Revised Markov modeling supports considering concurrent salpingo-oophorectomy at hysterectomy for benign indication for those aged 50 years or older and using estrogen therapy before age 50 years.
Abstract
OBJECTIVE:
To perform an updated Markov modeling to assess the optimal age for bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign indication.
METHODS:
We performed a literature review that assessed hazard ratios (HRs) for mortality by disease, age, hysterectomy with or without BSO, and estrogen therapy use. Base mortality rates were derived from national vital statistics data. A Markov model from reported HRs predicted the proportion of the population staying alive to age 80 years by 1-year and 5-year age groups at time of surgery, from age 45 to 55 years. Those younger than age 50 years were modeled as either taking postoperative estrogen or not; those 50 and older were modeled as not receiving estrogen. Computations were performed with R 3.5.1, using Bayesian integration for HR uncertainty.
RESULTS:
Performing salpingo-oophorectomy before age 50 years for those not taking estrogen yields a lower survival proportion to age 80 years than hysterectomy alone before age 50 years (52.8% [Bayesian CI 40.7–59.7] vs 63.5% [Bayesian CI 62.2–64.9]). At or after age 50 years, there were similar proportions of those living to age 80 years with hysterectomy alone (66.4%, Bayesian CI 65.0–67.6) compared with concurrent salpingo-oophorectomy (66.9%, Bayesian CI 64.4–69.0). Importantly, those taking estrogen when salpingo-oophorectomy was performed before age 50 years had similar proportions of cardiovascular disease, stroke, and people living to age 80 years as those undergoing hysterectomy alone or those undergoing hysterectomy and salpingo-oophorectomy at age 50 years and older.
CONCLUSION:
This updated Markov model argues for the consideration of concurrent salpingo-oophorectomy for patients who are undergoing hysterectomy at age 50 and older and suggests that initiating estrogen in those who need salpingo-oophorectomy before age 50 years mitigates increased mortality risk.
Approximately 300,000 hysterectomies are performed every year in the United States; as of 2018, 31.8% of American women aged 50 years and older have undergone hysterectomy.1 Controversy continues over whether to perform bilateral salpingo-oophorectomy (BSO) concurrent with hysterectomy for benign indication in those at average risk of breast and ovarian cancers. The controversy lies in how surgical menopause affects mortality2 from causes such as cancers of the breast, ovary, lung and colon, cardiovascular disease (CVD), and stroke,3–5 and whether or how these mortality effects are influenced by patient age at time of surgery and postoperative estrogen therapy use.6,7 Further, the decision regarding concurrent BSO with hysterectomy is influenced by reoperation rates, which was found to be up to 9% after hysterectomy alone to remove retained ovaries.8
In 2005, Parker and colleagues9 published a Markov model to assess the optimal age for BSO at the time of hysterectomy and found that BSO should be delayed to age 65 years to mitigate mortality. This article significantly affected the gynecology community and continues to be cited as justification to delay BSO to age 65 years, despite multiple large cohort studies subsequently published that challenge that age, including two subsequent articles by the Parker group.10,11 After careful review, it appears that Parker et al overestimated the cardiovascular risk in their model. They cite that based on the work of van der Schouw et al: “the risk of CHD [coronary heart disease] decreases 6% for each year oophorectomy is delayed after menopause.”9 However, the van der Schouw study only looked at menopause for any reason (natural, hysterectomy, or oophorectomy), did not look at oophorectomy after menopause for other reasons, and reported a decreased annual cardiovascular death risk of 2%, not 6%, per year menopause is delayed.12 These errors drove the conclusion that oophorectomy should be delayed until age 65 years. Despite this, the publication changed practice, causing a decrease in opportunistic salpingo-oophorectomy for years afterward.13 Although it is true that many women should avoid BSO with hysterectomy before the natural age of menopause, not removing ovaries in those aged 50 years and older can lead to potential increased mortality2 and reoperation.8
Markov models are beneficial statistical tools to predict outcomes when different medical decisions are made. However, they are best able to influence medical decision making when created using the most current population-based data. In light of these points and our concerns for the overestimation of cardiovascular risk in the original model, our objective was to provide an updated model that would reflect the intervening research and a corrected cardiovascular risk calculation.
METHODS
Markov models assume that, over time, individuals in a population transition through various discrete Markov states, with transition probabilities that may depend on the current time and state but not on any previous states.14 We modeled our patient population with the starting Markov state of being healthy and alive with at least one normally functioning ovary and a uterus. We then imposed one of two possible surgical interventions involving either hysterectomy alone or hysterectomy and BSO at various ages from 45 to 55 years. Those undergoing surgery before age 50 years were modeled as either undergoing hysterectomy and BSO with estrogen therapy after surgery compared with hysterectomy and BSO without estrogen therapy after surgery. The model then allowed prediction of proportions of the population staying alive to age 80 years, accounting for how surgery at various ages and whether the use of postoperative estrogen therapy would affect the risk of death from CVD, stroke, and ovarian, lung, colon and breast cancer. Mortality by differing diseases were treated as competing risks. Base rates of these diseases were established by Centers for Disease Control and Prevention (CDC) vital statistics data.15
Our first step was to recreate the original Markov model based on the description of methods in its 2005 publication.9 We used the same data reported on at that time, including the hazard ratios (HRs), mortality risk by age, and correction factor to convert 5-year mortality as reported in U.S. vital statistics to 1-year mortality. This allowed us to determine that we thoroughly understood the steps used to create the original model before modifying the inputs from current literature.
To update the model with current data, we next performed PubMed and Scopus searches with the following terms: oophorectomy, ovarian conservation, estrogen, coronary heart disease (CHD), cardiovascular disease (CVD), hip fracture, breast cancer, lung cancer, colorectal cancer, and stroke. Our literature review was conducted between 2005 and 2019, given the original Markov model was published in 2005, and we sought to update the research inputs used in our revised model. We manually reviewed the works cited sections of all relevant articles for more references that would be applicable to the study. We reviewed all abstracts of these articles, and completely read all articles addressing pertinent topics.
The original Markov model assessed death from ovarian cancer, breast cancer, CVD, stroke, and hip fracture. Our final updated model reports on all disease states excluding hip fracture, because the mortality from this condition in the original model was based on a calculated risk rather than HRs reported from population-based studies evaluating surgery type and age at surgery. As noted in the original article, direct mortality from hip fracture was not published, so the authors calculated it by multiplying the annual incidence of hip fracture by case-fatality rates as estimated by age-specific excess mortality. We, instead, wished to reflect the established risks as demonstrated in vital statistics and published patient cohorts; hence, we made the decision to drop this parameter in our updated model.
We specifically limited the model to mortality rather than morbidity risk in our effort to respond to and challenge the original model that has been practice changing. Of note, BSO compared with ovarian conservation at various ages affects mortality and morbidity trends similarly. All the studies from which we based our estimates of risk were large retrospective4,16–18 or prospective11,19,20 observational studies. The large majority of our included studies were based on the Rochester Epidemiology Project population,4,5,17 as well as the Nurse's Health Study,11 the Women's Health Initiative Observational Study,7,19 a Kaiser-based patient population,16 an Australian retrospective record-linkage study population,18 and the Breast Cancer Detection and Demonstration Project patient population.20 Although not all studies had information to control for base risk factors of CVD and various cancers, most did. We also used our base rates of disease as gleaned from the CDC vital statistics, as updated through 2017. All studies included patients who were at average risk for breast and ovarian cancer or they controlled for family history of these diseases; we excluded any studies that focused on patients with genetic or family histories that would predispose them to breast and ovarian cancer. Further, we added the mortality risks of lung and colon cancer to our revised model, because more has been published about these diseases since the original model.11,19,20
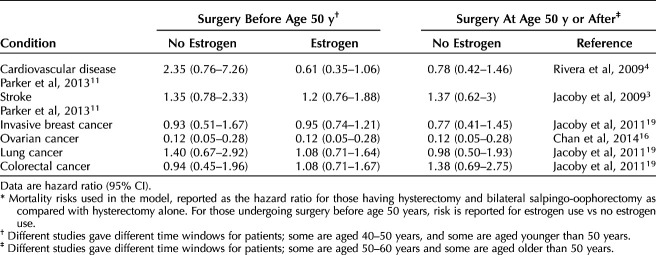
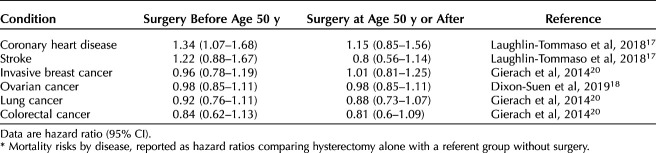
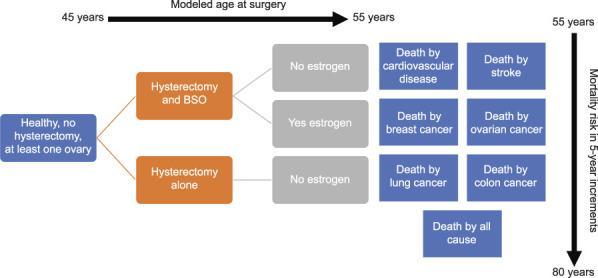
Our literature review yielded multiple references concerning risk of death associated with elective oophorectomy compared with ovarian conservation and the modifying effects that postoperative estrogen therapy has on mortality. After reviewing all pertinent studies, we chose those studies that reported risks as HRs to include in our revised Markov model. A summary of the most pertinent literature is included in Appendix 1 (available online at http://links.lww.com/AOG/C647), and Tables 1 and 2 highlight the hazards ratios and their CIs for disease states by hysterectomy compared with hysterectomy and BSO (Table 1)4,11,16,19 and by hysterectomy compared with referent group (no surgery) (Table 2)17,18,20 that were then incorporated into our revised model. With the updated literature and base rates of disease, we then proceeded to create and run our revised Markov model (Fig. 1 for schematic).
Table 1.
Hazard Ratios for Bilateral Salpingo-Oophorectomy and Hysterectomy Compared With Hysterectomy Alone*
Table 2.
Hazard Ratios for Hysterectomy Alone Compared With Referent Group*
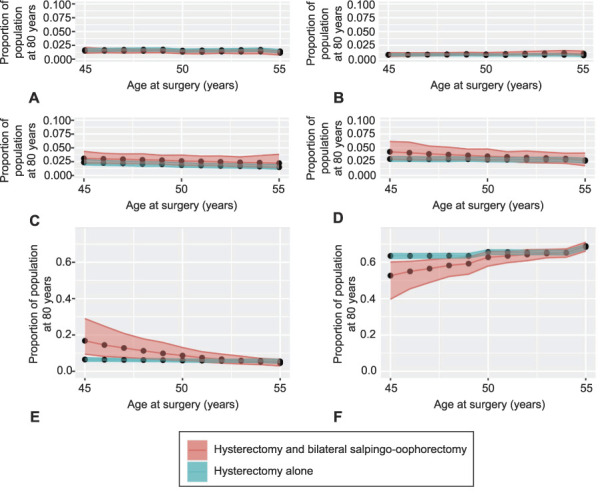
Fig. 1. Schematic of revised Markov model. This schematic demonstrates the initial Markov state of healthy, with uterus, and at least one ovary, and the interventions being surgery with hysterectomy alone vs hysterectomy and bilateral salpingo-oophorectomy (BSO) from age 45 to 55 years. Whether estrogen was used after surgery is modeled for those undergoing surgery before age 50 years. We report on death from cardiovascular disease, stroke, breast cancer, ovarian cancer, lung cancer, colon cancer, and all-cause. Our final outputs are proportion of patients alive to 80 years and dead from the previously listed diseases.
Rush. Remodeling Ovary Removal at Hysterectomy. Obstet Gynecol 2022.
Our model considered three groups of patients: those who underwent hysterectomy alone, those who had concurrent BSO without postoperative estrogen therapy, and those who underwent concurrent BSO before age 50 years and took postoperative estrogen therapy. For those younger than age 50 years who were modeled as taking estrogen therapy, the estrogen therapy was modeled as being taken up to at least age 50 years, consistent with the Rivera et al 2009 study, reporting estrogen therapy use to age 45 or 50 years.4 We modeled patients undergoing surgery at 45–55 years of age. Most articles provide HRs estimated for patients undergoing surgery within a range of ages (eg, 40–50, 50–60 years), rather than constrained to 1-year increments. Using quadratic approximation to be maximally flexible while also anchoring to 5-year reported HRs, we interpolated reference HRs and obtained transition probabilities for patients undergoing surgery at specific ages in 1-year increments. We performed several control calculations to assure that inference was not sensitive to the specific model assumptions. One control used a step-function rather than quadratic approximation to the HR, with a step at 50 years of age; we also repeated calculations without including HRs that had not been confirmed to be different from unity (ie, if the reported 95% CI contained 1). See Appendix 1 (http://links.lww.com/AOG/C647) for details of these alternatives.
For each modeled cohort, we simulated paths for 10,000 patients from age 55 to 80 in 5-year increments, with possible paths being alive or dead from CVD, stroke, ovarian cancer, lung cancer, colon cancer, or breast cancer based on age at hysterectomy and BSO and use of estrogen therapy. Markov model states were considered to be discrete, ie, death as being caused by only one of the studied diseases. Postoperative estrogen therapy was modeled as being either used or not used among those who underwent BSO before age 50 years. Five-year death rates by each disease for those who did not undergo hysterectomy or BSO (referent group) were obtained from the CDC15 to generate base transition probabilities. We used a referent group based on the literature comparing hysterectomy alone with no surgery to obtain the baseline risk of death from target conditions when hysterectomy alone was chosen. This referent group serves an important function, because ovarian compromise has been proposed even with hysterectomy alone, given reduced collateral ovarian blood flow after hysterectomy, among other proposed explanations.17 Further, we sought to compare those undergoing hysterectomy with those not having surgery, because other studies have demonstrated that those undergoing hysterectomy generally tend to have higher rates of comorbidities.17,19,21,22 Death rate by CVD, stroke, breast cancer, lung cancer, colon cancer, ovarian cancer, and overall survival rate for patients at age 80 years are used to compare the treatments.
Going beyond the modeling techniques previously reported, we sought to account for uncertainty in reported HRs and to propagate this uncertainty into inference statements about survival rates after various interventions. Briefly, we deployed a Bayesian integration strategy in which HR point estimates and CIs defined log-normal posterior distributions for these input parameters.23 We repeatedly simulated parameter states and evolved cohorts according to the induced transition probabilities, averaging target frequencies over the simulated states.
All statistical analyses were performed using built-in tools and custom scripts in R 3.5.1. We assess differences between survival rates by reporting 95% Bayesian CIs under different treatments and at different intervention ages. To support reproducibility, we include in Appendix 1 (http://links.lww.com/AOG/C647) a rendered R markdown literate program that includes all statistical computations. Given this is a model based on previously published population-based studies and CDC vital statistics, all with deidentified and publicly accessible data, this study is institutional review board exempt.
RESULTS
We were able to recreate the results reported in the original 2005 Markov model. In doing so, we were unable to verify the cardiovascular risk used in the original model. Parker et al's methods state that, “risk of CHD decreases 6% for each year oophorectomy is delayed after menopause, and the relative risk was adjusted accordingly from age 55 to age 65.”9 The source cited for this calculation is an article by van der Schouw et al, titled “Age at Menopause as a Risk Factor for Cardiovascular Mortality,” which found that, “each year the menopause is delayed decreases the annual hazard of cardiovascular death by 2%.”12 van der Schouw et al studied menopause for any reason, and those with hysterectomy with concomitant BSO were never evaluated separately.12 Further, van der Schouw et al never evaluated women undergoing BSO after menopause, so it would be impossible to have found an increased risk of CVD related to BSO after menopause, let alone the 6% increased risk per year that was used in the original Markov model. This falsely elevated the mortality risk by CVD that was associated with BSO without use of estrogen therapy and largely explained the recommendation drawn from the original model to delay concurrent BSO to age 65 years.
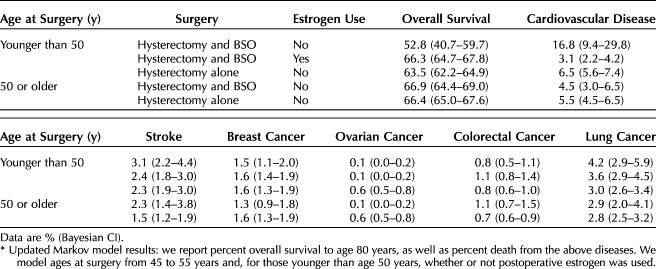
After modifying the original model to include contemporary research and calculating proportion alive to age 80 years based on age at surgery, we found that the age at which BSO can safely be performed without undue increased mortality was at age 50 years or older (Table 3). We also calculated risk of death from various causes based on age at surgery in 1-year increments, finding that 53 years was the age at which there was no increased risk of death associated with BSO (Fig. 2). When age at surgery occurs before 50, hysterectomy alone has higher overall survival to age 80 years than hysterectomy and BSO without estrogen (hysterectomy 63.5% [Bayesian 95% CI 62.2–64.9%] vs hysterectomy and BSO without estrogen 52.8% [Bayesian 95% CI 40.7–59.7%]). When the surgery is conducted at age 50 years and older, hysterectomy alone and hysterectomy and BSO have similar overall survival (hysterectomy 66.4% [Bayesian 95% CI 65.0–67.6%] vs hysterectomy and BSO 66.9% [Bayesian 95% CI 64.4–69.0%]).
Table 3.
Overall Survival to Age 80 Years and Mortality Risk of Disease by Age 80 Years*
Fig. 2. Long-term effects of surgery conducted at different ages without estrogen therapy. Modeled cohorts of 10,000 patients undergoing hysterectomy and bilateral salpingo-oophorectomy or hysterectomy alone between age 45 and 55 years were simulated from surgery to 80 years. We report percent survival to 80 years and percent death from cardiovascular disease in E‒F, then report percent death from disease (subplots) in A‒D. The simulation uses age-specific baseline death rates and a posterior random sampling of 500 settings of hazard ratios for various interventions. Black dots represent the average of marginal statistics over 500 simulated cohorts, and bands show 95% Bayesian posterior intervals. Death from breast cancer (A), colon cancer (B), stroke (C), lung cancer (D), and cardiovascular disease (E) and survival (F).
Rush. Remodeling Ovary Removal at Hysterectomy. Obstet Gynecol 2022.
Before age 50 years, patients who undergo hysterectomy and BSO without postoperative estrogen therapy are at increased risk of death from CVD and, thus, suffer lower overall survival to age 80 years. There is a slight increased risk of death from ovarian cancer in those who undergo hysterectomy alone before age 50 years, but this risk is not nearly as significant as the risk associated with CVD (Table 3). The absolute mortality risk difference from CVD in those undergoing hysterectomy and BSO without estrogen therapy and hysterectomy alone before age 50 years is 10.3%, favoring hysterectomy alone.
If surgery is performed after age 50 years, hysterectomy and BSO confers the same overall survival to age 80 years as performing hysterectomy alone before age 50 years. This holds true when evaluating CVD risk of death by age 80 years for hysterectomy and BSO compared with hysterectomy alone (4.5% [Bayesian 95% CI 3.0–6.5%] vs 5.5% [Bayesian 95% CI 4.5–6.5%]). In fact, hysterectomy and BSO at age 50 years or later has roughly the same or better overall survival compared with hysterectomy alone at any age and hysterectomy and BSO before age 50 years with or without estrogen therapy.
We further modeled the effect of estrogen therapy use after BSO in those younger than age 50 years. The increased risk of death from CVD was largely mitigated with estrogen therapy after BSO. Further, a continued benefit from ovarian cancer risk reduction was demonstrated, and invasive breast cancer risk was not affected (Table 3). We also considered the effects of ovarian preservation compared with BSO with or without estrogen therapy on mortality risk by lung and colorectal cancer. These diseases were not adversely affected by BSO, especially when those undergoing surgery before age 50 years were subsequently prescribed estrogen therapy.
Articles comparing hysterectomy alone and hysterectomy and BSO typically report age at surgery in ranges of 5- and 10-year increments; hence, we interpolated the HRs and simulated populations with age at surgery ranging from 45 to 55 in 1-year increments. We then simulated a cohort of 10,000 patients and, by generating 500 random settings of HRs, inferred overall survival and risks of death from various causes based on surgery age (Fig. 2). Plotting survival to age 80 years for those undergoing hysterectomy compared with hysterectomy and BSO, the intercept of the two lines crossed at age 53, when the survival rate between hysterectomy and hysterectomy and BSO are not significantly different.
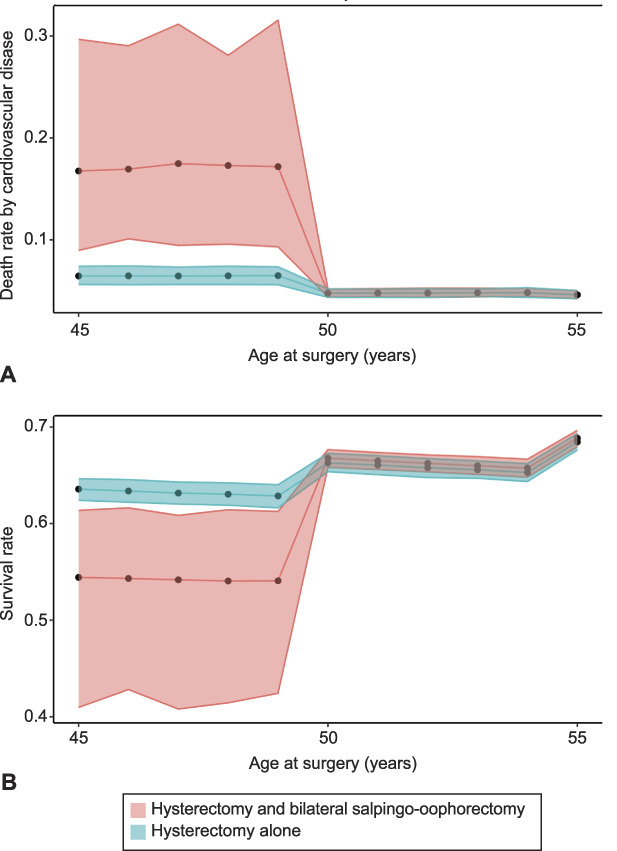
Overall survival was dictated almost exclusively by CVD mortality risk. Other than ovarian cancer, all other causes of death demonstrated percent risks whose CIs overlapped between the surgery groups before and after age 50 years, and with and without estrogen therapy. As an added assurance that our model captured the most pertinent risks, we reran the model considering mortality from just CVD and ovarian cancer, diseases whose CIs did not include 1. We also ran the model using a step-wise rather than a quadratic equation. Our results from these control calculations were the same on timing BSO with hysterectomy (Fig. 3); also see Appendix 1 (http://links.lww.com/AOG/C647) for additional details.
Fig. 3. Results of a supporting control computation for comparison analogous to Figure 2, but in which we have removed any factors for which prior work does not establish a non-null hazard ratio and we have assumed a step-function hazard ratio across the age-50-years threshold. Death rate by cardiovascular disease (A), survival (B).
Rush. Remodeling Ovary Removal at Hysterectomy. Obstet Gynecol 2022.
DISCUSSION
Certain articles capture the minds of their academic readership and continue to influence teaching and understanding of their target topics for years to come. The original Markov model published in 2005 is just such an article. At a time when BSO was becoming a standard prophylactic measure in women at high risk for breast and ovarian cancer,24–26 the original article made a strong and valid argument to conceptualize differently the practice of BSO in average-risk women at the time of hysterectomy for benign indication. After its publication, a notable decrease in BSO at the time of hysterectomy for benign indication occurred.13
However, intervening scholarship demonstrates in well-conducted large observational studies that BSO can safely be performed many years before age 65 years in average-risk populations. Further studies have also demonstrated that estrogen therapy can mitigate some of the negative effects of BSO in premenopausal patients when BSO cannot be avoided. And, perhaps most importantly, the erroneous calculation that delaying oophorectomy after menopause (which was never studied in the referenced articles) increased CVD death, elevated the overall risk and led to the inaccurate conclusion in the original Markov model to wait to age 65 years before BSO with hysterectomy for benign indication.
Our literature review on the topics of timing BSO with hysterectomy for benign indication, risks of death from CVD, stroke, ovarian cancer and breast cancer, use of estrogen therapy after BSO, and reoperation rates yielded a large body of work that was published subsequent to the original Markov model. Cardiovascular disease remains the largest contributor to increased risk of death with hysterectomy and BSO before age 50 years without estrogen therapy. Most indicative was the study by Rivera et al4 that demonstrated an increased risk of death associated with CVD when BSO was performed before age 45 years, but no such increased risk after that age. In that article, they also found that continued use of estrogen therapy after BSO to the age of 45 years or after mitigated the increased risk from CVD. Likewise, Jacoby et al19 in 2011 demonstrated in the women's health initiative observational cohort that BSO performed at the time of hysterectomy did not increase risk of cardiovascular death or morbidity, broken out by myocardial infarction, revascularization, heart failure and stroke. Estrogen therapy was common in that cohort overall, with more than 78% using estrogen therapy.
Parker et al,11 in both of their Nurses' Health Studies from 2009 to 2013, found that CVD was increased significantly in those who underwent BSO with hysterectomy before age 4510 or 50 years. The increased risk, however, was attenuated with advancing age at time of surgery and with postoperative estrogen therapy. To illuminate, Parker et al's 2013 follow-up study with the Nurses' Health Study cohort found the risk of CVD was higher for those undergoing surgery before age 50 years (HR 1.24, 95% CI 1.03–1.50) than at 50–59 years (HR 0.82, 95% CI 0.54–1.24) or after 60 years (HR 1.13, 95% CI 0.42–3.05).10 The same was found in the Gierach et al study of nearly 53,000 study participants, in which increased risk of CVD was again attenuated with later age at surgery. For instance, the risk of CVD when undergoing hysterectomy and BSO before 35 years of age was higher (HR 1.56, 95% CI 1.29–1.89) than when undergoing hysterectomy and BSO after the age of 55 years (1.11, 95% CI 1.00–1.21).20 Mytton et al27 also found that those undergoing hysterectomy and BSO between the ages of 35 and 40 years were at increased risk of CVD death but unfortunately did not have information on use of estrogen therapy. LaCroix et al and Manson et al evaluated the Nurse's Health study hysterectomy population as well and demonstrated that use of estrogen therapy after hysterectomy was protective against myocardial infarction,7 stroke, and all-cause CVD6; this benefit was best seen in younger patients using estrogen therapy, with that benefit disappearing with greater age.
Some have argued that hysterectomy alone with ovarian conservation also leads to increased risk of CVD. A 2018 report from Laughlin-Tommaso et al,17 with more than 20 years of follow-up, shows that risks of hyperlipidemia, hypertension, obesity, cardiac arrhythmias, and coronary artery disease were all significantly increased for patients undergoing hysterectomy at age 35 years or younger, compared with a referent group of same-aged patients without hysterectomy or BSO, but not for those having surgery who are older than age 50 years. Those undergoing hysterectomy with ovarian conservation were more likely to have preexisting cardiovascular risk factors, but, even controlling for these, the group undergoing hysterectomy with ovarian conservation before age 50 years still had increased risk of developing CVD and other metabolic dysfunction after surgery.17 The CARDIA (Coronary Artery Risk Development in Young Adults) and SWAN (Study of Women’s Health across the Nation) studies also found that those undergoing hysterectomy or hysterectomy and BSO had higher baseline CVD risk factors. They did not identify an increased rate of accumulating risk factors after surgery, but also did not study surgery at varying ages and further did not address CVD incidence outright.21,22 Although not well understood, it appears from these studies that patients undergoing hysterectomy and BSO have elevated cardiac risk factors. It is important to ensure that BSO is necessary in patients younger than 50 and, if so, that estrogen therapy may mitigate already increased CVD risk.
Bilateral salpingo-oophorectomy at the time of hysterectomy for benign indication must also be assessed in terms of effect on cancer risk. The most obvious risk reduction in performing BSO at hysterectomy is in ovarian cancer, which is still the fifth leading cause of cancer death in women in the United States.28 Although 20% of ovarian cancers are found to be associated with either a germline or somatic mutation,29 a large majority arise de novo and present as advanced disease with 30% 5-year overall survival.30 Performing BSO at hysterectomy, especially in average-risk patients aged 50 years or older, mitigates any increased mortality risk while greatly reducing ovarian cancer risk.19 Some have argued that the risk of ovarian cancer is still low in the average-risk population, but oophorectomy also confers decreased risk of breast cancer,11,19,31 all cancer,32 and, when performed at ages greater than 50 years, lung and colorectal cancers.31,32
To highlight the decreased risk of cancers in the setting of oophorectomy, we will review some of the studies addressing that question. Parker et al, in a 2009 prospective observational study in the Nurses' Health Study cohort, reviewed risks of ovarian, breast, lung and colorectal, as well as all-cause cancers in those who underwent hysterectomy alone compared with hysterectomy and BSO. There was reduced risk with BSO before age 45 years for breast cancer (HR 0.67, 95% CI 0.58–0.78), at any age for ovarian cancer (HR 0.03–0.07 depending on age at surgery), and before age 45 years for total cancer risk (0.86, 95% CI 0.79–0.95). Bilateral salpingo-oophorectomy did not affect colorectal cancer risk in this study, and was associated with increased lung cancer risk when performed before age 45 years (HR 1.34, 95% CI 1.05–1.72).10 Gaudet et al32 in a 2014 study using the cancer prevention study-II nutrition cohort similarly found that hysterectomy and BSO before age 55 years was associated with a lower risk of total cancer, and this positive trend held in those undergoing hysterectomy alone at less than age 45 years. Use of estrogen therapy mitigated any increased risk of cancer associated with BSO at younger ages. Specifically for breast cancer, use of estrogen therapy in those undergoing premenopausal surgery did not affect the risk of breast cancer.19,33
Our model demonstrates that postoperative estrogen use in those undergoing hysterectomy and BSO before the age of 50 years can mitigate the increased mortality risks from CVD and stroke, without effect on ovarian or breast cancer risk. This reflects the findings we used in multiple articles from multiple large cohorts with long follow-up.4,5,10,20,32,34 Repeatedly, in all studies addressed, when using estrogen therapy past or current, when evaluating patients undergoing surgery before age 45 or 50 years, estrogen therapy mitigated any increased risk of BSO concurrent with hysterectomy. This knowledge is important for those instances in which patients ultimately have to have ovaries removed for benign indication before the age of 50 years. It allows us to counsel them of the mortality reduction of using estrogen therapy until the natural age of menopause.
The limitations of our study are those inherent in using Markov modeling. Although the model does allow for incorporation of many population-based longitudinal observations, our model, just like the 2005 original, is only as good as the data used. It allows for the best estimation of overall survival and mortality risks and does not report on real human observation. This is an important point that must be considered when reflecting on the original and updated models. We also used large retrospective and prospective U.S. and Australian population-based studies to glean the HRs for our model. These studies largely sought to control for confounding risk factors, such as obesity, smoking, preexisting CVD, diabetes, hypertension, and hyperlipidemia. However, because they are all observational in nature, none of the studies can control for how each individual person is counseled or what they ultimately choose to do surgically with their doctor. Further, although the CDC vital statistics used for base rates of deaths was representative of the diversity of the U.S. population, most of the studies were not as representative, with White participants comprising 80–97% of those studied.
Likewise, our model focused on reviewing a previous model that addresses mortality alone and not the morbidity that might be associated with each surgical choice. There is certainly the morbidity of increased fracture risk, neurologic decline, sexual side effects, and vasomotor symptoms associated with BSO before age 50 years, and these morbidities in those undergoing BSO before age 50 are, again, mitigated by estrogen therapy.5,35–37 We hope that with improving the understanding of surgical sequelae and hormone therapy use, we might develop a model that can take individual risk factors into account and allow shared decision-making about surgery and hormone therapy. We hope in the meantime that others will continue to use the original data that allowed us to create this model to counsel patients carefully when deciding BSO at the time of hysterectomy for benign indication, and use of hormone therapy after surgery.
It should be noted that, while this article was under revision, a large Canadian observational study was published that supports the findings of our model.2 Their work sought to assess all-cause mortality as related to hysterectomy with ovarian conservation and hysterectomy with BSO, finding that the mortality risk associated with surgical choice was very different for those undergoing surgery before age 50 years compared with at or after age 50 years. An increased all-cause mortality associated with BSO between age 45 and 49 years (HR 1.16, 95% CI 1.04–1.30) was driven by noncancer death (HR 1.29, 95% CI 1.10–1.52). At age 50 years or later, BSO was not associated with increased all-cause death (HR 0.83, 95% CI 0.72–0.97). So, too, was the case with BSO at or after age 55 (HR 0.92, 95% CI 0.82–1.03). The group calculated weighted cumulative incidence of all-cause death at 20 years and found an absolute risk reduction of 1.9% for those undergoing BSO at or after age 50 years and 3.6% at or after age 55 years. This important work, based on a patient population of more than 200,000, again, supports our argument for consideration of BSO with hysterectomy at age 50 years or later.2
With updated data, revised statistical modeling, and Bayesian integration to account for HR uncertainties, our Markov model predicts that, at or after age 50 years, the increased risks associated with concurrent BSO with hysterectomy for benign indication are negated. This is in contrast to the 2005 Markov model that argued for ovarian preservation up to age 65 years. This update should have a profound effect on how we counsel patients who are undergoing hysterectomy for benign indication. We should counsel patients that having concurrent BSO with hysterectomy at age 50 years or older will not increase mortality and will avoid reoperation. For those younger than age 50 years, we should counsel that retaining ovaries leads to decreased mortality; though, if BSO is necessary, the increased mortality can be mitigated with estrogen therapy.
Footnotes
Presented at the Society for Academic Specialists in General Obstetrics and Gynecology Annual Meeting, held virtually, October 2020, with the posters being made available August 15, 2020–August 1, 2023; and at the inaugural Heartland Association for Gynecologic Oncology Meeting, held virtually, September 10–11, 2020.
Financial Disclosure The authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C648.
REFERENCES
- 1.Percentage of women aged ≥50 years who have had a hysterectomy, by race/ethnicity and year – National Health Interview Survey, United States, 2008 and 2018. MMWR Morb Mortal Wkly Rep 2019;68;935. doi: 10.15585/mmwr.mm6841a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cusimano MC, Chiu M, Ferguson SE, Moineddin R, Aktar S, Liu N, et al. Association of bilateral salpingo-oophorectomy with all cause and cause specific mortality: population based cohort study. Br Med J 2021;375:e067528. doi: 10.1136/bmj-2021-067528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacoby VL, Grady D, Sawaya GF. Oophorectomy as a risk factor for coronary heart disease. Am J Obstet Gynecol 2009;200:140.e1–9. doi: 10.1016/j.ajog.2008.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera CM Grossardt BR Rhodes DJ Brown RD Jr, Roger VL Melton LJ 3rd, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009;16:15–23. doi: 10.1097/gme.0b013e31818888f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol 2006;7:821–8. doi: 10.1016/S1470-2045(06)70869-5 [DOI] [PubMed] [Google Scholar]
- 6.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA 2011;305:1305–14. doi: 10.1001/jama.2011.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the women's health initiative randomized trials. JAMA 2013;310:1353–68. doi: 10.1001/jama.2013.278040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casiano ER, Trabuco EC, Bharucha AE, Weaver AL, Schleck CD, Melton LJ. 3rd, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol 2013;121:1069–74. doi: 10.1097/AOG.0b013e31828e89df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol 2005;106:219–26. doi: 10.1097/01.aog.0000167394.38215.56 [DOI] [PubMed] [Google Scholar]
- 10.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses' health study. Obstet Gynecol 2009;113:1027–37. doi: 10.1097/AOG.0b013e3181a11c64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses' Health Study. Obstet Gynecol 2013;121:709–16. doi: 10.1097/AOG.0b013e3182864350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans MJC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet 1996;347:714–8. doi: 10.1016/s0140-6736(96)90075-6 [DOI] [PubMed] [Google Scholar]
- 13.Novetsky AP, Boyd LR, Curtin JP. Trends in bilateral oophorectomy at the time of hysterectomy for benign disease. Obstet Gynecol 2011;118:1280–6. doi: 10.1097/AOG.0b013e318236fe61 [DOI] [PubMed] [Google Scholar]
- 14.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Mak 1993;13:322–38. doi: 10.1177/0272989X9301300409 [DOI] [PubMed] [Google Scholar]
- 15.United States Department of Health and Human Services, Centers for Disease Control and Prevention. United States Cancer Statistics - Mortality Data: 1999-2017, WONDER Online Database. Accessed December 13, 2019. https://wonder.cdc.gov/wonder/help/cancermort-v2017.html
- 16.Chan JK, Urban R, Capra AM, Jacoby V, Osann K, Whittemore A, et al. Ovarian cancer rates after hysterectomy with and without salpingo-oophorectomy. Obstet Gynecol 2014;123:65–72. doi: 10.1097/AOG.0000000000000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laughlin-Tommaso SK, Khan Z, Weaver AL, Smith CY, Rocca WA, Stewart EA. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause 2018;25:483–92. doi: 10.1097/GME.0000000000001043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon-Suen SC, Webb PM, Wilson LF, Tuesley K, Stewart LM, Jordan SJ. The association between hysterectomy and ovarian cancer risk: a population-based record-linkage study. J Natl Cancer Inst 2019;111:1097–103. doi: 10.1093/jnci/djz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacoby VL, Grady D, Wactawski-Wende J, Manson JE, Allison MA, Kuppermann M, et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the women's health initiative observational study. Arch Intern Med 2011;171:760–8. doi: 10.1001/archinternmed.2011.121 [DOI] [PubMed] [Google Scholar]
- 20.Gierach GL, Pfeiffer RM, Patel DA, Black A, Schairer C, Gill A, et al. Long-term overall and disease-specific mortality associated with benign gynecologic surgery performed at different ages. Menopause 2014;21:592–601. doi: 10.1097/GME.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews KA, Gibson CJ, El Khoudary SR, Thurston RC. Changes in cardiovascular risk factors by hysterectomy status with and without oophorectomy: study of Women's Health across the Nation. J Am Coll Cardiol 2013;62:191–200. doi: 10.1016/j.jacc.2013.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appiah D, Schreiner PJ, Bower JK, Sternfeld B, Lewis CE, Wellons MF. Is surgical menopause associated with future levels of cardiovascular risk factor Independent of antecedent levels? The CARDIA study. Am J Epidemiol 2015;182:991–9. doi: 10.1093/aje/kwv162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian data analysis. 1st ed. Chapman and Hall/CRC; 1995. [Google Scholar]
- 24.Domchek SM, Friebel TM, Neuhausen SL, Wagner T, Evans G, Isaacs C, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol 2006;7:223–9. doi: 10.1016/S1470-2045(06)70585-X [DOI] [PubMed] [Google Scholar]
- 25.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2002;346:1609–15. doi: 10.1097/00006254-200209000-00016 [DOI] [PubMed] [Google Scholar]
- 26.Olivier RI, Van Beurden M, Lubsen MAC, Rookus MA, Mooij TM, van de Vijver MJ, et al. Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow-up. Br J Cancer 2004;90:1492–7. doi: 10.1038/sj.bjc.6601692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mytton J, Evison F, Chilton PJ, Lilford RJ. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ 2017;356:j372. doi: 10.1136/bmj.j372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention, U.S. Cancer Statistics. United States cancer statistics: data visualizations. Accessed June 6, 2021. https://gis.cdc.gov/Cancer/USCS/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcancer%2Fdataviz%2Findex.htm#/AtAGlance/value,1,1,52,1,3,1 [Google Scholar]
- 29.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol 2016;2:482–90. doi: 10.1001/jamaoncol.2015.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surveillance Research Program. SEER*Explorer Application. Ovary: SEER relative survival rates by time since diagnosis, 2004–2017. Accessed December 13, 2019. https://seer.cancer.gov/explorer/application.html?site=61&data_type=4&graph_type=6&compareBy=race&chk_race_1=1&hdn_sex=3&age_range=1&stage=106&advopt_precision=1&advopt_show_ci=on&advopt_display=2#graphArea [Google Scholar]
- 31.Boggs DA, Palmer JR, Rosenberg L. Bilateral oophorectomy and risk of cancer in African American women. Cancer Causes Control 2014;25:507–13. doi: 10.1007/s10552-014-0353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudet MM, Gapstur SM, Sun J, Teras LR, Campbell PT, Patel AV. Oophorectomy and hysterectomy and cancer incidence in the cancer prevention study-II nutrition cohort. Obstet Gynecol 2014;123:1247–55. doi: 10.1097/AOG.0000000000000270 [DOI] [PubMed] [Google Scholar]
- 33.Robinson WR, Nichols HB, Tse CK, Olshan AF, Troester MA. Associations of premenopausal hysterectomy and oophorectomy with breast cancer among Black and White women: the Carolina Breast Cancer Study, 1993-2001. Am J Epidemiol 2016;184:388–99. doi: 10.1093/aje/kwv448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai JC, Chou YJ, Huang N, Chen HH, Wang KL, Wang CW, et al. The risk of stroke after bilateral salpingo-oophorectomy at hysterectomy for benign diseases: a nationwide cohort study. Maturitas 2018;114:27–33. doi: 10.1016/j.maturitas.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 35.Gallicchio L, Whiteman MK, Tomic D, Miller KP, Langenberg P, Flaws JA. Type of menopause, patterns of hormone therapy use, and hot flashes. Fertil Steril 2006;85:1432–40. doi: 10.1016/j.fertnstert.2005.10.033 [DOI] [PubMed] [Google Scholar]
- 36.Kritz-Silverstein D, Von Muhlen DG, Ganiats TG, Barrett-Connor E. Hysterectomy status, estrogen use and quality of life in older women: the Rancho Bernardo study. Qual Life Res 2004;13:55–62. doi: 10.1023/B:QURE.0000015318.00707.53 [DOI] [PubMed] [Google Scholar]
- 37.Kritz-Silverstein D, Von Mühlen DG, Barrett-Connor E. Hysterectomy and oophorectomy are unrelated to bone loss in older women. Maturitas 2004;47:61–9. doi: 10.1016/S0378-5122(03)00242-1 [DOI] [PubMed] [Google Scholar]