For undesired pregnancy of unknown location, immediate abortion initiation with mifepristone and misoprostol is associated with more rapid exclusion of ectopic pregnancy and pregnancy termination but lower abortion efficacy than delaying for diagnosis.
Abstract
OBJECTIVE:
To compare immediate initiation with delayed initiation of medication abortion among patients with an undesired pregnancy of unknown location.
METHODS:
This retrospective cohort study used electronic medical record data from the Planned Parenthood League of Massachusetts (2014–2019) for patients who requested medication abortion with a last menstrual period (LMP) of 42 days or less and pregnancy of unknown location (no gestational sac) on initial ultrasonogram. Clinicians could initiate medication abortion with mifepristone followed by misoprostol while simultaneously excluding ectopic pregnancy with serial serum human chorionic gonadotropin (hCG) testing (same-day-start group) or establish a diagnosis with serial hCG tests and repeat ultrasonogram before initiating treatment (delay-for-diagnosis group). We compared primary safety outcomes (time to diagnosis of pregnancy location [rule out ectopic], emergency department visits, adverse events, and nonadherence with follow-up) between groups. We also reported secondary efficacy outcomes: time to complete abortion, successful medication abortion (no uterine aspiration), and ongoing pregnancy.
RESULTS:
Of 5,619 medication abortion visits for patients with an LMP of 42 days or less, 452 patients had pregnancy of unknown location (8.0%). Three patients underwent immediate uterine aspiration, 55 had same-day start, and 394 had delay for diagnosis. Thirty-one patients (7.9%), all in the delay-for-diagnosis group, were treated for ectopic pregnancy, including four that were ruptured. Among patients with no major ectopic pregnancy risk factors (n=432), same-day start had shorter time to diagnosis (median 5.0 days vs 9.0 days; P=.005), with no significant difference in emergency department visits (adjusted odds ratio [aOR] 0.90, 95% CI 0.43–1.88) or nonadherence with follow-up (aOR 0.92, 95% CI 0.39–2.15). Among patients who proceeded with abortion (n=270), same-day start had shorter time to complete abortion (median 5.0 days vs 19.0 days; P<.001). Of those who had medication abortion with known outcome (n=170), the rate of successful medication abortion was lower (85.4% vs 96.7%; P=.013) and the rate of ongoing pregnancy was higher (10.4% vs 2.5%; P=.041) among patients in the same-day-start group.
CONCLUSION:
In patients with undesired pregnancy of unknown location, immediate initiation of medication abortion is associated with more rapid exclusion of ectopic pregnancy and pregnancy termination but lower abortion efficacy.
Patients present for abortion at increasingly earlier gestational ages, with 40% presenting at 6 weeks of gestation or less in the United States.1 Early presentation increases the likelihood that health care professionals will not visualize an intrauterine gestational sac on ultrasonogram and diagnose a pregnancy of unknown location.2 Patients with pregnancy of unknown location may eventually be diagnosed with intrauterine pregnancy, early pregnancy loss, or ectopic pregnancy.2 Because mifepristone and misoprostol are highly effective treatments for early abortion3–5 and early pregnancy loss,6 the most serious risk of initiating medication abortion in the setting of undesired pregnancy of unknown location is delaying the diagnosis and treatment of ectopic pregnancy, which can result in significant morbidity and mortality. Several studies also suggest an increased risk of ongoing pregnancy among those who initiate medication abortion with pregnancy of unknown location compared with those with a visualized intrauterine gestational sac.7,8
The benefit of immediate initiation of mifepristone and misoprostol for undesired pregnancy of unknown location is pregnancy termination without delay. Additionally, just as uterine aspiration can help distinguish an intrauterine pregnancy from an ectopic pregnancy, it is plausible that uterine emptying with medications may also facilitate diagnosis of pregnancy location more rapidly than expectant management with serial human chorionic gonadotropin (hCG) testing and ultrasonography. Furthermore, many patients prefer medical management over aspiration.9
Although current mifepristone labeling lists ectopic pregnancy as a contraindication, several national organizations allow immediate medication abortion for undesired pregnancy of unknown location when combined with serial hCG follow-up to exclude ectopic pregnancy (“same-day-start”).10–12 This retrospective cohort study compares the safety and efficacy of same-day start with the common practice of first confirming intrauterine pregnancy and then initiating treatment (“delay for diagnosis”).
METHODS
We performed a retrospective cohort study of all patients who presented to the Planned Parenthood League of Massachusetts, Boston, Worcester, or Springfield, from 2014 to 2019 requesting medication abortion with a reported gestational age by last menstrual period (LMP) of 42 days or less. We defined this population as the very early gestation, medication abortion–seeking population. Our primary interest was in the subpopulation of these patients who were found to have pregnancy of unknown location (no gestational sac) on initial ultrasonogram.
We identified patients using the current and previous Planned Parenthood League of Massachusetts electronic health record (EHR) systems (NextGen and Athenahealth). We used practice-management software to identify all patients who scheduled a medication abortion appointment and reported an LMP of 42 days or less at the time of scheduling. Of these patients, we then identified those diagnosed with pregnancy of unknown location on initial ultrasonogram.
Ultrasonographic images are uploaded into the EHR and undergo clinician interpretation of the ultrasonogram, including: 1) definite intrauterine pregnancy (gestational sac with yolk sac or fetal pole), 2) probable intrauterine pregnancy (small gestational sac with no yolk sac),2 3) pregnancy of unknown location (no intrauterine gestational sac and no evidence of ectopic pregnancy),2 4) early pregnancy loss,13 or 5) ectopic pregnancy. Ultrasonographic images for all those diagnosed with pregnancy of unknown location by their care-providing clinician were reviewed by one of our research physicians (D.D., A.G.) to confirm the interpretation and diagnosis. Any discrepancies in ultrasonogram interpretation were reviewed by the principal investigator (A.B.G.), who determined the final diagnosis. Demographic data were exported directly from the relevant EHR system to the research database. Detailed clinical data were manually extracted from the EHR onto a data-collection form for all patients with pregnancies of unknown location. Data from the written forms were then entered twice into the research database. All information was merged into a single database created for this study. This study was approved by the Partners Institutional Review Board.
The primary goal of the study was to compare outcomes between two management groups (same-day start and delay for diagnosis) for patients seeking medication abortion with pregnancy of unknown location on ultrasonogram who were very early in gestation by LMP. Patients in the delay-for-diagnosis group were followed with serial hCG testing and repeat ultrasonograms to confirm intrauterine pregnancy before initiating medication abortion. Patients in the same-day-start group initiated medication abortion by taking mifepristone on the day of presentation with pregnancy of unknown location while simultaneously initiating serial hCG testing to exclude ectopic pregnancy. In April 2014, we began offering same-day-start medication abortion for patients found to have pregnancy of unknown location, a positive urine hCG test result, no ectopic pregnancy symptoms, and no major ectopic pregnancy risk factors (intrauterine device in situ, prior tubal surgery, prior ectopic pregnancy). Initially, to be eligible for same-day start, patients had to have a certain LMP of 35 days or less. During the 5-year study period, eligibility expanded to include patients with a known LMP of 42 days or less. Patients who were managed with same-day start followed a standard protocol (Box 1). Importantly, decision making about whether to initiate medication abortion while simultaneously ruling out ectopic pregnancy compared with first confirming intrauterine pregnancy was left to the discretion of the physician or nurse practitioner. All patients with pregnancies of unknown location, whether managed with same-day start or delay for diagnosis, had a baseline serum hCG drawn on the day of presentation (day 1). Patients managed with same-day start took misoprostol 24–48 hours after mifepristone (day 2–3) and had a follow-up hCG drawn 48–72 hours after misoprostol (day 4–5); those managed with delay for diagnosis had a follow-up hCG drawn 48–72 hours after baseline (day 3–4). Regardless of group, management of patients with baseline hCG levels greater than 2,000 followed the same standard protocol described in Box 1. Those in the delay-for-diagnosis group with an initial hCG level less than 2,000, a doubling of their hCG level in 48–72 hours, and no ectopic pregnancy symptoms or major risk factors were presumed to have a normal intrauterine pregnancy and were scheduled for a repeat ultrasonogram and abortion when their hCG level was expected to be greater than 2,000. Those whose hCG level did not rise as expected or who were symptomatic or at high risk were managed on a case-by-case basis.
Box 1. Planned Parenthood League of Massachusetts Clinical Management Protocol for Patients Initiating Medication Abortion While Simultaneously Determining Pregnancy Location.
Day 1: Mifepristone 200 mg administered orally
• Serum hCG must be sent on the day of mifepristone (day 1). Management based on initial serum hCG level (results are obtained after mifepristone has already been administered and patient has left clinic).
• hCG of less than 2,000, the abortion can proceed as planned.
• hCG between 2,000 and 2,999, a diagnostic ultrasonogram must be performed on the day hCG results are obtained. If a diagnostic ultrasonogram cannot be performed that day or if the patient is symptomatic, the patient must be referred to an ED for ectopic pregnancy evaluation.
• hCG of more than 3,000 or if diagnostic ultrasonogram does not confirm IUP, the patient must be referred to an ED for ectopic pregnancy evaluation.
Day 2–3: Misoprostol 800 micrograms self-administered buccally
Day 4–5: Serum hCG testing (48–72 h after misoprostol)
-
• Serum hCG decline required to diagnose complete abortion.
50% decline 48–72 h after misoprostol (day 4–5 after mifepristone).
80% decline by 1 week after mifepristone.12
hCG, human chorionic gonadotropin; ED, emergency department; IUP, intrauterine pregnancy.
The primary outcomes focused on evaluating the safety of same-day-start mifepristone with pregnancy of unknown location and included 1) time to diagnosis, 2) significant adverse events, and 3) nonadherence with follow-up. Time to diagnosis was measured as the number of days between the initial diagnosis of pregnancy of unknown location and the final diagnosis of pregnancy location, meaning that ectopic pregnancy was excluded or diagnosed and treated. For patients in the delay-for-diagnosis group, the pregnancy location diagnosis was usually made by confirming pregnancy location on ultrasonogram; for patients in the same-day-start group, the diagnosis of pregnancy location was usually made by diagnosing a completed abortion by decline in serial hCG levels. According to Planned Parenthood League of Massachusetts protocols, a decline in serum hCG level of 50% within 48–72 hours after misoprostol or 80% within 1 week of taking mifepristone was used to diagnose complete abortion.14 Nonadherence with follow-up was defined as 30 days without clinical contact and without a definitive diagnosis. Patients could be coded as “adherent” if they complied with some follow-up, even if a definitive diagnosis was never achieved. Significant adverse events included ruptured ectopic pregnancy, hemorrhage (estimated blood loss 500 mL or more), blood transfusion, hospital admission, and abdominal surgical procedures (laparoscopy or laparotomy) occurring within 3 months of initial presentation.
We also considered secondary outcomes focused on efficacy among patients who received mifepristone and misoprostol, including 1) time to complete abortion (measured in days), 2) overall rate of successful medication abortion, and 3) frequency of ongoing pregnancy after a single dose of mifepristone and misoprostol. For time to complete abortion, patients with slowly declining hCG levels after mifepristone and misoprostol that eventually resolved without uterine aspiration were diagnosed as having completed abortions. Successful medication abortion was defined as a completed abortion with no uterine aspiration procedure, even if those individuals took additional doses of misoprostol or retook mifepristone and misoprostol. Ongoing pregnancy was defined as an ongoing pregnancy after a single dose of mifepristone and misoprostol, regardless of whether the patient then chose uterine aspiration or a repeat dose of mifepristone and misoprostol.
In addition, we identified and report the proportion of early pregnancies that presented as pregnancy of unknown location and were ultimately diagnosed as ectopic pregnancies. A diagnosis of ectopic pregnancy was defined as either a definitive or presumed ectopic pregnancy treated with either methotrexate or surgery. We included treated ectopic pregnancies that were diagnosed on initial presentation and those that were initially diagnosed as pregnancy of unknown location and later diagnosed as ectopic pregnancy. We did not review ultrasonographic images or abstract additional clinical data for patients diagnosed with intrauterine pregnancy, probable intrauterine pregnancy, or early pregnancy loss on initial ultrasonogram. For the patients who initially presented with pregnancy of unknown location and were later treated for ectopic pregnancy, we also describe ectopic pregnancy outcomes, ectopic pregnancy symptoms, and major risk factors.
Patient demographic and health characteristics were also collected, including ethnicity and race, insurance type, parity, gestational age by LMP (days), uncertain LMP, major ectopic pregnancy risk factors, and ectopic pregnancy symptoms. Race and Hispanic or Latinx ethnicity were self-reported by patients from a list of predefined options, including an “other” category (reported in this article as “none of the above”). A variable was created for Hispanic ethnicity and race, with the following categories: Hispanic, Asian (non-Hispanic), Black (non-Hispanic), White (non-Hispanic), none of the above (non-Hispanic), and declined or unknown. We considered race and ethnicity to be a potential confounder of the treatment group–clinical outcome relationships, because health care professional conscious and unconscious bias are conceptually plausible causal drivers of treatment-group allocation and could also be associated with loss to follow-up. Insurance type included private (includes group policy), public, and self-pay or other.
For our primary outcome, we estimated that, with 200 patients, we would have more than 99% power to detect a 5-day difference in time to diagnosis with various sample size allocations in each management group (1:1, 2:1, or 3:1). To detect a difference in successful abortion rates of 5% (93% vs 98%) with 80% power, we would require 400 patients. To complete adjusted analyses and maximize identification of ectopic pregnancies, we sought to identify at least 400 patients who were seeking medication abortion with no gestational sac visualized on initial ultrasonogram.
We compared the distribution of patient demographic and health characteristics by management group in the pregnancy of unknown location population using the Fisher exact test for categorical variables and Wilcoxon rank sum tests for continuous variables. Similarly, for the primary and secondary outcomes, we used bivariate analyses to describe the associations between the management groups and the outcome of interest. For the time-to-event outcomes (time to diagnosis and time to complete abortion), we created Kaplan-Meier survival curves for the probability of each outcome by days and formally compared curves using the Tarone-Ware test. For the remaining binary outcomes, we used χ2 tests to compare outcomes between management groups. Because patients with major ectopic pregnancy risk factors were not eligible for same-day start according to Planned Parenthood League of Massachusetts clinical protocols during the dates of this study, the 17 individuals with major ectopic pregnancy risk factors were excluded from all primary analyses.
Finally, we conducted adjusted analyses to estimate the relationship between management group and the primary outcomes of interest. For time to diagnosis, we used an adjusted accelerated failure time model with log-logistic distribution to estimate and compare the odds of being diagnosed before day “t” in the same-day-start and delay-for-diagnosis groups. Note that, in a log-logistic model, the odds ratio (OR) is assumed to be fixed for all days after the first day of follow-up (ie, t ∈ [1,∞]). For the binary safety outcomes, we used logistic regression models to estimate the OR for each outcome, comparing same-day start with delay for diagnosis. All models adjusted for the following covariates: Hispanic ethnicity and race, insurance type, gestational age by LMP, uncertain LMP, and presence of any ectopic pregnancy symptoms. Because there was missingness in the covariates (ranging from 0% to 17%), we used the mice R package to create 50 imputed data sets using multiple imputation by chained equations based on patient demographic and health characteristics, management group membership, and primary outcomes. The log-logistic and logistic regression models described above were fit on each imputed data set, and results were then combined according to Rubin's rules.15 All analyses were completed in R 3.6.0.
RESULTS
Of the 5,619 patients seeking medication abortion with an LMP of 42 days or less, four were diagnosed with ectopic pregnancy on the initial ultrasonogram (0.07%) (Fig. 1). Of the 452 patients with confirmed pregnancy of unknown location, 31 were eventually diagnosed with ectopic pregnancy, resulting in an overall incidence of ectopic pregnancy of 35 of 5,619 (0.6%, 95% CI 0.4–0.9%) and an ectopic pregnancy incidence among pregnancies of unknown location of 31 of 452 (6.9%, 95% CI 4.7–9.6%). We do not know whether there were additional ectopic pregnancies among those initially diagnosed with probable intrauterine pregnancy.
Fig. 1. Flowchart of participants. IUP, intrauterine pregnancy.
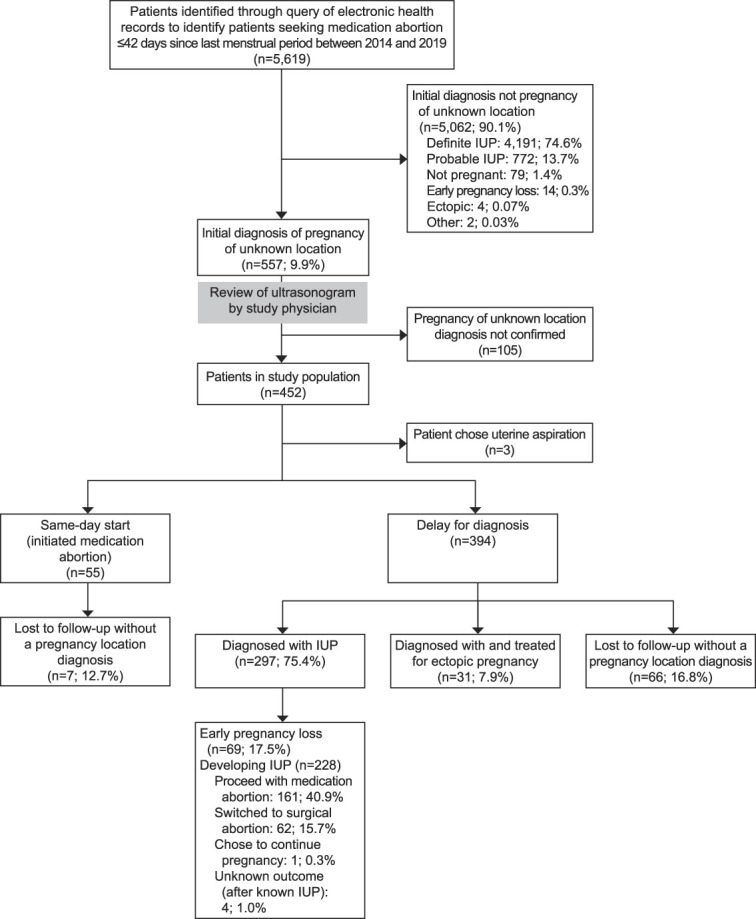
Goldberg. Undesired Pregnancy of Unknown Location. Obstet Gynecol 2022.
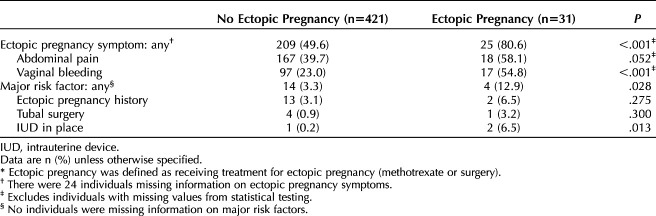
Eighty percent (n=25) of the patients with pregnancies of unknown location who had an eventual ectopic pregnancy (Table 1) reported ectopic pregnancy symptoms on their initial evaluation, including abdominal pain or vaginal bleeding, compared with 49.6% (n=209) of those with pregnancy of unknown location never treated for ectopic pregnancy (P<.001). Major ectopic pregnancy risk factors (prior ectopic pregnancy, tubal surgery, intrauterine device in place) were infrequent but also more common among those eventually diagnosed with ectopic pregnancy, compared with those without ectopic pregnancy (12.9% [n=4] vs 3.3% [n=14], P=.028).
Table 1.
Prevalence of Symptoms and Major Risk Factors by Ectopic Pregnancy* Status Among Patients With Initial Diagnosis of Pregnancy of Unknown Location (N=452)
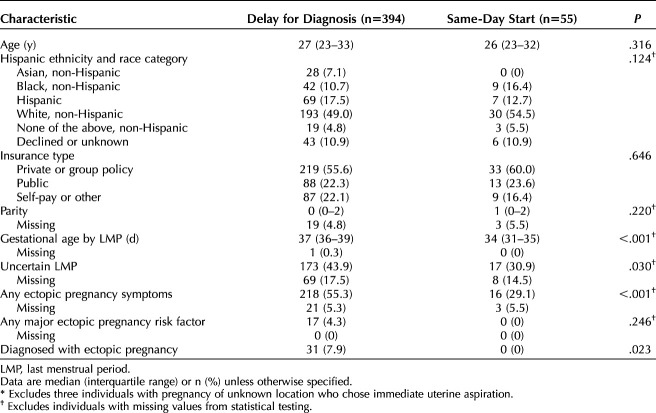
Three of the patients with pregnancies of unknown location switched to surgical abortion on the day of presentation and were excluded from the remaining analyses (n=449). Three hundred ninety-four (87.8%) patients were in the delay-for-diagnosis group, and 55 (12.2%) were in the same-day-start group. The median age of study participants was 27 years, with no difference in age distribution between the management groups (Table 2). There were no significant differences in the distribution of those of Hispanic ethnicity and race or an individual’s insurance status between the management groups (Table 2). Individuals in the delay-for-diagnosis group were slightly further along in gestation by LMP (median 37 days in the delay-for-diagnosis group vs 34 days in same-day-start group; P<.001), more likely to have an uncertain LMP (43.9% vs 30.9%; P=.03), and more likely to exhibit symptoms of ectopic pregnancy (55.3% vs 29.1%; P<.001). All 31 ectopic pregnancies were in the delay-for-diagnosis group (7.9% vs 0%; P=.023).
Table 2.
Description of Patient Characteristics for Patients With Pregnancy of Unknown Location Managed With Delay for Diagnosis Compared with Same-Day Start (n=449*)
In the delay-for-diagnosis group, 161 (40.9%) patients eventually proceeded with mifepristone and misoprostol and 62 (15.7%) switched to uterine aspiration. Overall, a total of 233 (52.0%) patients in the delay-for-diagnosis group never took mifepristone owing to spontaneous pregnancy loss, treatment for ectopic pregnancy, switch to uterine aspiration, or unknown reasons (Fig. 1).
Figure 2 presents our primary outcome, time to diagnosis, defined as the probability of identifying the pregnancy's location by days since initial diagnosis of pregnancy of unknown location, for the 432 patients with pregnancy of unknown location and no major ectopic pregnancy risk factors. The median time to diagnosis of pregnancy location was 5.0 days in the same-day-start group and 9.0 days in the delay-for-diagnosis group (P=.005). Eighty-eight (20.4%) individuals were censored at their dates of last clinical contact because they did not have a final diagnosis documented in the EHR.
Fig. 2. Time to diagnosis for patients with pregnancy of unknown location, managed with same-day start vs delay for diagnosis (n=432). The median time to diagnosis was 5.0 days (same-day start) vs 9.0 days (delay for diagnosis). Excludes 17 people with major ectopic pregnancy risk factors. Tarone-Ware test, P=.005.
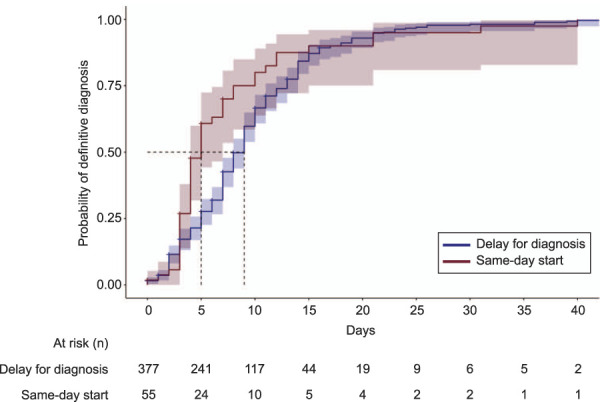
Goldberg. Undesired Pregnancy of Unknown Location. Obstet Gynecol 2022.
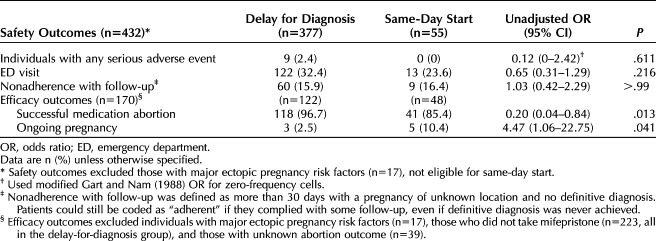
Table 3 details the remaining primary safety outcomes. Nine (2.4%) patients in the delay-for-diagnosis group had a serious adverse event documented; no patients in the same-day-start group had an adverse event (P=.611). The adverse events included a combination of ruptured ectopic pregnancy (n=4), hospitalization (n=4), hemorrhage (n=2), and unplanned major surgery (n=8). Emergency department visits were slightly higher in the delay-for-diagnosis group, but the difference was not statistically significant (32.4% vs 23.6% respectively; P=.216). There was no difference in nonadherence with follow-up between the groups (15.9% delay for diagnosis vs 16.4% same-day start; P=1.000).
Table 3.
Safety and Efficacy Outcomes for Patients With Pregnancy of Unknown Location Managed With Same-Day Start Compared With Delay for Diagnosis
The Kaplan-Meier curve for time to complete abortion includes only those individuals with no major ectopic pregnancy risk factors who we know proceeded with uterine evacuation either by mifepristone and misoprostol or suction curettage (n=270), thereby including the entire same-day-start group but excluding delay-for-diagnosis patients with a spontaneous completed miscarriage, a diagnosis of ectopic pregnancy, or a diagnosis of an intrauterine pregnancy with unknown pregnancy outcome. Figure 3 presents the probability of complete abortion by days since initial diagnosis with pregnancy of unknown location and shows a significantly shorter time to complete abortion in the same-day-start group (P<.001). Forty-four (16.3%) individuals were censored because they did not have a known time to complete abortion. Excluding censored individuals, the median time to complete abortion was 5.0 days in same-day-start and 19.0 days in delay for diagnosis.
Fig. 3. Time to complete abortion for patients with pregnancy of unknown location managed with same-day start vs delay for diagnosis (n=270). The median time to complete abortion was 5.0 days (same-day start) vs 19.0 days (delay for diagnosis). Excludes 17 people with major ectopic pregnancy risk factors and those in the delay-for-diagnosis group with a spontaneous completed miscarriage, a diagnosis of ectopic pregnancy, or a diagnosis of an intrauterine pregnancy with unknown pregnancy outcome. Tarone-Ware test, P<.001.
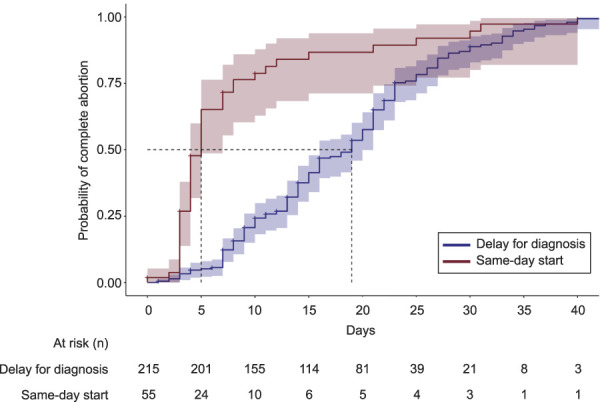
Goldberg. Undesired Pregnancy of Unknown Location. Obstet Gynecol 2022.
Table 3 details the remaining efficacy outcomes for success of medication abortion. By definition, this analysis includes only those individuals who received mifepristone (n=209), thereby including the entire same-day-start group but excluding the delay-for-diagnosis patients who never took mifepristone. We further excluded 39 (20.8% delay for diagnosis and 12.7% same-day start) patients with unknown abortion outcome (n=170). In this population, those in the same-day-start group had a lower rate of successful medication abortion (85.4% vs 96.7%; P=.013) and higher ongoing pregnancy rates (10.4% vs 2.5%; P=.041).
The odds of being diagnosed before day t (for any arbitrary day t) among those in the same-day-start group is 1.72 (95% CI 1.35–2.19; P<.001) times higher than for those in the delay-for-diagnosis group, adjusting for Hispanic ethnicity and race, insurance type, gestational age by LMP, uncertain LMP, and presence of any ectopic pregnancy symptoms. There was no difference in the adjusted odds of emergency department visit (OR 0.90, 95% CI 0.43–1.88; P=.784) or nonadherence (OR 0.92, 95% CI 0.39–2.15; P=.847) between patients in the same-day-start and delay-for-diagnosis groups. We did not fit an adjusted model for incidence of ectopic pregnancy or significant adverse events, because there were no ectopic pregnancies and no adverse events in the same-day-start group.
DISCUSSION
For patients with undesired pregnancies who have pregnancy of unknown location on initial ultrasonogram, we found that initiating medication abortion with mifepristone on the day of presentation, with simultaneous close serial hCG follow-up (same-day-start), was associated with: 1) shorter time to rule out ectopic pregnancy, 2) shorter time to completed abortion, and 3) a lower rate of successful medication abortion and a higher rate of ongoing pregnancy when compared with delay for diagnosis. Additionally, in the same-day-start group, we found no evidence of an increase in the rates of serious adverse events, emergency department visits, or nonadherence with follow-up. However, we were underpowered to detect smaller yet meaningful differences for these safety outcomes.
Although the most obvious benefit of immediate initiation of medication abortion is to meet patient demand for a timely abortion, we are unaware of other studies that have identified a diagnostic benefit to immediate initiation of mifepristone and misoprostol in the setting of pregnancy of unknown location. Although uterine aspiration with inspection of the aspirate may still be the fastest and most definitive way to both terminate an intrauterine pregnancy and rule out ectopic pregnancy, health care professionals may now consider using mifepristone and misoprostol in a similar diagnostic and therapeutic way.
Interestingly, we observed no ectopic pregnancies in our same-day-start group compared with 31 (7.9%) in the delay-for-diagnosis group. If ectopic pregnancy rates were similar between groups, we would have anticipated four ectopic pregnancies in the same-day-start group. The difference in the ectopic pregnancy rate between management groups may be due to confounding, where certain observed (ie, bleeding or pain) and unobserved patient characteristics influenced a clinician's decision to manage expectantly, and were also associated with ectopic pregnancy. Unfortunately, the fact that there were no ectopic pregnancies in the same-day-start group precluded us from an adjusted analysis. However, it also may be possible that administering mifepristone and misoprostol in the setting of very early (nonvisualized) ectopic pregnancy increases the likelihood of self-resolving tubal pregnancy. Two randomized trials from China collectively provided 1,021 patients with no visible gestational sac on ultrasonogram mifepristone and misoprostol for abortion.16,17 These studies had 98% follow-up and three suspected (but never confirmed or treated) ectopic pregnancies (0.3%).16,17 These suspected ectopic pregnancies may have been self-resolving tubal pregnancies. It is also possible that patients with ectopic pregnancy in our same-day-start group were more likely to be lost to follow-up and not have their ectopic pregnancies captured in our database. However, this is unlikely given that we observed similar rates of nonadherence with follow-up in both groups.
Although we observed numerous benefits to same-day-start medication abortion in the setting of pregnancy of unknown location, there are also risks. Similar to other authors,7,8 we found that initiating medication abortion in the setting of pregnancy of unknown location was associated with an increased risk of ongoing pregnancy compared with initiating medication abortion with a gestational sac visualized on ultrasonogram. It is unclear why this occurs, although perhaps with extremely early gestations progesterone levels are not yet high enough for the effects of mifepristone to be fully realized. Nonetheless, it is important that patients with pregnancy of unknown location who take mifepristone and misoprostol have close follow-up to identify and manage ongoing pregnancies in a timely fashion.
Additionally, some patients who present with undesired pregnancies of unknown location may never require an abortion. We found that 18% of patients in the delay-for-diagnosis group were eventually diagnosed with early pregnancy loss and 8% with ectopic pregnancy; thus, collectively, 26% did not require abortion. In the United States, patients may have health insurance that covers care for early pregnancy loss and ectopic pregnancy but does not cover abortion; thus, delaying treatment to determine a diagnosis may enable these patients to avoid the out-of-pocket expenses of abortion care.
Recently, a randomized trial compared expectant with active management of persistent pregnancy of unknown location. Active management included either uterine aspiration followed by methotrexate as needed or empiric methotrexate. The authors found that active management, with either treatment, was associated with a greater likelihood of successful pregnancy resolution and lower risk of unscheduled surgeries. They found randomization challenging because patients had strong management preferences.18
Patients seeking abortion have similarly strong preferences,19 and our data, generalizable to those with an LMP of 42 days or less with no major ectopic pregnancy risk factors, suggest that there is no reason to mandate that these patients with pregnancies of unknown location delay initiating abortion to first obtain a definitive diagnosis. In contrast, there is diagnostic and therapeutic benefit to administering mifepristone and misoprostol in the setting of undesired pregnancy of unknown location. Given that both management strategies are reasonably safe and effective, and that each carries benefits and risks, our data inform shared decision making and enable choices heavily weighted toward patient priorities and preferences.
Footnotes
This work was supported by the Society of Family Planning Research Fund [SFPRF12-MA7].
Financial Disclosure Alisa B. Goldberg was supported by the Society of Family Planning Research Fund [SFPRF12-MA7], which was awarded to the Planned Parenthood League of Massachusetts. She received royalties from UpToDate and was a consultant for Sanofi/Genzyme from 2018 to 2019. Danielle Roncari reports receiving payment from Organon as a Nexplanon trainer. The following authors are or were employed by Planned Parenthood League of Massachusetts during the study: Jennifer Fortin, Rebecca Hofer, and Alex Cottrill. The other authors did not report any potential conflicts of interest.
Presented at the National Abortion Federation Annual Meeting, held virtually, May 11–12, 2021.
The authors thank Julia Max, Katharine White, and Talcott Camp for their advice on study concept and design.
The findings and conclusions expressed in this article are those of the authors and do not necessarily reflect the views of Planned Parenthood Federation of America, Inc., or the Society of Family Planning Research Fund.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C678.
REFERENCES
- 1.Kortsmit K, Jatlaoui TC, Mandel MG, Reeves JA, Oduyebo T, Petersen E, et al. Abortion surveillance - United States, 2018. MMWR Surveill Summ 2020;69:1–29. doi: 10.15585/mmwr.ss6907a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnhart K, van Mello NM, Bourne T, Kirk E, Van Calster B, Bottomley C, et al. Pregnancy of unknown location: a consensus statement of nomenclature, definitions, and outcome. Fertil Steril 2011;95:857–66. doi: 10.1016/j.fertnstert.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapp N, Baldwin MK, Rodriguez MI. Efficacy of medication abortion prior to 6 gestational weeks: a systematic review. Contraception 2018;97:90–9. doi: 10.1016/j.contraception.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 4.Chen MJ, Creinin MD. Mifepristone with buccal misoprostol for medical abortion: a systematic review. Obstet Gynecol 2015;126:12–21. doi: 10.1097/AOG.0000000000000897 [DOI] [PubMed] [Google Scholar]
- 5.Heller R, Cameron S. Termination of pregnancy at very early gestation without visible yolk sac on ultrasound. J Fam Plann Reprod Health Care 2015;41:90–5. doi: 10.1136/jfprhc-2014-100924 [DOI] [PubMed] [Google Scholar]
- 6.Schreiber CA, Creinin MD, Atrio J, Sonalkar S, Ratcliffe SJ, Barnhart KT. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med 2018;378:2161–70. doi: 10.1056/NEJMoa1715726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstone P, Michelson J, Williamson E. Effectiveness of early medical abortion using low-dose mifepristone and buccal misoprostol in women with no defined intrauterine gestational sac. Contraception 2013;87:855–8. doi: 10.1016/j.contraception.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 8.Bizjak I, Fiala C, Berggren L, Hognert H, Sääv I, Bring J, et al. Efficacy and safety of very early medical termination of pregnancy: a cohort study. BJOG 2017;124:1993–9. doi: 10.1111/1471-0528.14904 [DOI] [PubMed] [Google Scholar]
- 9.Jones RK, Witwer E, Jerman J. Abortion incidence and service availability the United States, 2017. Accessed January 18, 2022. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017 [Google Scholar]
- 10.National Abortion Federation. Management of pregnancy of uncertain location. In: 2020 clinical policy guidelines for abortion care. National Abortion Federation; 2020. [Google Scholar]
- 11.Planned Parenthood Federation of America. Medical standards & guidelines. Planned Parenthood Federation of America; 2016. [Google Scholar]
- 12.Reid S, Condous G. Is there a need to definitively diagnose the location of a pregnancy of unknown location? The case for “no”. Fertil Steril 2012;98:1085–90. doi: 10.1016/j.fertnstert.2012.09.032 [DOI] [PubMed] [Google Scholar]
- 13.Doubilet PM, Benson CB, Bourne T, Blaivas M; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy, Barnhart KT, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med 2013;369:1443–51. doi: 10.1056/NEJMra1302417 [DOI] [PubMed] [Google Scholar]
- 14.Fiala C, Safar P, Bygdeman M, Gemzell-Danielsson K. Verifying the effectiveness of medical abortion; ultrasound versus hCG testing. Eur J Obstet Gynecol Reprod Biol 2003;109:190–5. doi: 10.1016/s0301-2115(03)00012-5 [DOI] [PubMed] [Google Scholar]
- 15.Rubin DB. Multiple imputation for nonresponse in surveys . John Wiley and Sons, Inc; 2004. [Google Scholar]
- 16.Li CL, Song LP, Tang SY, Zhou LJGY, He H, Mo XT, et al. Efficacy, safety, and acceptability of low-dose mifepristone and self-administered misoprostol for ultra-early medication abortion: a randomized controlled trial. Reprod Sci 2017;24:731–7. doi: 10.1177/1933719116669055 [DOI] [PubMed] [Google Scholar]
- 17.Li CL, Chen DJ, Song LP, Wang Y, Zhang ZF, Liu MX, et al. Effectiveness and safety of lower doses of mifepristone combined with misoprostol for the termination of ultra-early pregnancy: a dose-ranging randomized controlled trial. Reprod Sci 2015;22:706–11. doi: 10.1177/1933719114557897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnhart KT, Hansen KR, Stephenson MD, Usadi R, Steiner AZ, Cedars MI, et al. Effect of an active vs expectant management strategy on successful resolution of pregnancy among patients with a persisting pregnancy of unknown location: the ACT or NOT randomized clinical trial. JAMA 2021;326:390–400. doi: 10.1001/jama.2021.10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rørbye C, Nørgaard M, Nilas L. Medical versus surgical abortion: comparing satisfaction and potential confounders in a partly randomized study. Hum Reprod 2005;20:834–8. doi: 10.1093/humrep/deh643 [DOI] [PubMed] [Google Scholar]