Abstract
Introduction:
Endometriosis is a chronic inflammatory gynecological disorder that causes pelvic pain. Due to the heterogeneity of the disease, response to any treatment in an individual is variable. We aimed to develop in vitro testing that could be adapted for use in precision therapy for endometriosis. We piloted a personalized medicine approach by identifying predictive biomarkers while determining the effect of bazedoxifene (BZA) and medroxyprogesterone acetate (MPA) on the gene expression of a progesterone receptor (PR), an estrogen receptor (ER), and an aromatase (CYP19A1) enzyme in cells cultured from biopsies of endometriosis patients. The differential expression of the most common molecular targets in endometriosis therapy correlated with cellular response.
Methods:
Primary eutopic endometrial stromal cells were cultured from endometrial biopsies obtained in secretory phase from women between 24 and 42 years old with moderate-to-severe endometriosis (stages III and IV). Exclusion criteria included use of hormonal treatments and intrauterine contraception in the 6 months prior to surgery. Cells were treated either with BZA, MPA, or vehicle control. Total RNA was extracted from the treated and untreated cells. Differential expression of genes that are involved in the pathogenesis of endometriosis was determined by quantitative reverse transcriptase-polymerase chain reaction analysis.
Results:
After determining the baseline expression levels of PRA/B, ERα and CYP19A1, response to treatment was monitored using Ki-67 as a marker of cell proliferation. MPA was effective in blocking proliferation in the group expressing high levels of PRA/B. Endometrium expressing high levels of CYP19A1 preferentially responded to BZA, a selective estrogen receptor modulator known to block estrogen action in endometrium.
Conclusions:
PR expression may predict progestin resistance in endometriosis while CYP19A1 expression may indicate the need to block estrogen signaling.
Keywords: Bazedoxifene, Biomarkers, Endometriosis, Estrogen receptor, Medroxyprogesterone, Progesterone receptor
Introduction
Endometriosis is a chronic and recurrent disease characterized by the growth of endometrial-like tissue outside the uterus. Endometriosis affects approximately 10% of women of reproductive age and is a major cause of pelvic pain and subfertility (1, 2). The principal objective in treating endometriosis is symptom relief (3). In addition to relieving pain, the goals of treatment for patients with endometriosis are to prevent disease progression by reducing endometriotic implants through surgical treatment or medically induced atrophy of the implants (4, 5). Although some agents show efficacy in relieving pain, all differ in their side effects, making it difficult to achieve a balance between efficacy and safety. First-line treatment is typically a progestin, most commonly administered as a component of an oral contraceptive. Therapeutic efficacy has been demonstrated with danazol and gonadotro-pin-releasing hormone (GnRH) agonists; however, treatment with these agents is limited because of significant side effects (4, 6). Newer options for treatment of endometriosis, such as GnRH antagonists, aromatase inhibitors, and selective progesterone receptor modulators (SPRMs) appear to have great therapeutic potential; however, all act by mimicking or altering levels of sex steroid hormones (7).
Non-steroidal molecules that bind to estrogen receptors (ERs), and that can act as either estrogen agonists or antagonists depending on the target tissue, are known as selective estrogen receptor modulators (SERMs). For use in the treatment of endometriosis, SERMs that have strong estrogen receptor antagonist activity on the endometrium but agonist activity on bone and circulating lipoproteins are candidates for use in the treatment of endometriosis (8, 9). One such agent is bazedoxifene (BZA), which we have previously shown to have a therapeutic benefit on endometriosis in pre-clinical models (10-17). BZA not only blocks estrogen receptor function in the endometrium, it also leads to receptor degradation (13,18).
Endometriotic lesions are associated with hormonal abnormalities, including increased estrogen synthesis, altered estrogen metabolism, and progesterone resistance. These hormonal changes cause increased proliferation, inflammation, pain, and infertility. Molecular differences in the pathways that mediate hormonal signaling may allow us to predict optimal targets for treatment as well as lead to precise drug recommendations leading to more complete response. Progestins and SPRMs act through the progesterone receptor (PR) to decidualize endometriosis, eventually leading to atrophy. Acting (directly or indirectly) on estrogen receptors are drugs decreasing systemic and local estrogen synthesis (GnRH agonists, GnRH antagonists, aromatase inhibitors) or estrogen activity (SERMs).
The response of endometriosis to drug therapy is variable, and endometriosis is a disease where precision medicine approaches may be quite useful. Progesterone resistance may prevent the action of progestins in some women with endometriosis. Progesterone resistance has multiple etiologies, including epigenetic alterations in PR that can lead to diminished PR expression. Similarly, resistance to agents that block ovarian estrogen synthesis is associated with aromatase expression and local estrogen synthesis.
Cancer therapies that target specific molecular processes are expanding available treatments and leading to customized, precise, and more effective treatment. Given the current challenges in endometriosis therapy and the marked heterogeneity of disease, can we approach the treatment of endometriosis with what is now termed personalized medicine? Based on the differential expression levels of the most common molecular targets in endometriosis therapy, we conducted a pilot in vitro study to determine the ability to predict response to endometriosis therapies. We sought to identify predictive biomarkers for the personalized treatment of endometriosis.
Materials and methods
Compounds
Medroxyprogesterone 17-acetate (MPA), purchased from Sigma, Inc. was dissolved in dimethylsulfoxide (DMSO). BZA was supplied by Pfizer, Inc. and was dissolved in DMSO, which was used as the vehicle control.
Sample collection
The samples were collected from 11 patients who under-went surgery for diagnosis or treatment of endometriosis. All of the women were between 24 and 42 years old and had regular menstrual cycles. Endometrial biopsies in secretory phase were obtained from women with moderate-to-severe disease (stages III and IV) with the use of a Pipelle catheter (Cooper Surgical). All samples were collected in the same secretory phase of the menstrual cycle to assure standardization. The secretory phase was selected due to progesterone exposure to detect possible progestin resistance. In addition to standardization, we selected the secretory phase because it is the time of progesterone action. We hypothesized that it would be better to detect a lack of progestin response at the time of progesterone exposure. Exclusion criteria included the use of hormonal treatments, including a GnRH agonist or sex steroids, or the use of intrauterine contraception in the 6 months prior to surgery. Approval for the collection of specimens was obtained from the Yale University Human Investigations Committee.
Culture of primary eutopic endometrial stromal cells (ESCs)
The endometrium was finely minced and the cells dispersed by means of incubation in Hanks balanced salt solution containing HEPES (25 mmol/mL), 1% anti-anti, collagenase (1 mg/mL, 15 U/mg), and DNase (0.1 mg/mL; 1,500 U/mg) for 30 minutes at 37°C with agitation. During and at the end of the incubation, the tissue was pipetted gently to disperse the cells every 15 minutes. The cells were pelleted, washed, suspended in Ham F12: DMEM (1:1) containing 10% fetal bovine serum (FBS), 1% anti-anti, passed through a 40-mm cell strainer (Falcon), and plated into 75-cm2 Falcon tissue culture flasks (BD Biosciences). Primary eutopic endometrial stromal cells (ESCs) at 2-4 passages were used for further analysis. A well-characterized telomerase immortalized human endometrial stromal cell (HESC) line was used as a reference control (19). This cell line serves as a readily available and stable source to which we could compare endometriosis samples. It has a response to treatment with sex steroids, which is typical of normal endometrial cells (20-25). We chose the HESC line instead of primary cells because primary cells can change significantly over time and can vary due to the presence of other pathology. While HESCs used here are free of endometriosis as well as other concomitant diseases. They are immortalized so that they do not change over time. They provided a more consistent gene expression profile than the use of primary cells. Further, the response of these cells to estrogens and progestins is well characterized and replicates that of normal primary endometrial cells; deviations from the levels of the genes involved in this response were predicted to alter the response to treatment.
BZA and MPA treatment
ESCs from patients with endometriosis were harvested from the culture flask with the use of Trypsin/EDTA 0.05% and were plated in 6-well plates in 2 mL media at 37°C in 5% CO2 in a humidified environment as follows: phenol red-free Ham F12/Dulbecco’s Modified Eagle’s medium (DMEM) and 1% anti-anti. At 80% confluence, cells were placed in phenol red-free Ham F12/DMEM without FBS for 24 hours then treated with either BZA, MPA, or vehicle control at concentrations of 10−6 mol/L. Three wells for each sample were used, and all culture dishes were incubated in the presence of 5% CO2 at 37°C for another 24 hours. All experiments were conducted in triplicate.
mRNA isolation
The cells were treated with TRIzol reagent (Invitrogen), and lysates were kept on ice for 5 minutes, then 0.2 mL of chloroform (per 1 mL TRIzol) was added to each tube separately, and then homogenates were vortexed for 15 seconds. Samples were then incubated at room temperature for 3 minutes and centrifuged at 12,000 rpm at 4°C for 15 minutes. Next, the aqueous layer from each sample was transferred to a fresh tube, and the mRNA was precipitated by adding 0.5 mL of isopropyl alcohol (per 1 mL TRIzol) to each sample and incubated at RT for 10 minutes. All tubes were centrifuged at 12,000 rpm to form the mRNA pellets, which were then collected, washed with 75% ethanol, and dissolved in RNase-free water. The total mRNA was quantified by a NanoDrop spectrophotometer (ThermoFisher Scientific) and was immediately used for cDNA synthesis or stored at −80°C until use.
Quantitative real-time polymerase chain reaction analysis
Total mRNA (500 ng) was reverse transcribed in 20 pL reaction mixture using iScript cDNA Synthesis Kit (Bio-Rad Laboratories). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR Green (Bio-Rad) and optimized in the MyQ Single Color Real-Time PCR Detection System (Bio-Rad). The specificity of the amplified transcript and the absence of primer dimers were confirmed by a melting curve analysis. All products yielded the predicted melting temperature. Gene expression was normalized to the expression of beta-actin. Relative mRNA expression of each gene was calculated using the comparative cycle threshold method (also known as the 2−ΔΔCT) (26). The primers used are ERα: forward- CCACCAACCAGTGCACCATT, reverse- GGTCTTTTCGTATCCCACCTTTC; CYP19A1: forward- TGTCTCTTTGTTCTTCATGCTATTTCTC, reverse- TCACCAATAACAGTCTGGATTTCC; Ki-67: forward- CTTTGGGTGCGACTTGACG, reverse- GTCGACCCCGCTCCTTTT; and beta-actin: forward- GGACTTCGAGCAAGAGATGG, reverse- AGCACTGTGTTGGCGTACAG. All experiments were carried out in triplicate. Negative controls were run at the same time without reverse transcriptase in the reaction.
Statistical analysis
Results are presented as the mean ± SEM. Results from qRT-PCR were analyzed by Welch’s t-test. All statistical analyses were carried out using Graph Pad Prism 7.0 for Macintosh (Graph-Pad Software for Science Inc.). p<0.05 was considered statistically significant.
Results
Baseline expression of PRA/B, ERα and CYP19A1 (aromatase) varies in primary ESCs
The level of PRA/B, ERα and CYP19A1 (aromatase) mRNA in primary ESCs (n = 11) and HESC was determined using qPCR. The HESC line was derived from a subject without endometriosis, expresses ERα and PR A/B at normal levels and responds to sex steroids in a manner typical of normal endometrial stromal cells (19-25). These cells were used to provide a consistent standard for normalization. If the level of gene expression was more than two fold that measured in HESC, we defined those cells as having high expression. The experiment was done in triplicate. As expected, the expression of PRA/B in the high expression group were significantly higher than the low expression group (**p<0.01) (Fig. 1). In five samples, ERα was expressed more than two fold higher than the expression in HESCs. We similarly divided the samples into ERα high- versus low-expression groups. As expected, the expression of ERα in the high-expression group was significantly higher than the low-expression group (*p<0.05). Furthermore, we found that in three samples CYP19A1 was expressed at a level more than twofold higher than the expression in HESCs. Similarly, the expression of CYP19A1 in the high-expression group was significantly higher than the low-expression group (**p<0.01; Fig. 1). One cell sample expressed high levels of all three molecules. One expressed high levels of PR and ER. All others expressed high levels of only one molecule among ER, PR or CYP19A1. The baseline expression of these markers did not predict the baseline proliferation rate as measured by Ki-67 expression.
Fig. 1 -.
Difference in the baseline expression of PRA/B, ERα and CYP19A1 in primary endometrial stromal cells from women with endometriosis. Gene expression levels were normalized to their respective expression in HESC. As expected, levels of PRA/B, ERα and CYP19A1 in high expression groups were significantly higher than in the low expression groups (*p<0.05, **p<0.01). The terms “high and low” are relative to the expression of the same genes in HESC. The expression values in patient samples were normalized to the expression values in HESC. ER = estrogen receptor; HESC = human endometrial stromal cell.
Effects of MPA on endometrial stromal cell proliferation: PRA/B predicts response to MPA treatment
We sought to determine if the level of PR expression could be used to predict progesterone responsive endometriosis from progesterone resistant disease. As shown in Figure 2A, Ki-67 expression was significantly inhibited by MPA in the group expressing high levels of PRA/B compared with the group expressing low levels (*p = 0.037). Moreover, Ki-67 response was not significantly correlated with ERα or CYP19A1 expression, (p>0.05) (Fig. 2B, C). To confirm the decrease in proliferation predicted by Ki-67, the number of cells in each culture was compared. The cell number decreased by 19% in the high PR group treated with MPA, but did not decrease in the low PR group after MPA treatment.
Fig. 2 -.
Effect of medroxyprogesterone acetate (MPA) treatment on cell proliferation. The Y axis shows the levels of Ki67, a proliferation marker. Levels of proliferation in response to MPA treatment were correlated to the expression of PR and not to CYP19A1 or ERα. (A) MPA was significantly more effective in reducing proliferation in cells expressing high levels of PR compared to low PR (*p = 0.037). (B) ERα expression did determine the effectiveness of MPA treatment. proliferation in response to MPA was not significantly different based on ERα expression (p>0.05). (C) MPA effectiveness in reducing proliferation was not significantly different based on the level of CYP19A1 expression (p>0.05). CYP19A1 expression did determine the effectiveness of MPA treatment. Gene expression levels were normalized to that of HESC. ER = estrogen receptor; HESC = human endometrial stromal cell; PR = progesterone receptor.
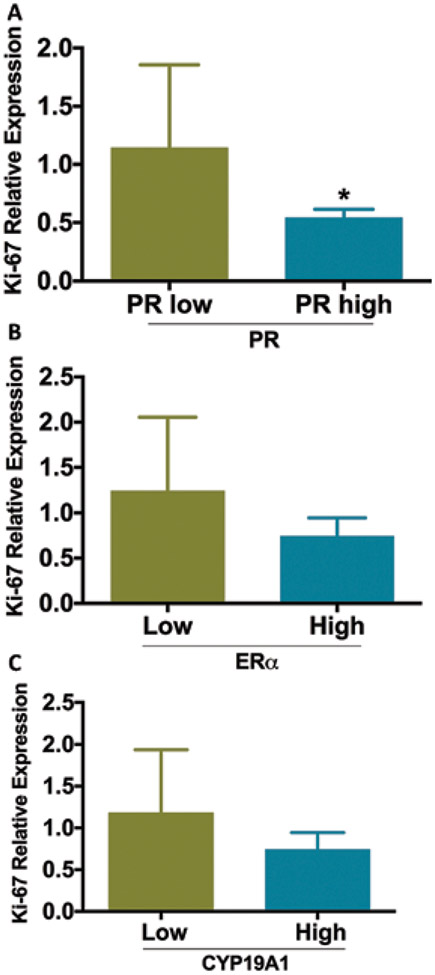
Effects of BZA on endometrial stromal cell proliferation: CYP19A1 predicts response to BZA treatment
We predicted that CYP19A elevations would predict the response to agents that modify aromatase (i.e., aromatase inhibitors) or ER (i.e., SERMs). In our in vitro model system, there is no added androgen to function as a CYP19A1 substrate; therefore, we chose to use an agent that would directly block and degrade the estrogen receptor. We imagine that other forms of estrogen blockade, such as GnRH agonists or aromatase inhibitors, could be useful in vivo; however, their mechanism of action involves multiple organs, and therefore are not suitable agents for use in this cell culture system. The effect of BZA on the proliferation marker Ki-67 was again used as a measure of cell response to treatment. As shown in Figure 3A, Ki-67 expression was not significantly inhibited by BZA in the group expressing high levels of PRA/B compared with the group of expressing low levels of PRA/B low expression (p>0.05). Moreover, Ki-67 expression was not significantly inhibited by BZA in the group expressing high levels of ERα compared to the group with low ERα expression (p>0.05; Fig. 3B). However, Ki-67 expression was significantly inhibited by BZA in the group expressing high levels of CYP19A1 compared to the group with low expression (*p = 0.026; Fig. 3C). To confirm the effect of BZA on cell proliferation as indicated by the molecular marker Ki-67, we measured the relative cell count between treated and untreated cells. BZA did not reduce the number of cells compared to control in the low ER group; however, BZA reduced the number of cells by 31% (p<0.01) in the high ER group.
Fig. 3 -.
Effect of bazedoxifene (BZA) treatment on cell proliferation. The Y axis shows the levels of Ki67, a proliferation marker. Levels of proliferation in response to BZA treatment were correlated to CYP19A1 and not to PRA/B or ERα. (A) There was no significant difference in proliferation in response to BZA based on the level of PR expression (p>0.05). (B) There was no significant difference in proliferation in response to BZA, based on the level of ERα expression (p>0.05). (C) BZA was far more effective in reducing proliferation in cells expressing high levels of CYP19A1 (aromatase) (*p = 0.0256). Gene expression levels were normalized to that of HESC. ER = estrogen receptor; HESC = human endometrial stromal cell; PR = progesterone receptor.
Discussion
Endometriosis is a chronic heterogeneous disease that does not consistently respond to all available medical therapies (27). While progestin-containing oral contraceptives are often used as first-line treatment, progestin resistance is common and leads to an inadequate or failed response (28). Similarly, aromatase expression in endometriosis can lead to resistance to estrogen-deprivation therapies, such as GnRH agonists (29-31). The ability to predict patient response could allow precise and individualized therapies for endometriosis; in theory, targeted therapy could be more effective and have a lower failure rate. Here, we used endometrial tissue from women with endometriosis to evaluate the common molecular pathways involved in the treatment of this disease. We hypothesized that we could predict response to individual therapies based on the molecular composition of the endometrium.
Progesterone and progestins relieve pain by limiting growth and inflammation in endometriosis, but a portion of patients with endometriosis and pelvic pain do not respond to treatment with progestins (7). Moreover, progesterone-induced molecular changes in the eutopic endometrial tissue of women with endometriosis are often blunted or undetectable (32). The effect of progestins on nuclear receptors, cytokines, and other mediators of proliferation with respect to progestin activity has been well studied (33, 34). MPA is effective in suppressing the growth of endometriotic implants by inhibiting cell proliferation (35). The molecular basis of progesterone resistance in endometriosis may be related to an overall reduction in the levels of progesterone receptors and the lack of progesterone receptor B (PRB) (32). The reduction of PR is largely epigenetic, and is regulated by PR gene methylation and microRNA inhibition (32, 36-40). In our study, we found that cells from women with high PR consistently responded to MPA therapy, while those with low PR were progestin resistant. We speculate that this is due to low PR in these women. Measuring PR in endometrial tissues or endometriosis may predict response, and thus eliminate the need for a trial of an ineffective therapy.
Similarly, endometriosis produces an aromatase enzyme in some patients; therefore, agents that lower ovarian estradiol production may not be effective (41, 42). GnRH agonists and antagonists are effective in treating endometriosis (43); however, the lack of response in some women may reflect high local estradiol production by aromatase. Here, high expression of aromatase predicted a favorable response to an agent known to directly block ER. We have previously shown that BZA is an effective endometriosis therapy in an animal model of the disease, where BZA blocked ER directly, independent of the circulating or local estradiol level, and led to ER degradation (11,13). In that model, decreased ER expression led to decreased estrogen-mediated proliferation and regression of disease.
The use of aromatase inhibitors leads to a low estrogen state for the treatment of endometriosis in clinical practice (41, 42). We speculate that aromatase inhibitors also may be effective in women with high CYP19A1 expression; unlike cell cultures, humans or intact animals would have a source of androgen production to serve as the substrate for this enzyme. Whether CYP19A1 can be used as a predictive biomarker for the efficacy of aromatase inhibitor treatment in endometriosis needs further investigation in intact animal experiments.
Surprisingly ERα expression did not correlate with the response to an estrogen blocking agent. BZA treatment was effective in both ER high and low expressers. Only a single patient sample showed very low ER expression, and we posit that all levels of ER seen in this study are sufficient to transduce estrogen signaling. Conversely, high levels of ERα did not result in a greater proliferation or response to therapy, which suggests that ER levels, within the observed range, are not the rate-limiting determinate of estrogen signaling.
In conclusion, our results suggest that aromatase expression may be a predictive biomarker for the success of aromatase or SERM treatment while PRA/B expression may be a predictive biomarker for progestogen treatment in endometriosis. Predictive biomarkers may allow precision therapy for endometriosis. This paradigm will need to be evaluated in clinical trials prior to implementation in practice.
Acknowledgments
We thank Dr. Clare Flannery for assistance with cell culture, Yuping Zhou for technical assistance, Dr. Pinar Kodaman and Dr. Valerie Flores for assistance with the collection of tissue samples.
Financial support:
This work was supported by NIH HD052668, Pfizer W5957335 and Jiangsu Province Government Scholarship for Overseas Studies (JS-2015-082).
Footnotes
Conflict of interest: None of the authors has financial interest related to this study to disclose.
References
- 1.Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–388. [DOI] [PubMed] [Google Scholar]
- 4.Rice VM. Conventional medical therapies for endometriosis. Ann N Y Acad Sci. 2002;955:343–352. [DOI] [PubMed] [Google Scholar]
- 5.Valle RF, Sciarra JJ. Endometriosis: treatment strategies. Ann N Y Acad Sci. 2003;997:229–239. [DOI] [PubMed] [Google Scholar]
- 6.Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis and treatment. Drugs. 2001;61(12):1735–1750. [DOI] [PubMed] [Google Scholar]
- 7.Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: an update for clinicians. Hum Reprod Update. 2006;12(2):179–189. [DOI] [PubMed] [Google Scholar]
- 8.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–629. [DOI] [PubMed] [Google Scholar]
- 9.Vigano P, Mangioni S, Odorizzi MP, Chiodini A, Rocca S, Chiodo I. Use of estrogen antagonists and aromatase inhibitors in endometriosis. Curr Opin Investig Drugs. 2003;4(10):1209–1212. [PubMed] [Google Scholar]
- 10.Han SJ, Begum K, Foulds CE, et al. The Dual Estrogen Receptor α Inhibitory Effects of the Tissue-Selective Estrogen Complex for Endometrial and Breast Safety. Mol Pharmacol. 2016;89(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naqvi H, Sakr S, Presti T, Krikun G, Komm B, Taylor HS. Treatment with bazedoxifene and conjugated estrogens results in regression of endometriosis in a murine model. Biol Reprod. 2014;90(6):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakr S, Naqvi H, Komm B, Taylor HS. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology. 2014; 155(4):1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulak J Jr, Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology. 2011;152(8):3226–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulak J Jr, Ferriani RA, Komm BS, Taylor HS. Tissue selective estrogen complexes (TSECs) differentially modulate markers of proliferation and differentiation in endometrial cells. Reprod Sci. 2013;20(2):129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor HS, Ohleth K. Using bazedoxifene plus conjugated estrogens for treating postmenopausal women: a comprehensive review. Menopause. 2012;19(4):479–485. [DOI] [PubMed] [Google Scholar]
- 16.Tosti C, Biscione A, Morgante G, Bifulco G, Luisi S, Petraglia F. Hormonal therapy for endometriosis: from molecular research to bedside. Eur J Obstet Gynecol Reprod Biol. 2017;209:61–66. [DOI] [PubMed] [Google Scholar]
- 17.Biankin AV. The road to precision oncology. Nat Genet. 2017;49(3):320–321. [DOI] [PubMed] [Google Scholar]
- 18.Ethun KF, Wood CE, Cline JM, Register TC, Appt SE, Clarkson TB. Endometrial profile of bazedoxifene acetate alone and in combination with conjugated equine estrogens in a primate model. Menopause. 2013;20(7):777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krikun G, Mor G, Alvero A, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291–2296. [DOI] [PubMed] [Google Scholar]
- 20.Penna I, Du H, Ferriani R, Taylor HS. Calpain5 expression is decreased in endometriosis and regulated by HOXA10 in human endometrial cells. Mol Hum Reprod. 2008;14(10):613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lockwood CJ, Kumar P, Krikun G, et al. Effects of thrombin, hypoxia, and steroids on interleukin-8 expression in decidualized human endometrial stromal cells: implications for long-term progestin-only contraceptive-induced bleeding. J Clin Endocrinol Metab. 2004;89(3):1467–1475. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Bocca S, Anderson S, et al. Modulation of the expression of the transcription factors T-bet and GATA-3 in immortalized human endometrial stromal cells (HESCs) by sex steroid hormones and cAMP. Reprod Sci. 2013;20(6):699–709. [DOI] [PubMed] [Google Scholar]
- 23.Du H, Daftary GS, Lalwani SI, Taylor HS. Direct regulation of HOXA10 by 1,25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol. 2005;19(9):2222–2233. [DOI] [PubMed] [Google Scholar]
- 24.Du H, Sarno J, Taylor HS. HOXA10 inhibits Kruppel-like factor 9 expression in the human endometrial epithelium. Biol Reprod. 2010;83(2):205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahin E rsoy G, Zhou Y, inan H, Taner CE, Cosar E, Taylor HS. Cigarette Smoking Affects Uterine Receptivity Markers. Reprod Sci. 2017;24(7):989–995. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 27.Becker CM, Gattrell WT, Gude K, Singh SS. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil Steril. 2017;108(1):125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632. [DOI] [PubMed] [Google Scholar]
- 29.Cho S, Mutlu L, Zhou Y, Taylor HS. Aromatase inhibitor regulates let-7 expression and let-7f-induced cell migration in endometrial cells from women with endometriosis. Fertil Steril. 2016;106(3):673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81(1):174–179. [DOI] [PubMed] [Google Scholar]
- 31.Bulun SE, Noble LS, Takayama K, et al. Endocrine disorders associated with inappropriately high aromatase expression. J Steroid Biochem Mol Biol. 1997;61(3-6):133–139. [PubMed] [Google Scholar]
- 32.Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1-2):94–103. [DOI] [PubMed] [Google Scholar]
- 33.Grandi G, Mueller MD, Bersinger NA, Facchinetti F, McKinnon BD. The association between progestins, nuclear receptors expression and inflammation in endometrial stromal cells from women with endometriosis. Gynecol Endocrinol. 2017;33(9):712–715. [DOI] [PubMed] [Google Scholar]
- 34.Grandi G, Mueller M, Bersinger N, et al. Progestin suppressed inflammation and cell viability of tumor necrosis factor-alpha-stimulated endometriotic stromal cells. Am J Reprod Immunol. 2016;76(4):292–298. [DOI] [PubMed] [Google Scholar]
- 35.Surrey ES, Halme J. Direct effects of medroxyprogesterone acetate, danazol, and leuprolide acetate on endometrial stromal cell proliferation in vitro. Fertil Steril. 1992;58(2):273–278. [PubMed] [Google Scholar]
- 36.Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone-hox gene interactions. Semin Reprod Med. 2010;28(l):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colón-Caraballo M, Monteiro JB, Flores I. H3K27me3 is an Epigenetic Mark of Relevance in Endometriosis. Reprod Sci. 2015;22(9):1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou M, Fu J, Xiao L, et al. miR-196a overexpression activates the MEK/ERK signal and represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Hum Reprod. 2016;31(11):2598–2608. [DOI] [PubMed] [Google Scholar]
- 39.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–111. [DOI] [PubMed] [Google Scholar]
- 41.Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis. Fertil Steril. 2012;98(6):1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Słopień R, Mczekalski B. Aromatase inhibitors in the treatment of endometriosis. Prz Menopauzalny. 2016;15(1):43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor HS, Giudice LC, Lessey BA, et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N Engl J Med. 2017;377(l):28–40. [DOI] [PubMed] [Google Scholar]