Abstract
Objective
To investigate how physical exercise (PE) would affect brain‐derived neurotrophic factor (BDNF) in randomized controlled trials (RCTs) of healthy subjects.
Methods
Seven databases (PubMed, Web of Science, Cochrane, Embase, PsycINFO, CINAHL, SPORTDiscus) were searched for RCTs assessing the effects of PE on serum and/or plasma BDNF until December 18, 2021. Meta‐analysis was performed by random‐effects method with standardized mean difference (SMD) and 95% confidence intervals (CIs). Subgroup analysis and meta‐regression analysis were conducted to investigate the potential source of heterogeneity. Trim and fill method, and leave‐one‐out cross‐validation were conducted.
Results
Eventually, 21 articles, involving 809 participants, were included in the meta‐analysis. Overall, both acute (5 trials, SMD: 1.20, 95% CI: 0.36 to 2.04, p = .005) and long‐term (17 trials, SMD: 0.68, 95% CI: 0.27 to 1.08, p = .001) PE had significant positive effects on BDNF levels. Via subgroup analysis, studies of long‐term PE with larger sample sizes, female participants, participants older than 60 years, and aerobic exercise contributed to a more pronounced improvement on BDNF levels than that found when all studies were combined.
Conclusion
Both acute and long‐term PE had significant positive effects on circulating BDNF in healthy subjects. This review suggests that acute exercise and long‐term aerobic exercise are powerful forms of PE to enhance neurotrophic effect, especially for female subjects or subjects over 60 years.
Keywords: brain‐derived neurotrophic factor, meta‐analysis, neurotrophic factor, physical exercise

1. INTRODUCTION
There is a tremendous amount of evidence that physical exercise (PE) can improve neurological function and counteract the risk of dementia (Larson et al., 2006; Voss et al., 2019). Among the potential mechanisms of PE‐enhancing cognitive effects, neurotrophic molecules such as brain‐derived neurotrophic factor (BDNF) (Erickson et al., 2012) and insulin‐like growth factor‐1, are important candidates. The increased expressions of BDNF (Gomez‐Pinilla et al., 2011; Neeper et al., 1996) were related to the beneficial effect of PE in neurogenesis and neuroplasticity. BDNF, first purified from pig brain (Barde et al., 1982), is a protein of the neurotrophin family promoting proliferation and survival of neurons (Park & Poo, 2013) as well as immunity and tissue repair (Kerschensteiner et al., 1999). BDNF is released by many tissues, including skeletal muscle (J. J. Walsh et al., 2015) in addition to the brain. BDNF plays an essential role in the structure and function of the brain via protecting cells and DNA from damage by down‐regulating oxidative stress (Hacioglu et al., 2016), modulating neurogenesis (Brown et al., 2003), promoting axonal and dendritic growth (Gonçalves et al., 2016), and modulating synaptic plasticity (Zenke et al., 2015). The bulk of available evidences have found that BDNF could improve the cognitive ability of both animal (Vaynman et al., 2004) and human models (Leckie et al., 2014). It was also found that BDNF improved cell signal transduction and restored learning and memory through amyloid‐independent mechanisms in rodent and primate models of Alzheimer's disease (Nagahara et al., 2009).
Existing randomized controlled trials (RCTs) of humans showed that the effects of PE on BDNF are inconsistent, with some finding increases (Rentería et al., 2020; Schmolesky et al., 2013) in BDNF after PE, while most of the others reporting no change (Arrieta et al., 2020; Baird et al., 2018; Forti et al., 2014) in circulating BDNF. This variability may be due to differences in dose parameters, such as type, intensity, and duration of PE. Meta‐analysis (Dinoff et al., 2017) based on pre‐post design showed that an acute bout of PE increases circulating BDNF transiently, while the effect of long‐term PE on neurotrophic molecules is still uncertain. Previous meta‐analyses (Dinoff et al., 2016; Szuhany et al., 2015) on the effect of exercise training on resting concentrations of BDNF in humans found that regular exercise training could enhance the response of BDNF to acute PE. Likewise, their analysis also focused only on estimating the association between exercise and BDNF concentrations through the change of BDNF levels from pre‐exercise to post‐exercise (non‐RCT), which is inefficient in clarifying the actual effect. In addition, one (Szuhany et al., 2015) of these two meta‐analyses included people with diseases that are known to have lower basal BDNF (i.e., Parkinson's disease, obesity, and metabolic syndrome). A recent review by E. I. Walsh et al. (2020) concluded that high‐intensity short‐term activities might effectively promote BDNF response but specific PE type and dose for optimal BDNF release is unclear. Consequently, in the changing context of gradual decline of physical and cognitive abilities in the normal aging process, the magnitude of the actual effects of PE on peripheral BDNF concentrations is still uncertain.
Considering the extensive attention of PE on BDNF and cognition, it is of great significance to collect the existing RCTs for a comprehensive meta‐analysis to determine: (1) the specific role of PE on BDNF under physiological condition for healthy subjects and (2) how do training protocols and characteristics of subjects influence the outcomes.
2. METHODS
2.1. Literature search
The systematic search was carried out following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) in this meta‐analysis (Moher et al., 2009). Seven electronic databases, including PubMed, Web of Science, Cochrane Library, Embase, PsycINFO, Cumulated Index to Nursing and Allied Health Literature (CINAHL), and SPORTDiscus, were searched from 1980 to December 8, 2021 for relevant articles, using the following search strategy: (Exercise OR “Physical Exercise” OR “Exercise Therapy”) AND (“Brain‐Derived Neurotrophic Factor” OR BDNF) AND “randomized controlled trial.” Detailed search strategy was shown in Table S1.
2.2. Study selection
Two researchers (YHW and HHZ) independently screened titles and abstracts, then reviewed full‐text for eligibility. The third researcher (SDC) arbitrated any discrepancies to reach consensus. We also conducted a manual search for references to eligible articles, relevant review articles, and systematic reviews. For selection, studies had to fulfill the following criteria: (1) being human RCTs with parallel or crossover; (2) the volunteers were healthy people; (3) using PE as the intervention treatment; the comparisons were exercise versus nonexercise control or exercise plus other intervention versus other intervention only; (4) the interested outcomes were BDNF in plasma or serum; and (5) have been published in English since 1980.
The exclusion criteria were (1) studies including people with diseases; (2) lacking net changes of neurotrophic biomarkers and their corresponding SDs as outcome measures or providing sufficient information to calculate them (mean changes of treatment [both intervention groups and control groups] ± SD).
2.3. Data extraction and quality assessment
The study selection, data extraction, and quality assessment were undertaken independently by two investigators (YHW and HHZ) with standard form. Eligible studies were reviewed and the following data were extracted: The first author's surname, publication year, study design, study location, sample size, participants age and gender, baseline body mass index (BMI) of participants, PE intervention (duration, type, intensity, frequency), and reported circulating BDNF levels. If one study contained two or more independent intervention strata (e.g., different types, intensity, frequency or duration of PE), it was treated as separate trials for analysis. All types of PE (i.e., aerobic exercise, resistance exercise, and multicomponent exercise) were included in this review. Intensity of exercise was classified by maximal heart rate (HRmax), maximal oxygen uptake (VO2peak), and repetition maximum (RM) according to American College of Sports Medicine (Haskell et al., 2007). Advanced data extraction was performed using Adobe Photoshop for studies that did not directly provide data but present their data in a graphic format, according to the protocol proposed by Gheibi et al. (2019). The methodological quality of selected studies was evaluated by the PEDro scale (Maher et al., 2003). The Grading of Recommendation, Assessment, Development and Evaluation (GRADE) system was used to assess the evidence level of each outcome (Goldet & Howick, 2013). According to the specific regulations of GRADE guidelines, study design dictates baseline quality of the evidence (RCTs are initially defined as high quality) but other factors could decrease (e.g., unexplained heterogeneity) or increase (e.g., a large magnitude of effect) the quality level (Goldet & Howick, 2013). Discrepancies were resolved by discussing with the third reviewer (SDC) to reach consensus.
2.4. Statistical analysis
The pooled effect sizes were defined as the standardized mean difference (SMD) with 95% confidence intervals (CIs) of net changes of the concentrations of BDNF. The heterogeneity among studies was evaluated using I 2 and Cochrane's Q test and there is heterogeneity when I 2 > 50% and p‐value < .1 for Q test (Higgins et al., 2003). Considering the existing heterogeneity between studies, the random effects model was used in pooling estimates of net changes. For studies in which either baseline or final mean and standard deviation (SD) of outcomes were not provided directly, advanced data extraction using the reported method proposed by Wan et al. (2014) was conducted.
Firstly, a primary meta‐analysis was conducted to establish the overall effect. Then subgroup analyses were performed to investigate the potential source of heterogeneity based on the sample size, region, gender, age, baseline BMI of participants, duration of intervention, and type and intensity of exercise (only conducted if more than five trials reported the same outcomes). Differences between groups and sources of heterogeneity were tested by meta‐regression analysis, with p‐value < .1 as statistically significant.
Both Begg's and Egger's regression tests as well as funnel plots were utilized to assess the publication bias, with a p‐value < .1 suggesting the presence of bias (Egger et al., 1997). If publication bias was encountered, the trim and fill method was performed (Duval & Tweedie, 2000). Sensitivity analysis using leave‐one‐out method was performed to investigate key studies that have substantial impact on the heterogeneity between studies (Serban et al., 2015), using p < .1 as the criterion. All analyses were performed using STATA version 11.0 (Stata Corp, College Station, TX, USA), with double data input to avoid input errors. p < .05 was deemed as statistically significant unless specified elsewhere.
3. RESULTS
3.1. Flow of study selection
The detailed flowchart of literature search and study selection is presented in Figure 1. A total of 1658 articles (183 from PubMed, 327 from web of science, 529 from Cochrane library, 489 from Embase, 74 from PsycINFO, 73 from CINAHL, and 40 from SPORTDiscus) were initially identified from the databases search. After excluding the duplicates and screening the titles and abstracts, 371 articles were left for full‐text review, of which 356 articles were further eliminated for the following reasons: 85 articles were non‐RCT design, participants of 98 studies were not healthy, 63 articles had improper intervention or control, assessable target outcomes were not reported in 66 articles, and 44 articles lack sufficient data for quantitative analysis. Additionally, reference lists of all eligible articles and relevant reviews (Azevedo et al., 2020; Dinoff et al., 2017; Marinus et al., 2019; Stigger et al., 2019) were screened and identified six eligible articles. Finally, 21 eligible studies involving 809 participants met inclusion criteria for final meta‐analysis.
FIGURE 1.
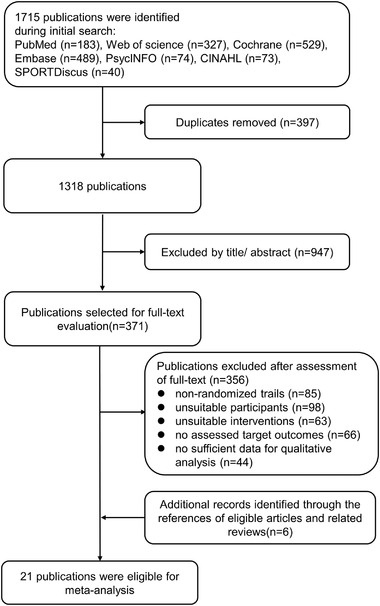
Flowchart of study selection through the review
3.2. Qualities of included studies and outcome measure evidences by GRADE
The methodological qualities of one study (Matura et al., 2017) was rated as excellent quality according to the PEDro scale (scores ≥ 9), while the remaining 20 studies were good quality (6‐8 scores) mostly because of lacking blindness (Table 1). The evidence quality of acute PE on BDNF levels was at a moderate level, while the quality of long‐term PE on BDNF levels was at a low level according to the GRADE system (Table S2).
TABLE 1.
Characteristics of included studies in this meta‐analysis (21 studies)
| Study | Design | Country | Subject | Comparators | Interventions | Duration of intervention | BDNF measure | Quality a |
|---|---|---|---|---|---|---|---|---|
| BDNF measured after acute exercise | ||||||||
| Arazi et al., 2021 | RP | Iran | Healthy older men ( N = 30) | Control (N = 10): Did not perform any exercise. | Resistance Exercise (N = 10): warm‐up (10 min); 6 exercises with 10 repetitions (30 min); cool‐down (5 min). Intensity: 65−70% of 1RM Aerobic Exercise (N = 10): warm‐up (10 min); running (3 × 10‐min with 120 s interval); cool‐down (5 min). Intensity: 65−75% of HRmax | 45 min | Serum | Good |
| Helm et al., 2017 | RP | USA | Neurologicaly intact subjects ( N = 54) | Rest (N = 27): quiet rest. | Resistance Exercise (N = 27): pedaling with high resistance (1 min); pedaling with resistance decreased by half (1 min); rest (1 min); pedaling with high resistance (1 min); pedaling with resistance decreased by half (1 min) Intensity: > 80% HRmax | 5 min | Serum | Good |
| Schmolesky et al., 2013 | RP | USA | Healthy adult males ( N = 45) | Control (N = 10): Remained seated and at rest during the exercise period. | Aerobic Exercise (N = 35): cycling (30 min). Intensity: 60–80% of HR reserve. | 30 min | Serum | Good |
| Urzi et al., 2019 b | RP | Slovenia | Healthy elderly women ( N = 20) | Control (N = 9): No changes in diet habits | Resistance Exercise (N = 11): warm‐up (10 min); 8 resistance exercises (35–40 min). Intensity: moderate | 45‐50 min | Plasma | Good |
| Winter et al., 2007 | RC | Germany | Male healthy sport students ( N = 27) | Relaxed (N = 27): Being sedentary. | Aerobic Exercise (N = 27): low impact running (40 min) Intensity: blood lactate level ≤ 2 mmol); Resistance Exercise (N = 27): 2 sprints of 3 min at increasing speed Intensity: blood lactate level > 10 mmol Wash‐out: at least 1 week apart | 40 min | Serum | Good |
| BDNF measured after long‐term exercise | ||||||||
| Arrieta et al., 2020 | RP, Sb | Spain | Men and women living in nursing homes ( N = 88) | Control (N = 45): Engaged in routine activities. | Multicomponent Exercise (N = 43): warm‐up (5 min); strength training, balance exercises, proprioceptive exercises, and stepping practice; deep breathing exercise (5 min). walking (5‐20 min/day). Intensity: 40−70% of 1RM Frequency: 2 sessions/week (except for walking) | 24 week | Serum | Good |
| Čekanauskaitė et al., 2020 | RP | Lithuania | Physically inactive healthy older men ( N = 33) | Control (N = 15): Maintain their daily living habits. | Aerobic Exercise (N = 18): warm‐up (15 min); yoga asanas (45 min), Himalayan kriya breathing exercises (25 min); relaxation in shavasana (corpse pose) (15 min). Intensity: n/a Frequency: 2 sessions/week | 10 week | Serum | Good |
| Cho & Roh, 2019 | RP | South Korea | Healthy women aged 65 years or older ( N = 37) | Control (N = 18): Maintained their activities of daily living. | Aerobic Exercise (N = 19): warm‐up and cool‐down (10 min); taekwondo training (50 min) Intensity: 50−80% of HRmax Frequency: 5 sessions/week | 16 week | Serum | Good |
| Forti et al., 2014 | RP | Belgium | Elderly volunteers ( N = 40) | Control (N = 20): Maintain daily activity levels. | Resistance Exercise (N = 20): warm‐up; progressive strength training; muscle stretching (60 min). Intensity: 50–80% of 1RM Frequency: 3 sessions/week | 12 week | Serum | Good |
| Goekint et al., 2010 | RP | Belgium | Untrained subjects ( N = 23) | Control (N = 8): Remained physically inactive. | Resistance Exercise (N = 15): warm‐up; six strength exercises (chest press, shoulder press, vertical traction, leg press, adductor strength, and abductor strength). Intensity: 50−80% 1RM Frequency: 3 sessions/week | 10 week | Serum | Good |
| Jeon & Ha, 2015 | RP | South Korea | Healthy junior high school students ( N = 20) | Control (N = 10): Continue their daily normal and sedentary activities. | Aerobic Exercise (N = 10): treadmill exercise until burned 200 kcal. Intensity: 40−60% of VO2max Frequency: 3 days/week | 8 week | Serum | Good |
| Jeon & Ha, 2017 | RP | South Korea | Male middle school students (N = 40) | Stretching group (N = 10): Performed whole‐body stretching at the same time. | Aerobic Exercise (N = 30): treadmill exercise until burned 200 kcal. Intensity: 40% (low intensity group, N = 10) / 55% (moderate intensity group, N = 10) / 70% (high intensity group, N = 10) of VO2max. Frequency: 4 times/week | 12 week | Serum | Good |
| Kim & Kim, 2018 | RP | South Korea | Sedentary elderly women (N = 26) | Control (N = 12): Make no changes to their diet and exercise habits. | Aerobic Exercise (N = 14): warm‐up (10 min); training (40 min); cool‐down (10 min). Intensity: 40−50% (1−4 week), 50−60% (5−8 week), 60−65% (9−12 week), and 65−70% (13−16 week) of HR reserve Frequency: 2 times/week | 16 week | Serum | Good |
| Ledreux et al., 2019 | RP | USA & Sweden | Healthy older individuals (N = 68) | Control (N = 39): Not described. | Aerobic Exercise (N = 29): aerobic exercise training following pre‐recorded video segments (35 min) Intensity: n/a Frequency: 5 days/week | 5 week | Serum | Good |
| Maass et al., 2016 | RP | Germany | Sedentary healthy older adults (N = 40) | Control (N = 19): Supervised progressive muscle relaxation/stretching training (45 min). Frequency: 2 times/week | Aerobic Exercise (N = 21): warm‐up (5 min); training (40 min); stretching (5 min). Intensity: 65–80% of HRmax Frequency: 3 days/week | 12 week | Serum, plasma | Good |
| Matura et al., 2017 | RP, Sb | Germany | Healthy older participants (N = 53) | Control (N = 24): Not to change their habitual physical activity. | Aerobic Exercise (N = 29): supervised cycle ergometer training (30 min). Intensity: 64 ± 9% of VO2max Frequency: 3 sessions/week | 12 week | Serum | Excellent |
| Nilsson et al., 2020 | RP | Sweden | Healthy older adults (N = 70) | Control (N = 21): Seated rest. | Aerobic Exercise (N = 49): warm‐up (5 min); aerobic activity (30 min). Intensity: 65−75% of HRmax Frequency: 3 days/week | 12 week | Serum | Good |
| Rentería, 2020 | RP | Mexico | Healthy young adult women (N = 17) | Control (N = 8): Maintain their regular physical activity habits. | Aerobic Exercise (N = 9): warm‐up; 3–5 cycling bouts of 30s+4‐min recovery. Intensity: 80% maximal aerobic power Frequency: 3 days/week | 4 week | Serum | Good |
| Schiffer, 2009 | RP | Germany | Healthy sports students (N = 36) | Control (N = 18): Continue their regular lifestyle. | Resistance Exercise (N = 9): 3 sets of 8–10 repetition of complete body work out. Intensity: 80% of 1RM Frequency: 3 times/week Aerobic Exercise (N = 9): ran (45 min). Intensity: 80% of HRmax Frequency: 3 times/week | 12 week | Plasma | Good |
| Seifert et al., 2010 | RP | Denmark | Sedentary male (N = 12) | Control (N = 5): Continue sedentary lifestyle; on a diet creating a negative energy balance of ∼600 kcal/day. | Aerobic Exercise (N = 7): cycling or running or swimming (60 min or until 600 kcal expenditure was reached). Intensity: 70% of HRmax Frequency: everyday | 12 week | Plasma | Good |
| Solianik et al., 2021 | RP | Lithuania | Healthy elderly (N = 30) | Control (N = 15): Maintain their daily routines. | Aerobic Exercise (N = 15): warm‐up (15 min); 8‐form Yang‐style tai chi (40 min); cool‐down (5 min). Intensity: n/a Frequency: 2 times/week | 10 week | Serum | Good |
| Urzi et al., 2019 b | RP | Slovenia | Healthy elderly women (N = 20) | Control (N = 9): No changes in diet habits. | Resistance Exercise (N = 11): warm‐up (10 min); 8 resistance exercises (35–40 min). Intensity: moderate | 12 week | Plasma | Good |
Abbreviations: BDNF, brain‐derived neurotrophic factor; HRmax, maximal heart rate; kcal, kilocalories; km, kilometer; min, minute; RC, randomized crossover; RM, repetition maximum; RP, randomized‐parallel; s, second; Sb, single blind; SB, single‐blinded; VO2max, maximal oxygen uptake; wk, week.
Better methodological quality is indicated by a higher PEDro score (9–10: excellent; 6–8: good; 4–5: fair; < 4: poor).
The study examined both acute and chronic effects of PE on BDNF levels.
3.3. Characteristics of included studies
Table 1 summarizes the characteristics of the included 21 studies. The final sample consisted of 809 unique participants, with mean age ranging from 15 to 84.9. Sample sizes ranged from 12 to 88 participants, with a median size of 36.5. The average baseline BMI value of participants ranged from 17.2 to 28.5. Fifteen studies reported gender composition of the participants, and 57.2% of the participants were male. These studies were carried out in different countries including Germany (n = 4), South Korea (n = 4), USA (n = 3), Belgium (n = 2), Lithuania (n = 2), Sweden (n = 2), and other five countries with single study. Different types (aerobic exercise: 16 trials; resistance exercise: 8 trials; multicomponent exercise: 1 trials) of PE were reported in those included studies. In addition, the intervention duration of long‐term PE ranged from 4 to 24 weeks.
3.4. Effect of acute PE on BDNF levels
Five studies (Arazi et al., 2021; Helm et al., 2017; Schmolesky et al., 2013; Urzi et al., 2019; Winter et al., 2007) examined the effect of acute PE on BDNF levels. The results of analysis showed that acute PE remarkably elevated the levels of BDNF (SMD: 1.20, 95% CI: 0.36 to 2.04, p = .005), with a high heterogeneity observed (p < .001, I 2 = 89.0%) (Table 2; Figure 2).
TABLE 2.
Results of subgroup analysis and publication bias stratified by study characteristics
| Heterogeneity | p 4 | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Trials | SMD (95% CI) | p 1 | I 2 (%) | p 2 | p 3 | Begg's value | Egger's value |
| BDNF, ng/ml (acute effect) | 5 | 1.20 (0.36, 2.04) | .005 | 89.0 | <.001 | – | – | |
| BDNF, ng/ml (long‐term effect) | 17 | 0.68 (0.27, 1.08) | .001 | 82.8 | <.001 | .030 | .473 | |
| Sample size | .890 | |||||||
| ≤ 20 | 6 | 0.33 (−0.12, 0.77) | .150 | 48.9 | .048 | .210 | .321 | |
| > 20 | 11 | 0.93 (0.34, 1.52) | .002 | 89.1 | <.001 | .008 | .073 | |
| Region | .581 | |||||||
| Conducted in Asia | 4 | 0.56 (0.05, 1.07) | .003 | 54.6 | .051 | – | – | |
| Conducted in Europe | 11 | 0.74 (0.11, 1.37) | .021 | 88.6 | <.001 | .193 | .561 | |
| Conducted in North American | 1 | 1.26 (0.21, 2.31) | .019 | – | – | – | – | |
| Gender | .081 | – | – | |||||
| Male | 3 | 0.35 (−0.07, 0.76) | .104 | 0 | .485 | – | – | |
| Female | 4 | 1.10 (0.53, 1.68) | <.001 | 42.0 | .160 | – | – | |
| Age | .287 | |||||||
| ≤ 60 years | 6 | 0.30 (−0.12, 0.73) | .163 | 45.4 | .066 | .348 | .313 | |
| > 60 years | 11 | 0.95 (0.35, 1.55) | .002 | 89.2 | <.001 | .020 | .071 | |
| Baseline BMI | .340 | |||||||
| < 25 | 4 | 0.42 (−0.03, 0.88) | .070 | 20.2 | .286 | – | – | |
| ≥ 25 | 8 | 0.65 (0.07, 1.24) | .029 | 85.8 | <.001 | .035 | .509 | |
| Duration | .447 | |||||||
| ≤ 8 weeks | 3 | 0.62 (0.05, 1.20) | .034 | 39.1 | .193 | – | – | |
| 8 < duration ≤ 12 weeks | 11 | 0.66 (0.09, 1.24) | .025 | 86.1 | <.001 | .108 | .826 | |
| > 12 weeks | 3 | −0.71 (−0.24, 1.66) | .141 | 84.7 | .001 | – | – | |
| Type of exercise | .169 | |||||||
| Aerobic exercise | 13 | 0.86 (0.37, 1.36) | .001 | 83.5 | <.001 | .030 | .473 | |
| Resistance exercise | 4 | 0.20 (−0.59, 0.98) | .626 | 71.3 | .015 | – | – | |
| Multicomponent exercise | 1 | –0.01 (−0.43, 0.41) | .960 | – | – | – | – | |
| Intensity of exercise | .915 | |||||||
| Low | 3 | 0.14 (−0.29, 0.58) | .518 | 21.8 | .278 | – | – | |
| Moderate | 4 | 0.75 (−0.79, 2.29) | .339 | 91.5 | <.001 | – | – | |
| High | 3 | 0.30 (−0.63, 1.23) | .523 | 65.0 | .058 | – | – | |
Note: p 1 value for net change; p 2 value for heterogeneity in the subgroup; p 3 value for heterogeneity between groups with meta‐regression, analyzed as categorical variables; p 4 value for publication bias (conducted only when trials > 5); significant p‐values are highlighted in bold prints.
Abbreviations: BDNF, brain‐derived neurotrophic factor; BMI, body mass index.
FIGURE 2.
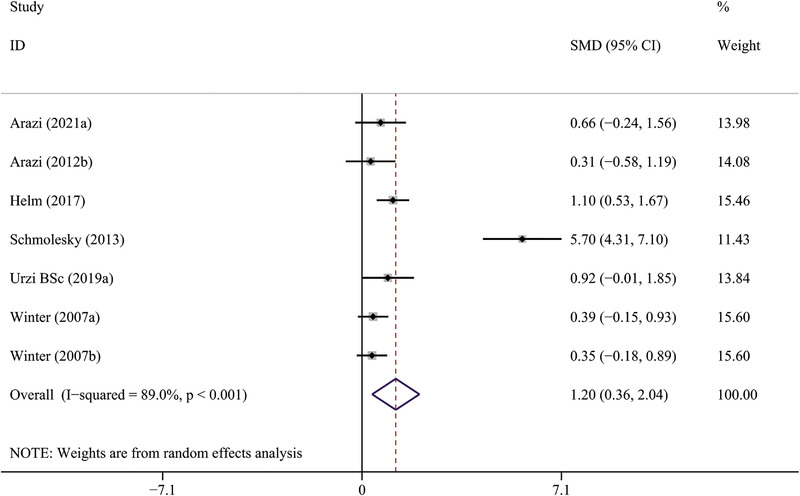
The forest plot of acute PE intervention on circulating BDNF levels
3.5. Effect of long‐term PE on BDNF levels
Seventeen studies (Arrieta et al., 2020; Čekanauskaitė et al., 2020; Cho & Roh, 2019; Forti et al., 2014; Goekint et al., 2010; Jeon & Ha, 2015, 2017; Kim & Kim, 2018; Ledreux et al., 2019; Maass et al., 2016; Matura et al., 2017; Nilsson et al., 2020; Rentería et al., 2020; Schiffer et al., 2009; Seifert et al., 2010; Solianik et al., 2021; Urzi et al., 2019) that reported the effect of long‐term PE on BDNF were included in the meta‐analysis. The primary meta‐analysis revealed that long‐term PE could significantly increase BDNF levels (SMD: 0.68, 95% CI: 0.27 to 1.08, p = .001), with a high heterogeneity observed (p = .000, I 2 = 82.8%) (Table 2; Figure 3).
FIGURE 3.
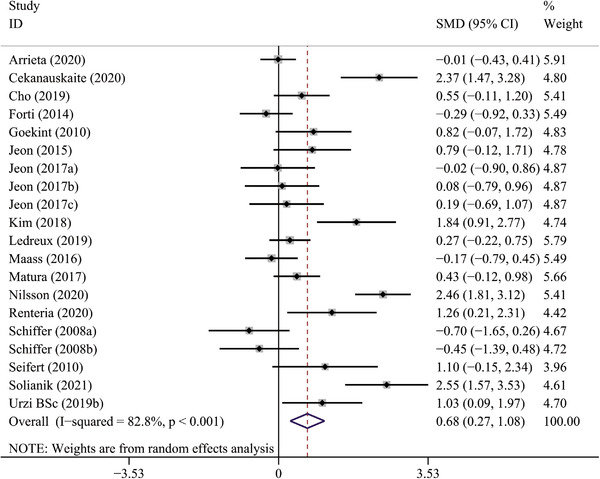
The forest plot of long‐term PE intervention on circulating BDNF levels
3.5.1. The results of subgroup analysis
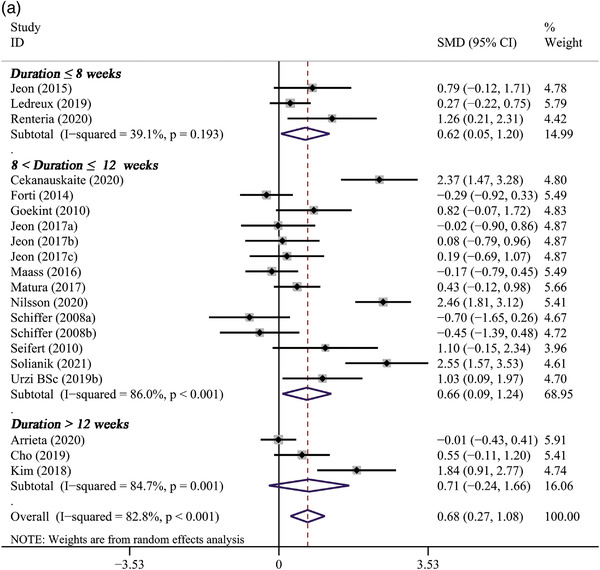
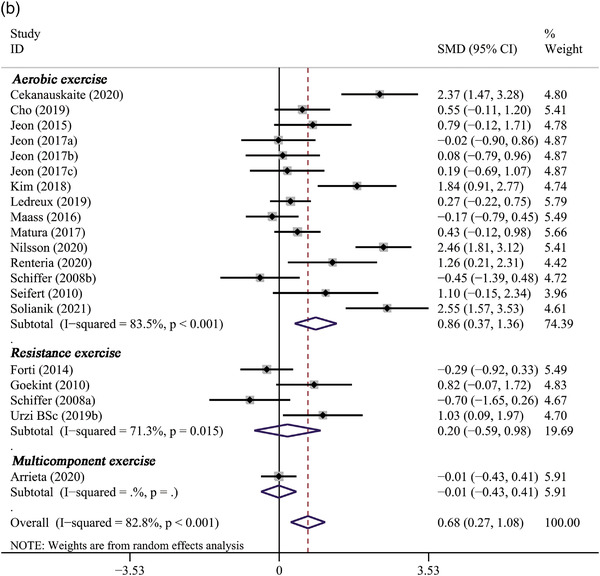
Subgroup analysis revealed that the pooled effect of long‐term PE on BDNF concentration was related to sample size, gender, age, baseline BMI, duration of PE, and type of PE. A significantly positive effect on BDNF levels was observed only in studies with larger sample sizes (n > 20) (11 trials, SMD: 0.93, 95% CI: 0.34 to 1.52, p = .002) but not in studies with smaller sample sizes (n ≤ 20) (6 trials, SMD: 0.33, 95% CI: −0.12 to 0.77, p = .150) (Figure 4A). It also indicated that the effect of PE intervention on BDNF levels was significant only in female participants (4 trials, SMD: 1.10, 95% CI: 0.53 to 1.68, p < .001) (Figure 4B). In addition, PE intervention remarkably elevated BDNF levels in participants over 60 years (11 trials, SMD: 0.95, 95% CI: 0.35 to 1.55, p = .002), whereas not in participants younger than 60 years (Figure 4C). Subgroup analysis based on baseline BMI of participants showed that BDNF levels significantly increased only in participants whose baseline BMI ≥ 25 (8 trials, SMD: 0.65, 95% CI: 0.07 to 1.24, p = .029), but not in participants whose baseline BMI < 25 (4 trials, SMD: 0.42, 95% CI: −0.03 to 0.88, p = .070) (Figure 4D). Significant improvements on BDNF concentrations were observed only in studies lasting less than 8 weeks (3 trials, SMD: 0.56, 95% CI: 0.01 to 1.12, p = .041) or between 8 and 12 weeks (11 trials, SMD: 0.47, 95% CI: 0.19 to 0.76, p = .001), but not in studies lasting more than 12 weeks (3 trials, SMD:1.34, 95% CI: −0.07 to 2.76, p = .063) (Figure 5A). Only aerobic exercise significantly elevated the levels of BDNF (13 trials, SMD: 0.86, 95% CI: 0.37 to 1.36, p = .001), while resistance exercise (4 trials, SMD: 0.20 95% CI: −0.59 to 0.98, p = .626) or multicomponent exercise (1 trials, SMD: −0.01, 95% CI: −0.43 to 0.41, p = .960) did not (Figure 5B).
FIGURE 4.
The subgroup analysis of long‐term PE intervention on circulating BDNF levels stratified by sample size (A), gender (B), age (C), and baseline BMI (D)
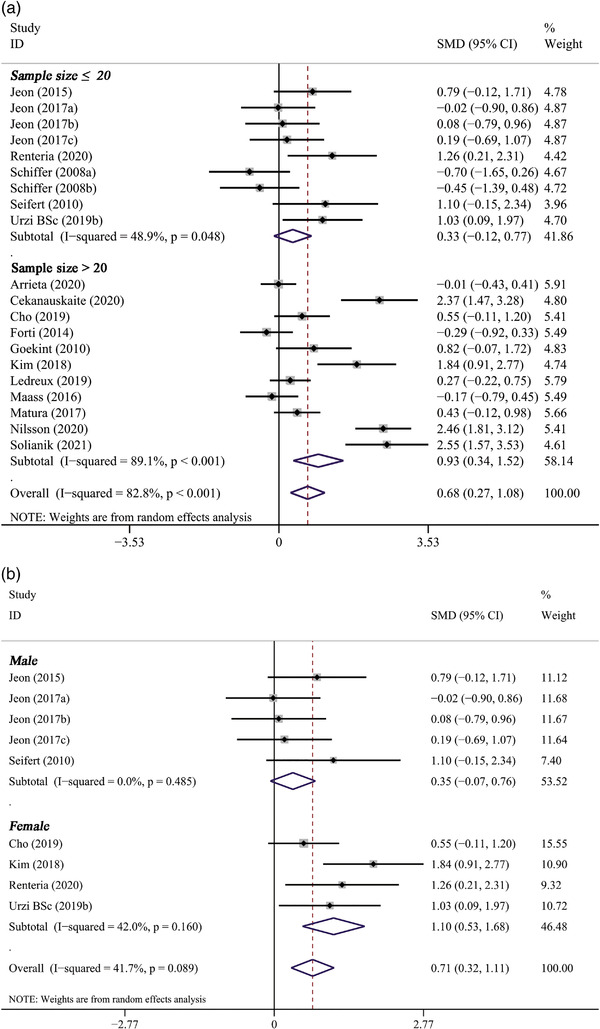
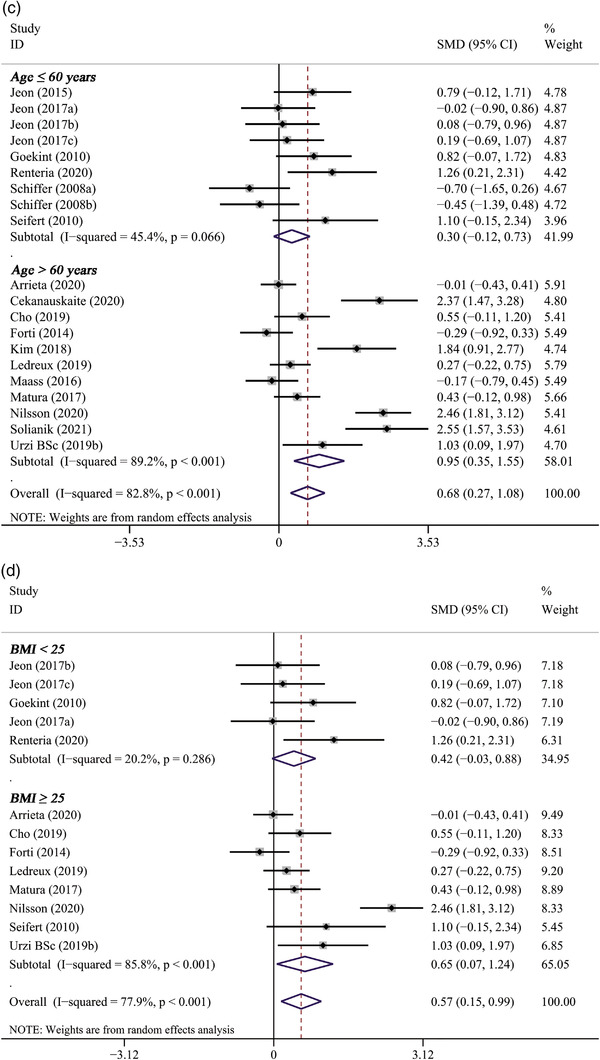
FIGURE 5.
The subgroup analysis of long‐term PE intervention on circulating BDNF levels stratified by duration of exercise (A) and type of exercise (B)
3.5.2. The results of meta‐regression
Meta‐regression analysis was conducted to explore the potential sources of heterogeneity. Among selected covariates, including sample size, region, gender, age, baseline BMI, duration of PE, type of PE, and intensity of PE, the results of meta‐regression analysis revealed that gender of participants was a potential confounder of the effect of long‐term PE intervention on the BDNF levels, with adjusted R 2 of 59.01% (p = .081) (Table 2).
3.5.3. Publication bias
Publication bias was suggested by Begg's test (p = .030), but not by Egger's test (p = .473) in the primary meta‐analysis (Table 2). Evidences also showed publication bias in the subgroup results of sample size > 20, age ≥ 60 years, baseline BMI ≥ 25, and aerobic exercise (Table 2). As shown in Table 3, for those results with publication bias indicated by Begg's and Egger's tests, the pooling estimates were recalculated using Duval and Tweedie's trim and fill method. The results of meta‐analyses remained unchanged or still statistically significant after being adjusted by trim and fill method, which confirmed and strengthened the evidence‐base regarding the effects of long‐term PE on BDNF (Table 3).
TABLE 3.
Trim and fill analysis and leave‐one‐out analysis
| SMD (95% CI) | p | |||||
|---|---|---|---|---|---|---|
| Trim and fill analysis | Trials (n) | Before adjusted | After adjusted | Before adjusted | After adjusted | Adjusted studies |
| BDNF, ng/ml (Long‐term effect) | 17 | 0.68 (0.27, 1.08) | Unchanged | .001 | Unchanged | – |
| Sample size > 20 | 11 | 0.93 (0.34, 1.52) | Unchanged | .002 | Unchanged | – |
| Age ≥ 60 years | 11 | 0.95 (0.35, 1.55) | 0.80 (0.17, 1.44) | .002 | .014 | 3 |
| BMI ≥ 25 | 8 | 0.65 (0.07, 1.24) | Unchanged | .029 | Unchanged | – |
| Aerobic exercise | 15 | 0.86 (0.37, 1.36) | Unchanged | .001 | Unchanged | – |
| SMD (95% CI) | P | |||||
|---|---|---|---|---|---|---|
| Leave‐one‐out cross validation | Trials (n) | Before adjusted | After adjusted | Before adjusted | After adjusted | Omitted studies |
| BDNF, ng/ml (Acute effect) | 5 | 1.20 (0.36, 2.04) | 0.60 (0.32, 0.88) | .005 | <.001 | 1 |
| BDNF, ng/ml (Long‐term effect) | 17 | 0.68 (0.27, 1.08) | 0.38 (0.13, 0.62) | .001 | .003 | 7 |
Note: Significant p‐values are highlighted in bold prints.
Abbreviations: BDNF, brain‐derived neurotrophic factor; BMI, body mass index; CI, confidence interval.
3.6. Sensitivity analysis
Regarding the robustness of overall effect sizes, we performed leave‐one‐out cross validation for sensitivity analysis. The results of leave‐one‐out cross validation suggested that one study in acute PE (Schmolesky et al., 2013) and seven studies in long‐term PE on BDNF (Arrieta et al., 2020; Čekanauskaitė et al., 2020; Forti et al., 2014; Kim & Kim, 2018; Nilsson et al., 2020; Schiffer et al., 2009; Solianik et al., 2021) contributed to the 96.2% and 73.2% of heterogeneity between studies, respectively. After excluding the mentioned trials, the pooled results of acute PE (SMD: 0.60, 95% CI: 0.32 to 0.88, p < .001, I 2 = 3.8%) and long‐term PE (SMD: 0.38, 95% CI: 0.13 to 0.62, p = .006, I 2 = 22.2%) remained significant elevated (Table 3).
4. DISCUSSION
This is the first meta‐analysis based on RCTs but not pre‐post design to estimate the effect of PE (both acute and long‐term) on the levels of circulating BDNF among healthy subjects. Overall, our present results mainly revealed that both acute and long‐term PE significantly increased BDNF levels, although study heterogeneity was very high for both acute and long‐term effects. Furthermore, studies of long‐term PE with larger sample sizes, female participants, participants older than 60 years, and aerobic exercise contributed to a more pronounced improvement on BDNF levels than that found when all studies were combined.
Acute PE is an effective stimulating factor to increase peripheral BDNF. A bulk of available evidence has reported that acute PE is associated with increased circulating BDNF (Dinoff et al., 2017; Szuhany et al., 2015). H. C. Cho et al. (2012) reported that progressive, maximum intensity treadmill exercise increased the entire peripheral BDNF levels including serum, plasma, and platelets. The release of BDNF from platelets can be changed by allergic airway inflammation (Lommatzsch, Schloetcke, et al., 2005), so acute PE may affect the release of BDNF from platelets by stimulating inflammatory response (Scheffer & Latini, 2020). Moreover, the transient BDNF response to acute exercise may induce a series of neuronal responses to improve cognitive function (Bechara, Lyne, & Kelly, 2014).
Overall, and in accordance with other studies (Dinoff et al., 2016; Szuhany et al., 2015), we observed a significant elevated effect of long‐term PE on BDNF levels. One of the possible mechanisms by which long‐term PE induced brain plasticity and cognitive enhancement is via stimulating an increase in the concentration of BDNF (Gligoroska & Manchevska, 2012). Usually, 99% of BDNF in circulation binds to platelets (E. I. Walsh et al., 2020), which are stored in the spleen for later release. After BDNF is released into plasma, it can bind with specific neural receptors (E. I. Walsh et al., 2020). PE can stimulate the release of BDNF from brain, skeletal muscle, platelets, and other tissues (J. J. Walsh & Tschakovsky, 2018), mainly by increasing blood circulation throughout the body and the release of platelets from the spleen (E. I. Walsh et al., 2020). Due to differences in the sample size of the included studies (ranged from 12 to 88), we stratified the results according to the sample size. Our subgroup analysis revealed that only studies with larger sample sizes displayed significant elevation in BDNF levels post long‐term PE. Small sample size studies may have sampling error and instability, and are more likely to draw false negative conclusions, namely type Ⅱ error (Akobeng, 2016). However, after excluding studies with small sample sizes, there are still high heterogeneity and publication bias among large sample studies. However, the results of subgroup analysis did not change after adjustment for publication bias by trim and fill method. Notably, through leave‐one‐out analysis, we found that 6 of the 7 literatures contributing most to heterogeneity were from large‐sample studies. These indicated that future large‐scale and well‐designed RCTs are still required to further examine our main findings.
In our study, we found that women were more likely to benefit from long‐term PE through subgroup analysis and meta‐regression. However, previous meta‐analysis (Szuhany et al., 2015) reported that the effect of long‐term regular exercise on peripheral BDNF levels was negatively correlated to the proportion of women included in the studies. Meanwhile, we also found that the elevation effect of PE on BDNF was only reflected in those older than 60 years. There is no doubt that gender and age are two important factors affecting the levels of BDNF. In addition, women and the elderly are also high‐risk groups of Alzheimer's disease and other diseases related to abnormal BDNF levels (Beam et al., 2018; Riedel et al., 2016). Weisbrod et al. (2019) also found that BDNF levels decreased in female rats after exposure to stress, but not in male mice. Our meta‐analysis could suggest that long‐term PE intervention might be effective for improving BDNF levels for people over 60‐years‐old and the female population. However, more females than males (n = 112/80) and more people over 60‐years‐old than under 60‐years‐old (n = 505/168) were included in the analyses, so future studies with equal sex and age ratios need to be replicated.
Based on current evidences, aerobic exercise has been proved to be successful in improving circulating BDNF (Cassilhas et al., 2012; Dinoff et al., 2016), while strength training seems to be mostly ineffective (Huang et al., 2014; Knaepen et al., 2010). A meta‐analysis also showed that aerobic exercise may contribute to increased levels of BDNF in neurological populations (Mackay et al., 2017). Consistent with previous studies, we also found that aerobic exercise, but not resistance training, increased circulating BDNF. Aerobic exercise is related to the improvement of endothelial function, insulin resistance, metabolic function, and cerebral blood flow, which are all associated with the increase of BDNF (Lemos et al., 2016; Zembron‐Lacny et al., 2016).
In our current study, we found that intensity of exercise did not influence the levels of BDNF. Most of studies admitted that the intensity of exercise is positively correlated with the increase of BDNF circulation levels. A systematic review reported that BDNF levels increase in an intensity‐dependent manner (Knaepen et al., 2010). Higher intensity of exercise is associated with hyperthermia, splenic response (Brunelli et al., 2012; Stewart et al., 2003), increased blood‐brain barrier permeability (Roh et al., 2017), and hypoxia. These are all related to the increase of BDNF release. Soya et al. found that acute treadmill running at low intensity (15 m/min) increased BDNF levels in the hippocampus of Wistar rats, but no increase was observed at moderate intensity (20 or 25 m/min) (Soya et al., 2007). Similarly, Gilder et al. (2014) found that serum BDNF increased by approximately 48% at 78% VO2max, but decreased at maximal exertion trial in healthy young men. Hence, the relationship between intensity of exercise and BDNF levels needs to be further investigated as a key point in clarifying the effect of PE on BDNF.
4.1. Limitations
There exist several limitations in this study. Firstly, 9 of the 21 included studies did not claim to adopt blinding, which resulted in the possibility of bias. The heterogeneity across studies and the limited sample size in some of the subgroups also made the interpretation of the results requiring to be cautious. In addition, some results showed publication bias, which might threaten the validity and interpretation of the effect; however, most original analyses remained unchanged after adjustment via trim and fill analysis. Lastly, only three included studies (Matura et al., 2017; Nilsson et al., 2020; Seifert et al., 2010) reported the results of fitness levels, making it impossible to explore the relationship between fitness levels and BDNF levels.
5. CONCLUSION
Taken together, both acute and long‐term PE significantly elevated circulating BDNF levels in healthy subjects. Long‐term aerobic exercise can lead to a more pronounced neurotrophic effect especially for female subjects or subjects over 60 years. Future large‐scale and high‐quality RCTs focusing on more detailed divisions of PE prescriptions and the importance of carefully considering the physiological response to PE will be of great necessity.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
All authors have contributed to the work in a meaningful way. Ya‐Hai Wang, Huan‐Huan Zhou, and Sidong Cui were involved in the conceptualization and design of the methodology. Ya‐Hai Wang and Huan‐Huan Zhou conducted the research, analyzed the data. Ya‐Hai Wang, Huan‐Huan Zhou, and Sidong Cui wrote the initial draft. Qiang Luo and Sidong Cui critically reviewed the manuscript. All authors agree with publication of the final version of the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2544
Supporting information
Supporting Information
ACKNOWLEDGMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Wang, Y.‐H. , Zhou, H.‐H. , Luo, Q. , & Cui, S. (2022). The effect of physical exercise on circulating brain‐derived neurotrophic factor in healthy subjects: A meta‐analysis of randomized controlled trials. Brain and Behavior, 12, e2544. 10.1002/brb3.2544
Ya‐Hai Wang and Huan‐Huan Zhou contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Akobeng, A. K. (2016). Understanding type I and type II errors, statistical power and sample size. Acta Paediatrica, 105(6), 605–609. 10.1111/apa.13384 [DOI] [PubMed] [Google Scholar]
- Arazi, H. , Babaei, P. , Moghimi, M. , & Asadi, A. (2021). Acute effects of strength and endurance exercise on serum BDNF and IGF‐1 levels in older men. BMC Geriatr, 21(1), 50. 10.1186/s12877-020-01937-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta, H. , Rezola‐Pardo, C. , Kortajarena, M. , Hervás, G. , Gil, J. , Yanguas, J. J. , Iturburu, M. , Gil, S. M. , Irazusta, J. , & Rodriguez‐Larrad, A. (2020). The impact of physical exercise on cognitive and affective functions and serum levels of brain‐derived neurotrophic factor in nursing home residents: A randomized controlled trial. Maturitas, 131, 72–77. 10.1016/j.maturitas.2019.10.014 [DOI] [PubMed] [Google Scholar]
- Azevedo, K. P. M. D. , De Oliveira, V. H. , Medeiros, G. C. B. S. D. , Mata, Á. N. D. S. , García, D. Á. , Martínez, D. G. , Leitão, J. C. , Knackfuss, M. I. , & Piuvezam, G. (2020). The effects of exercise on BDNF levels in adolescents: A systematic review with meta‐analysis. International Journal of Environmental Research and Public Health, 17(17), 6056. 10.3390/ijerph17176056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird, J. F. , Gaughan, M. E. , Saffer, H. M. , Sarzynski, M. A. , Herter, T. M. , Fritz, S. L. , Den Ouden, D. B. , & Stewart, J. C. (2018). The effect of energy‐matched exercise intensity on brain‐derived neurotrophic factor and motor learning. Neurobiology of Learning and Memory, 156, 33–44. 10.1016/j.nlm.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde, Y. A. , Edgar, D. , & Thoenen, H. (1982). Purification of a new neurotrophic factor from mammalian brain. Embo Journal, 1(5), 549–553. 10.1002/j.1460-2075.1982.tb01207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam, C. R. , Kaneshiro, C. , Jang, J. Y. , Reynolds, C. A. , Pedersen, N. L. , & Gatz, M. (2018). Differences between women and men in incidence rates of dementia and Alzheimer's disease. Journal of Alzheimer's Disease, 64(4), 1077–1083. 10.3233/jad-180141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara, R. G. , Lyne, R. , Kelly, Á. M. (2014). BDNF‐stimulated intracellular signalling mechanisms underlie exercise‐induced improvement in spatial memory in the male Wistar rat. Behavioural Brain Research, 275, 297–306. 10.1016/j.bbr.2013.11.015 [DOI] [PubMed] [Google Scholar]
- Brown, J. , Cooper‐Kuhn, C. M. , Kempermann, G. , Van Praag, H. , Winkler, J. , Gage, F. H. , & Kuhn, H. G. (2003). Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. European Journal of Neuroscience, 17(10), 2042–2046. 10.1046/j.1460-9568.2003.02647.x [DOI] [PubMed] [Google Scholar]
- Brunelli, A. , Dimauro, I. , Sgrò, P. , Emerenziani, G. P. , Magi, F. , Baldari, C. , Guidetti, L. , Di Luigi, L. , Parisi, P. , & Caporossi, D. (2012). Acute exercise modulates BDNF and pro‐BDNF protein content in immune cells. Medicine and Science in Sports and Exercise, 44(10), 1871–1880. 10.1249/MSS.0b013e31825ab69b [DOI] [PubMed] [Google Scholar]
- Cassilhas, R. C. , Lee, K. S. , Fernandes, J. , Oliveira, M. G. , Tufik, S. , Meeusen, R. , & de Mello, M. T. (2012). Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience, 202, 309–317. 10.1016/j.neuroscience.2011.11.029 [DOI] [PubMed] [Google Scholar]
- Čekanauskaitė, A. , Skurvydas, A. , Žlibinaitė, L. , Mickevičienė, D. , Kilikevičienė, S. , & Solianik, R. (2020). A 10‐week yoga practice has no effect on cognition, but improves balance and motor learning by attenuating brain‐derived neurotrophic factor levels in older adults. Experimental Gerontology, 138, 110998. 10.1016/j.exger.2020.110998 [DOI] [PubMed] [Google Scholar]
- Cho, H. C. , Kim, J. , Kim, S. , Son, Y. H. , Lee, N. , Jung, S. H. (2012). The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO2max performance in healthy college men. Neuroscience Letters, 519(1), 78–83. 10.1016/j.neulet.2012.05.025 [DOI] [PubMed] [Google Scholar]
- Cho, S. Y. , & Roh, H. T. (2019). Taekwondo enhances cognitive function as a result of increased neurotrophic growth factors in elderly women. International Journal of Environmental Research and Public Health, 962, 16(6). 10.3390/ijerph16060962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinoff, A. , Herrmann, N. , Swardfager, W. , & Lanctôt, K. L. (2017). The effect of acute exercise on blood concentrations of brain‐derived neurotrophic factor in healthy adults: A meta‐analysis. European Journal of Neuroscience, 46(1), 1635–1646. 10.1111/ejn.13603 [DOI] [PubMed] [Google Scholar]
- Dinoff, A. , Herrmann, N. , Swardfager, W. , Liu, C. S. , Sherman, C. , Chan, S. , & Lanctôt, K. L. (2016). The effect of exercise training on resting concentrations of peripheral brain‐derived neurotrophic factor (BDNF): A meta‐analysis. PLoS One, 11(9), e0163037. 10.1371/journal.pone.0163037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval, S. , & Tweedie, R. (2000). Trim and fill: A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics, 56(2), 455–463. 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Egger, M. , Davey Smith, G. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, K. I. , Miller, D. L. , & Roecklein, K. A. (2012). The aging hippocampus: Interactions between exercise, depression, and BDNF. The Neuroscientist, 18(1), 82–97. 10.1177/1073858410397054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti, L. N. , Njemini, R. , Beyer, I. , Eelbode, E. , Meeusen, R. , Mets, T. , & Bautmans, I. (2014). Strength training reduces circulating interleukin‐6 but not brain‐derived neurotrophic factor in community‐dwelling elderly individuals. Age (Dordr), 36(5), 9704. 10.1007/s11357-014-9704-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheibi, S. , Mahmoodzadeh, A. , Kashfi, K. , Jeddi, S. , & Ghasemi, A. (2019). Data extraction from graphs using adobe photoshop: Applications for meta‐analyses. International Journal of Endocrinology and Metabolism, 17(4), e95216. 10.5812/ijem.95216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder, M. , Ramsbottom, R. , Currie, J. , Sheridan, B. , & Nevill, A. M. (2014). Effect of fat free mass on serum and plasma BDNF concentrations during exercise and recovery in healthy young men. Neuroscience Letters, 560, 137–141. 10.1016/j.neulet.2013.12.034 [DOI] [PubMed] [Google Scholar]
- Gligoroska, J. P. , & Manchevska, S. (2012). The effect of physical activity on cognition—Physiological mechanisms. Mater Sociomed, 24(3), 198–202. 10.5455/msm.2012.24.198-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goekint, M. , De Pauw, K. , Roelands, B. , Njemini, R. , Bautmans, I. , Mets, T. , & Meeusen, R. (2010). Strength training does not influence serum brain‐derived neurotrophic factor. European Journal of Applied Physiology, 110(2), 285–293. 10.1007/s00421-010-1461-3 [DOI] [PubMed] [Google Scholar]
- Goldet, G. , & Howick, J. (2013). Understanding GRADE: An introduction. J Evid Based Med, 6(1), 50–54. 10.1111/jebm.12018 [DOI] [PubMed] [Google Scholar]
- Gomez‐Pinilla, F. , Zhuang, Y. , Feng, J. , Ying, Z. , & Fan, G. (2011). Exercise impacts brain‐derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. European Journal of Neuroscience, 33(3), 383–390. 10.1111/j.1460-9568.2010.07508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves, J. T. , Schafer, S. T. , & Gage, F. H. (2016). Adult neurogenesis in the hippocampus: From stem cells to behavior. Cell, 167(4), 897–914. 10.1016/j.cell.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Hacioglu, G. , Senturk, A. , Ince, I. , & Alver, A. (2016). Assessment of oxidative stress parameters of brain‐derived neurotrophic factor heterozygous mice in acute stress model. Iran Journal of Basic Medical Science, 19(4), 388–393. [PMC free article] [PubMed] [Google Scholar]
- Haskell, W. L. , Lee, I.‐M. , Pate, R. R. , Powell, K. E. , Blair, S. N. , Franklin, B. A. , Macera, C. A. , Heath, G. W. , Thompson, P. D. , & Bauman, A. (2007). Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise, 39(8), 1423–1434. 10.1249/mss.0b013e3180616b27 [DOI] [PubMed] [Google Scholar]
- Helm, E. E. , Matt, K. S. , Kirschner, K. F. , Pohlig, R. T. , Kohl, D. , & Reisman, D. S. (2017). The influence of high intensity exercise and the Val66Met polymorphism on circulating BDNF and locomotor learning. Neurobiology of Learning and Memory, 144, 77–85. 10.1016/j.nlm.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T. , Larsen, K. T. , Ried‐Larsen, M. , Møller, N. C. , & Andersen, L. B. (2014). The effects of physical activity and exercise on brain‐derived neurotrophic factor in healthy humans: A review. Scandinavian Journal of Medicine & Science in Sports, 24(1), 1–10. 10.1111/sms.12069 [DOI] [PubMed] [Google Scholar]
- Jeon, Y. K. , & Ha, C. H. (2015). Expression of brain‐derived neurotrophic factor, IGF‐1 and cortisol elicited by regular aerobic exercise in adolescents. J Phys Ther Sci, 27(3), 737–741. 10.1589/jpts.27.737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, Y. K. , & Ha, C. H. (2017). The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ Health Prev Med, 22(1), 27. 10.1186/s12199-017-0643-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner, M. , Gallmeier, E. , Behrens, L. , Leal, V. V. , Misgeld, T. , Klinkert, W. E. F. , Kolbeck, R. , Hoppe, E. , Oropeza‐Wekerle, R.‐L. , Bartke, I. , Stadelmann, C. , Lassmann, H. , Wekerle, H. , & Hohlfeld, R. (1999). Activated human T cells, B cells, and monocytes produce brain‐derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? Journal of Experimental Medicine, 189(5), 865–870. 10.1084/jem.189.5.865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. H. , & Kim, D. Y. (2018). Aquarobic exercises improve the serum blood irisin and brain‐derived neurotrophic factor levels in elderly women. Experimental Gerontology, 104, 60–65. 10.1016/j.exger.2018.01.024 [DOI] [PubMed] [Google Scholar]
- Knaepen, K. , Goekint, M. , Heyman, E. M. , & Meeusen, R. (2010). Neuroplasticity—Exercise‐induced response of peripheral brain‐derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Medicine (Auckland, N.Z.), 40(9), 765–801. 10.2165/11534530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Larson, E. B. , Wang, L. , Bowen, J. D. , McCormick, W. C. , Teri, L. , Crane, P. , & Kukull, W. (2006). Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Annals of Internal Medicine, 144(2), 73–81. 10.7326/0003-4819-144-2-200601170-00004 [DOI] [PubMed] [Google Scholar]
- Leckie, R. L. , Oberlin, L. E. , Voss, M. W. , Prakash, R. S. , Szabo‐Reed, A. , Chaddock‐Heyman, L. , Phillips, S. M. , Gothe, N. P. , Mailey, E. , Vieira‐Potter, V. J. , Martin, S. A. , Pence, B. D. , Lin, M. , Parasuraman, R. , Greenwood, P. M. , Fryxell, K. J. , Woods, J. A. , Mcauley, E. , Kramer, A. F. , & Erickson, K. I. (2014). BDNF mediates improvements in executive function following a 1‐year exercise intervention. Front Hum Neurosci, 8, 985. 10.3389/fnhum.2014.00985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledreux, A. , Håkansson, K. , Carlsson, R. , Kidane, M. , Columbo, L. , Terjestam, Y. , Ryan, E. , Tusch, E. , Winblad, B. , Daffner, K. , Granholm, A.‐C. , & Mohammed, A. K. H. (2019). Differential effects of physical exercise, cognitive training, and mindfulness practice on serum BDNF levels in healthy older adults: A randomized controlled intervention study. Journal of Alzheimer's Disease, 71(4), 1245–1261. 10.3233/jad-190756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos, J. R. Jr. , Alves, C. R. , De Souza, S. B. C. , Marsiglia, J. D. C. , Silva, M. S. M. , Pereira, A. C. , Teixeira, A. L. , Vieira, E. L. M. , Krieger, J. E. , Negrão, C. E. , Alves, G. B. , De Oliveira, E. M. , Bolani, W. , Dias, R. G. , & Trombetta, I. C. (2016). Peripheral vascular reactivity and serum BDNF responses to aerobic training are impaired by the BDNF Val66Met polymorphism. Physiological Genomics, 48(2), 116–123. 10.1152/physiolgenomics.00086.2015 [DOI] [PubMed] [Google Scholar]
- Lommatzsch, M. , Schloetcke, K. , Klotz, J. , Schuhbaeck, K. , Zingler, D. , Zingler, C. , Schulte‐Herbrüggen, O. , Gill, H. , Schuff‐Werner, P. , Virchow, J. C. (2005). Brain‐derived Neurotrophic Factor in Platelets and Airflow Limitation in Asthma. American Journal of Respiratory and Critical Care Medicine, 171(2), 115–120. 10.1164/rccm.200406-758oc [DOI] [PubMed] [Google Scholar]
- Maass, A. , Düzel, S. , Brigadski, T. , Goerke, M. , Becke, A. , Sobieray, U. , Neumann, K. , Lövdén, M. , Lindenberger, U. , Bäckman, L. , Braun‐Dullaeus, R. , Ahrens, D. , Heinze, H.‐J. , Müller, N. G. , Lessmann, V. , Sendtner, M. , & Düzel, E. (2016). Relationships of peripheral IGF‐1, VEGF and BDNF levels to exercise‐related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage, 131, 142–154. 10.1016/j.neuroimage.2015.10.084 [DOI] [PubMed] [Google Scholar]
- Mackay, C. P. , Kuys, S. S. , & Brauer, S. G. (2017). The effect of aerobic exercise on brain‐derived neurotrophic factor in people with neurological disorders: A systematic review and meta‐analysis. Neural Plasticity, 2017, 1. 10.1155/2017/4716197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher, C. G. , Sherrington, C. , Herbert, R. D. , Moseley, A. M. , & Elkins, M. (2003). Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical Therapy, 83(8), 713–721. 10.1093/ptj/83.8.713 [DOI] [PubMed] [Google Scholar]
- Marinus, N. , Hansen, D. , Feys, P. , Meesen, R. , Timmermans, A. , & Spildooren, J. (2019). The impact of different types of exercise training on peripheral blood brain‐derived neurotrophic factor concentrations in older adults: A meta‐analysis. Sports Medicine (Auckland, N.Z.), 49(10), 1529–1546. 10.1007/s40279-019-01148-z [DOI] [PubMed] [Google Scholar]
- Matura, S. , Fleckenstein, J. , Deichmann, R. , Engeroff, T. , Füzéki, E. , Hattingen, E. , Hellweg, R. , Lienerth, B. , Pilatus, U. , Schwarz, S. , Tesky, V. A. , Vogt, L. , Banzer, W. , & Pantel, J. (2017). Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: Results of the randomised controlled SMART trial. Transl Psychiatry, 7(7), e1172. 10.1038/tp.2017.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara, A. H. , Merrill, D. A. , Coppola, G. , Tsukada, S. , Schroeder, B. E. , Shaked, G. M. , Wang, L. , Blesch, A. , Kim, A. , Conner, J. M. , Rockenstein, E. , Chao, M. V. , Koo, E. H. , Geschwind, D. , Masliah, E. , Chiba, A. A. , & Tuszynski, M. H. (2009). Neuroprotective effects of brain‐derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nature Medicine, 15(3), 331–337. 10.1038/nm.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper, S. A. , Gómez‐Pinilla, F. , Choi, J. , & Cotman, C. W. (1996). Physical activity increases mRNA for brain‐derived neurotrophic factor and nerve growth factor in rat brain. Brain Research, 726(1–2), 49–56. 10.1016/0006-8993(96)00273-9 [DOI] [PubMed] [Google Scholar]
- Nilsson, J. , Ekblom, Ö. , Ekblom, M. , Lebedev, A. , Tarassova, O. , Moberg, M. , & Lövdén, M. (2020). Acute increases in brain‐derived neurotrophic factor in plasma following physical exercise relates to subsequent learning in older adults. Science Reports, 10(1), 4395. 10.1038/s41598-020-60124-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. , & Poo, M. M. (2013). Neurotrophin regulation of neural circuit development and function. Nature Reviews Neuroscience, 14(1), 7–23. 10.1038/nrn3379 [DOI] [PubMed] [Google Scholar]
- Rentería, I. , García‐Suárez, P. C. , Martínez‐Corona, D. O. , Moncada‐Jiménez, J. , Plaisance, E. P. , & JiméNez‐Maldonado, A. (2020). Short‐term high‐intensity interval training increases systemic brain‐derived neurotrophic factor (BDNF) in healthy women. Eur J Sport Sci, 20(4), 516–524. 10.1080/17461391.2019.1650120 [DOI] [PubMed] [Google Scholar]
- Riedel, B. C. , Thompson, P. M. , & Brinton, R. D. (2016). Age, APOE and sex: Triad of risk of Alzheimer's disease. Journal of Steroid Biochemistry and Molecular Biology, 160, 134–147. 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh, H. T. , Cho, S. Y. , Yoon, H. G. , & So, W. Y. (2017). Effect of exercise intensity on neurotrophic factors and blood‐brain barrier permeability induced by oxidative‐nitrosative stress in male college students. Int J Sport Nutr Exerc Metab, 27(3), 239–246. 10.1123/ijsnem.2016-0009 [DOI] [PubMed] [Google Scholar]
- Schiffer, T. , Schulte, S. , Hollmann, W. , Bloch, W. , & Strüder, H. K. (2009). Effects of strength and endurance training on brain‐derived neurotrophic factor and insulin‐like growth factor 1 in humans. Hormone and Metabolic Research, 41(3), 250–254. 10.1055/s-0028-1093322 [DOI] [PubMed] [Google Scholar]
- Schmolesky, M. T. , Webb, D. L. , & Hansen, R. A. (2013). The effects of aerobic exercise intensity and duration on levels of brain‐derived neurotrophic factor in healthy men. J Sports Sci Med, 12(3), 502–511. [PMC free article] [PubMed] [Google Scholar]
- Scheffer, D. D. L. , Latini, A. (2020). Exercise‐induced immune system response: Anti‐inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol Basis Dis, 1866(10), 165823. 10.1016/j.bbadis.2020.165823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert, T. , Brassard, P. , Wissenberg, M. , Rasmussen, P. , Nordby, P. , Stallknecht, B. , Adser, H. , Jakobsen, A. H. , Pilegaard, H. , Nielsen, H. B. , & Secher, N. H. (2010). Endurance training enhances BDNF release from the human brain. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 298(2), R372–R377. 10.1152/ajpregu.00525.2009 [DOI] [PubMed] [Google Scholar]
- Serban, C. , Sahebkar, A. , Ursoniu, S. , Andrica, F. , & Banach, M. (2015). Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: A systematic review and meta‐analysis of randomized controlled trials. Journal of Hypertension, 33(6), 1119–1127. 10.1097/hjh.0000000000000585 [DOI] [PubMed] [Google Scholar]
- Solianik, R. , Mickevičienė, D. , Žlibinaitė, L. , & Čekanauskaitė, A. (2021). Tai chi improves psychoemotional state, cognition, and motor learning in older adults during the COVID‐19 pandemic. Experimental Gerontology, 150, 111363. 10.1016/j.exger.2021.111363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya, H. , Nakamura, T. , Deocaris, C. C. , Kimpara, A. , Iimura, M. , Fujikawa, T. , Chang, H. , McEwen, B. S. , & Nishijima, T. (2007). BDNF induction with mild exercise in the rat hippocampus. Biochemical and Biophysical Research Communications, 358(4), 961–967. 10.1016/j.bbrc.2007.04.173 [DOI] [PubMed] [Google Scholar]
- Stewart, I. B. , Warburton, D. E. , Hodges, A. N. , Lyster, D. M. , & McKenzie, D. C. (2003). Cardiovascular and splenic responses to exercise in humans. J Appl Physiol (1985), 94(4), 1619–1626. 10.1152/japplphysiol.00040.2002 [DOI] [PubMed] [Google Scholar]
- Stigger, F. S. , Zago Marcolino, M. A. , Portela, K. M. , & Plentz, R. D. M. (2019). Effects of exercise on inflammatory, oxidative, and neurotrophic biomarkers on cognitively impaired individuals diagnosed with dementia or mild cognitive impairment: A systematic review and meta‐analysis. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 74(5), 616–624. 10.1093/gerona/gly173 [DOI] [PubMed] [Google Scholar]
- Szuhany, K. L. , Bugatti, M. , & Otto, M. W. (2015). A meta‐analytic review of the effects of exercise on brain‐derived neurotrophic factor. Journal of Psychiatric Research, 60, 56–64. 10.1016/j.jpsychires.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzi, F. , Marusic, U. , Ličen, S. , & Buzan, E. (2019). Effects of elastic resistance training on functional performance and myokines in older women‐a randomized controlled trial. Journal of the American Medical Directors Association, 20(7), 830–834.e2.e832. 10.1016/j.jamda.2019.01.151 [DOI] [PubMed] [Google Scholar]
- Vaynman, S. , Ying, Z. , & Gomez‐Pinilla, F. (2004). Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience, 20(10), 2580–2590. 10.1111/j.1460-9568.2004.03720.x [DOI] [PubMed] [Google Scholar]
- Voss, M. W. , Soto, C. , Yoo, S. , Sodoma, M. , Vivar, C. , & van Praag, H. (2019). Exercise and hippocampal memory systems. Trends in Cognitive Sciences, 23(4), 318–333. 10.1016/j.tics.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, E. I. , Smith, L. , Northey, J. , Rattray, B. , & Cherbuin, N. (2020). Towards an understanding of the physical activity‐BDNF‐cognition triumvirate: A review of associations and dosage. Ageing Research Reviews, 60, 101044. 10.1016/j.arr.2020.101044 [DOI] [PubMed] [Google Scholar]
- Walsh, J. J. , Edgett, B. A. , Tschakovsky, M. E. , & Gurd, B. J. (2015). Fasting and exercise differentially regulate BDNF mRNA expression in human skeletal muscle. Applied Physiology, Nutrition and Metabolism, 40(1), 96–98. 10.1139/apnm-2014-0290 [DOI] [PubMed] [Google Scholar]
- Walsh, J. J. , & Tschakovsky, M. E. (2018). Exercise and circulating BDNF: Mechanisms of release and implications for the design of exercise interventions. Applied Physiology, Nutrition and Metabolism, 43(11), 1095–1104. 10.1139/apnm-2018-0192 [DOI] [PubMed] [Google Scholar]
- Wan, X. , Wang, W. , Liu, J. , & Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology [Electronic Resource], 14, 135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod, A. S. , Barry, E. S. , Graham, A. M. , Eklund, M. , & Grunberg, N. E. (2019). Decreased BDNF in female but not male rats after exposure to stress: A sex‐sensitive rat model of stress? Stress (Amsterdam, Netherlands), 22(5), 581–591. 10.1080/10253890.2019.1617692 [DOI] [PubMed] [Google Scholar]
- Winter, B. , Breitenstein, C. , Mooren, F. C. , Voelker, K. , Fobker, M. , Lechtermann, A. , Krueger, K. , Fromme, A. , Korsukewitz, C. , Floel, A. , & Knecht, S. (2007). High impact running improves learning. Neurobiology of Learning and Memory, 87(4), 597–609. 10.1016/j.nlm.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Zembron‐Lacny, A. , Dziubek, W. , Rynkiewicz, M. , Morawin, B. , & Woźniewski, M. (2016). Peripheral brain‐derived neurotrophic factor is related to cardiovascular risk factors in active and inactive elderly men. Brazilian Journal of Medical and Biological Research, 49(7). 10.1590/1414-431x20165253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke, F. , Agnes, E. J. , & Gerstner, W. (2015). Diverse synaptic plasticity mechanisms orchestrated to form and retrieve memories in spiking neural networks. Nature Communication, 6, 6922. 10.1038/ncomms7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


