Abstract
Maintaining genome stability involves coordination between different subcellular compartments providing cells with DNA repair systems that safeguard against environmental and endogenous stresses. Organisms produce the chemically reactive molecule formaldehyde as a component of one-carbon metabolism, and cells maintain systems to regulate endogenous levels of formaldehyde under physiological conditions, preventing genotoxicity, among other adverse effects. Dysregulation of formaldehyde is associated with several diseases, including cancer and neurodegenerative disorders. In the present review, we discuss the complex topic of endogenous formaldehyde metabolism and summarize advances in research on formaldehyde dysregulation, along with future research perspectives.
Keywords: Formaldehyde, One Carbon Metabolism, Mitochondrial DNA, DNA Damage
1. Introduction
Eukaryotic cells divide and proliferate through a series of highly regulated biochemical processes that promote growth and preserve the redox state of the cells, while maintaining genomic stability. These activities rely in part on one-carbon metabolism, a series of enzymatic reactions providing cells with building blocks that sustain protein, lipid and nucleic acid biosynthesis. One-carbon metabolism is an important source of endogenous formaldehyde; other physiological processes are known to contribute to formaldehyde levels, as well. This article provides a summary of background information on formaldehyde metabolism and then outlines progress in understanding the roles of formaldehyde in the context of mammalian cells and mitochondrial metabolism. This review also makes use of results obtained through work with the yeast Schizosaccharomyces pombe system.
2. Sources of Endogenous Formaldehyde
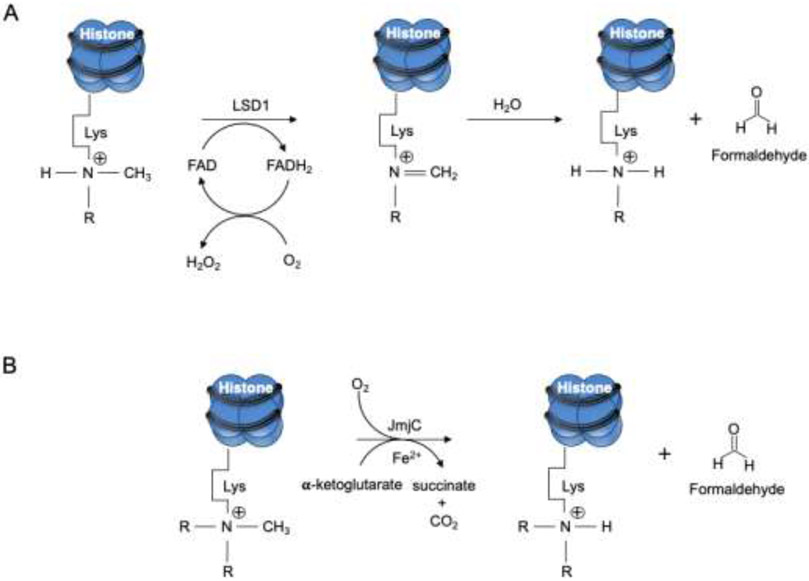
Formaldehyde is produced from a range of sources and metabolic processes (Fig. 1). Demethylations of DNA, RNA and histones release formaldehyde that in turn regulates the activity of DNA methyltransferases (DNMTs). DNMTs are vital enzymes, and their deletion is linked to lethal phenotypes in early embryogenesis and postnatal development [1, 2]. Histone demethylation occurs also through the action of the superfamily of Jumonji C (JmjC)-containing oxygenases and lysine-specific demethylase 1 (LSD1) (Fig. 2). These enzymes demethylate histone lysine residues, the latter through an oxidative mechanism that requires the cofactors flavin adenine nucleotide, α-ketoglutarate and Fe (II).
Fig. 1.
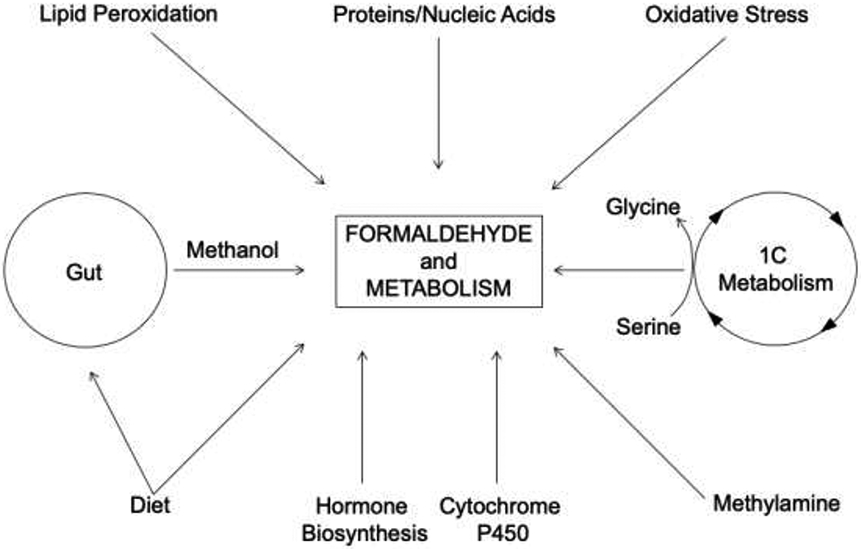
Endogenous sources of formaldehyde. Scheme showing key metabolic processes that generate formaldehyde in mammalian cells.
Fig. 2.
Demethylation of histones produces formaldehyde. A) Lysine specific demethylase (LSD1) and B) Jumonji C (JmjC) domain removal of methyl groups from lysine (Lys) residues in histones. Reactions in both A and B release formaldehyde.
Methanol is also a source of formaldehyde [3, 4]. Endogenous methanol derives from different physiological processes, such as activity of intestinal microbiota and the methylation reaction between S-adenosylmethionine (SAM) and carboxylate groups of amino acids in proteins [5]. Methanol is oxidized to formaldehyde and further degraded by multiple families of enzymes that include catalase, cytoplasmic alcohol dehydrogenase 5 (ADH5), and the mitochondrial aldehyde dehydrogenase 2 (ALDH2) [3, 6], and requires flavin adenine dinucleotide [7]. Hydrogen peroxide, for example, is naturally produced by a nicotinamide adenine dinucleotide phosphate (NADPH)-electron transfer mechanism, and accounts for the direct oxidation of methanol by catalase. Additionally, hydroxyl-radicals generated from the Fenton reaction of hydrogen peroxide support oxidation of methanol, generating formaldehyde and formate [8-11]. Cytochrome P450 monooxygenases also participate in production of formaldehyde [12-14]. These enzymes are responsible for oxidation of glycerol to formaldehyde in a NADPH-dependent process.
Lipid oxidation contributes to formaldehyde production, as a result of malondialdehyde (MDA) metabolism [15, 16]. In addition, semicarbazide-sensitive amine oxidase (SSAO) catalyzes deamination of endogenous methylamine resulting in release of formaldehyde [17-21]. This reaction, coordinated by SSAO, produces the corresponding aldehyde, hydrogen peroxide and ammonia [21-25]. Methylamine also derives from deamidation of adrenaline, as mediated by monoamine oxidase-A (MAO-A), and can be further metabolized to formaldehyde by SSAO. The general mechanism through which formaldehyde is generated by oxidative deamination of methylamine is:
where hydrogen peroxide and ammonia are released [22, 26]. Finally, formaldehyde is generated by the innate immune system where activation of neutrophils and monocytes engage the myeloperoxidase system, producing reactive oxygen species [16, 27].
An important function of formaldehyde is in support of one-carbon metabolism, a series of reactions in the cytoplasm as well as in mitochondria (Fig. 3) [28]. One-carbon metabolism supports proliferation and genome stability through biosynthesis of essential cellular components, such as purines and pyrimidines, and provides antioxidant defenses. During this process, serine is cleaved to glycine and formaldehyde by serine hydroxymethyl transferase (SHMT). There are two isoforms of SHMT, SHMT1 and SHMT2, that localize, respectively, to the cytoplasm and mitochondria in mammalian cells. The result of SHMT activity is formation of N5, N10-methylene tetrahydrofolate (CH2-THF); this metabolite is produced as an essential substrate for other enzymes, such as thymidylate synthase, N5, N10-methylene tetrahydrofolate reductase and N5, N10-methylene tetrahydrofolate dehydrogenase. These enzymes carryout methylation reactions. Serine catabolism that forms formaldehyde (Fig. 3) also occurs through the action of methylene-THF dehydrogenase 1 (MTHFD 1), and in mitochondria due to methylene-THF 2 (MTHFD 2), or 2 like (MTHFD 2L), and methylene-THF dehydrogenase 1 like (MTHFD1L). Another route of formaldehyde formation involves the reaction of formaldehyde with cysteine and histidine [29]. The reactions result in formation of timonacic and spinacine, respectively. Timonacic is considered a reservoir of formaldehyde in the human body; decomposition of timonacic by ADH5 into formate and formaldehyde fuels one-carbon metabolism.
Fig. 3.
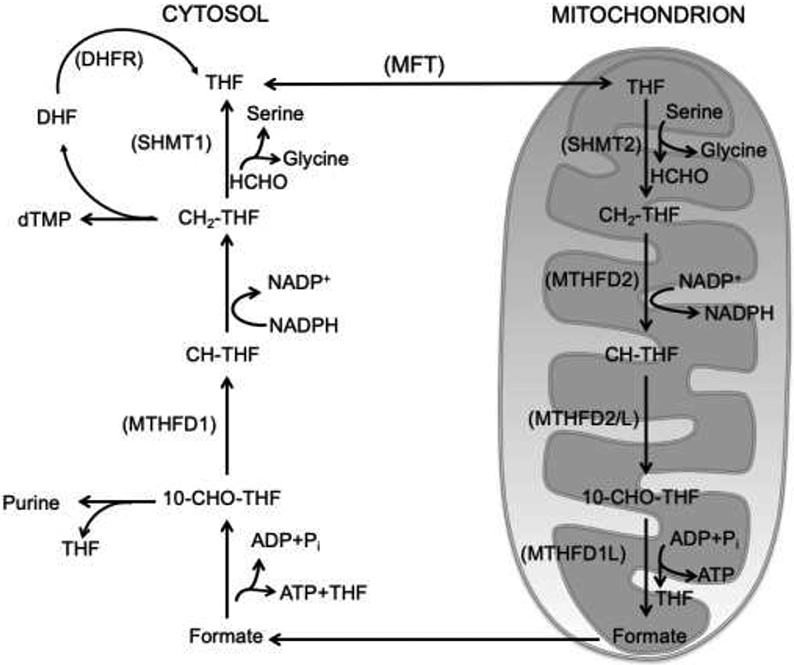
Simplified scheme illustrating formaldehyde reactions involved in canonical one-carbon metabolism. Distinct cytoplasmic and mitochondrial one-carbon metabolism reactions enable eukaryotic cells to support biochemical processes, such as nucleotide, amino acid and methyl group biosynthesis, essential for survival and DNA metabolism. The figure was adapted from reference 28.
3. Regulation of Formaldehyde
Despite of the range of processes through which formaldehyde is generated, physiological levels are maintained by multiple metabolic systems (Fig. 4). A product of these systems is formate (i.e., formic acid) that is either excreted in the urine or further oxidized to carbon dioxide and water. Formaldehyde clearance involves several different pathways, including the cytochrome P450 monooxygenases (CYPs). CYPs represent a superfamily of enzymes acting on exogenous and endogenous molecules, such as methanol and formaldehyde. These enzymes are widely expressed and localized to the endoplasmic reticulum and mitochondria. CYPs can activate molecular oxygen and stimulate the addition of a single oxygen atom in organic reactions [30-32]. The heme iron of CYPs binds oxygen, and iron linked to the thiolate sulfur group of cysteine, and stimulates splitting of molecular oxygen. The basic reaction catalyzed by CYPs is:
where one oxygen atom is reduced to water and the other oxygen atom is activated and transferred to a substrate molecule, yielding the hydroxylated product, SOH [4, 33-35]. Microsomal enzymes, such as NADPH-cytochrome P450 oxidoreductase and cytochrome b5 are essential to coordinate CYPs functions [36].
Fig. 4.
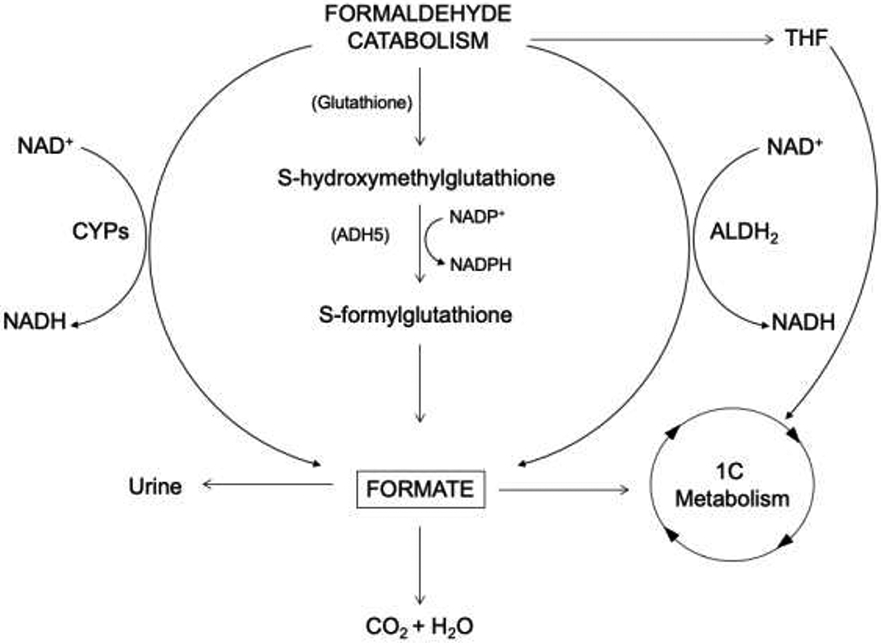
Catabolism of formaldehyde. Scheme showing key clearance systems to prevent accumulation of formaldehyde in mammalian cells.
One-carbon metabolism is engaged to safeguard physiological concentrations of formaldehyde [37, 38] through a mechanism that links the glutathione-antioxidant system and the ALDH system. Initially, glutathione reacts with formaldehyde producing S-hydroxymethylglutathione that is further oxidized to S-formylglutathione in a NADP+-dependent manner by ADH5. Subsequently, S-formylglutathione enters the one-carbon metabolism cycle as formate, due to the activity of another enzyme, S-formylglutathione hydrolase (Fig. 4). ADH5 is capable of promoting oxidation of formaldehyde to formate in cells with defective mitochondrial folate metabolism, allowing formaldehyde to be used in the one-carbon metabolism cycle under these circumstance. Finally, the cytoplasm contains multiple ALDH isoforms that oxidize formaldehyde to formate.
4. Reactions of Formaldehyde with DNA and DNA Repair Mechanisms
Formaldehyde has an electrophilic carbon that reacts with electron-rich amino- and thiol-groups forming covalent adducts through nucleophilic substitutions. Thus, formaldehyde can form the Schiff base intermediate in a pathway leading to covalent bond formation with a protein or nucleobase in DNA. As a consequence, formaldehyde can influence DNA, RNA and proteins by forming mono-adducts [39], nucleic acid-protein crosslinks and protein-protein crosslinks [40]. In an example of these reactions, the exocyclic amine N6 of adenine acts as a nucleophile attacking formaldehyde; this in turn promotes alkylation of adenine in DNA or RNA [41]. The resulting intermediates can further react with other amine groups and generate nucleic acid adducts. DNA fragmentations, including single-strand and double-strand DNA breaks (DSBs), are additional lesions reported after cellular exposure to formaldehyde [42-45]. DNA lesions may be produced in response to formaldehyde-induced oxidative stress that results in oxidative-induced damage to DNA bases. Alkylated bases can be removed by base excision repair (BER) enzymes or can be subject to spontaneous base loss leaving abasic sites. Strand break intermediates produced after base removal or during the BER of oxidized bases could account for mitochondrial strand breaks.
Mitochondria do not possess the full set of DNA repair pathways available in the nucleus, but mitophagy as well as the BER pathway appear to be central mechanisms to address a variety of mitochondrial DNA small lesions. These small lesions may cause mitochondrial stress and trigger the activity of damage-associated molecular patterns (DAMPs) that activate innate immunity and mitophagy. In this context, the E3 ubiquitin ligase (parkin) and the ubiquitin kinase (PINK1) are crucial and function by removing damaged mitochondria. Recently, Youle and coworkers reported a strong inflammatory phenotype in both parkin−/− and Pink1−/− mice following exhaustive exercise and in Parkin−/− mutator mice [46]. These mice with deficient mitophagy develop severe inflammation due to accumulation of mutations in mitochondrial DNA (mtDNA). Interestingly, the inflammatory process caused by exhaustive exercise or mtDNA mutation was completely rescued by loss of STING, a key regulator of the type I interferon response to cytosolic DNA. These results support a role for PINK1- and parkin-mediated mitophagy in containing innate immunity [46]. Yet, it remains to be established whether mitochondria have a dedicated repair pathway for DNA-DSBs. It is known, however, that linear mtDNA, produced from DSBs, are digested by mitochondrial genome maintenance exonuclease I (MGME1) and the exonuclease activity of DNA polymerase γ (POLG).
To date, mtDNA replication stalling emerges as the primary cause of DSBs in mitochondria, and this is associated with the generation of mtDNA deletions [47, 48]. Several mutations in the mitochondrial helicase Twinkle and the replicative POLG have been also associated with promutagenesis features. Recently, it was reported that Twinkle upregulation promotes mtDNA replication stalling, accumulation of replicative intermediates, and mitochondrial genome destabilization linked to large-scale mtDNA deletions [49]. There are also reports that arrested mtDNA repair can result in formation of DNA protein crosslinks (DPCs), i.e., covalent trapping of a repair enzyme on mtDNA, essentially leading to more severe DNA damage than the original lesion [50, 51]. The mechanism by which formaldehyde induces mtDNA-DSBs is unknown.
In other important DNA alteration systems, formaldehyde is released in close proximity to DNA. An example of this is the release of formaldehyde from histones and methylated nucleobases following methyl group removal by the AlkB monooxygenase enzyme system. These enzymes attack the methyl group and other alkylation adducts in DNA through an AlkB mediated oxidative process . The overall result of the reaction is removal of methyl adducts from DNA and release of formaldehyde in proximity to DNA where reactions with nucleobases or abasic sites can occur.
As already noted, DNA is vulnerable to formaldehyde linked formation of structural and chemical alterations such as alkylated nucleobases, abasic sites, and adducts with chemicals, including formaldehyde. In addition, inter-strand crosslinks, strand breaks and DPCs are lesions found in DNA that appear to be promoted by formaldehyde. DPCs are bulky structures that likely affect genomic stability by stalling replication fork progression and other DNA transactions. The abundance of DPCs formation appears to be high in cells. DPC lesions smaller than approximately 8 to 11 kDa (proteolytic products), can be resolved by repair pathways, whereas other pathways are activated when DPCs have larger dimensions [52-54]. Stingele and coworkers described a DPC repair pathway that removes DPCs either during S-phase of the cell cycle by a DNA-dependent protease system, called Spartan (SPRTN/DVC1) [55-58], or through a replication-coupled proteolysis process that degrades DPCs into smaller peptides. DPCs also can be bypassed by translesion DNA polymerases (TLS) (Fig. 5). These mechanisms circumvent replisome disassembly. In this theme, the MCM2-7 helicase together with CDC45 and the GINS complex, form a DNA replicative helicase (CMG) that can bypass a leading strand DPC when DPC proteolysis is blocked. This process is facilitated by another helicase, RTEL1, that unwinds DNA, generating a single strand DNA region beyond the DPC; this allows CMG and DNA replication to bypass the DPC [54].
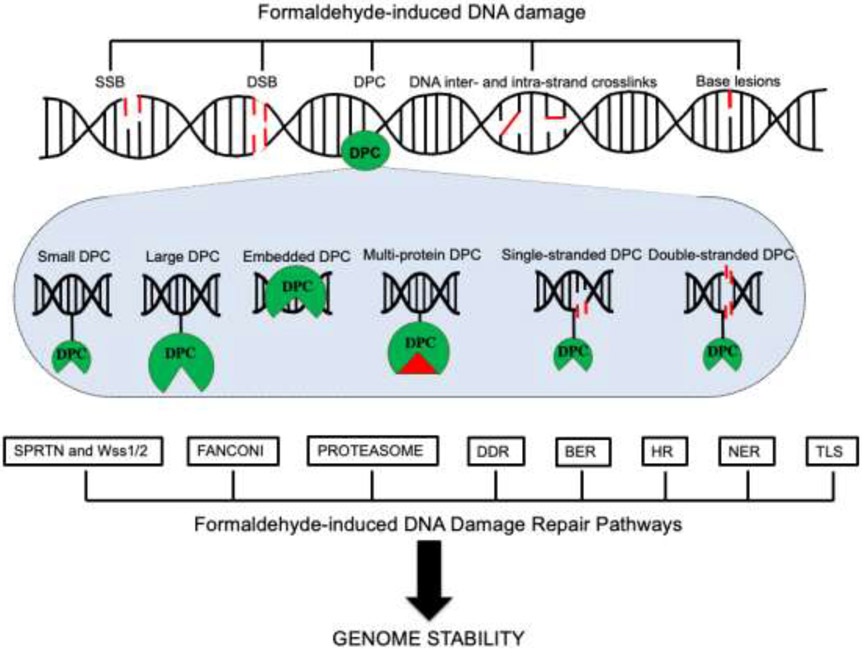
Fig. 5.
Summary of formaldehyde-induced DNA damage and repair. Endogenous formaldehyde can produce different lesions, such as single-strand (SSB) and double-strand DNA breaks (DSB), DNA-protein crosslinks (DPCs), base lesions, and DNA inter- and intra-strand crosslinks. Specialized DNA repair pathways are activated following formaldehyde-induced DNA damage to safeguard genome stability. Formaldehyde-DNA-induced lesions are repaired by DNA damage response repair pathway (DDR), homologous recombination (HR), nucleotide excision repair (NER), base excision repair (BER) and translesion synthesis (TLS). DPCs are predominantly resolved by DPCs-proteolysis repair systems that involve SPRTN and Wss1/2, the Fanconi complex and the proteasome system.
Formaldehyde-induced DPCs activate the Fanconi anemia protein complex facilitating repair of the DNA lesion [59, 60]. Cells deficient in the Fanconi anemia pathway are hypersensitive to formaldehyde treatment. Overall, multiple DPC DNA repair pathways, reflecting the range of lesions induced by formaldehyde, are involved in repairing formaldehyde-induced lesions. Based on studies in mammalian systems and in yeast [55, 56, 61] these are BER, NER, HR, TLS, Fanconi anemia repair and DPC repair (Fig. 5).
5. Formaldehyde Dysregulation and Mitochondrial DNA Damage
A few reports have linked formaldehyde to the mitochondrial compartment [62, 63]. Evidence from microarray analysis indicates that deregulation of intracellular formaldehyde alters expression of many nuclear genes, including those linked to formaldehyde metabolism [45]. Increased intracellular formaldehyde has the potential to induce DPCs, making nucleic acids and proteins more vulnerable to degradation and in some cases inducing DPC signalling. These lesions in nuclear DNA are repaired by systems related to BER and by other repair pathways (Fig. 5) [64-67]. Mitochondria have such proteasomal-based systems [61, 65]. These are coupled to both BER and NER. Consequently, mtDNA transactions may be vulnerable to formaldehyde-induced DPCs [50, 68-70]. Consistent with this notion, we have identified in both published and preliminary experiments, accumulation of nuclear strand break markers γH2AX and p53BP1 in mtDNA after formaldehyde treatment [45]. Given the well-known crosslinking effects of formaldehyde and the production of mtDNA-protein crosslinks, this might readily explain formaldehyde-induced strand break formation. A surprising observation, however, is that DSB repair is not well established within mitochondria [69, 70], and we did not expect to find strong accumulation of DSB proteins in the mtDNA. The results suggested that signalling of mitochondrial strands breaks to the nucleus must be robust. One explanation is that mitochondrial strand break signalling could be mediated by the innate immune system. The pattern-recognition receptors of the innate immune system are activated by nucleic acids, triggering multiple cellular signalling systems that are components of the cytosolic innate immune response.
DNA strand breaks in the cytoplasm are detected by the DNA sensing system that involves cyclic GMP-AMP synthase (cGAS). cGAS functions upstream of stimulator of interferon genes, STING, and naturally is inactive in cells. After binding to DNA, cGAS becomes activated, and the second messenger cyclic GMP-AMP (cGAMP) is produced. cGAMP activates STING, and stimulates TANK-binding kinase (TBK1). This cascade of events is followed by a series of phosphorylation reactions that trigger the expression of interferon-stimulated genes that coordinate the well-recognized anti-viral innate immune response (Fig. 6) [71-76]. Interestingly, strand breaks induced by formaldehyde within mitochondrial DNA may cause mitochondrial DNA to leak into the cytoplasm, serving as a trigger for the innate immune response, and may be eventually destroyed by the 3' repair exonuclease 1 (TREX1). TREX1 plays a central role as a cytosolic 3' exonuclease that degrades cytosolic DNA to prevent activation of intracellular DNA sensors, inflammation, and genome instability. However, TREX1 does not directly degrade damaged 3' termini containing 3' obstructive groups [77], such as a DPC.
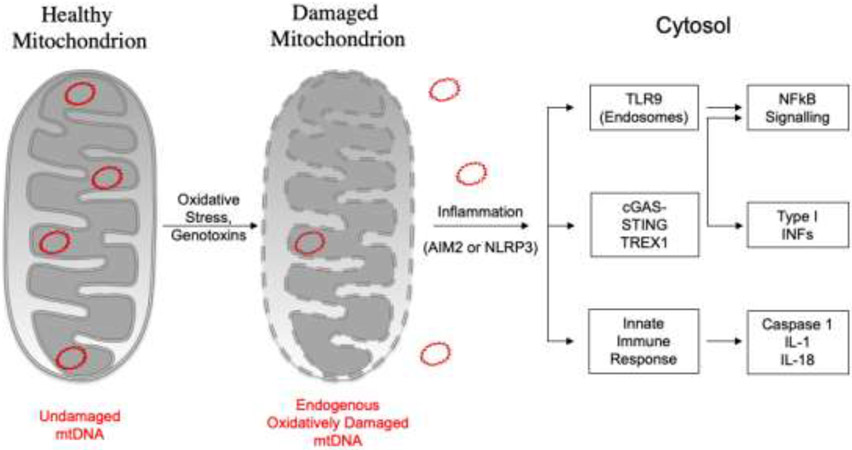
Fig. 6.
Inflammatory signalling pathways activated by mtDNA. In mammalian cells, the release of mtDNA into the cytosol induces different inflammatory signalling pathways, including endosomal localized TLR9, cGAS-STING or the cytosolic inflammasome (AIM2 or NLRP3).
There are a number of Mendelian inborn metabolism error syndromes related to uncontrolled immune responses, known as type I interferonopathies. These conditions are associated with mutations in enzymes directly or indirectly involved in clearance of cytosolic DNA and DNA-RNA hybrids [78]. Recently, Yang and coworkers reported that knock-down of N-glycanase 1 (Ngly1), a deglycosylation enzyme that plays a role in immune homeostasis, activates DNA- and RNA-sensing pathways. They observed leakage of both mtDNA and RNA into the cytoplasm of Ngly1−/− cells, and this was associated with impaired mitophagy. To date, a broad range of autoimmune and inflammatory diseases, including Aicardi-Goutières syndrome and systemic lupus erythematosus, have been associated to DNA damage and deficiency in cytosolic nucleic acid metabolizing enzymes, such as TREX1 [79]. In addition, Wu and coworkers showed that in Tfam+/− cells, doxorubicin and PARP-9 knockdown caused mtDNA leakage into the cytoplasm. This release was a consequence of mtDNA damage and involved IFN–JAK–STAT signalling and interferon type I induction. In this system, mtDNA stress was shown to enhance nuclear DNA repair capacity, with PARP-9 playing a role in this type of DNA damage response [80]. Importantly, depletion of the mtDNA quality control enzyme, endonuclease G-like 1 (EXOG), exacerbated interferon type I induction in Tfam+/− cells, suggesting involvement of EXOG in innate immune signalling [81]. It is noteworthy that newly synthesized mtDNA is particularly vulnerable to oxidation and nuclease activity due to its close proximity to the respiratory chain complex before packaging into nucleoids. This can generate oxidatively-induced mtDNA damage, promoting DNA leakage into the cytosol and activating the NLRP3 inflammasome (Fig. 6) [82]. An interface between formaldehyde and the innate immune system and mitochondria has been appreciated [81, 83, 84]. Mitochondria participate in innate immunity at several levels, such that this innate immune system can regulate energy requirement responses within the mitochondria [85, 86].
6. Survey of Formaldehyde in Disease
Formaldehyde is classified as a carcinogen and is associated with many human illnesses, including neurodegenerative disorders and age-related diseases. In the last decade, in vitro and in vivo studies, indicated that formaldehyde excess reduces global DNA methylation by interfering with DNMT functions, and this has been associated with memory loss and age-related damage to neurons [87]. Formaldehyde-induced norepinephrine deficiency also has been reported to be related with cognitive decline syndromes [88].
A hallmark of the rare pediatric neurodegenerative genetic disease, known as Sarcosinemia, is the presence of high levels of sarcosine in the blood and urine. Sarcosinemia is associated with severe mental retardation. It is a recessive disorder with loss-of-function mutations in the sarcosine dehydrogenase gene (SARDH) that result in a reduction of the enzymatic activity of SARDH in mitochondria. Under this condition, sarcosine is not converted to formaldehyde and glycine, leading to sarcosine accumulation. Increased formaldehyde has been linked to Ruijs-Aalfs syndrome, a genetic condition characterized by genomic instability and progeria-like-features. Maternal and fetal aldehyde catabolism cooperates with the Fanconi anemia DNA repair pathway to preserve development [89], and mice deficient in this repair system spontaneously develop bone marrow toxicity and leukemia. Short life span and multisystem disorders are also associated with deficiency of formaldehyde clearance in mice and humans [90-92]. Prostate and bladder cancer patients have higher levels of urinary formaldehyde compared to healthy subjects and elevated concentrations of formaldehyde have been measured in different tissues from patients with breast or lung tumors.
7. Perspectives
Metabolic homeostasis of formaldehyde is preserved by mechanisms that prevent formaldehyde dysregulation through controlled formaldehyde generation and degradation processes. Formaldehyde excess has detrimental effects on genome stability, posing a threat to normal cellular functions. DNA repair mechanisms that become activated when formaldehyde metabolism is altered are the subject of ongoing investigation. The response to the apparent formaldehyde-induced mitochondrial strand breaks may involve mitotophagy and cytoplasmic DNA signalling pathways functioning in part in the innate immune system; these possibilities are under investigation, as are potential roles of cytoplasmic DNA degradation enzymes, such as TREX1.
Acknowledgments
We thank William C. Copeland, Fred W. Perrino, Michael B. Fessler and Paul W. Doetsch for discussions and comments on the manuscript; we also thank Julie K. Horton and William Beard for comments. This work was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences-NIH, Project Number Z01-ES050159.
Abbreviations:
- DNMTs
DNA methyltransferases
- JmjC
Jumonji C
- LSD1
lysine-specific demethylase 1
- SAM
S-adenosylmethionine
- ADH5
alcohol dehydrogenase 5
- ALDH2
mitochondrial aldehyde dehydrogenase 2
- NADPH
nicotinamide adenine dinucleotide phosphate
- MDA
malondialdehyde
- SSAO
semicarbazide-sensitive amine oxidase
- MAO-A
monoamine oxidase-A
- SHMT
serine hydroxymethyl transferase
- THF
tetrahydrofolate
- CH2-THF
N5, N10-methylene tetrahydrofolate
- MTHFD 1
methylene-THF dehydrogenase 1
- MTHFD 2
methylene-THF 2
- MTHFD 2L
methylene-THF 2 like
- MTHFD1L
methylene-THF dehydrogenase 1 like
- CYPs
cytochrome P450 monooxygenases
- DSBs
double-strand DNA breaks
- BER
base excision repair
- DAMPs
damage-associated molecular patterns
- PINK1
PTEN-induced kinase
- mtDNA
mitochondrial DNA
- STING
stimulator of interferon genes
- MGME1
mitochondrial genome maintenance exonuclease 1
- DPCs
DNA-protein crosslinks
- POLG
DNA polymerase γ
- AlkB
alkane monooxygenase
- SPRTN
Spartan
- TLS
translesion DNA polymerases
- MCM2-7
minichromosome maintenance protein complex
- CMG
CDC45-MCM-GINS complex
- RTEL1
regulator of telomere elongation helicase 1
- NER
nucleotide excision repair
- HR
homologous recombination
- cGAS
cyclic GMP-AMP synthase
- cGAMP
messenger cyclic GMP-AMP
- TBK1
TANK-binding kinase
- TREX1
3' repair exonuclease 1
- Ngly1
N-glycanase 1
- EXOG
endonuclease G-like 1
- SARDH
sarcosine dehydrogenase gene
- DDR
DNA damage response repair pathway
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Li E, Bestor TH, Jaenisch R, Targeted mutation of the DNA methyltransferase gene results in embryonic lethality, Cell, 69 (1992) 915–926. [DOI] [PubMed] [Google Scholar]
- [2].Okano M, Bell DW, Haber DA, Li E, DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development, Cell, 99 (1999) 247–257. [DOI] [PubMed] [Google Scholar]
- [3].MacAllister SL, Choi J, Dedina L, O'Brien PJ, Metabolic mechanisms of methanol/formaldehyde in isolated rat hepatocytes: carbonyl-metabolizing enzymes versus oxidative stress, Chem. Biol. Interact, 191 (2011) 308–314. [DOI] [PubMed] [Google Scholar]
- [4].Dorokhov YL, Shindyapina AV, Sheshukova EV, Komarova TV, Metabolic methanol: molecular pathways and physiological roles, Physiol. Rev, 95 (2015) 603–644. [DOI] [PubMed] [Google Scholar]
- [5].Diliberto EJ, Axelrod J, Regional and subcellular distribution of protein carboxymethylase in brain and other tissues, J. Neurochem, 26 (1976) 1159–1165. [DOI] [PubMed] [Google Scholar]
- [6].Pocker Y, Li H, Kinetics and Mechanism of Methanol and Formaldehyde Interconversion and Formaldehyde Oxidation Catalyzed by Liver Alcohol Dehydrogenase, Adv. Exp. Med. Biol, (1991) 315–325. [DOI] [PubMed] [Google Scholar]
- [7].Frisell WR, Mackenzie CG, Separation and purification of sarcosine dehydrogenase and dimethylglycine dehydrogenase, J. Biol. Chem, 237 (1962) 94–98. [PubMed] [Google Scholar]
- [8].Lambert CE, Shank RC, Role of formaldehyde hydrazone and catalase in hydrazine-induced methylation of DNA guanine, Carcinogenesis, 9 (1988) 65–70. [DOI] [PubMed] [Google Scholar]
- [9].Tephly TR, The toxicity of methanol, Life Sci., 48 (1991) 1031–1041. [DOI] [PubMed] [Google Scholar]
- [10].Skrzydlewska E, Toxicological and metabolic consequences of methanol poisoning, Toxicol. Mech. Methods, 13 (2003) 277–293. [DOI] [PubMed] [Google Scholar]
- [11].Sweeting JN, Siu M, Wiley MJ, Wells PG, Species- and strain-dependent teratogenicity of methanol in rabbits and mice, Reprod. Toxicol, 31 (2011) 50–58. [DOI] [PubMed] [Google Scholar]
- [12].Guo L, Zeng XY, Wang DY, Li GQ, Methanol metabolism in the Asian corn borer, Ostrinia furnacalis (Guenee) (Lepidoptera: Pyralidae), J. Insect Physiol, 56 (2010) 260–265. [DOI] [PubMed] [Google Scholar]
- [13].Wang SP, He GL, Chen RR, Li F, Li GQ, The involvement of cytochrome P450 monooxygenases in methanol elimination in Drosophila melanogaster larvae, Arch. Insect Biochem. Physiol, 79 (2012) 264–275. [DOI] [PubMed] [Google Scholar]
- [14].Xu M, Tang H, Rong Q, Zhang Y, Li Y, Zhao L, Ye G, Shi F, Lv C, The Effects of Formaldehyde on Cytochrome P450 Isoform Activity in Rats, Biomed. Res. Int, (2017) 6525474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li FX, Lu J, Xu YJ, Tong ZQ, Nie CL, He RQ, Formaldehyde-mediated chronic damage may be related to sporadic neurodegeneration, Prog. Biochem. Biophys, 35 (2008) 393–400. [Google Scholar]
- [16].Nakamura J, Shimomoto T, Collins LB, Holley DW, Zhang ZF, Barbee JM, Sharma V, Tian X, Kondo T, Uchida K, Yi XW, Perkins DO, Willis MS, Gold A, Bultman SJ, Evidence that endogenous formaldehyde produces immunogenic and atherogenic adduct epitopes, Sci. Rep, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boor PJ, Trent MB, Lyles GA, Tao M, Ansari GAS, Methylamine Metabolism to Formaldehyde by Vascular Semicarbazide-Sensitive Amine Oxidase, Toxicology, 73 (1992) 251–258. [DOI] [PubMed] [Google Scholar]
- [18].Yu PH, Zuo DM, Oxidative Deamination of Methylamine by Semicarbazide-Sensitive Amine Oxidase Leads to Cytotoxic Damage in Endothelial-Cells - Possible Consequences for Diabetes, Diabetes, 42 (1993) 594–603. [DOI] [PubMed] [Google Scholar]
- [19].Obata T, Diabetes and semicarbazide-sensitive amine oxidase (SSAO) activity: A review, Life Sci., 79 (2006) 417–422. [DOI] [PubMed] [Google Scholar]
- [20].Yu PH, Involvement of cerebrovascular semicarbazide-sensitive amine oxidase in the pathogenesis of Alzheimer's disease and vascular dementia, Med. Hypotheses, 57 (2001) 175–179. [DOI] [PubMed] [Google Scholar]
- [21].Yu PH, Wright S, Fan EH, Lun ZR, Gubisne-Harberle D, Physiological and pathological implications of semicarbazide-sensitive amine oxidase, Biochim. Biophys. Acta Proteins Proteom, 1647 (2003) 193–199. [DOI] [PubMed] [Google Scholar]
- [22].O'Sullivan J, Unzeta M, Healy J, O'Sullivan MI, Davey G, Tipton KF, Semicarbazide-sensitive amine oxidases: Enzymes with quite a lot to do, Neurotoxicology, 25 (2004) 303–315. [DOI] [PubMed] [Google Scholar]
- [23].Li H, Luo WH, Lin JJ, Lin ZX, Zhang Y, Assay of plasma semicarbazide-sensitive amine oxidase and determination of its endogenous substrate methylamine by liquid chromatography, J. Chromatogr. B Analyt. Technol. Biomed. Life Sci, 810 (2004) 277–282. [DOI] [PubMed] [Google Scholar]
- [24].Jalkanen S, Salmi M, VAP-1 and CD73, endothelial cell surface enzymes in leukocyte extravasation, Arterioscler. Thromb. Vasc. Biol, 28 (2008) 18–26. [DOI] [PubMed] [Google Scholar]
- [25].Nunes SF, Figueiredo IV, Pereira JS, de Lemos ET, Reis F, Teixeira F, Caramona MM, Monoamine oxidase and semicarbazide-sensitive amine oxidase kinetic analysis in mesenteric arteries of patients with type 2 diabetes, Physiol. Res, 60 (2011) 309–315. [DOI] [PubMed] [Google Scholar]
- [26].Kazachkov M, Chen K, Babiy S, Yu PH, Evidence for in vivo scavenging by aminoguanidine of formaldehyde produced via semicarbazide-sensitive amine oxidase-mediated deamination, J. Pharmacol. Exp. Ther, 322 (2007) 1201–1207. [DOI] [PubMed] [Google Scholar]
- [27].Anderson MM, Hazen SL, Hsu FF, Heinecke JW, Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation, J. Clin. Invest, 99 (1997) 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zheng Y, Lin TY, Lee G, Paddock MN, Momb J, Cheng Z, Li Q, Fei DL, Stein BD, Ramsamooj S, Zhang G, Blenis J, Cantley LC, Mitochondrial One-Carbon Pathway Supports Cytosolic Folate Integrity in Cancer Cells, Cell, 175 (2018) 1546–1560.e1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pietzke M, Burgos-Barragan G, Wit N, Tait-Mulder J, Sumpton D, Mackay GM, Patel KJ, Vazquez A, Amino acid dependent formaldehyde metabolism in mammals, Commun. Chem, 3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kumar D, de Visser SP, Sharma PK, Derat E, Shaik S, The intrinsic axial ligand effect on propene oxidation by horseradish peroxidase versus cytochrome P450 enzymes, J. Biol. Inorg. Chem, 10 (2005) 181–189. [DOI] [PubMed] [Google Scholar]
- [31].Zanger UM, Schwab M, Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation, Pharmacol. Ther, 138 (2013) 103–141. [DOI] [PubMed] [Google Scholar]
- [32].Konstandi M, Johnson EO, Lang MA, Consequences of psychophysiological stress on cytochrome P450-catalyzed drug metabolism, Neurosci. Biobehav. Rev, 45 (2014) 149–167. [DOI] [PubMed] [Google Scholar]
- [33].Anzenbacher P, Anzenbacherova E, Cytochromes P450 and metabolism of xenobiotics, Cell. Mol. Life Sci, 58 (2001) 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cederbaum AI, Methodology to assay CYP2E1 mixed function oxidase catalytic activity and its induction, Redox Biol., 2 (2014) 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bart AG, Scott EE, Structural and functional effects of cytochrome b(5) interactions with human cytochrome P450 enzymes, J. Biol. Chem, 292 (2017) 20818–20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stavropoulou E, Pircalabioru GG, Bezirtzoglou E, The Role of Cytochromes P450 in Infection, Front. Immunol, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pontel LB, Rosado IV, Burgos-Barragan G, Garaycoechea JI, Yu R, Arends MJ, Chandrasekaran G, Broecker V, Wei W, Liu LM, Swenberg JA, Crossan GP, Patel KJ, Endogenous Formaldehyde Is a Hematopoietic Stem Cell Genotoxin and Metabolic Carcinogen, Mol. Cell, 60 (2015) 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Burgos-Barragan G, Wit N, Meiser J, Dingler FA, Pietzke M, Mulderrig L, Pontel LB, Rosado IV, Brewer TF, Cordell RL, Monks PS, Chang CJ, Vazquez A, Patel KJ, Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism, Nature, 548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McGhee JD, Von Hippel PH, Formaldehyde as a probe of DNA structure. 4. Mechanism of the initial reaction of formaldehyde with DNA, Biochemistry, 16 (1977) 3276–3293. [DOI] [PubMed] [Google Scholar]
- [40].Lu K, Ye W, Gold A, Ball LM, Swenberg JA, Formation of S-[1-(N2-deoxyguanosinyl)methyl]glutathione between glutathione and DNA induced by formaldehyde, J. Am. Chem. Soc, 131 (2009) 3414–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Delrue I, Verzele D, Madder A, Nauwynck HJ, Inactivated virus vaccines from chemistry to prophylaxis: merits, risks and challenges, Expert Rev. Vaccines, 11 (2012) 695–719. [DOI] [PubMed] [Google Scholar]
- [42].Grafstrom RC, Fornace AJ, Autrup H, Lechner JF, Harris CC, Formaldehyde Damage to DNA and Inhibition of DNA-Repair in Human Bronchial Cells, Science, 220 (1983) 216–218. [DOI] [PubMed] [Google Scholar]
- [43].Kumari A, Owen N, Juarez E, McCullough AK, BLM protein mitigates formaldehyde-induced genomic instability, DNA Repair, 28 (2015) 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].J JV, Betancourt N, Carranza-Rosales P, Viveros-Valdez E, Guzmán-Delgado N, López-Márquez F, Moran J, Formaldehyde Induces DNA Strand Breaks on Spermatozoa and Lymphocytes of Wistar Rats, Cytol. Genet, 51 (2017) 65–73. [PubMed] [Google Scholar]
- [45].Nadalutti CA, Stefanick DF, Zhao ML, Horton JK, Prasad R, Brooks AM, Griffith JD, Wilson SH, Mitochondrial dysfunction and DNA damage accompany enhanced levels of formaldehyde in cultured primary human fibroblasts, Sci. Rep, 10 (2020) 5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, Cai H, Borsche M, Klein C, Youle RJ, Parkin and PINK1 mitigate STING-induced inflammation, Nature, 561 (2018) 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [47].Wanrooij S, Luoma P, van Goethem G, van Broeckhoven C, Suomalainen A, Spelbrink JN, Twinkle and POLG defects enhance age-dependent accumulation of mutations in the control region of mtDNA, Nucleic Acids Res., 32 (2004) 3053–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF, Oxidative stress induces degradation of mitochondrial DNA, Nucleic Acids Res., 37 (2009) 2539–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nadalutti CA, Ciesielski GL, Oliveira MT, Jacobs HT, Griffith JD, Kaguni LS, Structural rearrangements in the mitochondrial genome of Drosophila melanogaster induced by elevated levels of the replicative DNA helicase, Nucleic Acids Res., 46 (2018) 3034–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Caston RA, Demple B, Risky repair: DNA-protein crosslinks formed by mitochondrial base excision DNA repair enzymes acting on free radical lesions, Free Radic. Biol. Med, 107 (2017) 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cline SD, Mitochondrial DNA damage and its consequences for mitochondrial gene expression, Biochim. Biophys. Acta, 1819 (2012) 979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ide H, Shoulkamy MI, Nakano T, Miyamoto-Matsubara M, Salem AMH, Repair and biochemical effects of DNA-protein crosslinks, Mutat. Res, 711 (2011) 113–122. [DOI] [PubMed] [Google Scholar]
- [53].Duxin JP, Dewar JM, Yardimci H, Walter JC, Repair of a DNA-Protein Crosslink by Replication-Coupled Proteolysis, Cell, 159 (2014) 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sparks JL, Chistol G, Gao AO, Räschle M, Larsen NB, Mann M, Duxin JP, Walter JC, The CMG Helicase Bypasses DNA-Protein Cross-Links to Facilitate Their Repair, Cell, 176 (2019) 167–181.e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stingele J, Schwarz MS, Bloemeke N, Wolf PG, Jentsch S, A DNA-Dependent Protease Involved in DNA-Protein Crosslink Repair, Cell, 158 (2014) 327–338. [DOI] [PubMed] [Google Scholar]
- [56].Lopez-Mosqueda J, Maddi K, Prgomet S, Kalayill S, Marinovic-Terzic I, Terzic J, Dikic I, SPRTN is a mammalian DNA-binding metalloprotease that resolves DNA-protein crosslinks, Elife, 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stingele J, Bellelli R, Alte F, Hewitt G, Sarek G, Maslen SL, Tsutakawa SE, Borg A, Kjaer S, Tainer JA, Skehel JM, Groll M, Boulton SJ, Mechanism and Regulation of DNA-Protein Crosslink Repair by the DNA-Dependent Metalloprotease SPRTN, Mol. Cell, 64 (2016) 688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Stingele J, Bellelli R, Boulton SJ, Mechanisms of DNA-protein crosslink repair, Nat. Rev. Mol. Cell Biol, 18 (2017) 563–573. [DOI] [PubMed] [Google Scholar]
- [59].Noda T, Takahashi A, Kondo N, Mori E, Okamoto N, Nakagawa Y, Ohnishi K, Zdzienicka MZ, Thompson LH, Helleday T, Asada H, Ohnishi T, Repair pathways independent of the Fanconi anemia nuclear core complex play a predominant role in mitigating formaldehyde-induced DNA damage, Biochem. Biophys. Res. Comm, 404 (2011) 206–210. [DOI] [PubMed] [Google Scholar]
- [60].Rosado IV, Langevin F, Crossan GP, Takata M, Patel KJ, Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway, Nat. Struct. Mol. Biol, 18 (2011) 1432–1434. [DOI] [PubMed] [Google Scholar]
- [61].Anandarajan V, Noguchi C, Oleksak J, Grothusen G, Terlecky D, Noguchi E, Genetic investigation of formaldehyde-induced DNA damage response in Schizosaccharomyces pombe, Curr Genet, 66 (2020) 593–605. [DOI] [PubMed] [Google Scholar]
- [62].Van Buskirk JJ, Frisell WR, Inhibition by formaldehyde of energy transfer and related processes in rat liver mitochondria. II. Effects on energy-linked reactions in intact mitochondria and phosphorylating particles, Arch. Biochem. Biophys, 132 (1969) 130–138. [DOI] [PubMed] [Google Scholar]
- [63].Zerin T, Kim JS, Gil HW, Song HY, Hong SY, Effects of formaldehyde on mitochondrial dysfunction and apoptosis in SK-N-SH neuroblastoma cells, Cell Biol. Toxicol, 31 (2015) 261–272. [DOI] [PubMed] [Google Scholar]
- [64].Prasad R, Horton JK, Wilson SH, Requirements for PARP-1 covalent crosslinking to DNA (PARP-1 DPC), DNA Repair, 89 (2020) 102824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Reinking HK, Hofmann K, Stingele J, Function and evolution of the DNA-protein crosslink proteases Wss1 and SPRTN, DNA Repair, 88 (2020) 102822. [DOI] [PubMed] [Google Scholar]
- [66].Sun Y, Saha S, Wang W, Saha LK, Huang S-YN, Pommier Y, Excision repair of topoisomerase DNA-protein crosslinks (TOP-DPC), DNA Repair, 89 (2020) 102837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Quiñones JL, Thapar U, Wilson SH, Ramsden DA, Demple B, Oxidative DNA-protein crosslinks formed in mammalian cells by abasic site lyases involved in DNA repair, DNA Repair, 87 (2020) 102773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Phillips AF, Millet AR, Tigano M, Dubois SM, Crimmins H, Babin L, Charpentier M, Piganeau M, Brunet E, Sfeir A, Single-Molecule Analysis of mtDNA Replication Uncovers the Basis of the Common Deletion, Mol. Cell, 65 (2017) 527–538.e526. [DOI] [PubMed] [Google Scholar]
- [69].Moretton A, Morel F, Macao B, Lachaume P, Ishak L, Lefebvre M, Garreau-Balandier I, Vernet P, Falkenberg M, Farge G, Selective mitochondrial DNA degradation following double-strand breaks, PLoS One, 12 (2017) e0176795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhao LL, Mitochondrial DNA degradation: A quality control measure for mitochondrial genome maintenance and stress response, DNA Repair, 45 (2019) 311–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A, Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses, J. Leukoc. Biol, 75 (2004) 995–1000. [DOI] [PubMed] [Google Scholar]
- [72].Atianand MK, Fitzgerald KA, Molecular basis of DNA recognition in the immune system, J. Immunol, 190 (2013) 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Demaria S, Vanpouille-Box C, TREX1 is a checkpoint for innate immune sensing of DNA damage that fosters cancer immune resistance, Emerg. Top. Life Sci, 1 (2017) 509–515. [DOI] [PubMed] [Google Scholar]
- [74].Huang KW, Liu TC, Liang RY, Chu LY, Cheng HL, Chu JW, Hsiao YY, Structural basis for overhang excision and terminal unwinding of DNA duplexes by TREX1, PLoS Biol., 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mukherjee S, Abdisalaam S, Bhattacharya S, Srinivasan K, Sinha D, Asaithamby A, Mechanistic link between DNA damage sensing, repairing and signaling factors and immune signaling, DNA Repair, 115 (2019) 297–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Grieves JL, Fye JM, Harvey S, Grayson JM, Hollis T, Perrino FW, Exonuclease TREX1 degrades double-stranded DNA to prevent spontaneous lupus-like inflammatory disease, PNAS, 112 (2015) 5117–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Harrigan JA, Fan J, Momand J, Perrino FW, Bohr VA, Wilson DM 3rd, WRN exonuclease activity is blocked by DNA termini harboring 3' obstructive groups, Mech. Ageing Dev, 128 (2007) 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Uggenti C, Lepelley A, Crow YJ, Self-Awareness: Nucleic Acid–Driven Inflammation and the Type I Interferonopathies, Annu. Rev. Immunol, 37 (2019) 247–267. [DOI] [PubMed] [Google Scholar]
- [79].Yang K, Huang R, Fujihira H, Suzuki T, Yan N, N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1, J. Exp. Med, 215 (2018) 2600–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wu Z, Oeck S, West AP, Mangalhara KC, Sainz AG, Newman LE, Zhang X-O, Wu L, Yan Q, Bosenberg M, Liu Y, Sulkowski PL, Tripple V, Kaech SM, Glazer PM, Shadel GS, Mitochondrial DNA stress signalling protects the nuclear genome, Nat. Metab, 1 (2019) 1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS, Mitochondrial DNA stress primes the antiviral innate immune response, Nature, 520 (2015) 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin X.-j., Wong J, Ding S, Seki E, Schnabl B, Hevener AL, Greenberg HB, Kisseleva T, Karin M, New mitochondrial DNA synthesis enables NLRP3 inflammasome activation, Nature, 560 (2018) 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].West AP, Shadel GS, Ghosh S, Mitochondria in innate immune responses, Nat. Rev. Immunol, 11 (2011) 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Key J, Maletzko A, Kohli A, Gispert S, Torres-Odio S, Wittig I, Heidler J, Barcena C, Lopez-Otin C, Lei YJ, West AP, Munch C, Auburger G, Loss of mitochondrial ClpP, Lonp1, and Tfam triggers transcriptional induction of Rnf213, a susceptibility factor for moyamoya disease, Neurogenetics, 21 (2020) 187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Riley JS, Tait SW, Mitochondrial DNA in inflammation and immunity, EMBO Rep., 21 (2020) e49799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhao L, Sumberaz P, Mitochondrial DNA Damage: Prevalence, Biological Consequence, and Emerging Pathways, Chem. Res. Toxicol, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tulpule K, Dringen R, Formaldehyde in brain: an overlooked player in neurodegeneration?, J. Neurochem, 127 (2013) 7–21. [DOI] [PubMed] [Google Scholar]
- [88].Mei YF, Jiang C, Wan Y, Lv JH, Jia JP, Wang XM, Yang X, Tong ZQ, Aging-associated formaldehyde-induced norepinephrine deficiency contributes to age-related memory decline, Aging Cell, 14 (2015) 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Oberbeck N, Langevin F, King G, de Wind N, Crossan GP, Patel KJ, Maternal Aldehyde Elimination during Pregnancy Preserves the Fetal Genome, Mol. Cell, 55 (2014) 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nakamura J, Holley DW, Kawamoto T, Bultman SJ, The failure of two major formaldehyde catabolism enzymes (ADH5 and ALDH2) leads to partial synthetic lethality in C57BL/6 mice, Genes and Environment, 42 (2020) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Oka Y, Hamada M, Nakazawa Y, Muramatsu H, Okuno Y, Higasa K, Shimada M, Takeshima H, Hanada K, Hirano T, Kawakita T, Sakaguchi H, Ichimura T, Ozono S, Yuge K, Watanabe Y, Kotani Y, Yamane M, Kasugai Y, Tanaka M, Suganami T, Nakada S, Mitsutake N, Hara Y, Kato K, Mizuno S, Miyake N, Kawai Y, Tokunaga K, Nagasaki M, Kito S, Isoyama K, Onodera M, Kaneko H, Matsumoto N, Matsuda F, Matsuo K, Takahashi Y, Mashimo T, Kojima S, Ogi T, Digenic mutations in ALDH2 and ADH5 impair formaldehyde clearance and cause a multisystem disorder, AMeD syndrome, Science Advances, 6 (2020) eabd7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Shen X, Wang R, Kim MJ, Hu Q, Hsu CC, Yao J, Klages-Mundt N, Tian Y, Lynn E, Brewer TF, Zhang Y, Arun B, Gan B, Andreeff M, Takeda S, Chen J, Park JI, Shi X, Chang CJ, Jung SY, Qin J, Li L, A Surge of DNA Damage Links Transcriptional Reprogramming and Hematopoietic Deficit in Fanconi Anemia, Mol. Cell, 80 (2020) 1013–1024.e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]