Abstract
Introduction:
Children with benign epilepsy with centrotemporal spikes (BECTS) have rare seizures emerging from the motor cortex, which they outgrow in adolescence and additionally may have language deficits of unclear etiology. We piloted the use of transcranial magnetic stimulation paired with EMG and EEG (TMS-EMG, TMS-EEG) to test the hypotheses that net cortical excitability decreases with age and that use-dependent plasticity predicts learning.
Methods:
We assessed language and motor learning in 14 right-handed children with BECTS. We quantified two TMS metrics of left motor cortex excitability: the resting motor threshold (rMT; measure of neuronal membrane excitability) and amplitude of the N100 evoked potential (an EEG measure of GABAergic tone). To test plasticity, we applied 1 Hz repetitive TMS to the motor cortex to induce long term depression (LTD)-like changes in EMG and EEG evoked potentials.
Results:
Children with BECTS tolerate TMS; no seizures were provoked. rMT decreases with age, but is elevated above maximal stimulator output for half the group. N100 amplitude decreases with age after controlling for rMT. Motor cortex plasticity correlates significantly with language learning and at a trend level with motor learning.
Conclusions:
TMS is safe and feasible for children with BECTS, and TMS-EEG provides more reliable outcome measures than TMS-EMG in this group due to many children having unmeasurably high rMTs. Net cortical excitability decreases with age and motor cortex plasticity predicts not only motor but also language learning, suggesting a mechanism by which motor cortex seizures may interact with language development.
Keywords: Benign Epilepsy with Centrotemporal Spikes (BECTS), Transcranial Magnetic Stimulation with Electromyogram (TMS-EMG), Transcranial Magnetic Stimulation with Electroencephalogram (TMS-EEG), Plasticity, Language Learning
Benign Epilepsy with Centrotemporal Spikes (BECTS) accounts for 15–25% of all pediatric epilepsy cases. Children have focal seizures involving the face and arm typically during sleep, and the EEG shows sleep-potentiated spikes in a centrotemporal distribution1 arising from the sensorimotor cortex.2 Seizures are rare and resolve during adolescence. Intelligence quotients (IQs) are typically within the normal range,3 and patients have favorable social and occupational outcomes in adulthood.4 Many BECTS patients forego anti-seizure medications. However, despite its name, the BECTS syndrome is not entirely “benign.” Detailed neuropsychological profiles reveal deficits in many domains, including language,5 attention,6 and fine motor skills,7 though with significant individual variability. Studies suggest that BECTS patients have near-normal language abilities at time of diagnosis, with deficits developing over the course of the epilepsy5,8 and possibly persisting into adulthood,9,10 suggesting that the epilepsy may be associated with progressive cognitive impairment.
The etiology of neurocognitive comorbidities in BECTS is unclear. Many clinicians hypothesize that interictal spikes contribute to cognitive problems, either via transient disruptions in perception, processing, and reactivity during the day11 or via disruption of memory consolidation in sleep.12 Several (though not all5) studies identify a correlation between cognition and diurnal13 and nocturnal14,15 spike burden. BECTS may also affect cognition by altering functional brain organization. Lateralization of language to the left hemisphere is either delayed or permanently disrupted and the degree of disruption correlates with language performance.10,16–22 Xiao et al.,23 for instance, demonstrated that spikes enhance connectivity between the language and motor cortices, offering a potential mechanism by which spikes modify brain organization and cognition.
Transcranial magnetic stimulation (TMS) is a noninvasive form of brain stimulation that allows practical measures of cortical excitability and plasticity in epilepsy patients. A magnetic coil generates an extracranial magnetic field, which induces a focal intracranial electrical current, thus depolarizing underlying neurons. In its most common embodiment, TMS is applied over the motor cortex such that cortical activation can be confirmed and quantified in the form of motor evoked potentials (MEPs) recorded by surface EMG. For assay of non-motor cortex, the EEG response to TMS - termed TMS-EEG evoked potentials (TEPs) – can be measured. Only a few studies describe TMS-EEG in children and no such studies exist in pediatric epilepsy. Here, we present a pilot TMS-EMG-EEG study of children with BECTS assessing the feasibility of this methodology for studying pediatric epilepsy; we focused on two hypotheses.
First, given the predictable resolution of BECTS in late childhood, we hypothesized that net cortical excitability declines with age and applied TMS to characterize the balance of cortical excitation versus inhibition. We measured the resting motor threshold (rMT): the stimulation intensity necessary to elicit MEPs in 50% of trials. rMT is the most frequently reported measure of cortical excitability in TMS studies. The rMT may represent ion-channel-mediated excitability as it is increased by sodium channel antagonists24 but unchanged by γ-aminobutyric acid (GABA) agents. The rMT decreases in an age-dependent fashion in healthy children, stabilizing in adolescence.25 A prior TMS-EMG study in BECTS children26 did not identify a difference in rMT compared to controls. As GABA-A receptor mutations have been implicated in BECTS,27 we also measure the N100 TEP: a TMS-EEG indicator of mixed GABA-A and GABA-B tone. The N100 TEP decreases with GABA-A positive allosteric modulators (alprazolam, diazepam) and increases with GABA-B positive allosteric modulators (baclofen).28
Cortical plasticity often is defined as a use-dependent change in synaptic strength driven by neuronal firing patterns; it is the cellular underpinning of behavioral learning.29 High-frequency direct neuronal stimulation strengthens excitatory synapses, a process called long-term potentiation (LTP), while low-frequency stimulation weakens excitatory synaptic strength, called long-term depression (LTD). High- or low-frequency stimulation with repetitive TMS (rTMS) respectively excites or inhibits cortex, representing an approximation of LTP and LTD in humans.30,31 Accordingly, we also hypothesized that use-dependent plasticity mechanisms become saturated by frequent spikes in BECTS, thereby interfering with learning. Given concerns for seizure potentiation or induction32 with high-frequency TMS, we evaluated whether the magnitude of LTD-like changes induced by low-frequency rTMS correlate with learning.
METHODS
Subjects:
Right-handed, English-speaking children ages 5–12 years with BECTS were recruited from the Neurology Clinic or Neurodiagnostics Lab of Lucile Packard Children’s Hospital. Children had a history of at least one focal motor seizure affecting the face and/or arm and an EEG with sleep-potentiated spikes predominantly in a centrotemporal distribution. Exclusion criteria included a history of a severe neurologic disorder (i.e. neonatal encephalopathy, other seizure disorder, stroke), focal neurologic deficits on exam, or a history of prematurity. Imaging was not a prerequisite, but children with abnormal imaging as part of clinical care were excluded. The study protocol was approved by the Stanford University Institutional Review Board (IRB), but testing in a control population was not approved. Written consent was obtained from parents and assent from children. We recorded age of first seizure, medication use, epilepsy duration and number of lifetime seizures.
Neuropsychological Testing
Testing occurred on a separate day from TMS. We measured language learning with the learning slope subscore of the California Verbal Learning Test – Children’s Version (CVLT-C).33 We defined motor learning as improvement across trials of the Grooved Pegboard Test (Lafayette Instrument) with the dominant right hand:
We measured IQ using the Wechsler Abbreviated Scale of Intelligence – Second Edition (WASI-II),34 and imputed it for the single 5-year-old subject based on his Wechsler Preschool and Primary Scale of Intelligence score.35 We assessed attention using the Test of Variables of Attention.36
Transcranial Magnetic Stimulation
Set-Up:
Subjects underwent TMS in the morning or early afternoon. The TMS click was masked with noise-cancelling headphones. Alertness was confirmed and maintained via observation of the subject and EEG. To ensure consistent stimulation site delivery, the subject’s head and TMS coil were cross-registered to a representative MRI using the infrared-based netBrain Neuronavigation system. Stimulation was delivered using an EB Neuro ATES STM9000 magnetic stimulator with a butterfly coil held tangentially to the skull, with the handle oriented posteriorly, 45 degrees from midline. EMG recordings were measured from the abductor pollicis brevis (APB) muscle using the Electrical Geodesic, Inc Polygraphic Data acquisition system (sampled at 1000 Hz, low pass filtered at 1 Hz). EEG recordings were obtained with a 256 MicroCel Electrical Geodesic, Inc sensor net. Elefix conductive EEG paste was administered to 48 preselected electrodes, including the standard 10–20 montage, additional electrodes surrounding C3 and C4, and the bilateral mastoids (Figure 1). EEG data was referenced to Cz and impedances were kept below 5 kΩ. To minimize TMS artifact, EEG filters were left off and data was sampled at 1 kHz with fast recovery.
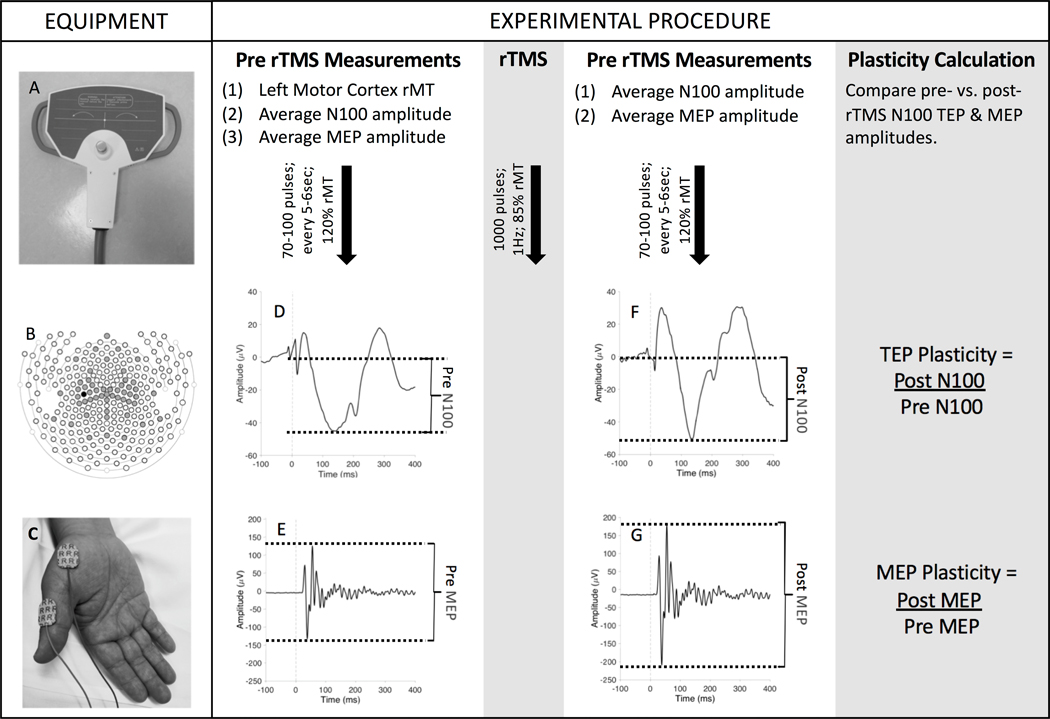
Figure 1. Summary of TMS-EMG-EEG Methodology:
EQUIPMENT: (A) Subjects received TMS to the left motor cortex using a 70mm butterfly coil. (B) A 257-lead EGI cap was placed and paste was applied to the 48 electrodes illustrated in gray (standard 10–20 montage plus additional central coverage); the TMS-EEG Evoked Potential (TEP) of interest (the N100 amplitude) was measured at the C3 electrode (black circle). (C) Motor evoked potentials (MEPs) were recorded from the right abductor pollicis brevis. EXPERIMENTAL PROCEDURE: Before repetitive TMS (rTMS), a resting motor threshold (rMT) was established. Subjects then received 70–100 single pulses of TMS to the left motor cortex. Resulting (D) N100 TEPs and (E) MEPs were grand averaged across all trials and the amplitude of this averaged waveform was calculated (baseline-to-peak for TEPs; peak-to-peak for MEPs). Baseline excitability was quantified by the rMT and pre-rTMS N100 TEP amplitude. Next, rTMS was administered to the left motor cortex. After rTMS administration, subjects again received 70–100 single pulses of TMS, and mean (F) N100 TEP and (G) MEP amplitudes were derived. Cortical plasticity was measured by calculating the ratio of post-rTMS to pre-rTMS TEP and MEP amplitudes.
Excitability Protocol:
Resting Motor Threshold (rMT):
The motor hotspot was identified as the location which evoked the largest MEPs from the right APB.37 The rMT of the left motor cortex was the minimal stimulation intensity to evoke a visible muscle twitch with time-locked EMG correlate on at least 5 out of 10 trials. In subjects with rMT greater than maximal stimulator output (MSO), we were also unable to elicit a twitch with the muscle contracted; for these subjects, we thus considered rMT as 100% MSO.
N100 Amplitude:
We administered 70–100 TMS pulses at 120% of the rMT (up to 100% MSO); we adjusted pulse number as needed with the goal of obtaining at least 60 high-quality trials per child.38 Pulses were separated by at least 5 seconds.
Plasticity Protocol:
We administered 70–100 TMS stimuli at 120% rMT, with pulses spaced by at least 5 seconds, immediately before and after rTMS. The rTMS train consisted of 1000 pulses at 1 Hz at 85% rMT.39
Data Processing
Processing of EMG Data:
EMG data were examined in MATLAB (R2017b, The MathWorks, Inc., Natick, MA, USA) using the EEGLAB toolbox.40 Trials with baseline EMG activity were rejected. The peak-to-peak amplitude of the MEP in the 100ms after stimulation was calculated for each trial and averaged across trials of each condition (pre/post rTMS).
Processing of EEG Data:
EEG analysis was performed in MATLAB using the TMS-EEG signal analyzer (TESA), an open-source extension of EEGLAB.40,41 We analyzed the pre- and post-rTMS pulses separately. Order of operations for EEG-TMS analysis were the following: (1) Data was segmented −500ms to +500ms. (2) EEG from −5 to 20ms was replaced with constant data to eliminate the majority of the TMS pulse artifact. (3) Data was baseline corrected using EEG preceding the pulse by −100ms to −6ms. (4) Data were visually inspected for profound artifacts (flatlining or noise unrelated to the TMS machine). Bad channels (mean of 2.7±1.5 per recording) were removed; electrodes with surrounding neighbors were interpolated. Bad trials were removed (average remaining trials 73±12). (5) TESA fast independent component analysis (fast ICA) was implemented to correct for TMS-induced muscle artifact, eye blinks, other muscle artifact and electrical noise. (6) EEG data was band-pass filtered from 1–100 Hz and band-stop filtered from 59–61 Hz. (7) A second fast ICA was performed to remove remaining artifacts. (8) Data was referenced to the average of all channels, TMS pulse artifact was linearly interpolated and data was baseline corrected to the level observed at −100 to −6ms before the pulse. (9) TEPs were averaged across all trials and the mean TEPs were then visualized. The amplitude of the largest negative peak at approximately 100ms (window 80–180ms) was calculated for condition (pre/post rTMS).
Quantification of Excitability:
rMT and the baseline, pre-rTMS N100 peak amplitude were calculated (Table 1).
Table 1:
TMS Measures of Excitability and Plasticity
| TMS Parameter | TMS Protocol | Definition | Likely Mechanism |
|---|---|---|---|
| Motor Threshold | Single pulses of TMS applied at varying intensities until individual threshold is defined. | Strength of TMS stimulus necessary to achieve a MEP (muscle twitch) >50μV in 50% of trials. Measured as a % MSO. If a MEP cannot be elicited, than rMT=100% MSO | Motor neuron membrane excitability |
| N100 Peak | Single pulses of TMS applied at 120% rMT. | Amplitude of the maximal negative peak seen on EEG at approximately 100msec after spTMS pulse | GABAA&B mediated intracortical and network inhibition |
| MEP Plasticity | Single pulses of TMS applied at 120% rMT followed by 1000 pulses of rTMS administered at 1 Hz at 85% rMT. Then single pulses TMS at 120% rMT applied a second time. |
Amplitude of MEP/N100 Peak After rTMS Amplitude of MEP/N100 Peak Prior to rTMS |
Long-term synaptic potentiation or depression. |
| TEP Plasticity |
MEP = Motor evoked potential; rMT=resting Motor Threshold; TEP = TMS-EEG Evoked Potential
Quantification of Plasticity:
The ratio of the mean MEP and N100 TEP amplitudes preceding and following rTMS were used to quantify plasticity (Table 1):
Statistical Analysis
Descriptive statistics (Table 2) were calculated for demographic, clinical, neurocognitive, and TMS data and are presented as mean ± standard deviation for normally distributed data and median [IQR] for non-normally distributed data. We compared MEP and TEP amplitudes before and after rTMS with a paired t-test. We then log transformed the following variables to better approximate a normal distribution: N100 amplitude, MEP plasticity, TEP plasticity, and Change in Pegboard Speed. Since 50% of children had a rMT greater than 100% MSO (and hence we were stimulating below their true rMT), we considered rMT as both a continuous and binary value (Elicitable rMT < 100% MSO < Supramaximal rMT).
Table 2:
Demographic, Clinical, Neurocognitive & TMS Data
| Demographics (n=14) | |
| Age (years) (mean+/−SD) | 9.2 +/−2.4 |
| Gender (% male) | 71% |
| Epilepsy Features (n=14) | |
| Seizure Medication Use | 50% |
| Age at First Seizure (years old) (mean+/−SD) | 7.2 +/−2.9y |
| Epilepsy Duration (years) (mean+/−SD) | 2.1 +/− 1.4yr |
| Lifetime Seizures (% with ≤5; % ≤10; % >10) | 50%; 21%; 29% |
| Predominant Spike Side on Diagnostic EEG
(Left/Right/Bilateral) |
14%; 43%; 43% |
| Neurocognitive Scores (n=14) | |
| IQ (mean, SD) | 103 +/− 13 |
| Inattention (n, %) | 5 (42%) |
| Language Learning (mean z-score, SD) | −0.14 +/− 1.0 |
| Motor Learning (% mean improvement, SD) | 12 +/− 16% |
| Excitability Measurements | |
| rMT(%MSO) (n=14) (median, IQR) | 98% [77 to 100%] |
| Supramaximal rMT(%) (rMT > 100% MSO) |
50% |
| N100 Amplitude (uV) (n=12) | −61 +/− 44 |
| Plasticity Measurements *** | |
| % MEP Change (n=6) (mean, SD) | +37% +/− 52% |
| % N100 Change** (n=9) (mean, SD) | −24 +/− 37% |
A negative number indicates that the N100 peak shrinks (approaches zero)
Wilcoxon Rank Sign test showed that the change in motor evoked potential (MEP) and TMS-EEG Evoked Potential (TEP) amplitude were ns (p>0.05).
For the first hypothesis, we tested the relationship between age and: (1) rMT (both continuous and binary); and (2) N100 amplitude using Pearson’s Correlation coefficient for continuous variables and student’s t-test for binary variables. Since TEP amplitude depends on stimulation intensity,42 and stimulation intensity cannot be normalized in individuals with a supramaximal rMT, we also investigated the impact of age on N100 amplitude after stratifying by presence/absence of supramaximal rMT using linear regression. In secondary analyses, we investigated the impact of medication use on excitability and the impact of age, medication use, and baseline excitability (N100 amplitude) on plasticity using student’s t-test for parametric and Wilcoxon Rank-Sum for non-parametric continuous data, and Fisher’s exact test for categorical data. For our second hypothesis, we first calculated Pearson’s correlation coefficient between learning (motor and language) and plasticity (MEP and TEP). We then controlled for IQ and attention using linear regression. Two-tailed p-values < 0.05 were considered significant, though we explored trends with p-values < 0.1.
RESULTS
Recruitment & Participation:
Fourteen children underwent neuropsychological testing and establishment of rMT. Two refused TMS beyond rMT determination due to scalp discomfort and three others did not finish the rTMS protocol due to inability to sit still. Therefore, we had rMT data for 14 subjects, N100 amplitude data for 12 subjects, and plasticity data for 9 subjects. Children otherwise tolerated TMS well. Importantly, no child had a seizure or substantial activation of spikes, even though we stimulated the “epileptogenic cortex” and half the group was unmedicated.
Demographic, Clinical, Neurocognitive and TMS Data (Table 2):
Children were 5–12 years-old and predominantly male. Half the group used seizure medications: five took levetiracetam, one sulthiame, and one a combination of levetiracetam and valproate. IQ and language-learning (CVLT-C) z-scores (mean = −0.14, SD=1) were normally distributed. Consistent with prior literature6, nearly half of the children had significant inattention. Children with BECTS had high rMT, with 7 of 14 (50%) having rMT greater than MSO (supramaximal rMT), and large N100 TEPs (−61μV ± 44 μV). rTMS did not significantly alter MEP (mean increase of 71.16 μV ± 11.7 μV, t=−1.56, DF=5, p=0.18) or N100 amplitude (mean decrease of 4.31 μV ± 20.16, t=0.64, DF=8, p=0.54).
Determinants of Cortical Excitability
Effect of Age:
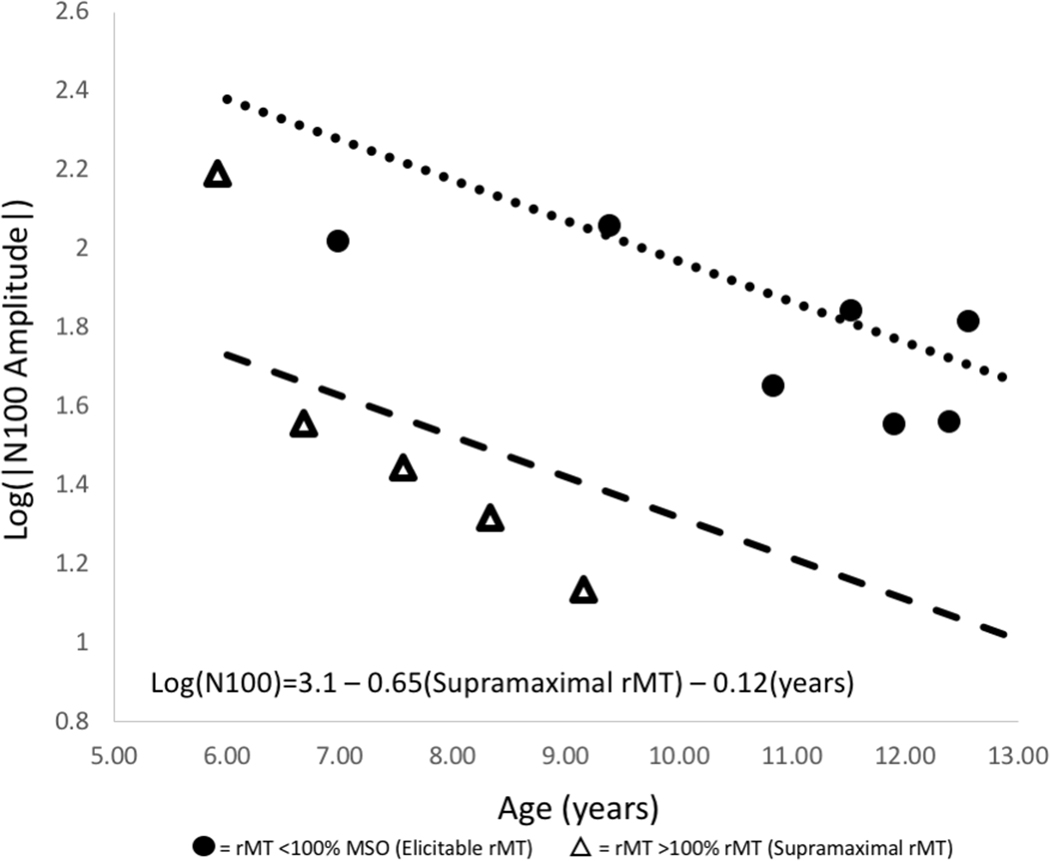
The rMT decreases with age (r=−0.64, 95% CI −0.87 to −0.17, p=0.01). Children with supramaximal rMTs are significantly younger (7.34 ± 1.24 years) than those with elicitable rMTs (10.79 ± 1.85 years) (t=3.93, df=12, p=0.002) and have smaller N100 amplitudes (−50.99 ± 59.49 μV in supramaximal vs. −67.66 +/− 32.04μV in elicitable rMT group, ns). We stratified by presence/absence of supramaximal rMT to account for the fact that children with supramaximal rMT received only “subthreshold” stimulation, which is known to have an impact on TEP amplitude.42 Supporting this concern, children with supramaximal rMTs have significantly smaller N100 amplitudes (ß=−0.65, 95% CI −1.08 to −0.23, p =0.007) than children with elicitable rMT of similar ages. After controlling for supramaximal rMT, N100 TEP amplitude decreases with age (ß=−0.12, 95% CI −0.21 to −0.03, p=0.02) (Figure 2).
Figure 2. N100 TEP Amplitude Decreases with Age:
N100 amplitude decreases as a function of age after controlling for whether rMT was elicitable (<100% MSO) or supramaximal (>100% MSO). N100 TEP amplitude increases as a function of stimulation intensity within a given individual. rMT is used to normalize stimulation intensity across individuals, but this cannot be done for those with supramaximal rMTs. Consequently, subjects with supramaximal rMTs (triangles) have smaller N100 amplitudes than those with elicitable rMTs (circles). Age becomes a significant predictor of N100 TEP amplitude after stratifying by rMT.
Effect of Medication:
Excitability measurements do not vary significantly with medication use. The rMT is lower (median difference 21%, 95% CI 0–26%, p=0.13) and N100 amplitude is larger (mean difference −13.97 μv, 95% CI −72.30 to 44.33 μV, p=0.61) in unmedicated compared to medicated children, but neither difference is significant.
Determinants of Cortical Plasticity:
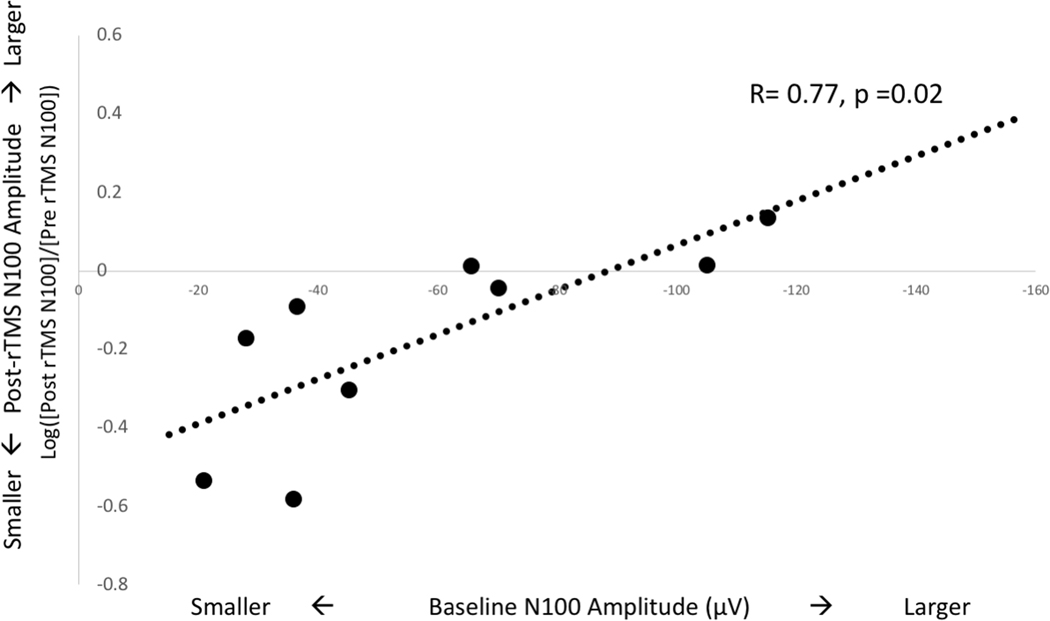
Of the nine subjects who underwent rTMS, 6 had elicitable rMTs and hence MEP data. MEP and TEP plasticity do not correlate with age. TEP plasticity differs with medication use: N100 amplitude decreases in medicated children (−60% ± 36%) but remains stable in unmedicated children (+1.32% ± 21.6%) after rTMS (mean difference 59.80%, 95% CI 27.53–92.04%, p=0.003). Finally, there is a strong correlation between baseline N100 amplitude and TEP plasticity (r=0.78, 95% CI 0.26–0.95, p = 0.01): rTMS induces an increase in N100 amplitude in those with larger baseline N100 peaks and a decrease in those with smaller peaks (Figure 3). Baseline MEP amplitudes do not correlate significantly with MEP plasticity.
Figure 3. N100 TEP Plasticity Depends on Baseline Excitability:
There is a strong relationship between baseline excitability and TEP plasticity. Children with smaller initial N100 TEP amplitudes experience a decrease in N100 amplitude after 1 Hz rTMS (circles below x-axis) while those with larger initial N100 TEP amplitudes have an increase in N100 amplitude after 1 Hz rTMS (circles above x-axis).
Cortical Plasticity & Learning
Plasticity and Motor Learning:
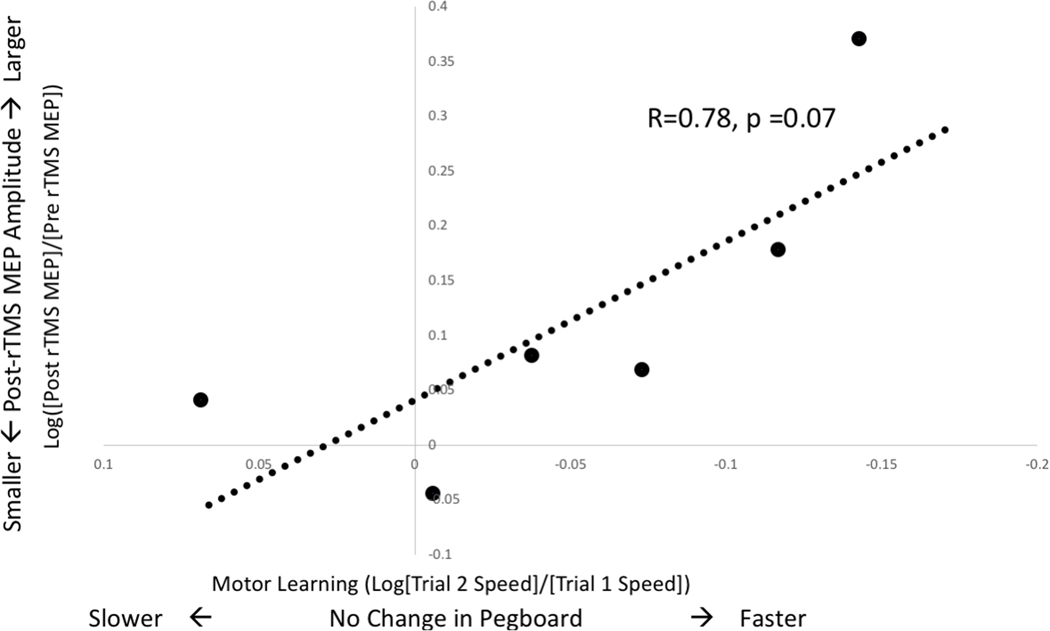
Children with the most substantial increases in MEP amplitude after rTMS also have the greatest increases in pegboard speed (Figure 4). The estimated correlation between MEP plasticity and motor learning is strong, but non-significant (r=0.78, 95% CI −0.09 to 0.97, p=0.07). The estimated correlation between motor learning and TEP plasticity is only moderate (r=0.58, 95% CI −0.13 to 0.90, p=0.11).
Figure 4. MEP Plasticity Correlates with Motor Learning:
MEP plasticity, a change from baseline amplitude, is represented here as movement away from the x-axis. MEP amplitude increases with rTMS (values above the x-axis) for most children and this change correlates with greater improvements in motor speed.
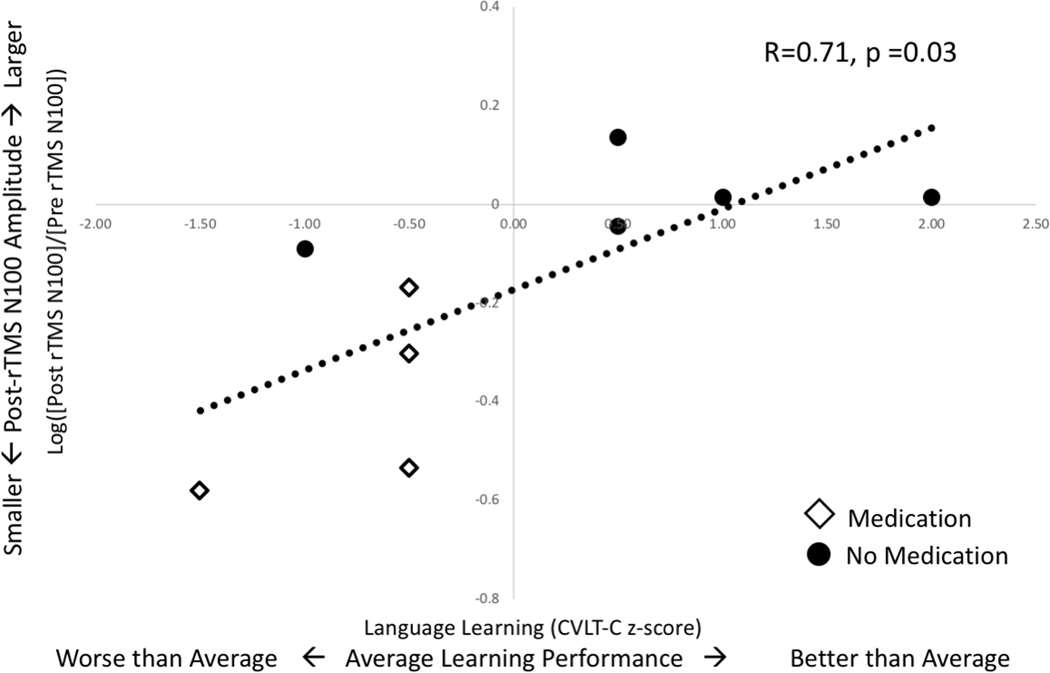
Plasticity and Language Learning:
TEP plasticity varies across subjects from a 74% decrease to a 37% increase in amplitude. The estimated correlation between TEP plasticity and language learning is strong (r=0.71, 95% CI 0.09 to 0.93, p=0.03) (Figure 5). Decreased N100 amplitude correlates with worse language learning, while increased N100 amplitude correlates with better language learning (ß=3.09, 95% CI 0.37 to 5.82, p=0.03). TEP plasticity predicts language learning after controlling for attention (ß=3.07, 95% CI 0.04 to 6.10, p=0.05) or IQ (ß=3.43, 95% CI −0.01 to 6.89, p=0.05). The estimated correlation between language learning and MEP plasticity is weak (r=0.40, 95% CI −0.61 to 0.92, p=0.46).
Figure 5. TEP Plasticity Correlates with Language Learning:
TEP plasticity, a change from baseline amplitude, is represented here as movement away from the x-axis. A decrease in N100 TEP amplitude after rTMS (values below x-axis) correlates with worse language learning scores while an increase in N100 TEP amplitude correlates with better scores. The trend line is fitted to all 9 points. However, it is notable that medicated (diamonds) and unmedicated (circles) children cluster differently with regard to these measurements.
Medication use may confound the relationship between language learning and plasticity. TEP plasticity differs significantly in medicated vs. unmedicated subjects. While medication use does not significantly correlate with language learning scores, 80% of the children with the lowest language learning scores were taking medication (Figure 5). If medication is included in the linear model, the relationship between language learning and plasticity becomes non-significant (ß =2.45, 95% CI −3.25 to 8.14, p=0.33).
DISCUSSION
In this pilot study, we used TMS-EMG and TMS-EEG to measure the trajectory of cortical excitability in children with BECTS and to assess whether motor cortex plasticity correlates with learning ability. TMS did not induce seizures or worsen EEG abnormalities, but our cohort had unusually high rMT, limiting MEP collection. In contrast, children had well-defined TEPs, suggesting that TMS-EEG may be a more robust tool in the pediatric epilepsy population. We find that rMT and N100 amplitude decrease in an age-dependent fashion, supporting the hypothesis that developmentally-mediated changes in cortical excitability continue in BECTS, and may explain the eventual resolution of this syndrome. We also find that TMS measurements of plasticity following 1 Hz rTMS correlate with behavioral learning: increased N100 amplitude correlates with improved language learning while increased MEP amplitude correlates at the trend level with improved motor learning.
Excitability
We find that rMT in BECTS subjects is much higher than previously reported in pediatric cohorts, but that it decreases in an age-dependent fashion similar to healthy children.43–48 Half our subjects have no elicitable MEPs, even with muscle activation; in contrast, an active MT can typically be established even in young children49 and rMT can be quantified in 80–95% of children over 6 years.43,44,50 The TMS literature in pediatric epilepsy is sparse, but in line with our experience, rMT exceeded MSO in >20% of children with focal epilepsy in the hemisphere opposite the epilepsy focus.45 Elevated rMT in BECTS could be caused by compensatory inhibition of the motor cortex to prevent seizure propagation. Alternatively, rMT elevation could be secondary to altered motor cortex connectivity that has been previously been reported in BECTS51; in healthy subjects, primary motor to premotor connectivity explains a substantial percent of rMT variance.52 As we did not have IRB approval to enroll controls, we must consider whether the elevated rMT is secondary to technical issues with our device; however, the healthy adult subjects undergoing TMS with the same equipment and same operator had a mean rMT of 64.5%. Furthermore, we confirmed a similar rMT in one BECTS subject using a Magventure MagPro device (83 vs. 89%).
rMT is lower in unmedicated children compared to those taking medications, though this difference is not significant. Levetiracetam,53,54 valproate,55 and sulthiame56 increase rMT in adults. One prior TMS study in BECTS26 showed that unmedicated patients had a rMT similar to controls that increased significantly after valproate initiation. Our results trend in the same direction and may fall short of significance due to insufficient power. A second consideration is that medicated children are more likely to have had multiple (>5) lifetime seizures (OR 15, CI 1.03–218, p=0.05) and hence may have increased cortical excitability compared to the unmedicated children; such baseline differences could obscure medication effect.
In contrast to rMT, we reliably obtained TEPs in every subject. There are very few studies of TMS-EEG in children, so our data contributes to this literature.42,48,50,57,58 Similar to the previous reports of healthy children, children with BECTS have high-amplitude TEPs 50 and 100ms after stimulation.42,48 BECTS children show an age-dependent decrease in N100 amplitude after controlling for rMT. This contrasts with healthy children42 in whom the age-dependent decrease in N100 amplitude is lost after correcting for rMT. The N100 peak is thought to represent a mixture of GABA tone28 – it is decreased by GABA-A and increased by GABA-B positive allosteric modulators – so the age-dependent decrease in N100 amplitude may represent increasing activity of GABA-A or decreasing activity of GABA-B receptors. A large body of work in animals and more limited literature in humans shows that many components of GABA transmission, including composition of the alpha-subunit of the GABA-A receptor, changes throughout childhood and adolescence (for review, Kilb 201259). Mutations in the gamma-subunit of the GABA-A receptor27 have also been identified in a small subset of BECTS patients, with one mutation decreasing GABA-A current. The change in N100 amplitude in our sample may represent an increase in GABA-A activity due to developmentally-mediated changes in GABA-A receptor subunits; such changes could contribute to the spontaneous resolution of BECTS in adolescence. This hypothesis could be tested by assessing the longitudinal impact of GABA-A modulators on N100 peak amplitude in children with BECTS.
Plasticity
In our subjects, rTMS induces a moderate increase in MEP amplitude and a small decrease in N100 amplitude, though neither change reaches significance. In adults, 1 Hz rTMS is considered a well-established protocol for reduction of cortical excitability, because it reduces MEP amplitude in approximately 80–90% of adults.60,61 In contrast, 80% of our subjects showed facilitation of the MEP. Response to 1 Hz rTMS has not been well described in children,62,63 but a study using continuous theta burst stimulation (which is typically inhibitory in adults) found that one-third of children with autism spectrum disorder had “paradoxical facilitation.”30 The impact of 1 Hz rTMS on the N100 peak is less documented, even in adults. Casula et al.39 report that N100 amplitude increases and MEP amplitude decreases after 1 Hz rTMS in healthy adults and conclude that this pair of findings indicates that 1 Hz rTMS increases cortical inhibition. A study assessing the impact of rTMS on children with ADHD50 found no change in MEPs and a decrease in N100 amplitude. The authors conclude that the N100 peak may be a more sensitive measure of cortical plasticity than MEPs and speculate that 1 Hz rTMS leads to an overall decrease in cortical inhibition in these children. Our results align with the previous pediatric population and suggest that low-frequency (1 Hz) stimulation may be excitatory in children, or at least children with neurologic disease.
Why would cortical response to low frequency stimulation differ in children with BECTS from that reported in adults? One consideration is medication effect. There is evidence that response to 1 Hz rTMS on MEP amplitude in healthy adults is governed by homeostatic mechanisms, switching from inhibitory to facilitatory when baseline excitability is decreased before application of rTMS (i.e. by seizure medication administration64 or inhibitory transcranial direct cortical stimulation65). Anti-seizure medications also modulate response to 1 Hz rTMS in adults with epilepsy, with inhibition of MEPs when drug levels are low and facilitation when levels are high.64 In our sample, N100 amplitude decreases after rTMS in medicated but not unmedicated subjects. Since greater N100 amplitude may represent greater inhibition, the decrease in N100 amplitude seen in our medicated subjects may represent a similar facilitatory response to 1 Hz rTMS as seen in adults. However, it is also notable that TEP plasticity of the N100 varies with the baseline N100 amplitude (Figure 3), such that children with the largest baseline N100 amplitudes have the greatest increases in N100 amplitude after rTMS; this suggests that homeostatic regulation of plasticity may be diminished in BECTS. A third consideration is that children may respond differently than adults to rTMS due to maturational brain differences, for example in GABA transmission as described above.59 Investigating the response of healthy children to 1 Hz rTMS could better elucidate this.
Plasticity & Learning
Our data support our a-priori hypothesis that MEP and TEP plasticity are biomarkers for the plasticity that subserves learning. Children with the greatest increase in MEP amplitude also have the greatest improvement in motor performance (Figure 4), though this finding was only at the trend level. Several studies found no correlation between MEP plasticity and motor learning in healthy adults66–68; these studies focused on high-frequency rTMS protocols that increase cortical excitability and hence model neuronal LTP. It is possible therefore that response to low-frequency rTMS is a superior biomarker of the plasticity underlying motor learning. Supporting this, motor learning enhances response to subsequent inhibitory rTMS more than excitatory rTMS, and furthermore, inhibitory rTMS enhances subsequent motor learning more prominently than excitatory rTMS.69 Since rTMS increased MEPs in 5 of 6 children, we cannot not determine if only magnitude (change from baseline) of plasticity matters or if directionality (i.e., increase vs. decrease in MEP amplitude) is also relevant. A larger study could examine the relevance of this directionality and could additionally test if rTMS improves motor skills in children with BECTS.
The estimated correlation between TEP plasticity and language learning is robust (Figure 5). Children with the greatest decrease in N100 amplitude after rTMS performed the worst on the language task, while those with stable or increased N100 amplitudes had better language learning. While the theoretical relationship between motor cortex plasticity and motor learning is clear, it is less obvious as to why motor cortex plasticity should correlate with language learning. We investigated this correlation, because children with BECTS have spiking from their motor cortex and well-described difficulties with language.5 Xiao et al.23 found that increased connectivity between the primary motor and inferior frontal gyri after centrotemporal spikes correlates with worse performance on language tasks in children with BECTS. A potential explanation for our findings is that the primary motor cortex must be relatively inhibited during language tasks so that language-specific brain regions can be preferentially activated. Ability to maintain higher GABA tone (larger N100 amplitude) in the motor cortex even during spikes may improve language learning. It is also notable that language learning correlates strongly with TEP but not MEP plasticity, potentially because TEPs more purely represent cortico-cortical interactions and therefore may be more sensitive to the brain processes necessary for language.
There are two important caveats to our findings on TEP plasticity and language learning. First, as illustrated in Figure 5, medications may confound the relationship, as those on medication had worse language learning and a larger decrease in N100 amplitude. This is an important observation as the benefits and drawbacks of pharmacologic therapy for BECTS is an area of clinical debate. Several studies have measured the impact of medications on TEP amplitudes,28,70,71 but none have focused on the impact of these drugs on cortical plasticity. Since medication use is significantly associated with lifetime seizure frequency, we questioned whether medication use was simply a proxy for disease severity. We think this is unlikely, however, as neither medication use nor TEP plasticity correlate with IQ. A second concern is that baseline N100 amplitude may confound the relationship between plasticity and learning: those with larger baseline N100 amplitudes perform better at the language learning task and have larger increases in N100 amplitude after rTMS. The fact that baseline N100 amplitude correlates strongly with TEP plasticity in BECTS is interesting in and of itself, as it suggests that children with lower baseline inhibition may have lost their ability to maintain inhibition; this could be explored in future experiments comparing N100 amplitude to spike frequency.
Limitations
This study has important limitations. Foremost, we only describe correlations between TMS measurements and clinical factors within a group of children with BECTS, and hence the methodology is not adequate to draw conclusions about causality. Follow-up studies testing these correlations in children and BECTS and healthy controls will permit more robust mechanistic conclusions. A second concern is our small sample size. Our estimated correlations are robust but imprecise and require confirmation in larger studies that would allow for appropriate controlling of confounders such as specific seizure medications or medication levels. However, within our group, 5 of 7 medicated children only took levetiracetam, making our population slightly more homogenous. Similarly, the small sample size prevented us from controlling for the laterality of a subject’s spike waves. Spike laterality could be an important confounder in our population, as TMS measurements are known to differ in the affected hemisphere in adults with focal epilepsy.72 While the majority of our subjects had unilateral spikes on the initial diagnostic EEG, the laterality of spike waves was not consistent between the diagnostic and study EEG, suggesting bilateral (or shifting) involvement for many participants as has previously been reported.73,74 Future larger studies stimulating both hemispheres and assessing children over time will be needed to assess the impact of spike laterality on TMS measurements. Finally, institutional rules did not permit a control group of normal children. Despite these limitations, our study contributes important information on feasibility and measurement variance of TMS-EEG data in pediatric epilepsy patients, a previously unstudied population.
Conclusion
Children with BECTS are at high risk for developing cognitive problems but therapies are limited and typically initiated only after significant academic difficulties have arisen. This pilot study suggests that TMS-EEG measurements correlate with cognition and learning. In the future, TMS-EEG biomarkers could be developed to identify children at highest risk for learning problems (before they clinically manifest) and to assess the impact of pharmacological treatment on brain processes underlying both seizure control and learning. Such biomarkers would permit more tailored, proactive treatment for patients with this common condition.
Acknowledgments
Conflicts of Interest and Funding Sources: F. Baumer is supported by a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083) and (UL1 TR 001085). A. Rotenberg is supported by NIH RO1 NS088583-01A1, R01 MH100186-01A1, and RO1 NS100766-01A1 awards, the Mass Life Sciences Centers Research Matching Grant Program, the Boston Children’s Hospital Translational Research Program, and the Roche Sponsored Research Agreement. R. Fisher is supported by the James and Carrie Anderson Fund, the Maslah Saul MD Chair, the Steve Chen Research Fund and the Susan Horngren Fund for Epilepsy. Dr. Fisher consults for Medtronic and holds stock options in Avails Medical, Eysz, Cerebral Therapeutics, Irody, Smart Monitor and Zeto (none related to this publication). K. Pfeifer, A. Fogarty, D. Pena, C. Rolle, and J. Wallace have no funding or conflicts to disclose.
REFERENCES
- 1.Wirrell EC. Benign epilepsy of childhood with centrotemporal spikes. Epilepsia. 1998;39 Suppl 4:S32–41. [DOI] [PubMed] [Google Scholar]
- 2.Eom T-H, Shin J-H, Kim Y-H, Chung S-Y, Lee I-G, Kim J-M. Distributed source localization of interictal spikes in benign childhood epilepsy with centrotemporal spikes: A standardized low-resolution brain electromagnetic tomography (sLORETA) study. J Clin Neurosci. 2017;38:49–54. [DOI] [PubMed] [Google Scholar]
- 3.Miziara CSMG, de Manreza MLG, Mansur L, et al. Impact of benign childhood epilepsy with centrotemporal spikes (BECTS) on school performance. Seizure. 2012;21:87–91. [DOI] [PubMed] [Google Scholar]
- 4.Camfield CS, Camfield PR. Rolandic epilepsy has little effect on adult life 30 years later: a population-based study. Neurology. 2014;82:1162–1166. [DOI] [PubMed] [Google Scholar]
- 5.Smith AB, Bajomo O, Pal DK. A meta-analysis of literacy and language in children with rolandic epilepsy. Dev Med Child Neurol. 2015;57:1019–1026. [DOI] [PubMed] [Google Scholar]
- 6.Kavros PM, Clarke T, Strug LJ, Halperin JM, Dorta NJ, Pal DK. Attention impairment in rolandic epilepsy: Systematic review. Epilepsia. 2008;49:1570–1580. [DOI] [PubMed] [Google Scholar]
- 7.Ayaz M, Kara B, Soylu N, Ayaz AB. Fine motor skills in children with rolandic epilepsy. Epilepsy Behav. 2013;29:322–325. [DOI] [PubMed] [Google Scholar]
- 8.Vannest J, Tenney JR, Altaye M, et al. Impact of frequency and lateralization of interictal discharges on neuropsychological and fine motor status in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2016;57:e161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monjauze C, Tuller L, Hommet C, Barthez MA, Khomsi A. Language in benign childhood epilepsy with centro-temporal spikes abbreviated form: rolandic epilepsy and language. Brain Lang. 2005;92:300–308. [DOI] [PubMed] [Google Scholar]
- 10.Monjauze C, Broadbent H, Boyd SG, Neville BGR, Baldeweg T. Language deficits and altered hemispheric lateralization in young people in remission from BECTS. Epilepsia. 2011;52:79–84. [DOI] [PubMed] [Google Scholar]
- 11.Holmes GL, Lenck-Santini P-P. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav E&B. 2006;8:504–515. [DOI] [PubMed] [Google Scholar]
- 12.Verrotti A, Filippini M, Matricardi S, Agostinelli MF, Gobbi G. Memory impairment and Benign Epilepsy with centrotemporal spike (BECTS): A growing suspicion. Brain Cogn. 2014;84:123–131. [DOI] [PubMed] [Google Scholar]
- 13.Riva D, Vago C, Franceschetti S, et al. Intellectual and language findings and their relationship to EEG characteristics in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2007;10:278–285. [DOI] [PubMed] [Google Scholar]
- 14.Ebus SCM, Overvliet GM, Arends JB a. M, Aldenkamp AP. Reading performance in children with rolandic epilepsy correlates with nocturnal epileptiform activity, but not with epileptiform activity while awake. Epilepsy Behav E&B. 2011;22:518–522. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Zhang X, Han Q, Guo J, Wang C. Cognition in Chinese children with benign childhood epilepsy with centrotemporal spikes (BCECTS). Neurosci Lett. 2012;507:1–4. [DOI] [PubMed] [Google Scholar]
- 16.Bedoin N, Ferragne E, Lopez C, Herbillon V, De Bellescize J, des Portes V. Atypical hemispheric asymmetries for the processing of phonological features in children with rolandic epilepsy. Epilepsy Behav. 2011;21:42–51. [DOI] [PubMed] [Google Scholar]
- 17.Datta AN, Oser N, Bauder F, et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2013;54:487–494. [DOI] [PubMed] [Google Scholar]
- 18.Lillywhite LM, Saling MM, Simon Harvey A, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50:2276–2284. [DOI] [PubMed] [Google Scholar]
- 19.Vannest J, Szaflarski JP, Eaton KP, et al. Functional Magnetic Resonance Imaging Reveals Changes in Language Localization in Children With Benign Childhood Epilepsy With Centrotemporal Spikes. J Child Neurol. 2013;28:435–445. [DOI] [PubMed] [Google Scholar]
- 20.Besseling RMH, Jansen JFA, Overvliet GM, et al. Reduced structural connectivity between sensorimotor and language areas in rolandic epilepsy. PLoS One. 2013;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besseling RMH, Overvliet GM, Jansen JFA, et al. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res. 2013;107:253–262. [DOI] [PubMed] [Google Scholar]
- 22.Besseling RMH, Jansen JFA, Overvliet GM, et al. Delayed convergence between brain network structure and function in rolandic epilepsy. Front Hum Neurosci. 2014;8:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao F, An D, Lei D, et al. Real-time effects of centrotemporal spikes on cognition in rolandic epilepsy. Neurology. 2016;86:544–551. [DOI] [PubMed] [Google Scholar]
- 24.Ziemann U, Reis J, Schwenkreis P, et al. TMS and drugs revisited 2014. Clin Neurophysiol. 2015;126:1847–1868. [DOI] [PubMed] [Google Scholar]
- 25.Säisänen L, Julkunen P, Lakka T, Lindi V, Könönen M, Määttä S. Development of corticospinal motor excitability and cortical silent period from mid-childhood to adulthood - a navigated TMS study. Neurophysiol Clin. 2018;48:65–75. [DOI] [PubMed] [Google Scholar]
- 26.Nezu A, Kimura S, Ohtsuki N, Tanaka M. Transcranial magnetic stimulation in benign childhood epilepsy with centro-temporal spikes. Brain Dev. 1997;19:134–137. [DOI] [PubMed] [Google Scholar]
- 27.Reinthaler EM, Dejanovic B, Lal D, et al. Rare variants in γ-aminobutyric acid type A receptor genes in rolandic epilepsy and related syndromes. Ann Neurol. 2015;77:972–986. [DOI] [PubMed] [Google Scholar]
- 28.Premoli I, Castellanos N, Rivolta D, et al. TMS-EEG Signatures of GABAergic Neurotransmission in the Human Cortex. J Neurosci. 2014;34:5603–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. [DOI] [PubMed] [Google Scholar]
- 30.Oberman LM, Pascual-Leone A, Rotenberg A. Modulation of corticospinal excitability by transcranial magnetic stimulation in children and adolescents with autism spectrum disorder. Front Hum Neurosci. 2014;8:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pascual-Leone A, Freitas C, Oberman L, et al. Plasticiy as an Intrinsic Property of Human Brain. Brain Topogr. 2011;24:302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruno V, Fossataro C, Garbarini F. Report of seizure induced by 10 Hz rTMS over M1. Brain Stimul. 2018;11:454–455. [DOI] [PubMed] [Google Scholar]
- 33.Horton AM, Russo AA, Bigler ED. The california verbal learning test -- children’s version (CVLT-C). Arch Clin Neuropsychol. 1996;11:171-171. [Google Scholar]
- 34.McCrimmon AW, Smith AD. Review of the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II). J Psychoeduc Assess. 2013;31:337–341. [Google Scholar]
- 35.Syeda MM, Climie EA. Test Review: Wechsler Preschool and Primary Scale of Intelligence–Fourth Edition. J Psychoeduc Assess. 2014;32:265–272. [Google Scholar]
- 36.Greenberg LM. The Test of Variables of Attention. 2011. [Google Scholar]
- 37.Anand S, Hotson J. Transcranial magnetic stimulation: Neurophysiological applications and safety. Brain Cogn. 2002;50:366–386. [DOI] [PubMed] [Google Scholar]
- 38.Kerwin LJ, Keller CJ, Wu W, Narayan M, Etkin A. Test-retest reliability of transcranial magnetic stimulation EEG evoked potentials. Brain Stimul. 2018;11:536–544. [DOI] [PubMed] [Google Scholar]
- 39.Casula EP, Tarantino V, Basso D, et al. Low-frequency rTMS inhibitory effects in the primary motor cortex: Insights from TMS-evoked potentials. Neuroimage. 2014;98:225–232. [DOI] [PubMed] [Google Scholar]
- 40.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- 41.Rogasch NC, Sullivan C, Thomson RH, et al. Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: A review and introduction to the open-source TESA software. Neuroimage. 2017;147:934–951. [DOI] [PubMed] [Google Scholar]
- 42.Bender S, Basseler K, Sebastian I, et al. Transcranial magnetic stimulation evokes giant inhibitory potentials in children. Ann Neurol. 2005;58:58–67. [DOI] [PubMed] [Google Scholar]
- 43.Garvey MA, Ziemann U, Bartko JJ, Denckla MB, Barker CA, Wassermann EM. Cortical correlates of neuromotor development in healthy children. Clin Neurophysiol. 2003;114:1662–1670. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hameed MQ, Dhamne SC, Gersner R, et al. Transcranial Magnetic and Direct Current Stimulation in Children. Curr Neurol Neurosci Rep. 2017;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallett M. Transcranial Magnetic Stimulation: A Primer. Neuron. 2007;55:187–199. [DOI] [PubMed] [Google Scholar]
- 47.Ferreri F, Guerra A, Vollero L, et al. Age-related changes of cortical excitability and connectivity in healthy humans: non-invasive evaluation of sensorimotor network by means of TMS-EEG. Neuroscience. 2017;357:255–263. [DOI] [PubMed] [Google Scholar]
- 48.Määttä S, Könönen M, Kallioniemi E, et al. Development of cortical motor circuits between childhood and adulthood: A navigated TMS-HdEEG study. Hum Brain Mapp. 2017;38:2599–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajapakse T, Kirton A. Non-invasive brain stimulation in children: Applications and future directions. Transl Neurosci. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helfrich C, Pierau SS, Freitag CM, Roeper J, Ziemann U, Bender S. Monitoring cortical excitability during repetitive transcranial magnetic stimulation in children with ADHD: a single-blind, sham-controlled TMS-EEG study. PLoS One. 2012;7:e50073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Y, Ji G-J, Zang Y-F, et al. Local Activity and Causal Connectivity in Children with Benign Epilepsy with Centrotemporal Spikes. Yao D, ed. PLoS One. 2015;10:e0134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosso C, Perlbarg V, Valabregue R, et al. Anatomical and functional correlates of cortical motor threshold of the dominant hand. Brain Stimul. 2017;10:952–958. [DOI] [PubMed] [Google Scholar]
- 53.Sohn YH, Kaelin-Lang A, Jung HY, Hallett M. Effect of levetiracetam on human corticospinal excitability. Neurology. 2001;57:858–863. [DOI] [PubMed] [Google Scholar]
- 54.Reis J, Wentrup A, Hamer HM, et al. Levetiracetam influences human motor cortex excitability mainly by modulation of ion channel function—a TMS study. Epilepsy Res. 2004;62:41–51. [DOI] [PubMed] [Google Scholar]
- 55.Cantello R, Civardi C, Varrasi C, et al. Excitability of the human epileptic cortex after chronic valproate: A reappraisal. Brain Res. 2006;1099:160–166. [DOI] [PubMed] [Google Scholar]
- 56.Japaridze N, Muthuraman M, Dierck C, et al. Neuronal networks in epileptic encephalopathies with CSWS. Epilepsia. 2016;57:1245–1255. [DOI] [PubMed] [Google Scholar]
- 57.Bruckmann S, Hauk D, Roessner V, et al. Cortical inhibition in attention deficit hyperactivity disorder: new insights from the electroencephalographic response to transcranial magnetic stimulation. Brain. 2012;135:2215–2230. [DOI] [PubMed] [Google Scholar]
- 58.Määttä S, Säisänen L, Kallioniemi E, et al. Maturation changes the excitability and effective connectivity of the frontal lobe: A developmental TMS-EEG study. Hum Brain Mapp. January 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kilb W. Development of the GABAergic System from Birth to Adolescence. Neurosci. 2012;18:613–630. [DOI] [PubMed] [Google Scholar]
- 60.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. [DOI] [PubMed] [Google Scholar]
- 61.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp brain Res. 2000;133:425–430. [DOI] [PubMed] [Google Scholar]
- 62.Allen CH, Kluger BM, Buard I. Safety of Transcranial Magnetic Stimulation in Children: A Systematic Review of the Literature. Pediatr Neurol. 2017;68:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of Noninvasive Brain Stimulation in Children and Adolescents. Brain Stimul. 2015;8:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fregni F, Boggio PS, Valle AC, et al. Homeostatic effects of plasma valproate levels on corticospinal excitability changes induced by 1Hz rTMS in patients with juvenile myoclonic epilepsy. Clin Neurophysiol. 2006;117:1217–1227. [DOI] [PubMed] [Google Scholar]
- 65.Siebner HR, Lang N, Rizzo V, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Voti P, Conte A, Suppa A, et al. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp Brain Res. 2011;212:91–99. [DOI] [PubMed] [Google Scholar]
- 67.López-Alonso V, Cheeran B, Fernández-del-Olmo M. Relationship Between Non-invasive Brain Stimulation-induced Plasticity and Capacity for Motor Learning. Brain Stimul. 2015;8:1209–1219. [DOI] [PubMed] [Google Scholar]
- 68.Vallence A-M, Kurylowicz L, Ridding MC. A comparison of neuroplastic responses to non-invasive brain stimulation protocols and motor learning in healthy adults. Neurosci Lett. 2013;549:151–156. [DOI] [PubMed] [Google Scholar]
- 69.Jung P, Ziemann U. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci. 2009;29:5597–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Premoli I, Rivolta D, Espenhahn S, et al. NeuroImage Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS – EEG. Neuroimage. 2014;103:152–162. [DOI] [PubMed] [Google Scholar]
- 71.Premoli I, Biondi A, Carlesso S, Rivolta D, Richardson MP. Lamotrigine and levetiracetam exert a similar modulation of TMS-evoked EEG potentials. Epilepsia. 2017;58:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badawy RAB, Curatolo JM, Newton M, Berkovic SF, Macdonell RAL. Changes in cortical excitability differentiate generalized and focal epilepsy. Ann Neurol. 2007;61:324–331. [DOI] [PubMed] [Google Scholar]
- 73.Panayiotopoulos CP, Michael M, Sanders S, Valeta T, Koutroumanidis M. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. [DOI] [PubMed] [Google Scholar]
- 74.Tenney JR, Glauser T, Altaye M, et al. Longitudinal stability of interictal spikes in benign epilepsy with centrotemporal spikes. Epilepsia. 2016;57:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]