Abstract
Alterations in neurovascular coupling have been associated with various ocular, cerebral, and systemic vascular disorders. In the eye, changes in vessel caliber by dynamic vessel analysis have been used to measure neurovascular coupling following a light flicker stimulus. Here, we present a new protocol for quantifying light-flicker induced hyperemia in the C57/Bl6J mouse retina using laser speckle flowgraphy (LSFG). Our protocol was adapted from protocols used in human subjects. By acquiring continuous time series data, we detected significant increase in blood flow. These responses are maintained with low variability over multiple imaging sessions, indicating these methods may be applied in serial studies of neurovascular coupling.
Keywords: laser speckle flowgraphy, retinal blood flow, neurovascular coupling
Neurovascular coupling, the process by which microvessels respond to the metabolic demands of neural tissues, is a critical component of blood flow regulation in the cerebrovascular system (Iadecola, 2017). This process has been extensively studied in humans (Fondi et al., 2018) as well as preclinical models, for example in the cat eye (Riva et al., 1991), where similar neurovascular responses as those found in the brain can be examined non-invasively with retinal imaging modalities. Exposure to light flicker induces retinal vasodilation and hyperemic response, in which retinal microvessels vasodilate to meet the increased metabolic demands of stimulated retinal neurons (Riva et al., 2005). Because the retinal vascular and neuronal structures are considered an extension of the cerebrovascular system, retinal hyperemic responses are believed to be representative of neurovascular health of the brain. Indeed, alterations in light flicker-induced retinal hyperemic response have been associated with a variety of disorders, such as glaucoma (Riva et al., 2004), macular degeneration (Lanzl et al., 2011), Alzheimer’s disease (Kotliar et al., 2017), late-life depression (Geraets et al., 2020), cognitive dysfunction (Rensma et al., 2020), diabetes (Nguyen et al., 2009) and obesity (Kotliar et al., 2011). The vast majority of flicker-induced hyperemia studies utilize dynamic vessel analysis (DVA) to examine changes in retinal vessel caliber during vasodilation and vasoconstriction (Albanna et al., 2018). However, the magnitude of arterial and venous dilation is small and on the order of 3–5% and 4–9%, respectively (Hammer et al., 2012; Kotliar et al., 2017; Lanzl et al., 2011). The even lower responses in mice, with both arterial and venous dilation in the 1–2% range (Albanna et al., 2018) may represent a limitation of this approach for animal studies. A very recent study with a custom laser Doppler Fourier domain-optical coherence tomography (FD-OCT) system reported volumetric retinal blood flow increases by 30–40% in arteries and veins (Kallab et al., 2021). These findings suggest that blood flow measurements may reveal substantial responses in animal models. However, this instrumentation is not widely available.
Recent developments in laser speckle-based imaging methods, namely laser speckle contrast imaging (LSCI) and laser speckle flowgraphy (LSFG), have made dynamic measurement of relative blood flow possible in humans and in small animal models (Patel et al., 2021; Remer et al., 2019; Wada et al., 2016). Both LSCI and LSFG quantify the interference patterns (“laser speckle”) produced by light reflecting off of blood cells passing through the field of the laser (Sugiyama, 2014; Sugiyama et al., 2010). Unlike custom-built devices, the LSFG-NAVI device (Softcare Co., Ltd.) is commercially available for both clinical and small animal research, and FDA-approved for use in human subjects. It has been used clinically to study not only numerous ocular and systemic disorders (Aizawa et al., 2011; Arimura et al., 2018; Luft et al., 2016; Shiba et al., 2017; Shiga et al., 2016; Yata et al., 2020), but also light flicker-induced retinal hyperemia in healthy control subjects (Fondi et al., 2018) and mice (Hanaguri et al., 2020). Similar magnitudes of blood flow increase of 20–25% have been reported by LSFG in humans and mice with light flicker stimulation (Fondi et al., 2018; Hanaguri et al., 2020).
We recently reported methods for acquiring serial LSFG blood flow measurements in C57/Bl6J mice under normal conditions and in a model of hypertension, with minimal inter-operator and inter-session variability (Tamplin et al., 2021). Here, we developed novel protocols to permit continuous data acquisition over a 100 s period, per established DVA protocols for neurovascular coupling (Kotliar et al., 2017). Given that human studies have deployed DVA for detecting retinal hyperemia and that responses in mice by DVA are minute and unlikely to produce robust data, we developed a new protocol to measure hyperemia by LSFG in mice.
All experimental procedures were approved by the University of Iowa Institutional Animal Care and Use Committee, and were in compliance with the Institute of Laboratory Animal Resource, National Academy of Science standards for the care and use of laboratory animals. Mice were housed in a room with controlled temperature (23 °C) and a dark/light cycle of 12 hours, with free access to water and standard rodent chow. All measurements were performed between 9 AM and 12 AM to avoid circadian variations. For this study, 5 male and 5 female C57/Bl6J mice (Jackson Laboratories) aged 12 to 14 weeks were imaged in 4 sessions over a 5-day period.
Before the imaging procedure, mice were anesthetized by IP injection of 17.5 mg/mL ketamine/2.5 mg/mL xylazine solution (VetaKet/AnaSed, 0.1 mL/20 g body weight). Mice were placed on an infrared heating pad controlled by a rectal temperature probe (PhysioSuite; Kent Scientific), to maintain core body temperature at 37 °C. Five successive measurements of intraocular pressure (IOP) were taken in the right eye using a handheld tonometer (Tonolab TV02; Icare). Eyes were dilated with 0.5% topical tropicamide and all experiments were performed under photopic conditions in an animal laboratory with fluorescent lights. Blood pressure was recorded in anesthetized mice immediately before image acquisition using an automatic tail-cuff plethysmography system (CODA Monitor; Kent Scientific).
Blood flow measurements were obtained using the laser speckle flowgraphy device LSFG-Micro (Softcare Co., Ltd.), a charge-coupled device camera (700 × 480 px) attached to a diode laser (830 nm wavelength) and microscope (SZ61TR; Olympus Corporation). The principles of LSFG have been described elsewhere (Sugiyama, 2014; Sugiyama et al., 2010). Briefly, as the incident laser light is scattered against blood cells moving through the retinal vessels, a speckle pattern is produced. The characteristics of this pattern are used to calculate mean blur rate, a measure of relative blood flow velocity, at each pixel of the image. Mean blur rate has been validated as an accurate measure of flow velocity by comparison to hydrogen gas clearance and microsphere methods (Aizawa et al., 2014).
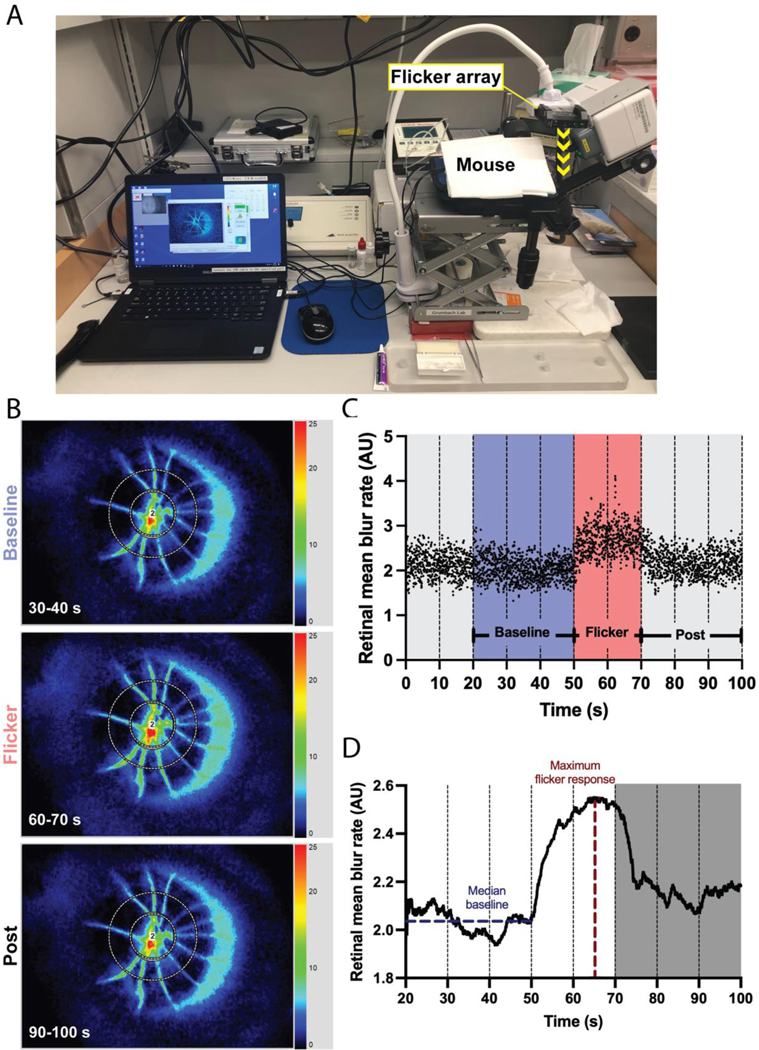
To invoke hyperemic response, diffuse light flicker stimulation was applied using a white light emitting-diode (LED) array (KICK LED, Rift Labs; 5000 K, 1200 lx) maintained in a fixed position above the mouse eye (yellow arrow in Fig. 1A). The positioning of the flicker array ensured exposure of the retina to flicker stimulus was consistent for each mouse, without affecting the LSFG measurements. Blood flow measurements were acquired continuously (30 fps) for 100 s, based on established dynamic vessel analyzer protocols (Kotliar et al., 2017; Lanzl et al., 2011): 50 s of baseline recording, 20 s of flicker stimulus (10 Hz), and 30 s of post-stimulus measurement. Temporal composites of the 3.2 × 2.5 mm2 imaged area (Fig. 1B) were automatically generated every 10 s to permit manual analysis, resulting in 10 composite images and a continuum of 3000 data points (Fig. 1C).
Figure 1: Continuous blood flow acquisition and processing methods.
(A) LSFG acquisition setup with position of the 10 Hz flicker array above the mouse eye relative to the LSFG-Micro camera. (B) Representative composite images acquired during baseline (30–40 s, top), flicker stimulus (60–70 s, middle) and post-flicker cessation (90–100 s, bottom) corresponding to the time series in (C,D). (C) Raw blood flow data acquired continuously at 30 fps over 100 s, resulting in 3000 data points. Time periods of interest for analysis are marked. (D) Processing of data shown in (C) result in a smooth response curve.
To analyze blood flow, data files were opened in the LSFG Analyzer software (v. 3.5.0.0; Softcare Co., Ltd.) to generate a composite image representing the average blood flow at each pixel over the 10 s recording period (Fig. 1B). A region of interest covering the retinal vasculature, but not the optic nerve head, was placed on each composite scan following our previously established annulus-based analysis (Tamplin et al., 2021). Briefly, a circular region of interest was placed centered on and encompassing the optic nerve head and any artifactual blur surrounding the structure. A second concentric band was placed starting at the edge of the first, and extending circumferentially to cover the retinal vasculature (Fig. 1B). The band set was saved and applied to the remaining images in the time series. Mean MBR (or, MA) in the outer band containing the retinal vasculature was calculated at each frame of the time series. Once bands were placed on all 10 composite images of the time series, the data were batch processed using the Analyzer software. This process results in a spreadsheet containing the time in seconds and mean flow value (MA) for each frame, by region of interest.
In order to reduce measurement noise caused by the mouse’s rapid heart rate, illustrated by the periodic data scatter in Fig. 1C, raw continuous data were processed using a custom Python script to generate a smoothed response curve (Fig. 1D). The first 20 s of adaptation (t = 0–20 s) were removed from the series. The following 30 s (t = 20–50 s) preceding the flicker stimulus were used for baseline calculations. Outliers were removed by calculating the mean and standard deviation of baseline flow; values outside the mean ± 3 standard deviations were removed. Then, the data were smoothed using the 129 exponential moving average (EMA) function (in Python, pandas.DataFrame.ewm) (McKinney W., 2010), defined as: EMAt = α(yt) + (1 − α)(EMAt-1), where yt is the 131 observation at time period t, EMAt is the EMA value at time t, and the smoothing factor α = 2/(n + 1) for span n. Here, the span n was set to 120, meaning 120 frames (or, 4 s of data) were used to define the smoothing window (Kotliar et al., 2017). After the data were smoothed, the median of the baseline period (blue line in Fig. 1D), the maximum of the response curve (after t = 50 s; red line in Fig. 1D), and the time to reach the maximum flicker response were calculated. Percent flicker response was defined as .
Statistical analyses were performed using GraphPad Prism (version 8.0.0; GraphPad Software). Blood flow levels at baseline and during the maximal flicker response were compared by paired t-test (two-tailed). Variations due to biological sex across sessions were determined using 2-way ANOVA with Sidak’s multiple comparisons test. Differences in measures between sessions were calculated using one-way ANOVA with Tukey’s multiple comparisons test. The coefficient of variability was defined as 100% x (stdev/mean); for intersession variability, the standard deviation and mean across all 4 sessions were used.
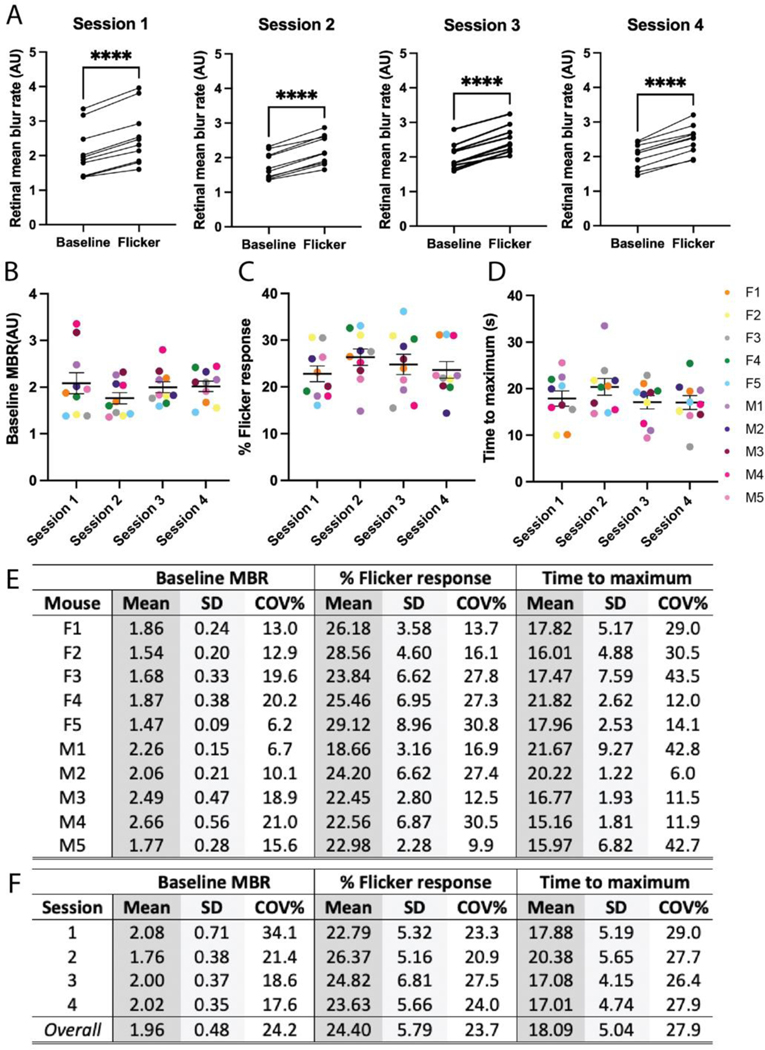
Representative images taken during baseline, flicker stimulus, and post-flicker stimulus in the same mouse are shown in Fig. 1B. The corresponding raw data series and processed curve are provided in Fig. 1C and 1D, respectively. Statistically significant increases in retinal blood flow (MBR) in response to the flicker stimulus were observed at all four sessions (p < 0.0001, Fig. 2A), indicating successful detection of light flicker-induced hyperemia at each session.
Figure 2: Blood flow characteristics with corresponding subject and session variability.
(A) Pairwise comparison of median baseline and maximal flicker response in 10 C57/Bl6J mice over four sessions. Significant increases in blood flow were observed across all sessions (p < 0.0001). Median baseline blood flow (B), maximal percent flicker response (C), and time to reach maximum response (D) calculated at each session. (E) Calculated mean, standard deviation, and intersession variability for all 10 mice, by each measure, across the four sessions. (F) Intrasession variability calculated for each measure. For (A-E) mice are codified by biological sex (F, female; M, male) and color-coded by mouse. **** p < 0.0001 by paired t-test (two-tailed) in A. (B-E) differences due to biological sex nonsignificant (p > 0.05) by 2-way ANOVA with Sidak’s multiple comparisons test; differences between sessions nonsignificant (p > 0.05) by one-way ANOVA with Tukey’s multiple comparisons test.
Across all four sessions, the average baseline MBR was 2.25 AU in male mice and 1.68 AU in female mice (Fig. 2B). The average percent flicker response across all four sessions was 22.17% in male mice and 26.6% in female mice (Fig. 2C). The mean value for each mouse, averaged across the four sessions, is reported in Fig. 2E. No significant differences were observed due to biological sex (2-way ANOVA with Sidak’s multiple comparisons); therefore, subsequent analyses combined data from males and females. The combined mean baseline MBR ranged between 1.76–2.08 across the four sessions; no significant difference between sessions was observed (Fig. 2B, F). The combined mean percent flicker response ranged between 22.79–26.37% across the four sessions, and did not vary significantly between sessions (Fig. 2C, F).
To establish variability in blood flow, the coefficient of variation was calculated for each measure. For individual mice, intersession variability ranged between 6.2–21.0% for baseline flow, and 9.9–30.8% for flicker response (Fig. 2E). Group intrasession variability ranged between 17.6–34.1% for baseline flow, and 20.9–27.5% for flicker response (Fig. 2F). Intersession variability across the four sessions was 24.2% for baseline flow, and 23.7% for flicker response.
Next, the time to reach the maximum flicker response, previously reported as a response latency parameter for staging Alzheimer’s disease (Kotliar et al., 2017), was calculated. The mean time to maximal flicker response ranged between 17.01–20.38 s across the four sessions (Fig. 2F). For individual mice, the intersession variability ranged between 6.0–43.5%. Group intrasession variability ranged between 26.4–29.0%. Intersession variability across the four sessions was 27.9%.
Here, we presented new protocols for acquiring time series-based blood flow data to study light flicker-induced retinal hyperemia by LSFG as a measure of neurovascular coupling. While previous studies in mice were based on changes in vessel caliber by dynamic vessel analysis, or considered flow changes in non-continuous scans of the optic nerve head (Hanaguri et al., 2020), we introduce an easily-adaptable protocol for acquiring continuous measurements of blood flow over the entire mouse retina. Continuous measurement ensures the window for maximal flicker response is not missed by improper timing of scans, or by the 15–30 second processing and setup time required between discrete scan acquisition.
First, we adapted a protocol to enable continuous measurement using LSFG, which detected significant increases in blood flow in response to a light flicker stimulus. We show significant increases in blood flow during flicker light stimulus, reaching maximal response over a range of time, that would be more difficult to accurately assess with the traditional 4 s scans requiring significant processing time between test intervals. We also describe the measurement variability for flicker response in C57/Bl6J mice. These results were consistent across four sessions performed over a five-day period. Baseline blood flow was similarly constant across sessions, in keeping with our previous results using the discrete scan protocol (Tamplin et al., 2021).
Importantly, this protocol can be altered to measure any region of interest in the retina. By changing the band type and location in the Analyzer software interface, similar data could be acquired in areas such as the optic nerve head, individual retinal vessels or retinal regions. Changing the band selection applied with this protocol would allow for future, more granular analyses of individual vessel response.
Several aspects of retinal blood flow in mice remain to be explored in future studies. For example, in humans, sex-related differences in blood flow by LSFG have been reported (Yanagida et al., 2015), however, similar studies in mice are currently missing. Additionally, different anesthesia protocols, i.e. inhalation versus injected anesthetics, can differentially affect ocular blood flow (Moult et al., 2017). The optimal choice of anesthetic and its impact on retinal hyperemic response in mice remains to be established. Furthermore, the intensity of light stimulus used here was higher than in previous studies of flicker-induced retinal hyperemia in mice (Albanna et al., 2018; Hanaguri et al., 2020); further investigation into the role of magnitude of light flicker intensity on blood flow results may be needed.
Acknowledgements
The authors thank Drs. Kenji Okamoto, Noriyoshi Takahashi, and Takeshi Shirakawa 219 (Softcare Co., Ltd.) for their assistance with the LSFG device.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa N, Nitta F, Kunikata H, Sugiyama T, Ikeda T, Araie M, Nakazawa T, 2014. Laser speckle and hydrogen gas clearance measurements of optic nerve circulation in albino and pigmented rabbits with or without optic disc atrophy. Investigative ophthalmology & visual science 55, 7991–7996. [DOI] [PubMed] [Google Scholar]
- Aizawa N, Yokoyama Y, Chiba N, Omodaka K, Yasuda M, Otomo T, Nakamura M, Fuse N, Nakazawa T, 2011. Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVI in patients with glaucoma. Clin Ophthalmol 5, 1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanna W, Kotliar K, Lüke JN, Alpdogan S, Conzen C, Lindauer U, Clusmann H, Hescheler J, Vilser W, Schneider T, Schubert GA, 2018. Non-invasive evaluation of neurovascular coupling in the murine retina by dynamic retinal vessel analysis. PloS one 13, e0204689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura T, Shiba T, Takahashi M, Kumashiro S, Osamura H, Matsumoto T, Sakai K, Hori Y, 2018. Assessment of ocular microcirculation in patients with end-stage kidney disease. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 256, 2335–2340. [DOI] [PubMed] [Google Scholar]
- Fondi K, Bata AM, Luft N, Witkowska KJ, Werkmeister RM, Schmidl D, Bolz M, Schmetterer L, Garhofer G, 2018. Evaluation of flicker induced hyperemia in the retina and optic nerve head measured by Laser Speckle Flowgraphy. PloS one 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraets AFJ, van Agtmaal MJM, Stehouwer CDA, Sörensen BM, Berendschot T, Webers CAB, Schaper NC, Henry RMA, van der Kallen CJH, Eussen S, Koster A, van Sloten TT, Köhler S, Schram MT, Houben A, 2020. Association of Markers of Microvascular Dysfunction With Prevalent and Incident Depressive Symptoms: The Maastricht Study. Hypertension 76, 342–349. [DOI] [PubMed] [Google Scholar]
- Hammer M., Heller T., Jentsch S., Dawczynski J., Schweitzer D., Peters S., Schmidtke KU., Muller UA., 2012. Retinal vessel oxygen saturation under flicker light stimulation in patients with nonproliferative diabetic retinopathy. Investigative ophthalmology & visual science 53, 4063–4068. [DOI] [PubMed] [Google Scholar]
- Hanaguri J, Yokota H, Watanabe M, Kuo L, Yamagami S, Nagaoka T, 2020. Longitudinal stability of retinal blood flow regulation in response to flicker stimulation and systemic hyperoxia in mice assessed with laser speckle flowgraphy. Scientific reports 10, 19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, 2017. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 96, 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallab M, Hommer N, Tan B, Pfister M, Schlatter A, Werkmeister RM, Chua J, Schmidl D, Schmetterer L, Garhöfer G, 2021. Plexus-specific effect of flicker-light stimulation on the retinal microvasculature assessed with optical coherence tomography angiography. Am J Physiol Heart Circ Physiol 320, H23–h28. [DOI] [PubMed] [Google Scholar]
- Kotliar K, Hauser C, Ortner M, Muggenthaler C, Diehl-Schmid J, Angermann S, Hapfelmeier A, Schmaderer C, Grimmer T, 2017. Altered neurovascular coupling as measured by optical imaging: a biomarker for Alzheimer’s disease. Scientific reports 7, 12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotliar KE, Lanzl IM, Schmidt-Trucksass A, Sitnikova D, Ali M, Blume K, Halle M, Hanssen H, 2011. Dynamic retinal vessel response to flicker in obesity: A methodological approach. Microvascular research 81, 123–128. [DOI] [PubMed] [Google Scholar]
- Lanzl IM, Seidova SF, Maier M, Lohmann C, Schmidt-Trucksass A, Halle M, Kotliar KE, 2011. Dynamic retinal vessel response to flicker in age-related macular degeneration patients before and after vascular endothelial growth factor inhibitor injection. Acta ophthalmologica 89, 472–479. [DOI] [PubMed] [Google Scholar]
- Luft N, Wozniak PA, Aschinger GC, Fondi K, Bata AM, Werkmeister RM, Schmidl D, Witkowska KJ, Bolz M, Garhöfer G, Schmetterer L, 2016. Ocular Blood Flow Measurements in Healthy White Subjects Using Laser Speckle Flowgraphy. PloS one 11, e0168190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney W.o., 2010. Data structures for statistical computing in python, Proceedings of the 9th Python in Science Conference, pp. 51–56. [Google Scholar]
- Moult EM, Choi W, Boas DA, Baumann B, Clermont AC, Feener EP, Fujimoto JG, 2017. Evaluating anesthetic protocols for functional blood flow imaging in the rat eye. Journal of biomedical optics 22, 16005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT., Kawasaki R., Wang JJ., Kreis AJ., Shaw J., Vilser W., Wong TY., 2009. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care 32, 20752080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DD, Dhalla AH, Viehland C, Connor TB, Lipinski DM, 2021. Development of a Preclinical Laser Speckle Contrast Imaging Instrument for Assessing Systemic and Retinal Vascular Function in Small Rodents. Transl Vis Sci Technol 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer I, Pierre-Destine LF, Tay D, Golightly LM, Bilenca A, 2019. In vivo noninvasive visualization of retinal perfusion dysfunction in murine cerebral malaria by camera-phone laser speckle imaging. J Biophotonics 12, e201800098. [DOI] [PubMed] [Google Scholar]
- Rensma SP, van Sloten TT, Houben A, Köhler S, van Boxtel MPJ, Berendschot T, Jansen JFA, Verhey FRJ, Kroon AA, Koster A, Backes WH, Schaper N, Dinant GJ, Schalkwijk CG, Henry RMA, Wolfs EML, van Heumen MJA, Schram MT, Stehouwer CDA, 2020. Microvascular Dysfunction Is Associated With Worse Cognitive Performance: The Maastricht Study. Hypertension 75, 237–245. [DOI] [PubMed] [Google Scholar]
- Riva CE, Harino S, Shonat RD, Petrig BL, 1991. Flicker evoked increase in optic nerve head blood flow in anesthetized cats. Neurosci Lett 128, 291–296. [DOI] [PubMed] [Google Scholar]
- Riva CE, Logean E, Falsini B, 2005. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Progress in retinal and eye research 24, 183–215. [DOI] [PubMed] [Google Scholar]
- Riva CE, Salgarello T, Logean E, Colotto A, Galan EM, Falsini B, 2004. Flicker-evoked response measured at the optic disc rim is reduced in ocular hypertension and early glaucoma. Investigative ophthalmology & visual science 45, 3662–3668. [DOI] [PubMed] [Google Scholar]
- Shiba T, Takahashi M, Matsumoto T, Hori Y, 2017. Relationship between Metabolic Syndrome and Ocular Microcirculation Shown by Laser Speckle Flowgraphy in a Hospital Setting Devoted to Sleep Apnea Syndrome Diagnostics. Journal of diabetes research 2017, 3141678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga Y, Kunikata H, Aizawa N, Kiyota N, Maiya Y, Yokoyama Y, Omodaka K, Takahashi H, Yasui T, Kato K, Iwase A, Nakazawa T, 2016. Optic Nerve Head Blood Flow, as Measured by Laser Speckle Flowgraphy, Is Significantly Reduced in Preperimetric Glaucoma. Current eye research 41, 1447–1453. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, 2014. Basic Technology and Clinical Applications of the Updated Model of Laser Speckle Flowgraphy to Ocular Diseases. Photonics 1. [Google Scholar]
- Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S, 2010. Use of laser speckle flowgraphy in ocular blood flow research. Acta ophthalmologica 88, 723–729. [DOI] [PubMed] [Google Scholar]
- Tamplin MR, Broadhurst KA, Vitale AH, Hashimoto R, Kardon RH, Grumbach IM, 2021. Longitudinal Testing of Retinal Blood Flow in a Mouse Model of Hypertension by Laser Speckle Flowgraphy. Transl Vis Sci Technol 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Higashide T, Nagata A, Sugiyama K, 2016. Longitudinal Changes in Optic Nerve Head Blood Flow in Normal Rats Evaluated by Laser Speckle Flowgraphy. Investigative ophthalmology & visual science 57, 5568–5575. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Iwase T, Yamamoto K, Ra E, Kaneko H, Murotani K, Matsui S, Terasaki H, 2015. Sex-Related Differences in Ocular Blood Flow of Healthy Subjects Using Laser Speckle Flowgraphy. Investigative ophthalmology & visual science 56, 4880–4890. [DOI] [PubMed] [Google Scholar]
- Yata K., Hashimoto R., Masahara H., Oyamada M., Maeno T., 2020. Changes in choroidal circulation and pulse waveform in a case of pregnancy-induced hypertension with serous retinal detachment. American journal of ophthalmology case reports 20, 100911. [DOI] [PMC free article] [PubMed] [Google Scholar]