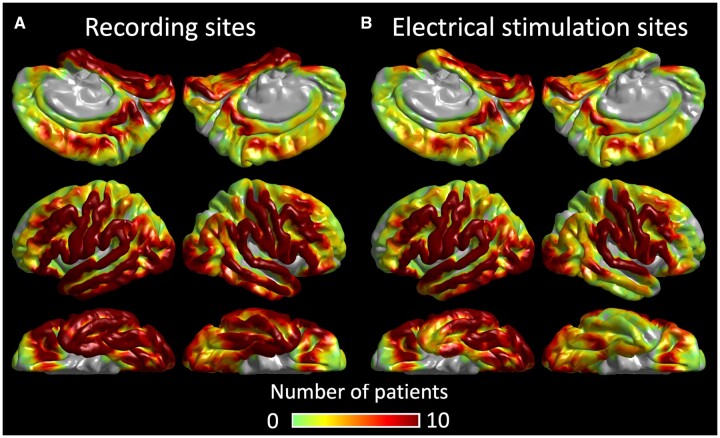
Before implanting intracranial electrodes, we acquired 3 T MRI, including a T1-weighted spoiled gradient-echo volumetric scan and fluid‐attenuated inversion recovery scan.38 We co-registered electrodes with a three‐dimensional surface image39,40 We spatially normalized all electrode locations of all patients to the FreeSurfer averaged image (http://surfer.nmr.mgh.harvard.edu).37,39,41Figure 1 shows the distribution of electrodes included in our iEEG analysis.
Figure 1.
Distribution of intracranial electrodes. (A) The FreeSurfer surface image presents the distribution of artefact-free electrode sites included in the present study (6886 sites). Colour indicates the number of patients at each cortical point. (B) The distribution of sites assessed by electrical stimulation mapping (5203 sites).
Electrical stimulation mapping
We previously described our ESM protocol in detail.6,39 We stimulated a pair of neighbouring electrode sites with a frequency of 50 Hz, pulse width of 0.3 ms and train duration of ≤5 s. Figure 1 shows the distribution of 5203 electrode sites assessed by ESM. We initially set the stimulus intensity at 3 mA, increased it to 6 and 9 mA in a stepwise manner until a clinical symptom or after-discharge was noted. We kept the stimulus intensity below the after-discharge threshold once identified in a given patient. During each stimulation trial, a given patient was instructed to answer auditory questions such as ‘What flies in the sky?’ or to name pictures presented by a neuropsychologist (R.R.) blinded to the results of naming-related spectral responses. The neuropsychologist asked each patient what made them fail to respond when needed. Additional tasks (e.g. syllable repetition) specified the functional role of each stimulated site. Sites at which stimulation induced the following symptoms were defined as ESM-defined language areas: (i) speech arrest: inability to vocalize; (ii) auditory receptive aphasia: failure to understand auditory questions; (iii) auditory expressive aphasia: intact vocalization, successful understanding of auditory questions, but failure to provide a relevant answer; and (iv) visual expressive aphasia: intact vocalization but failure to name pictures. We visualized the group-level probability of stimulation-induced symptoms at each cortical point on the FreeSurfer averaged surface image.39
Resective surgery
All patients underwent epilepsy surgery within 24 h after the completion of extra-operative iEEG recording. Our primary intention was to completely remove the presumed epileptogenic zone consisting of seizure onset zone and the neighbouring structural lesion while maximally preserving the eloquent areas.36,38,42 The spatial extent of ESM-defined language areas and the spatiotemporal profiles of naming-related high gamma augmentation were available before the surgery. We considered that ESM would localize the regions essential for language, whereas naming-related high gamma augmentation would localize those involved in language.20,28 With the patient and family, before the intracranial electrode placement as well as at least a day before the surgery, we discussed the pros and cons of the complete and incomplete resection of the presumed epileptogenic zone in case the language areas were spatially overlapped with the seizure onset zone.36
Based on the intraoperative photograph taken immediately before the dural closure, the FreeSurfer script computed the resection size, defined as the proportion of resected tissue among the hemisphere (% of the hemisphere).38 We previously reported that the resection size estimated with an intraoperative photograph was highly concordant with that based on postoperative MRI.42
Neuropsychological assessment
A neuropsychologist (A.C.), being blinded to the results of any iEEG analysis, evaluated the preoperative and postoperative language function using the Clinical Evaluation of Language Fundamentals, fourth edition.27 We computed the age-corrected Core Language Score, Receptive Language Index and Expressive Language Index [average: 100; standard deviation (SD): 15] for patients aged between 4 and 21 years (Supplementary Fig. 1). Since a given patient was expected to gradually recover from postoperative language impairment, if any, as a function of time, we treated the interval between surgery and postoperative neuropsychological assessment (Table 1) as a covariate in the multivariate regression analysis that follows.
Table 1.
Patient profile
| n | 65 |
| Mean age, years | 14.6 |
| Age range, years | 5–44 |
| Interval between surgery and postoperative language assessment, months (IQR) | 3.6 (2.3) |
| Female, % | 47.7 |
| Right-handedness, % | 81.5 |
| Language-dominant hemisphere, % | Left: 92.3 |
| Side of resected hemisphere, % | Left: 58.5 |
| Mean number of pre/postoperative anti-epileptic drugs | 2.3/2.0 |
| Seizure onset zone, n (%) | |
| Frontal | 17 (26.2) |
| Temporal | 32 (49.2) |
| Parietal | 22 (33.8) |
| Occipital | 13 (20.0) |
| Not availablea | 7 (10.8) |
| MRI-visible cortical lesion, n (%) | 37 (56.9) |
| Aetiology, n (%) | |
| Tumour | 13 (20.0) |
| Dysplasia | 20 (30.8) |
| Hippocampal sclerosis | 4 (6.2) |
| Inflammation | 2 (3.1) |
| Gliosis alone | 27 (41.5) |
IQR = interquartile range.
aThe extent of cortical resection was guided by interictal epileptiform discharges as well as the MRI lesion.
Naming tasks
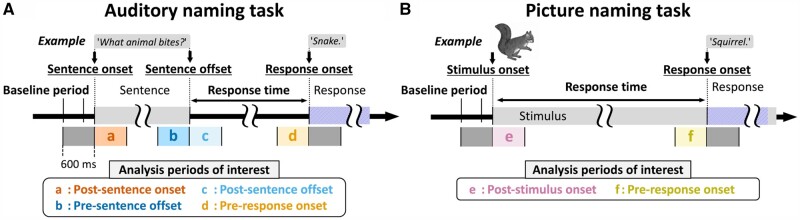
We assigned auditory and picture naming tasks to patients during interictal iEEG recording to localize the sites involved in language based on the time-frequency analysis.28,29 We have previously described the task parameters in detail.29,39 We synchronized iEEG traces, stimulus presentations and patient behaviours using a photosensor and microphones.28 For the auditory naming task, we instructed patients to overtly provide an answer for each of up to 100 audible sentence questions. We measured the percentage of correct answers and the response time defined as the interval between stimulus offset and response onset (Fig. 2). We excluded trials from time-frequency analysis if a patient failed to provide a relevant answer or the response time was longer than 2 SD from the individual mean.43
Figure 2.
Naming tasks and analysis periods of interest. (A) Auditory naming task. Each patient was instructed to verbally answer a brief sentence question (median duration of sentence stimuli: 1.8 s; range: 1.2–2.4 s). (B) Picture naming task. Each patient named an object presented on a monitor. Each 600-ms analysis period of interest is highlighted in colour and labelled as a to f.
For the picture naming task, we asked patients to overtly name an object presented on a liquid-crystal display monitor (up to 60 common objects such as ‘dog’ and ‘tree’). We measured the percentage of correct answers and the response time defined as the interval between stimulus onset and response onset. We aligned iEEG traces to stimulus onset and response onset (Fig. 2).
Measurement of naming-related high gamma responses
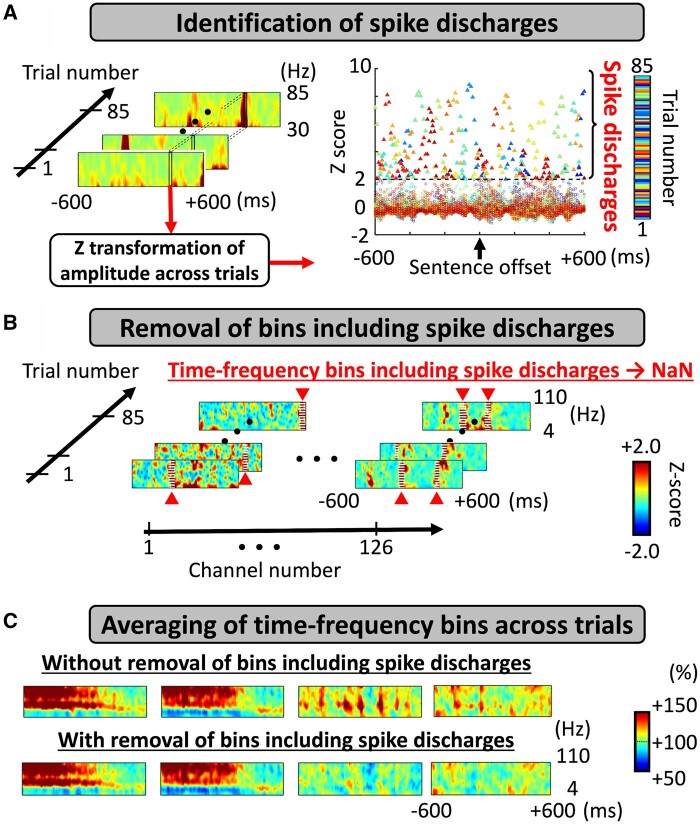
At each artefact-free channel, we determined the temporal profiles of iEEG high gamma (70–110 Hz) amplitude augmentation during the auditory naming task. We measured amplitude modulations during 1200-ms epochs centred at sentence onset, sentence offset and response onset, whereas picture naming-related responses during 1200-ms epochs centred at stimulus onset and response onset. Figure 2 presents a specific set of analysis periods of interest for each task. Using the Morlet wavelet method44 implemented in the FieldTrip (https://www.fieldtriptoolbox.org/tutorial/timefrequencyanalysis/), we transformed iEEG voltage data into time-frequency bins with a fixed wavelet duration of 32 ms, sliding in 10 ms steps. An amplitude measure was assigned to each of the all 10-ms bins ranging from alpha to high gamma bands (Fig. 3).
Figure 3.
Preprocessing to remove pathological components from time-frequency analysis. (A) Identification of randomly occurring spike discharges. We identified within-trial time-frequency bins showing a broadband (30–85 Hz) amplitude >2 SD from the across-trial mean (i.e. z-score of >2).45,46 (B) Removal of bins including spike discharges. We treated such time-frequency bins including an excessive broadband amplitude as missing values (i.e. NaN: Not a Number). (C) Averaging of time-frequency bins across trials. We computed the averaged amplitude modulations (i.e. percentage change) as compared to that during the 400-ms baseline period prior to the stimulus onset.39 Here, the time-frequency matrices present auditory naming-related spectral responses time-locked to stimulus offset at four electrode sites. Top: Across-trial averaged data before bins including spike discharges excluded; several matrices exhibit episodes of brief broadband augmentation attributed to randomly occurring spike discharges. Bottom: Across-trial averaged data after excluding bins with spike discharges. In the present study, we adopted the time-frequency data presented in the lower row. Amplitude scale: 100% indicates no change in amplitude compared to the baseline, whereas 110 and 90% indicate 10% increase and decrease, respectively.
We minimized the unwanted direct effect of interictal spikes on naming-related high gamma modulations by removing the time-frequency bins showing an excessive and irregular increase in broadband amplitude. If the amplitude averaged across 30–85 Hz at a given time in a trial was >2 SD from the mean amplitude across all trials in each patient, we treated the whole time-frequency bins at the corresponding time on that trial as missing values.45,46 We believe this analytic approach can effectively extract and eliminate the pathological high gamma component carried by interictal spike discharges, which would randomly occur without being time-locked to stimuli or responses (Fig. 3).
We subsequently determined when naming-related high gamma augmentation reached significance at each channel. We tested the null hypothesis that the high gamma amplitude percentage change compared to the baseline period would be zero at each 10-ms bin using a non-parametric permutation one-sample t-test (500 permutations; random sign swapping).47–49 A two-sided 5% significance level was used with a false discovery rate correction for repeated comparisons for 121 bins in a 1200-ms period (Fig. 2). We treated bins showing amplitude augmentation for at least three consecutive high gamma cycles (i.e. >33 ms) as significant high gamma augmentation. We finally computed auditory naming-related high gamma activity (percentage change in amplitude relative to the baseline) averaged across the four analysis periods of interest (Fig. 2A) at sites showing significant high gamma augmentation. Likewise, we computed picture naming-related high gamma activity averaged across the two analysis periods of interest (Fig. 2B). These high gamma values were treated as the summary measure reflecting the degree of local task-related cortical activation and incorporated in the subsequent multivariate regression analysis.
Statistical analysis: intracranial EEG high gamma and electrical stimulation mapping
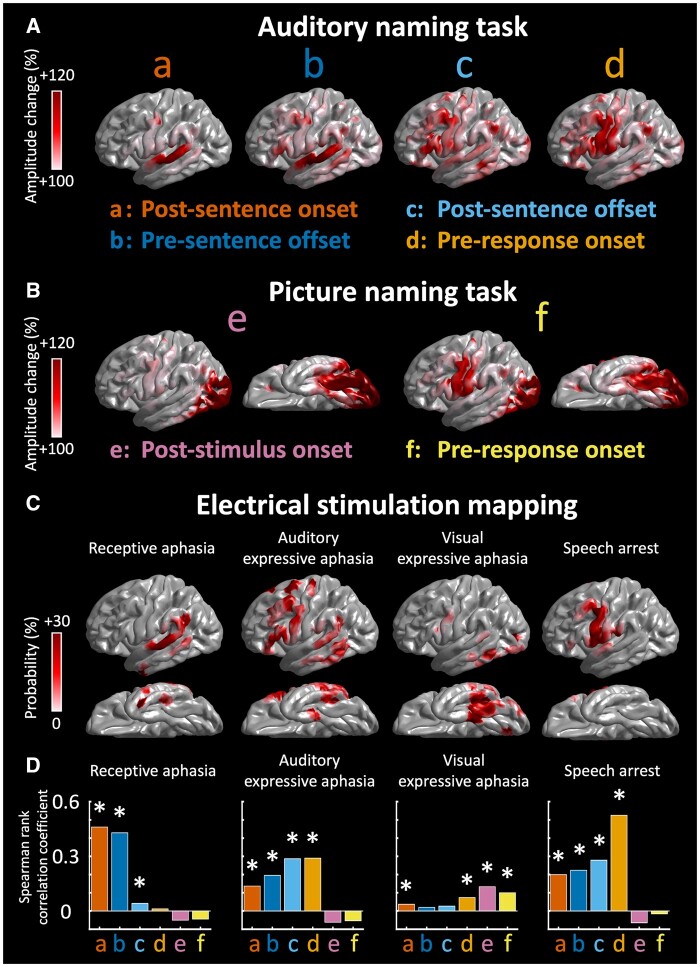
We determined whether the temporal profile of naming-related high gamma augmentation would account for language symptoms elicited by ESM. Using the Spearman’s rank correlation coefficient, we visualized how well the degree of significant high gamma augmentation during a specific 600-ms analysis period relative to stimulus and response would be correlated to the probability of each of the ESM-induced language symptoms (Fig. 4).
Figure 4.
Naming-related high gamma augmentation and ESM. (A) The spatial distribution of auditory naming-related high gamma augmentation during each 600-ms analysis period of interest: a, post-sentence onset; b, pre-sentence offset; c, post-sentence offset and d, pre-response onset. (B) The distribution of picture naming-related high gamma augmentation: e, post-stimulus onset; f, pre-response onset. (C) The spatial distribution of the probability of ESM-induced symptoms, including receptive aphasia, auditory expressive aphasia, visual expressive aphasia and speech arrest. (D) The bar charts show the strength of correlation between modality-specific high gamma augmentation (at sites with a z-score of ≥2) and the probability of each ESM-induced symptom. *Positive correlation with a Bonferroni-adjusted P < 0.05.
Statistical analysis: intracranial EEG high gamma and neuropsychological data
Aim 1: Multivariate linear regression analysis determined whether high gamma-based mapping would predict postoperative language performance independently of epilepsy and neuroimaging data available preoperatively.50 We used MATLAB 2020a Statistics and Machine Learning Toolbox (MathWorks, Natick, MA, USA) and set significance at P < 0.05. The predictor variables included: no. 1, ‘maximum resected high gamma (%)’ defined as the high gamma percentage change highest among sites included in the resected language-dominant hemispheric region; no. 2, the resection size of the language-dominant hemispheric cortex (%); no. 3, age at surgery (years); no. 4, sex (1 if female); no. 5, interval between surgery and postoperative neuropsychological assessment (months); no. 6, number of oral anti-epileptic drugs taken preoperatively (reflecting the severity of epilepsy-burden, including cognitive impairment);42,51 no. 7, MRI-visible cortical lesion (1 if present); no. 8, seizure onset zone location (1 if frontal or temporal); and no. 9, preoperative neuropsychological score. With a sample size of 65, a power of 0.8, an alpha of 0.05 and nine predictors incorporated, the regression model was anticipated to detect a moderate effect size of f2 of 0.28. We assumed that the test–retest reproducibility would be comparable across patients.52,53
Statistical analysis: Utility of intracranial EEG low gamma, beta and alpha modulations
Aim 2: As an ancillary analysis, we determined whether task-related modulations of iEEG frequency bands lower than high gamma would predict postoperative language performance. Using the aforementioned time-frequency analysis to measure naming-related high gamma augmentation, we computed low gamma augmentation (30–50 Hz), beta attenuation (12–30 Hz) and alpha attenuation (8–12 Hz) during each naming task. Similar to what we did in the high gamma band analysis, we treated bins showing amplitude attenuation lasting for at least three consecutive cycles (i.e. >300 ms for 10-Hz alpha activity) as significant attenuation. The multivariate linear regression analysis likewise determined whether ‘maximum resected low gamma’, ‘maximum resected beta’ or ‘maximum resected alpha’ would predict postoperative language performance independently of the covariates mentioned previously.
Machine learning: intracranial EEG amplitude modulations and neuropsychological data
Aim 3: We generated the machine learning-based atlas visualizing the sites, which, if resected, would lead to a postoperative decline in language function. For this purpose, we used the ensemble learning algorithm supported by Statistics and Machine Learning Toolbox implemented in the MATLAB R2020a (https://www.mathworks.com/help/stats/ensemble-algorithms.html). To accurately predict patients with a postoperative decline in Core Language Score by more than five points (i.e. reflecting the lower 25 percentile in our study cohort), we generated the boosted tree ensemble model initially based on the auditory naming-related amplitude modulations. The model incorporated the following 17 predictors measured during the auditory naming task: no. 1–4, ‘maximum resected high gamma augmentation (%)’, here defined as the high gamma percentage change, in each of the four analysis periods (Fig. 2A), highest among electrode sites within the resected region; no. 5–8, ‘maximum resected low gamma augmentation (%)’; no. 9–12, ‘maximum resected beta attenuation (%)’; no. 13–16, ‘maximum resected alpha attenuation (%)’; and no. 17, language dominance of the resected hemisphere (1 if resection involved the dominant hemisphere). Thereby, we linearly zero-centred auditory naming-related amplitude modulations (i.e. 0% reflects no augmentation or attenuation compared to the baseline).54 We selected the Gentle Adaptive Boosting as the ensemble learning algorithm.55 We subsequently used the Bayesian optimization algorithm with the expected-improvement acquisition function, which automatically selected the best set of values of the following hyperparameters through 100 iterations: ‘maximum number of splits’, ‘number of learners’ and ‘number of predictors to sample’. The algorithm outline of the Bayesian optimization used in this study is described at https://www.mathworks.com/help/stats/bayesian-optimization-algorithm.html.
We evaluated the prediction performance of our boosted tree ensemble model in predicting patients who developed such a postoperative Core Language Score decline, using accuracy and area under the receiver operating characteristic curve. We used a 5-fold cross-validation procedure, where the patient data were split randomly into five sets. The algorithm was trained on four sets (i.e. 80% of the patients) and tested on the other set (i.e. the remaining 20%). The testing process was iterated five times, and the averaged accuracy was reported.
Using the MATLAB ‘predictorImportance’ function (https://www.mathworks.com/help/stats/compactregressionensemble.predictorimportance.html),56 we determined the relative importance of each variable in predicting patients developing a >5-point decline in Core Language Score (Fig. 5C).
Figure 5.
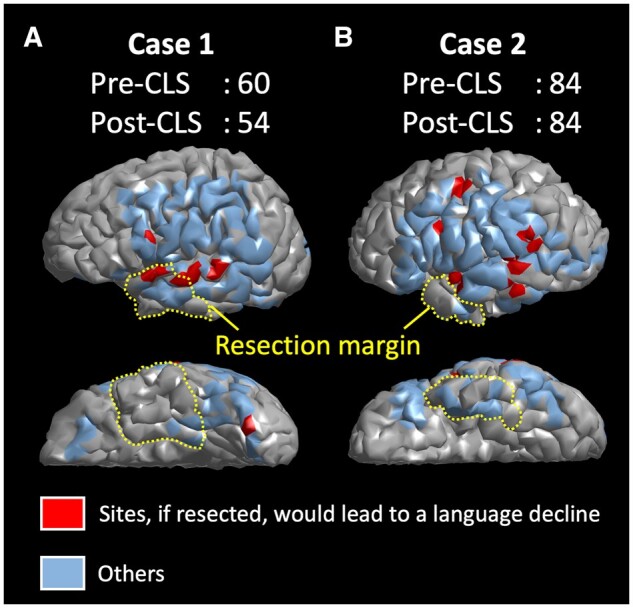
Spatial relationship between cortical sites predicting postoperative cognitive decline and resection margin. The cortical surface in each individual 3D brain image is highlighted whether a given site, if resected, would result in a >5-point Core Language Score decline. A given model prediction (i.e. red: >5-point decline; blue: otherwise) was projected onto triangular meshes within a 5-mm radius of each electrode site. Postoperative cognitive outcome of each electrode site was predicted by the boosted tree ensemble model incorporating auditory naming-related iEEG amplitude variables in addition to the dominant hemisphere variable. The yellow dotted lines denote the resection margin in a given patient. (A) Case 1: The cortical sites predicted to result in a >5-point Core Language Score decline (coloured in red) were indeed resected, and the language decline was observed postoperatively. (B) Case 2: The cortical sites predicted to result in such a >5-point decline were preserved, and no language decline was observed.
Simulation of our machine learning-based model using virtual resection of electrical stimulation mapping-defined language areas
Our boosted tree ensemble model predicted whether virtual resection of a given electrode site would result in a >5-point Core Language Score decline in all 65 study patients. The model was designed to predict the clinical consequence associated with resectioning sites in which naming-related spectral responses ranged strictly within those observed in the training data (i.e. a procedure referred to as interpolation).57 On the FreeSurfer averaged surface image, we have provided a group-level atlas that visualizes the probability of a >5-point Core Language Score decline resulting from virtual resection of given cortical points (Fig. 5A). We computed the relative risk of language impairment resulting from virtual resection of ESM-defined language sites compared to that of the others.58 It is feasible to hypothesize that a given patient would develop a substantial language impairment if an ESM-defined language site were surgically removed instead of being preserved.
We likewise generated the boosted tree ensemble model incorporating picture naming-related amplitude modulations. We assessed the model performance in predicting patients who developed a postoperative >5-point decline in Core Language Score (Fig. 5B and D). The picture naming-based model incorporated the following nine predictors: no. 1 to 2, ‘maximum resected high gamma augmentation (%)’, in each of the two analysis periods (Fig. 2B), highest among electrode sites within the resected region; no. 3 to 4, ‘maximum resected low gamma augmentation (%)’; no. 5 to 6, ‘maximum resected beta attenuation (%)’; no. 7 to 8, ‘maximum resected alpha attenuation (%)’; and no. 9, language dominance of the resected hemisphere.
Data and code availability
All data and code are available on request to the corresponding author (E.A.). We are pleased to reanalyse the data based on readers’ specific suggestions to improve the language mapping method.
Results
Patient profiles
A total of 65 patients satisfied the inclusion and exclusion criteria [age: 5–44 years; 6886 artefact-free electrode sites in total; 105.9 per patient on average (SD: ±17.9)]. Because of the time constraint during the extra-operative iEEG recording, a single patient failed to complete the auditory naming task and six the picture naming task. Thus, 64 (105.7 ± 17.9) and 59 patients (104.2 ± 22.2) contributed to the analysis of auditory and picture naming-related amplitude modulations (Fig. 1A). A total of 5203 artefact-free electrode sites were assessed by ESM (Fig. 1B).
Resective surgery involved a total of 1938 electrode sites (mean: 29.8 sites per patient; SD: ±24.5). Of the resected electrodes, 1798 electrodes (92.8%) implanted on the brain surface without structural abnormalities (e.g. tumours) were analysed in the standardized brain. The frontal [proportion: 27.8% (95% CI: 25.7–29.9%)] and temporal lobes [42.5% (95% CI: 40.2–44.9%)] had a greater probability of including resected electrode sites compared to the parietal [17.7% (95% CI: 15.9–19.5%)] and occipital lobes [12.0% (95% CI: 10.5–13.5%); Table 1].
Fifty-two patients underwent both pre- and postoperative assessments using the Clinical Evaluation of Language Fundamentals (Supplementary Fig. 1). One of the 52 patients completed the postoperative evaluation of receptive language function alone due to the time constraint (Supplementary Table 1). The mean postoperative changes in Core Language Score, Receptive Language Index and Expressive Language Index standard scores were −0.1 (SD: ±9.8 in 51 patients), −1.4 (SD: ±10.6 in 52 patients) and −0.1 (SD: 9.3 in 51 patients). Fourteen of the 51 patients showed a decline in Core Language Score by more than five standard points. Three of these 14 patients underwent resection of the right-hemispheric focus.
Concordance between electrical stimulation mapping and intracranial EEG high gamma-based mapping
Figure 4 demonstrates the spatial concordance between language areas defined by ESM and those by high gamma-based mapping. The probability of ESM-induced receptive aphasia at a given cortical point was highly correlated to the degree of significant auditory naming-related high gamma augmentation during the 600-ms periods after sentence onset (rho: +0.46; P < 0.001) and before sentence offset (rho: +0.43; P < 0.001). Likewise, the probability of ESM-induced auditory expressive aphasia was correlated to auditory naming-related high gamma augmentation during the 600-ms periods after sentence offset (rho: +0.29; P < 0.001) and before response onset (rho: +0.29; P < 0.001). The probability of ESM-induced visual expressive aphasia was correlated to picture naming-related high gamma augmentation during the 600-ms period after stimulus onset (rho: +0.13; P < 0.001). The probability of ESM-induced speech arrest was highly correlated to auditory naming-related high gamma augmentation during the 600-ms period before response onset (rho: +0.53; P < 0.001).
Multivariate regression models incorporating intracranial EEG high gamma augmentation
Multivariate regression models incorporating auditory naming-related high gamma augmentation predicted the postoperative changes in Core Language Score (r2 = 0.37; P = 0.015), Receptive Language Index (r2 = 0.43; P = 0.003), and Expressive Language Index (r2 = 0.32; P = 0.048; Supplementary Table 2). Higher ‘maximum resected high gamma’ was independently associated with greater decline in Core Language Score (β = −0.09; t = −3.03; P = 0.004) and Expressive Language Index (β = −0.08; t = −2.63; P = 0.01), but not in Receptive Language Index (β = −0.04; t = −1.20; P = 0.24). In other words, each 1% amplitude increase at the resected site showing the largest high gamma response resulted in a more severe postoperative decline in Core Language Score by 0.09.
Multivariate regression models incorporating picture naming-related high gamma augmentation likewise predicted the postoperative changes in Receptive Language Index (r2 = 0.50; P < 0.001), but not in Core Language Score (r2 = 0.29; P = 0.109) or Expressive Language Index (r2 = 0.27; P = 0.160; Supplementary Table 3). Higher ‘maximum resected high gamma’ was independently associated with greater decline in Receptive Language Index (β = −0.04; t = −2.25; P = 0.030), but not in Core Language Score (β = −0.04; t = −1.82; P = 0.077) or Expressive Language Index (β = −0.04; t = −1.67; P = 0.103).
Multivariate regression models incorporating iEEG lower frequency band modulations
Each of the multivariate regression models incorporating naming-related low gamma augmentation, beta attenuation and alpha attenuation during auditory or picture naming task likewise predicted the postoperative changes in Core Language Score, Receptive Language Index and Expressive Language Index (P < 0.05; r2 ranging from 0.32 to 0.50). The details are presented in Supplementary Tables 4–9.
Machine learning-based prediction of postoperative neuropsychological performance
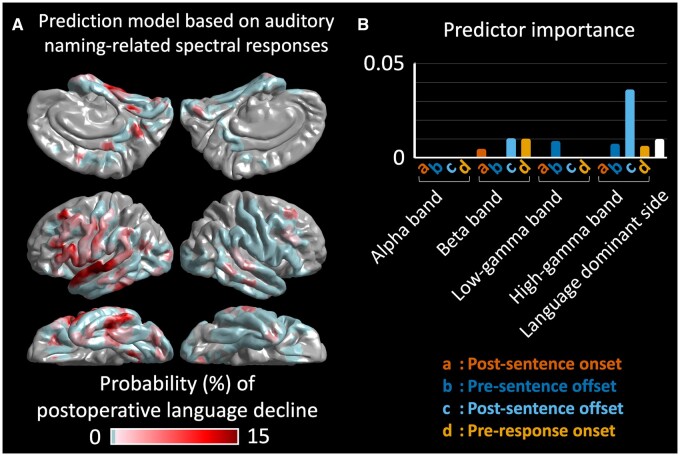
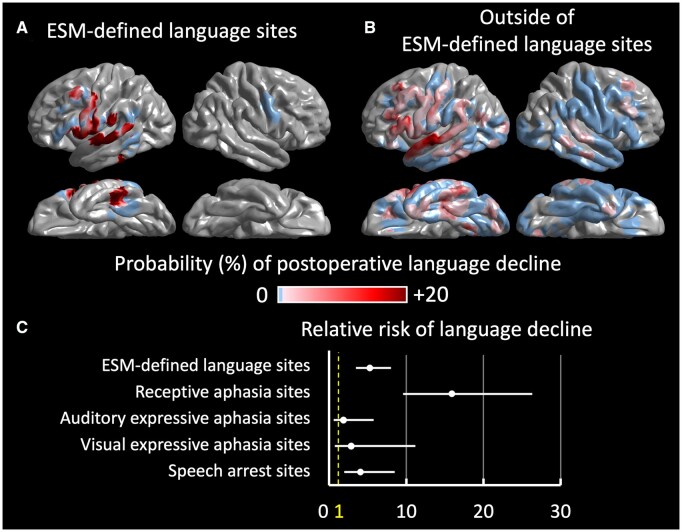
The boosted tree ensemble model, incorporating the aforementioned 16 auditory naming-related iEEG amplitude variables in addition to the dominant hemisphere variable, predicted patients showing a postoperative >5-point Core Language Score decline with an accuracy of 0.80 and area under the curve of 0.65. As shown in Fig. 5, this model can highlight cortical sites predicted to result in such a >5-point decline, if resected, on the individual surface image. Figure 6A visualizes the group-level probability of a >5-point Core Language Score decline resulting from the virtual resection of a given cortical point on the FreeSurfer averaged surface image. This group-level atlas suggests that resection of the left hemispheric regions, particularly the posterior portions of the temporal neocortices, would increase the risk of language decline. Figure 6B visualizes the relative contribution of the 17 variables mentioned previously to the prediction model. Resection of sites showing high gamma augmentation during the 600-ms period after stimulus offset had the most substantial contribution to the improved prediction. The relative risk of language decline related to virtual resection of ESM-defined language sites, compared to that of sites outside, was 5.27 (95% CI: 3.47–8.02; Fig. 7). Virtual resection of ESM-defined receptive language sites maximally increased the relative risk up to 15.9 (95% CI: 9.6–26.3).
Figure 6.
Three-dimensional brain atlas visualizing the group-level probability of postoperative language decline. (A) The averaged surface image presents the group-level probability of a >5-point Core Language Score decline resulting from the virtual resection of a given cortical point. The group-level probability was computed with the boosted tree ensemble model incorporating auditory naming-related iEEG amplitude modulation variables in addition to a dominant hemisphere variable. The model predicted such a Core Language Score decline with an accuracy of 0.80 and area under the curve of 0.65. (B) Bar charts visualize the relative contribution of each variable to the prediction model providing the atlas.
Figure 7.
Relative risk of language decline related to the virtual resection of ESM-defined language sites. The boosted tree ensemble model, incorporating auditory naming-related spectral responses, presents the group-level probability of a >5-point Core Language Score decline resulting from virtual resection of a given electrode site. (A) Virtual resection of an electrical stimulation mapping (ESM)-defined language site (i.e. cortical sites with ESM-induced receptive aphasia, auditory expressive aphasia, visual expressive aphasia or speech arrest). (B) Virtual resection of a site outside the ESM-defined language areas. (C) The relative risk (95% CI) of language decline related to the virtual resection of an ESM-defined language site, compared to that of a site outside.
The boosted tree ensemble model, incorporating the eight-picture naming-related iEEG amplitude variables in addition to the dominant hemisphere variable, failed to predict patients showing a >5-point Core Language Score decline with significance (accuracy: 0.73; area under the curve: 0.50).
Discussion
Significance of our prediction models
This prospective study clarified the relationship between resection of sites showing naming-related iEEG amplitude modulations within the language-dominant hemisphere and postoperative changes in objectively measured neuropsychological performance. We have generated the prediction models that neurosurgeons can use before and during the surgical procedures at their own epilepsy centres. Our innovative machine learning-based prediction model runs on an ordinary laptop computer with MATLAB installed (Supplementary material). All variables incorporated in the prediction models can be determined before the completion of resective surgery. In other words, we did not use measures available only after surgery. It is reasonable to expect that seizure control and reduction of anti-epileptic drugs after surgery would be associated with improved postoperative language development.59–62 However, such postoperative measures can play only a correlative role in characterizing the language function after surgery but cannot be used to predict future symptoms.
Independent utility of high gamma modulations in predicting neuropsychological outcomes
High gamma-based mapping had a robust predictive value independent of the effects of each patient’s epilepsy and neuroimaging profiles. Expressly, the regression-based model indicated that greater naming-related high gamma augmentation in the resected dominant hemispheric region accurately predicted a more severe decline in language function after surgery. In other words, there was a biological gradient between naming-related high gamma augmentation and underlying language function.20,28,29 In the multivariate regression analysis, we made the best effort to control the effects of covariate factors previously reported to be associated with neuropsychological decline. Investigators have indicated that the risk factors of postoperative language decline include more extensive resection, older age, absence of an MRI-visible lesion and higher preoperative neuropsychological performance.32,63–65 We found that a higher preoperative Receptive Language Index was independently associated with a higher risk of postoperative decline in this index (Supplementary Tables 2 and 3). A previous study of 875 adults with drug-resistant epilepsy reported that higher preoperative naming ability was independently associated with postoperative declines in naming.32 These findings are consistent with the notion that patients with high baseline cognitive function may still have functionally viable tissues proximal to the epileptogenic zone. With the effect of preoperative Receptive Language Index controlled in the present study, picture naming-related high gamma activity was found to be an independent predictor of postoperative declines in Receptive Language Index (Supplementary Table 3).
Our multivariate analysis also took into account the time interval between surgery and postoperative neuropsychological assessment (Table 1). It is plausible to expect that a given patient recovers and develops language skills as a function of time after surgery. Nonetheless, we failed to find a significant effect of the time interval on neuropsychological performance. This finding could be attributed to a restricted range of time post-surgery relative to the typical time course of recovery due to physiologic recovery or reorganization. Repeated neuropsychological testing of the same individuals after surgery may be needed to accurately assess the effect of time post-surgery on functional recovery.
Auditory naming-related high gamma activity predicted the postoperative changes in core and expressive language scores, whereas the picture naming-related activity predicted the receptive language score (Supplementary Tables 2 and 3). It remains uncertain why two different naming-based mappings predicted language outcomes differently. Previous iEEG and functional MRI studies have reported that the frontal lobe of the language-dominant hemisphere is more extensively and intensively activated by auditory naming tasks, whereas the ventral temporal-occipital regions are preferentially activated by picture naming task.29,66 Previous ESM studies have suggested that the left frontal lobe exerts lexical retrieval, whereas the posterior temporal and occipital regions play roles in the perception and semantic understanding of language stimuli.3–5,39,67–69
We have successfully generated the regression- and machine learning-based models predicting postoperative language outcomes without relying on the ESM data (Fig. 6). Such models are clinically significant because not all patients can have the comprehensive ESM completed for various reasons. Clinicians may not be able to initiate the ESM while patients are prone to develop stimulation-induced seizures due to the reduction or discontinuation of anti-epileptic drugs.19 Children may not sustain attentiveness or participation during an hour-long ESM mapping.8 Our prediction model based on iEEG high gamma augmentation triggered by naming tasks would be clinically useful because it could partially complement ESM assessment before resective surgery.
In turn, we assessed the use of our machine learning-based model using the simulated incidence of language impairment resulting from virtual resection of ESM-defined language sites (Fig. 7). According to the simulated data, resection of an ESM-defined language site, if performed, would have increased the relative risk of a >5-point Core Language Score decline by 5.27. Specifically, virtual resection of ESM-defined receptive language sites maximally increased the relative risk up to 15.90. Our machine learning-based model may provide prognostic information additive to the ESM because it inferred that resection of ESM language-negative sites in the left perisylvian regions could still result in a postoperative impairment. A meta-analysis of 15 studies suggests that high gamma mapping may exhibit language areas more extensively than ESM does in paediatric cohorts.7 Because we performed ESM using bipolar stimulation, we cannot rule out the possibility that only one of the pair of electrode sites would have been responsible for the symptom elicited during ESM.
The innovation of our intracranial EEG analysis and prediction model
Our novel machine learning-based model visualizes the site, which, if removed, would result in a language impairment at the group and individual levels. The group-level 3D atlas (Fig. 6) visualizes the probability of a postoperative language decline resulting from resection of given cortical sites. The atlas can be readily used for counselling and education of patients, students and healthcare providers. The individual-level model (Fig. 5) can be used to simulate the postoperative language outcome resulting from the planned resection for a given patient. With a 5-fold cross-validation used, the accuracy of predicting patients developing a >5-point Core Language Score decline was found to be 0.80. Our auditory naming-based model indicated that high gamma activity during a 600-ms period immediately after the stimulus offset most strongly contributed to the accurate prediction of postoperative language outcomes (Fig. 6B). This observation is consistent with the hypothesis that incorporation of the timing of task-related neural activation would improve the accuracy of preoperative language mapping.
Additional large and diverse datasets will provide an outstanding opportunity to externally validate our prediction models.70 At several tertiary epilepsy centres, we currently collect the iEEG, MRI and neuropsychological datasets, characterized by different electrode types (e.g. depth electrodes), iEEG sampling approach (e.g. more restricted spatial sampling), age group (e.g. adult-dominant cohorts) and spoken language (i.e. other than English).71 ‘Maximum resected high gamma’ is a continuous measure easy to compute regardless of the spatial extent of iEEG sampling in a given patient. Investigators can test the utility of our analytic approach in patients undergoing stereotactic EEG recording in the future.
In the current study, our innovative analysis systematically excluded the time-frequency bins affected by interictal spike discharges, which would randomly take place without being time-locked to stimuli or responses (Fig. 3). This method effectively minimized the observation of high gamma augmentation not attributed to task-related neural activations.28 Interictal spike discharges are accompanied by a temporary boost of broadband amplitude, including a 30–85 Hz band.45,46 Thus, interictal spike discharges, if not removed from the analysis, may undesirably inflate the high gamma amplitudes at non-eloquent cortices, particularly within the seizure onset zone.72 We are pleased to share our MATLAB code with investigators who want to replicate our analytic method.
Methodological considerations
The present study excluded the direct effect of interictal spike discharges on the measurement of naming-related high gamma augmentation at each electrode site. Still, our time-frequency analysis may not consider the indirect effects of a slow wave accompanying a given spike. Previous iEEG studies reported that slow-wave discharges immediately following spike discharges appeared to reduce task-related high gamma activation in trials affected by spike- and slow-wave discharges.73,74 The optimal criteria for exclusion of the effects of epileptiform discharges remain to be determined.
We designed our machine learning-based model to predict postoperative language outcomes without relying on the results of ESM, Wada test or functional MRI. We took this analytic approach because substantial proportions of children with drug-resistant epilepsy are expected to have difficulty completing these tests satisfactorily.8,30,31 Wada test is an invasive diagnostic procedure, which has not been commonly performed in young children across epilepsy centres.30 A previous study reported that the success rate of functional MRI-based language mapping ranged from 34 to 67% in healthy children 5–7 years of age.31 In the current study, five patients were left-handed, had a developmental lesion in the left neocortex, and were presumed to have a right-hemispheric language dominance (Supplementary Fig. 2). In contrast, the remaining 60 patients were presumed to have a left hemispheric dominance. ESM failed to localize expressive or receptive language areas in the left hemisphere of these five patients mentioned previously (Supplementary Fig. 3); thus, we do not find evidence that our analysis falsely lateralized the language-dominant hemisphere. However, the exact accuracy of our language dominance lateralization remains uncertain since neither functional MRI nor Wada test was performed systematically in our patient cohort in which 84.6% were younger than 18 years of age (Supplementary Fig. 1). Historical evidence supports our analytic approach to estimate the dominant hemisphere based on the handedness and left hemispheric neocortical MRI lesion.33–35 A previous study of 445 patients with epilepsy reported that only 9.1% of the 11 left-handed patients with early left hemispheric neocortical lesions had a left hemispheric dominance based on the Wada test, whereas only 6.2% of the remaining 434 patients had a right-hemispheric dominance.35 Strictly according to this data35, it is plausible to hypothesize that our analysis may have falsely lateralized the language-dominant hemisphere in <10% of patients. We believe this error rate is within an acceptable range. Functional MRI-based mapping was previously reported to falsely lateralize the language-dominant hemisphere in 9–21% of adults with drug-resistant focal epilepsy when the Wada test was treated as the gold standard procedure.75–77 Previous studies reported that small subsets of patients showed a decline in language-related scores after surgical resection of the non-dominant right hemisphere.78–80
Several factors may account for the suboptimal performance of picture naming-related iEEG amplitude variables in predicting postoperative changes in Core Language Score in the present study. We designed the picture naming task to be feasible for young children with drug-resistant epilepsy, and given patients were assigned to name common objects such as ‘cat’. Thus, the cognitive load of our picture naming might have been too low to activate areas supporting language-related function extensively. In our previous iEEG study of 10 patients, we found that the spatial extent of the left frontal lobe high gamma augmentation was greater when patients were required to name ambiguous drawings than unambiguous common objects.81 Yet, we are afraid that such a challenging assignment may reduce the success rate of task completion in young children.
The selection of iEEG analysis epochs may be associated with the reported performance of our machine learning-based prediction models. The analysis epochs for auditory naming-related iEEG responses included the period immediately after sentence offset when lexical retrieval is expected to occur.39,82 High gamma responses during this analysis epoch most strongly contributed to the improved prediction of postoperative changes in Core Language Score in the present study (Fig. 6B). Conversely, the picture naming analysis epochs included those immediately after stimulus onset and before response onset alone (Fig. 2B). Thus, iEEG responses during these analysis epochs may reflect perceptual and motor processes, in addition to lexical retrieval in the visual domain. Our previous iEEG study reported that left hemispheric dominance of naming-related high gamma augmentation was less prominent during picture than auditory naming.29
IEEG recording inevitably suffers from a sampling limitation. Thus, we are aware of the possibility that an unsampled cortical site may have generated the true maximum spectral responses. Investigators have looked for non-invasive neurophysiological biomarkers to localize the language areas throughout the cortical convexity. However, the unavoidable occurrence of electromyographic artefacts originating from ocular and temporal muscles during spontaneous saccades and overt responses make the non-invasive high gamma-based language mapping challenging.83,84 Our multivariate regression analysis indicated the utility of naming-related alpha/beta attenuation in predicting postoperative language outcomes (Supplementary Tables 6–9). Our study supports the potential role of task-related alpha/beta attenuation measured noninvasively in presurgical evaluation.85
The prediction performance of our machine learning-based model inevitably depends on the characteristics of the training data and the strength of model fitness to those data. A large proportion of our study patients had cortical resection involving the frontal or temporal lobe. Thus, our group-level atlas (Fig. 6) is expected to provide a more reliable prediction for patients with frontal or temporal lobe epilepsy than those with parietal or occipital lobe epilepsy. It is not reasonable to expect that a single diagnostic test would have a very high diagnostic accuracy approaching 100% in predicting the postoperative language function. Seizure control after surgery is associated with better cognitive development,32,63,64,86 but clinicians do not have the seizure outcome before surgery. Thus, further studies also incorporating epilepsy biomarkers capable of predicting postoperative seizure outcome are warranted to improve our prediction model. The promising iEEG candidate biomarkers include high-frequency oscillations87,88 and cross-frequency coupling between high-frequency oscillations and slow waves.42,89
The present study did not include patients aged of 4 years or younger. We expect that substantial proportions of such young patients will fail to complete an overt naming task satisfactorily. Thus, one would need to establish the language mapping without relying on the child’s attentive participation. Investigators have successfully recorded task-free high gamma augmentation associated with spontaneous cooing and babbling90 and during passive listening.7,91 Measurement of spectral responses to single-pulse electrical stimulation also has the potential to localize the network supporting speech and language92–94 Additional measures are expected to improve the accuracy of machine learning-based prediction models.
Supplementary Material
Acknowledgements
We are grateful to Karin Halsey, BS, REEGT and Jamie MacDougall, RN, BSN, CPN at Children’s Hospital of Michigan for the collaboration and assistance in performing the studies described previously.
Funding
This work was supported by NIH grants NS064033 (to E.A.) and NS089659 (to J.W.J.).
Competing interests
The authors have no conflicts of interest to report. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- ESM
electrical stimulation mapping
- iEEG
intracranial electroencephalography
References
- 1. Rosenow F, Lüders H.. Presurgical evaluation of epilepsy. Brain. 2001;124(Pt 9):1683–1700. [DOI] [PubMed] [Google Scholar]
- 2. So EL, Alwaki A.. A guide for cortical electrical stimulation mapping. J Clin Neurophysiol. 2018;35(2):98–105. [DOI] [PubMed] [Google Scholar]
- 3. Hamberger MJ, Seidel WT, Mckhann GM, Perrine K, Goodman RR.. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128(Pt 11):2742–2749. [DOI] [PubMed] [Google Scholar]
- 4. Tate MC, Herbet G, Moritz-Gasser S, Tate JE, Duffau H.. Probabilistic map of critical functional regions of the human cerebral cortex: Broca’s area revisited. Brain. 2014;137(Pt 10):2773–2782. [DOI] [PubMed] [Google Scholar]
- 5. Ojemann G, Ojemann J, Lettich E, Berger M.. Cortical language localization in left, dominant hemisphere: An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71(3):316–326. [DOI] [PubMed] [Google Scholar]
- 6. Kojima K, Brown EC, Rothermel R, et al. Multimodality language mapping in patients with left-hemispheric language dominance on Wada test. Clin Neurophysiol. 2012;123(10):1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arya R, Horn PS, Crone NE.. ECoG high-gamma modulation versus electrical stimulation for presurgical language mapping. Epilepsy Behav. 2018;79:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schevon CA, Carlson C, Zaroff CM, et al. Pediatric language mapping: Sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia. 2007;48(3):539–545. [DOI] [PubMed] [Google Scholar]
- 9. Sinai A, Bowers CW, Crainiceanu CM, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128(Pt 7):1556–1570. [DOI] [PubMed] [Google Scholar]
- 10. Schalk G, Leuthardt EC, Brunner P, Ojemann JG, Gerhardt LA, Wolpaw JR.. Real-time detection of event-related brain activity. Neuroimage. 2008;43(2):245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Towle VL, Yoon H-A, Castelle M, et al. ECoG gamma activity during a language task: Differentiating expressive and receptive speech areas. Brain. 2008;131(Pt 8):2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kapeller C, Korostenskaja M, Prueckl R, et al. CortiQ-based real-time functional mapping for epilepsy surgery. J Clin Neurophysiol. 2015;32(3):e12–e22. [DOI] [PubMed] [Google Scholar]
- 13. Crone NE, Sinai A, Korzeniewska A.. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. [DOI] [PubMed] [Google Scholar]
- 14. Lachaux J-P, Axmacher N, Mormann F, Halgren E, Crone NE.. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98(3):279–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS.. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28(45):11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leszczyński M, Barczak A, Kajikawa Y, et al. Dissociation of broadband high-frequency activity and neuronal firing in the neocortex. Sci Adv. 2020;6(33):eabb0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scheeringa R, Fries P, Petersson K-M, et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69(3):572–583. [DOI] [PubMed] [Google Scholar]
- 18. Nishida M, Juhász C, Sood S, Chugani HT, Asano E.. Cortical glucose metabolism positively correlates with gamma-oscillations in nonlesional focal epilepsy. Neuroimage. 2008;42(4):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arya R, Aungaroon G, Vera AZ, et al. Fosphenytoin pre-medication for pediatric extra-operative electrical stimulation brain mapping. Epilepsy Res. 2018;140:171–176. [DOI] [PubMed] [Google Scholar]
- 20. Kojima K, Brown EC, Rothermel R, et al. Clinical significance and developmental changes of auditory-language-related gamma activity. Clin Neurophysiol. 2013;124(5):857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Babajani-Feremi A, Holder CM, Narayana S, et al. Predicting postoperative language outcome using presurgical fMRI, MEG, TMS, and high gamma ECoG. Clin Neurophysiol. 2018;129(3):560–571. [DOI] [PubMed] [Google Scholar]
- 22. Arya R, Roth C, Leach JL, et al. Neuropsychological outcomes after resection of cortical sites with visual naming associated electrocorticographic high-gamma modulation. Epilepsy Res. 2019;151:17–23. [DOI] [PubMed] [Google Scholar]
- 23. Crone NE, Miglioretti DL, Gordon B, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;121(Pt 12):2271–2299. [DOI] [PubMed] [Google Scholar]
- 24. Crone NE, Miglioretti DL, Gordon B, Lesser RP.. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(Pt 12):2301–2315. [DOI] [PubMed] [Google Scholar]
- 25. Miller KJ, Leuthardt EC, Schalk G, et al. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007;27(9):2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fukuda M, Juhász C, Hoechstetter K, Sood S, Asano E.. Somatosensory-related gamma-, beta- and alpha-augmentation precedes alpha- and beta-attenuation in humans. Clin Neurophysiol. 2010;121(3):366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Semel E, Wiig E, Secord W.. Clinical evaluation of language fundamentals 4th edn (CELF-4). Psychological Corporation; 2003.
- 28. Kambara T, Sood S, Alqatan Z, et al. Presurgical language mapping using event-related high-gamma activity: The Detroit procedure. Clin Neurophysiol. 2018;129(1):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakai Y, Sugiura A, Brown EC, et al. Four‐dimensional functional cortical maps of visual and auditory language: Intracranial recording. Epilepsia. 2019;60(2):255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jayakar P, Gaillard WD, Tripathi M, et al. ; Task Force for Paediatric Epilepsy Surgery, Commission for Paediatrics, and the Diagnostic Commission of the International League Against Epilepsy . Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia. 2014;55(4):507–518. [DOI] [PubMed] [Google Scholar]
- 31. Rajagopal A, Byars A, Schapiro M, Lee GR, Holland SK.. Success rates for functional MR imaging in children. Am J Neuroradiol. 2014;35(12):2319–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Busch RM, Floden DP, Prayson B, et al. Estimating risk of word-finding problems in adults undergoing epilepsy surgery. Neurology. 2016;87(22):2363–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rasmussen T, Milner B.. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299(1):355–369. [DOI] [PubMed] [Google Scholar]
- 34. Akanuma N, Alarcón G, Lum F, et al. Lateralising value of neuropsychological protocols for presurgical assessment of temporal lobe epilepsy. Epilepsia. 2003;44(3):408–418. [DOI] [PubMed] [Google Scholar]
- 35. Möddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T.. Atypical language lateralization in epilepsy patients. Epilepsia. 2009;50(6):1505–1516. [DOI] [PubMed] [Google Scholar]
- 36. Asano E, Juhász C, Shah A, Sood S, Chugani HT.. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132(Pt 4):1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Motoi H, Jeong J-W, Juhász C, et al. Quantitative analysis of intracranial electrocorticography signals using the concept of statistical parametric mapping. Sci Rep. 2019;9(1):17385- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Motoi H, Miyakoshi M, Abel TJ, et al. Phase‐amplitude coupling between interictal high‐frequency activity and slow waves in epilepsy surgery. Epilepsia. 2018;59(10):1954–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakai Y, Jeong J, Brown EC, et al. Three- and four-dimensional mapping of speech and language in patients with epilepsy. Brain. 2017;140(5):1351–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stolk A, Griffin S, R van der M, et al. Integrated analysis of anatomical and electrophysiological human intracranial data. Nat Protoc. 2018;13(7):1699–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghosh SS, Kakunoori S, Augustinack J, et al. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11years of age. Neuroimage. 2010;53(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuroda N, Sonoda M, Miyakoshi M, et al. Objective interictal electrophysiology biomarkers optimize prediction of epilepsy surgery outcome. Brain Commun. 2021;3(2):fcab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ervin B, Buroker J, Rozhkov L, et al. High-gamma modulation language mapping with stereo-EEG: A novel analytic approach and diagnostic validation. Clin Neurophysiol. 2020;131(12):2851–2860. [DOI] [PubMed] [Google Scholar]
- 44. Tallon-Baudry C, Bertrand O, Tallon-Baudry C, Bertrand O.. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3(4):151–162. [DOI] [PubMed] [Google Scholar]
- 45. Gardner AB, Worrell GA, Marsh E, Dlugos D, Litt B.. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin Neurophysiol. 2007;118(5):1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Staba RJ, Wilson CL, Bragin A, Fried I, Engel J.. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88(4):1743–1752. [DOI] [PubMed] [Google Scholar]
- 47. Maris E, Oostenveld R.. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Meth. 2007;164(1):177–190. [DOI] [PubMed] [Google Scholar]
- 48. Cohen MX. Analyzing neural time series data: Theory and practice. The MIT Press; 2014. [Google Scholar]
- 49. Bassez I, Ricci K, Vecchio E, et al. The effect of painful laser stimuli on EEG gamma-band activity in migraine patients and healthy controls. Clin Neurophysiol. 2020;131(8):1755–1766. [DOI] [PubMed] [Google Scholar]
- 50. Draper NR, Smith H.. Applied regression analysis. John Wiley & Sons; 1966:90–407. [Google Scholar]
- 51. Kwan P, Brodie MJ.. Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet. 2001;357(9251):216–222. [DOI] [PubMed] [Google Scholar]
- 52. Paslawski T. The Clinical Evaluation of Language Fundamentals (CELF-4): A review. Can J Sch Psychol. 2005;20(1-2):129–134. [Google Scholar]
- 53. Eadie P, Nguyen C, Carlin J, Bavin E, Bretherton L, Reilly S.. Stability of language performance at 4 and 5 years: Measurement and participant variability. Int J Lang Commun Disord. 2014;49(2):215–227. [DOI] [PubMed] [Google Scholar]
- 54. Iacobucci D, Schneider MJ, Popovich DL, Bakamitsos GA.. Mean centering helps alleviate “micro” but not “macro” multicollinearity. Behav Res Methods. 2016;48(4):1308–1317. [DOI] [PubMed] [Google Scholar]
- 55. Friedman J, Hastie T, Tibshirani R.. Additive logistic regression: A statistical view of boosting (with discussion and a rejoinder by the authors). Ann Stat. 2000;28(2):337–407. [Google Scholar]
- 56. AlSkaif T, Dev S, Visser L, Hossari M, van Sark W.. A systematic analysis of meteorological variables for PV output power estimation. Renew Energy. 2020;153:12–22. [Google Scholar]
- 57. Quinlan JR. Learning with continuous classes. In: 5th Australian Joint Conference on Artificial Intelligence. 1992;92:343–348. [Google Scholar]
- 58. Altman DG. Practical statistics for medical research. Chapman & Hall/CRC Press; 1991. [Google Scholar]
- 59. Smith ML, Lah S, Elliott I.. Pediatric epilepsy surgery: Neuropsychological outcomes and measurement issues. In: Neuropsychology in the care of people with epilepsy. John Libbey Eurotext; 2011:239–247. [Google Scholar]
- 60. Ryvlin P, Cross JH, Rheims S.. Epilepsy surgery in children and adults. Lancet Neurol. 2014;13(11):1114–1126. [DOI] [PubMed] [Google Scholar]
- 61. Gröppel G, Dorfer C, Mühlebner-Fahrngruber A, et al. Improvement of language development after successful hemispherotomy. Seizure. 2015;30:70–75. [DOI] [PubMed] [Google Scholar]
- 62. Hoppe C, Porębska I, Beeres K, et al. Parents’ view of the cognitive outcome one year after pediatric epilepsy surgery. Epilepsy Behav. 2019;101(Pt A):106552. [DOI] [PubMed] [Google Scholar]
- 63. Jayakar P, Jayakar A, Libenson M, et al. ; International League Against Epilepsy . Epilepsy surgery near or in eloquent cortex in children—Practice patterns and recommendations for minimizing and reporting deficits. Epilepsia. 2018;59(8):1484–1491. [DOI] [PubMed] [Google Scholar]
- 64. Vakharia VN, Duncan JS, Witt J, Elger CE, Staba R, Engel J.. Getting the best outcomes from epilepsy surgery. Ann Neurol. 2018;83(4):676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Helmstaedter C, Beeres K, Elger C, Kuczaty S, Schramm J, Hoppe C.. Cognitive outcome of pediatric epilepsy surgery across ages and different types of surgeries: A monocentric 1-year follow-up study in 306 patients of school age. Seizure. 2020;77:86–92. [DOI] [PubMed] [Google Scholar]
- 66. Hamberger MJ, Habeck CG, Pantazatos SP, Williams AC, Hirsch J.. Shared space, separate processes: Neural activation patterns for auditory description and visual object naming in healthy adults. Hum Brain Mapp. 2014;35(6):2507–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schäffler L, Lüders HO, Beck GJ.. Quantitative comparison of language deficits produced by extraoperative electrical stimulation of Broca’s, Wernicke’s, and Basal temporal language areas. Epilepsia. 1996;37(5):463–475. [DOI] [PubMed] [Google Scholar]
- 68. Chang EF, Breshears JD, Raygor KP, Lau D, Molinaro AM, Berger MS.. Stereotactic probability and variability of speech arrest and anomia sites during stimulation mapping of the language dominant hemisphere. J Neurosurg. 2017;126(1):114–121. [DOI] [PubMed] [Google Scholar]
- 69. Forseth KJ, Kadipasaoglu CM, Conner CR, Hickok G, Knight RT, Tandon N.. A lexical semantic hub for heteromodal naming in middle fusiform gyrus. Brain. 2018;141(7):2112–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jordan MI, Mitchell TM.. Machine learning: Trends, perspectives, and prospects. Science. 2015;349(6245):255–260. [DOI] [PubMed] [Google Scholar]
- 71. Ikegaya N, Motoi H, Iijima K, et al. Spatiotemporal dynamics of auditory and picture naming-related high-gamma modulations: A study of Japanese-speaking patients. Clin Neurophysiol. 2019;130(8):1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jacobs J, Kobayashi K, Gotman J.. High-frequency changes during interictal spikes detected by time-frequency analysis. Clin Neurophysiol. 2011;122(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brown EC, Matsuzaki N, Asano E.. The transient effect of interictal spikes from a frontal focus on language-related gamma activity. Epilepsy Behav. 2012;24(4):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu S, Parvizi J.. Cognitive refractory state caused by spontaneous epileptic high-frequency oscillations in the human brain. Sci Transl Med. 2019;11(514):eaax7830. [DOI] [PubMed] [Google Scholar]
- 75. Woermann FG, Jokeit H, Luerding R, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61(5):699–701. [DOI] [PubMed] [Google Scholar]
- 76. Benke T, Köylü B, Visani P, et al. Language lateralization in temporal lobe epilepsy: A comparison between fMRI and the Wada test. Epilepsia. 2006;47(8):1308–1319. [DOI] [PubMed] [Google Scholar]
- 77. Janecek JK, Swanson SJ, Sabsevitz DS, et al. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: Rates and predictors of discordance. Epilepsia. 2013;54(2):314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Walton NH, Goodsman C, McCarter R, Sandeman DR, Bird JM.. An analysis of neuropsychological change scores following selective temporal resection of the non-dominant temporal lobe. Seizure. 1999;8(4):241–245. [DOI] [PubMed] [Google Scholar]
- 79. Sabsevitz DS, Swanson SJ, Hammeke TA, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60(11):1788–1792. [DOI] [PubMed] [Google Scholar]
- 80. Powell HWR, Richardson MP, Symms MR, et al. Preoperative fMRI predicts memory decline following anterior temporal lobe resection. J Neurol Neurosurg Psychiatry. 2008;79(6):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cho-Hisamoto Y, Kojima K, Brown EC, Matsuzaki N, Asano E.. Gamma activity modulated by naming of ambiguous and unambiguous images: Intracranial recording. Clin Neurophysiol. 2015;126(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sonoda M, Silverstein B, Jeong J, et al. Six-dimensional dynamic tractography atlas of language connectivity in the developing brain. Brain. 2021;144(11):3340–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY.. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58(3):429–441. [DOI] [PubMed] [Google Scholar]
- 84. Carl C, Açık A, König P, Engel AK, Hipp JF.. The saccadic spike artifact in MEG. Neuroimage. 2012;59(2):1657–1667. [DOI] [PubMed] [Google Scholar]
- 85. Hirata M, Goto T, Barnes G, et al. Language dominance and mapping based on neuromagnetic oscillatory changes: Comparison with invasive procedures: Clinical article. J Neurosurg. 2010;112(3):528–538. [DOI] [PubMed] [Google Scholar]
- 86. Meador KJ. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002;58(8 Suppl 5):S21–S26. [DOI] [PubMed] [Google Scholar]
- 87. Jacobs J, Staba R, Asano E, et al. High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol. 2012;98(3):302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zijlmans M, Jiruska P, Zelmann R, Leijten FS, Jefferys JG, Gotman J.. High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71(2):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Weiss SA, Orosz I, Salamon N, et al. Ripples on spikes show increased phase-amplitude coupling in mesial temporal lobe epilepsy seizure-onset zones. Epilepsia. 2016;57(11):1916–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cho-Hisamoto Y, Kojima K, Brown EC, Matsuzaki N, Asano E.. Cooing- and babbling-related gamma-oscillations during infancy: Intracranial recording. Epilepsy Behav. 2012;23(4):494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Swift JR, Coon WG, Guger C, et al. Passive functional mapping of receptive language areas using electrocorticographic signals. Clin Neurophysiol. 2018;129(12):2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Matsumoto R, Nair DR, LaPresto E, et al. Functional connectivity in the human language system: A cortico-cortical evoked potential study. Brain. 2004;127(Pt 10):2316–2330. [DOI] [PubMed] [Google Scholar]
- 93. Mitsuhashi T, Sonoda M, Jeong J, et al. Four-dimensional tractography animates neural propagations via distinct interhemispheric pathways. Clin Neurophysiol. 2021;132(2):520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Silverstein BH, Asano E, Sugiura A, Sonoda M, Lee M-H, Jeong J-W.. Dynamic tractography: Integrating cortico-cortical evoked potentials and diffusion imaging. Neuroimage. 2020;215:116763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code are available on request to the corresponding author (E.A.). We are pleased to reanalyse the data based on readers’ specific suggestions to improve the language mapping method.