Abstract
Background:
The benefit of fetal echocardiograms (FE) to detect severe congenital heart diseases (SCHD) in the setting of a normal second-trimester ultrasound is unclear. We aimed to assess whether the increase in SCHD detection rates when FE are performed for risk factors in the setting of a normal ultrasound was clinically significant to justify the resources needed.
Methods:
This is a multicenter, population-based, retrospective cohort study, including all singleton pregnancies and offspring in Quebec (Canada) between 2007 and 2015. Administrative health care data were linked with FE clinical data to gather information on prenatal diagnosis of CHD, indications for FE, outcomes of pregnancy and offspring, postnatal diagnosis of CHD, cardiac interventions, and causes of death. The difference between the sensitivity to detect SCHD with and without FE for risk factors was calculated using generalized estimating equations with a noninferiority margin of 5 percentage points.
Results:
A total of 688 247 singleton pregnancies were included, of which 30 263 had at least one FE. There were 1564 SCHD, including 1071 that were detected prenatally (68.5%). There were 12 210 FE performed for risk factors in the setting of a normal second-trimester ultrasound, which led to the detection of 49 additional cases of SCHD over 8 years. FE referrals for risk factors increased sensitivity by 3.1 percentage points (95% CI, 2.3–4.0; P<0.0001 for noninferiority).
Conclusions:
In the setting of a normal second-trimester ultrasound, adding a FE for risk factors offered low incremental value to the detection rate of SCHD in singleton pregnancies. The current ratio of clinical gains versus the FE resources needed to screen for SCHD in singleton pregnancies with isolated risk factors does not seem favorable. Further studies should evaluate whether these resources could be better allocated to increase SCHD sensitivity at the ultrasound level, and to help decrease heterogeneity between regions, institutions and operators.
Keywords: congenital heart disease, echocardiography, pregnancy, prenatal diagnosis, risk factors
Clinical Perspective.
It has been advocated that pregnancies at higher risk of congenital heart disease (CHD) should be referred for a detailed fetal echocardiogram (FE), including in the setting of a completely normal second-trimester ultrasound. The benefit of FE to detect severe CHD (SCHD) in this setting is unclear. The authors assessed whether the increase in SCHD detection rates when FE are performed for risk factors in the setting of a normal ultrasound was clinically significant to justify the resources needed. This was a multicenter, population-based, retrospective cohort study, including all singleton pregnancies between 2007 and 2015 in Quebec (Canada). The difference between the sensitivity to detect SCHD with and without FE for risk factors was calculated. A total of 688 247 singleton pregnancies were included, of which 30 263 had at least one FE. There were 1564 SCHD, including 1071 that were detected prenatally (68.5%). There were 12 210 FE performed for risk factors in the setting of a normal second-trimester ultrasound, which corresponded to >4 out of 10 pregnancies receiving a FE. FE referrals for risk factors increased sensitivity by 3.1 percentage points (95% CI, 2.3–4.0, P<0.0001 for noninferiority). The authors concluded that in the setting of a normal second-trimester ultrasound, adding a FE for risk factors offered low incremental value to the detection rate of SCHD in singleton pregnancies.
See Editorial by Hornberger and Simpson
Fetal echocardiography is an important diagnostic tool for the prenatal diagnosis of congenital heart disease (CHD). It is, however, resource intensive and generally only available in specialized centers. Selecting pregnancies that require this evaluation warrants careful consideration. It has been advocated that pregnancies at higher risk of CHD should be referred for a detailed fetal echocardiogram (FE), including in the setting of a completely normal second-trimester ultrasound,1,2 a recommendation that was endorsed in the 2014 fetal cardiology guidelines developed by the American Heart Association.3 These FE indications include high-risk maternal and fetal factors, such as aneuploidy, but also frequent situations with a moderately increased risk of CHD, such as pregestational diabetes, maternal medication, and family history of CHD.3
The added value of FE as a screening tool has been questioned and previous studies have cast doubt on the usefulness of FE to significantly increase detection rate of severe CHD (SCHD).4–7 However, these were small studies with a limited number of cases which were inadequately powered to examine the impact of FE screening of increased risk pregnancies in overall prenatal detection of SCHD. We have recently shown in a simulation study that the increase in detection rate incurred by FE could be limited by the low prevalence of SCHD and the high absolute number of SCHD cases in low-risk pregnancies.8 Clinical experience also suggests there may be marginal benefit of performing FE when a second-trimester ultrasound performed by those sufficiently trained reports no CHD, at the cost of high resource utilization. However, this has not been substantiated.
Our main objective was to assess whether FE increases prenatal detection of SCHD when used as a screening tool for at risk singleton pregnancies following a normal second-trimester ultrasound. Our hypothesis was that the increase in sensitivity would be low, and we used a noninferiority design to test whether the increases in the overall detection rate of SCHD was <5 percentage points. To assess this, we determined the sensitivity and negative predictive value of the prenatal detection of SCHD, we calculated the number needed to test (NNT) to detect a SCHD according to FE indications, and we measured the increase in overall sensitivity incurred by FE performed for risk factors after a normal second-trimester ultrasound in singleton pregnancies.
Methods
Design and Population
This is a multicenter, population-based, retrospective cohort study, including all singleton pregnancies in Quebec (Canada) encountered from 2007 to 2015, with follow-up of the offspring for 2 years. Administrative health care data were used to identify eligible pregnancies and derive offspring outcomes, while all 4 tertiary pediatric cardiology health care centers contributed detailed FE data. We previously published details on the study protocol.9 In Quebec, there is universal health care insurance that covers all pregnancy care and follow-ups, including second-trimester ultrasound, fetal cardiology referrals, FE, health care costs for delivery and all health care costs relating to the offspring.
This study was approved by the institutional review board of each participating center and by the Commission d’accès à l’information du Québec. The need for individual informed consent was waived. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Selection Criteria
We included all singleton pregnancies in Quebec for which a billing code for a second-trimester ultrasound was retrievable between 2007 and 2015, as well as their offspring. We excluded multiple pregnancies because it was difficult to clearly link each fetus’s data with the corresponding offspring. We also excluded offspring who could not be linked to a specific pregnancy.
Administrative Health Care Data Collection
Administrative healthcare data were obtained from government databanks housed at the Ministry of Health and Social Services, the Régie de l’assurance maladie du Québec and the Institut de la statistique du Québec. Prenatal data included billing, interventions, deliveries, information on termination of pregnancy and intrauterine death, and all relevant International Classification of Disease, Tenth edition diagnostic codes. Maternal data were collected from the first second-trimester ultrasound to delivery. Postnatal data for the offspring included information on livebirth or stillbirth, all billing codes (inpatient, outpatients, diagnostic services, interventions and imaging), all discharge summary International Classification of Disease, Tenth edition diagnostic codes, types and timing of percutaneous or surgical interventions, and information on pediatric cardiology follow-ups.
Death and stillbirth certificates were retrieved for all eligible pregnancies and offspring. The primary and secondary causes of death were recorded, which included autopsy reports (when performed).
Clinical Data Collection
Clinical data on all FE performed in Quebec during the study period was obtained from FE databanks housed in each participating center. We collected data on the date, indications, results, and CHD descriptions. We completed data with manual chart review when the banked data were incomplete or ambiguous. Only the first FE done for a specific pregnancy was included in the analysis.
Data Linkage
Clinical data on FE and prenatal administrative health care data were linked using the unique identifier number of the health care system in Quebec. Linkage was validated and completed using the name and date of birth. The Ministry of Health holds a mother and child link that was used to identify and link each offspring with their mother. The date of birth was used to assign each offspring to their respective pregnancy.
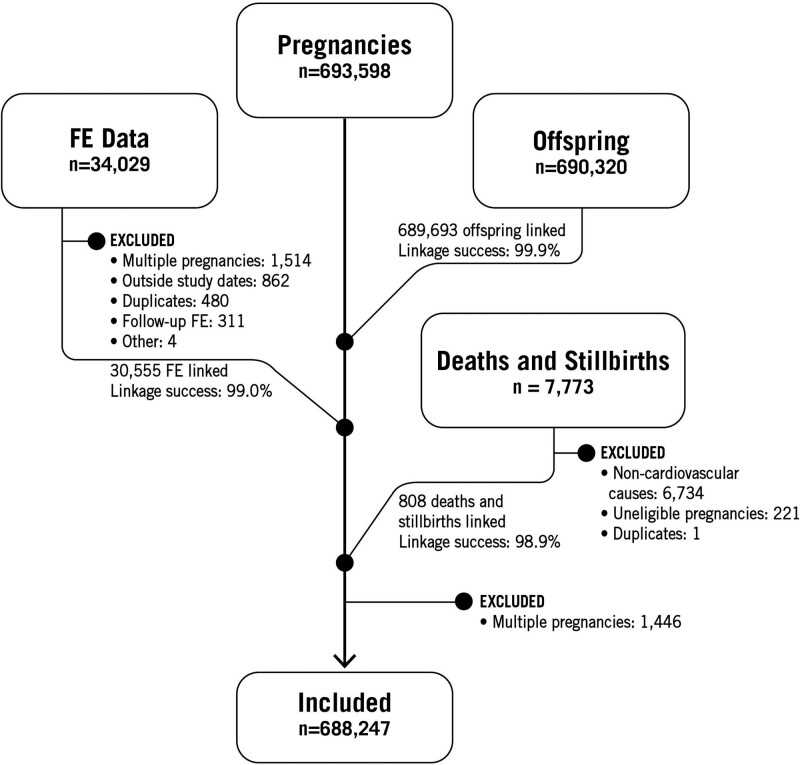
Death and stillbirth certificates were linked using probabilistic matching based on the name and date of birth of the offspring, and the name and date of birth of the mother that were available on the certificates. Because of the complexity of this linkage, death and stillbirth certificates were first screened for cardiovascular causes (see Figure 1 and Table S1). Death and stillbirth certificates with a primary or secondary diagnosis related to a cardiovascular cause were then linked to the appropriate pregnancy and offspring for further analysis.
Figure 1.
Study flowchart. FE indicates fetal echocardiograms.
Of note, in Quebec, infants delivered <20 weeks of gestation and weighing <500 grams will not appear on a birth, stillbirth of death certificate. Hence, we considered that pregnancies with no mother-child link and no other indication of a specific pregnancy outcome were the results of a miscarriage, intrauterine death, or termination of pregnancy.
Linkages based on personal identifiers were performed by a dedicated team at the Institut de la statistique du Québec and the research dataset used for analysis was deidentified.
Primary Outcome
The primary outcome was the presence of SCHD. It was defined as either of the following: a CHD that led to termination of pregnancy or intrauterine death, a CHD that required percutaneous or surgical intervention within the first month of life, a CHD that led to cardiovascular mortality within the first 6 months of life, or a complex CHD that was included in one of the following categories: functionally univentricular heart, transposition of the great arteries, common arterial trunk, double outlet right ventricle, anomaly of atrioventricular or ventriculo-arterial connection, congenital aortic valvar atresia, congenital pulmonary atresia, or tetralogy of Fallot. Diagnostic CHD data sources were cross-matched and each CHD was attributed the most precise diagnostic code possible based on the upcoming 11th version of the classification of diseases.10 They were then categorized based on their severity.9 The detailed algorithm used to determine the primary outcome is presented in the Supplemental Methods and Table S2.
Fetal Echocardiography Indication and Inference on Prenatal Detection
The indications for performing FE were retrieved from FE clinical databanks as described above. When multiple indications were noted, we used a hierarchical list to attribute the main indication. This list of indications was based on the likelihood of a suspicion of CHD at the second-trimester ultrasound. For example, for a FE with multiple indications such as suspicion of cardiac anomaly and pregestational diabetes, we attributed the indication suspicion of cardiac anomaly to that specific FE. The hierarchical list of indications is presented in the Table S3.
Our objective was to determine the incremental value of FE for risk factor in the setting of a normal second-trimester ultrasound. We specifically analyzed data for the following indications: isolated increased nuchal translucency, family history of CHD, maternal diabetes, and maternal medication.
During the study period, the usual trajectory of care in Quebec is to obtain 4-chamber, outflow tract and sagittal cardiac views at the second-trimester ultrasound,11 and to refer to fetal cardiology when an abnormal cardiac anatomy is suspected. Most centers following pregnancies in our population were adhering to the 2014 American Heart Association guidelines3 and were thus also referring pregnant women for a FE when maternal and fetal risk factors were present. Complicated and higher risk pregnancies were often also referred for a level 2 obstetric ultrasound to maternal and fetal medicine specialists. Because all centers performing FE were included in the study and contributed all their data for the study period, we inferred that any pregnancy not referred for a FE had a trajectory of care such that no suspicion of cardiac anomaly was present at any point.
When calculating the incremental value of FE for risk factors, we excluded from our analysis any FE performed before the second-trimester ultrasound or on the same day, as it was not possible to ascertain whether any anomaly detected on the FE would have been suspected during the ultrasound, had the ultrasound been performed before the FE. We also excluded pregnancies with first-trimester and early second-trimester FE when performed before the second trimester ultrasound, although this practice was exceptional during the study period in our population.
Statistical Analysis
The unit of analysis was a pregnancy. If a woman had more than one pregnancy during the study period, each pregnancy was included. We present descriptive analyses as numbers and percentages. We calculated sensitivity (ratio of participants with a suspicion of SCHD among participants with SCHD) and negative predictive values (ratio of participants without SCHD among participants without a suspicion of SCHD) using 2×2 contingency tables. We used generalized estimating equations to calculate 95% CI. We calculated the NNT as the number of FE divided by the number of SCHD detected prenatally, stratified by FE indications. The incremental value of performing FE was calculated using 2×2 contingency tables, comparing the results with and without FE for risk factors. The increment in sensitivity and its 95% CI were calculated using generalized estimating equations. Our hypothesis was that the detection rate without FE for risk factors was not inferior to the detection rate with FE for risk factors. This was tested by assessing whether the upper limit of the 95% CI of the increment in sensitivity excluded the predefined boundary of 5 percentage point. We also tested a mixed effect logistic regression that considered the potential effect of including pregnant women more than once when they had >1 pregnancy during the study period. This approach yielded virtually identical results compared with generalized estimating equations and was not pursued further (data not shown). The threshold of statistical significance was set at P<0.05. All analyses were performed on SAS for Windows version 9.4 (SAS Institute, Cary, NC).
Results
Figure 1 presents the flow chart for this study. We identified 693 598 potentially eligible pregnancies and 690 320 potentially eligible offspring during the study period. Of the offspring, 689 693 (99.9%) could be successfully linked to a specific pregnancy. We extracted clinical data on 34 029 potentially eligible FE. Of these, we excluded 1514 multiple pregnancies, 862 FE done outside the study period, 480 duplicate records, 311 follow-up FE, and 4 FE for other reasons. Of the 30 858 eligible FE, we were able to link 30 555 of them (99.0%) with eligible pregnancies. We further excluded 1446 multiple pregnancies following the linkage of clinical and administrative data. We, therefore, included in our analysis the remaining 688 247 pregnancies, 30 263 of which had at least one FE.
Rates of Severe CHD
We identified 1564 SCHD (2.3 cases per 1000 pregnancies), 1071 of which were detected before birth (68.5%). Table 1 presents the type of SCHD and the prenatal detection rate by SCHD types. The most common types of SCHD were functionally univentricular hearts (421 cases, 26.9%), tetralogy of Fallot and double outlet right ventricles (397 cases, 25.4%), and transposition of the great arteries (244 cases, 15.6%). The sensitivity of FE was 97.7% (95% CI, 96.8%–98.6%).
Table 1.
Prenatal Detection Rates of Severe Congenital Heart Diseases
NNT by Fetal Echocardiography Indications
We identified 12 210 pregnancies with FE performed for common isolated risk factors in the setting of a normal second-trimester ultrasound (40.3% of pregnancies with at least one FE). These were family history of CHD (5392, or 44.0% of all pregnancies referred for risk factors), maternal diabetes (3933, 32.1%), isolated increased nuchal translucency (2029, 16.6%), and maternal medication (856, 7.0%).
Table 2 presents the NNT to diagnose one case of SCHD, stratified by indications of FE. There were 1071 SCHD identified prenatally, for an overall NNT of 28. FE performed for risk factors in the setting of a normal second-trimester ultrasound detected 49 SCHD that were not detected at the second-trimester ultrasound (4.6% of SCHD detected prenatally, 3.1% of all SCHD). The NNT for all FE for risk factors was 249. Family history of CHD and maternal diabetes had NNT of 234 and 246, respectively.
Table 2.
Number Needed to Test to Identify One Case of Severe Congenital Heart Disease by Fetal Echocardiography Indications
Increase in Sensitivity Incurred by Fetal Echocardiography for Risk Factors
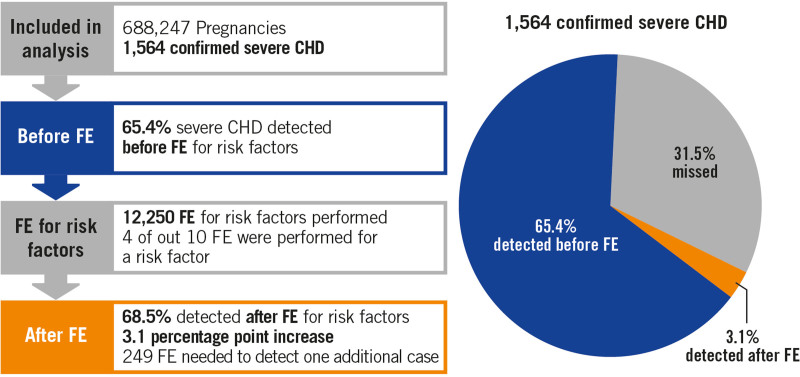
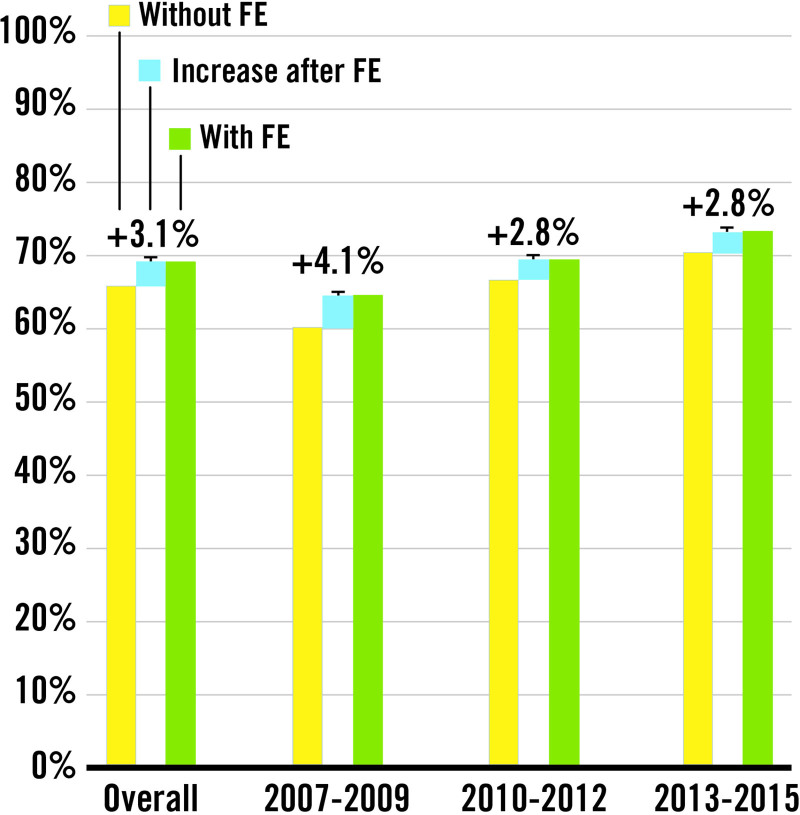
Figure 2 displays a summary of the results. Table 3 details the sensitivity to detect SCHD with and without FE referrals for risk factors. The sensitivity without FE for risk factor was 65.3% (95% CI, 63.0%–67.7%). FE referrals for risk factors increased the sensitivity for detecting SCHD by 3.1 percentage points (95% CI, 2.3–4.0) to 68.5% (95% CI, 66.2–70.). The P value for noninferiority was P<0.0001, considering our predefined threshold of 5 percentage point difference. Figure 3 shows that the increase in sensitivity incurred by performing FE for risk factors declined over time from 4.1 percentage points (95% CI, 2.3–5.8) in 2007 to 2009 to 2.8 percentage points (95% CI, 1.4–4.2) in 2013 to 2015. The negative predictive value was very high (>99.9 %), both with and without FE for risk factors. The addition of FE for risk factors enabled detection of 7.1 additional cases per 100 000 pregnancies, a number that slightly declined from 8.2 to 6.5 per 100 000 pregnancies during the study period.
Figure 2.
Graphical summary of the main study results. CHD indicates congenital heart disease; and FE, fetal echocardiogram.
Table 3.
Prenatal Screening Parameters of Severe Congenital Heart Diseases
Figure 3.
Increase in sensitivity incurred by fetal echocardiograms for risk factors. FE indicates fetal echocardiogram.
Discussion
We conducted a multicenter, population-based, retrospective cohort study that included all singleton pregnancies in Quebec between 2007 and 2015. We found that systematic referral for FE for an isolated risk factor in the absence of an anomaly in the second-trimester ultrasound increased overall detection rates of SCHD by 3.1 percentage points and led to the detection of 7.1 additional SCHD cases per 100 000 pregnancies. Over 8 years, only 49 additional cases of SCHD were detected among >12 000 FE performed. Considering our study covers the 2007 to 2015 period, it is possible that a continued rise in second-trimester ultrasound sensitivity after 2015 would decrease the number of detected SCHD even further.
This is the largest population-based study assessing the yield of FE to increase sensitivity of prenatal detection of SCHD following a reportedly normal second-trimester ultrasound. We previously reported a similar increase in sensitivity in a simulation study. We showed that for an ultrasound sensitivity of 65%, the expected increase in sensitivity by adding FE was 2.1 percentage points, with ≈4 additional SCHD cases found per 100 000 pregnancies. This small increase could be attributed to the low prevalence of SCHD in the population and the high absolute number of SCHD in low-risk pregnancies.6
We acknowledge that the roles of FE and fetal cardiology consultations are much broader than simply screening for CHD, and we stress that FE and prenatal cardiology consultations play an important role in the trajectory of care of pregnant women. With that in mind, our results should be interpreted strictly for what they are: the expected increase in sensitivity to detect SCHD by referring high-risk singleton pregnancy with normal second-trimester ultrasound will be limited to a few percentage points. In other words, when FE is viewed as a screening tool for increasing detection rates of SCHD, its impact on detection rate will be marginal, despite important resource utilization. Hence, it may represent only a small part of the solution to tackle low detection rates.
We want to emphasis that, in our population, the overall detection rate of these potentially fragile and unstable infants remained below 70%. Furthermore, despite the small increase in percentage points incurred by FE, it still led to the detection of 49 SCHD that would not have been detected otherwise. Our current results and previous mathematical modeling inform us that we still have an imperfect prenatal CHD detection model. Nevertheless, using FE to screen pregnancies at moderately increased risk was resource intensive, yet offered limited gains. We have previously shown that to obtain NNT that are in a reasonable range, we should target pregnancies that have risk ratio of CHD above 10—probably above 20—compared with low-risk pregnancies. These risk ratios are far higher than those observed in the most frequent FE indications when no cardiac anomaly was detected at the second-trimester ultrasound.
Our results confirm previous findings of smaller studies. Starikov et al7 evaluated the benefit of performing FE after a normal second-trimester ultrasound. They found that only 1 case of CHD could be identified out of 481 normal ultrasound.7 Garg et al5 performed FE in 302 consecutive pregnant women with gestational diabetes and found no pre- or postnatal evidence of CHD in any offspring. Bernard et al12 reviewed 114 pregnancies complicated by diabetes and found that all 6 SCHD were identified at the second-trimester ultrasound. Additional FE led to the detection of 3 CHD that were deemed not clinically significant in the postnatal period.12 Wright et al13 found that 25% of 2389 FE in their center were done for diabetes or a family history of CHD. They found that 4.6% of CHD were detected because of these risk factors.13 This is slightly higher than the 3.2% reported in our study, but the authors included all CHD.
Given that the majority of SCHD occur in pregnancies without risk factors, data suggests that the first steps to increase detection rates are improving the sensitivity of the second-trimester ultrasound and reducing regional variability.14 We believe that the ideal prenatal CHD model is one where ultrasound and FE form a pair in which the first is a screening tool with high sensitivity, and the second is a diagnostic tool with high sensitivity and high specificity. This model in which FE is reserved for fetuses with suspected cardiac anomaly or for instances where all normal cardiac views have not been well seen has been shown to be effective previously in some Canadian regions.15 The very high negative predictive value (>99.9%) informs us that the absence of a suspicion of a cardiac anomaly on the second-trimester ultrasound is correct >99.9% of the time. Nevertheless, sensitivity remains suboptimal, and our efforts should concentrate on the factors at play when SCHD are missed.
We found a SCHD prevalence of 2.3 cases per 1000 pregnancies. Previous studies have found similar incidence rates of SCHD, ranging from 1.7 to 2.3 cases per 1000.16–19 The SCHD most observed was the broad category of functionally univentricular heart, with an incidence of 0.55 cases per 1000. This is higher than previously reported birth prevalence in children,19 but similar to that observed when terminations of pregnancies are considered,18 or in older cohorts of patients with low rates of termination of pregnancy.17 We found an overall prenatal detection rate of 68.5%, and a steady increase in detection rate from 2007 to 2015, which is comparable to previous observations in Canada and elsewhere.14,16,19–21 The current analysis did not aim to specifically assess factors that influenced SCHD detection rates. Other studies have shown that they vary according to geographic region, volume of ultrasound per medical center, type of cardiac view at the ultrasound, type of CHD, and sonographer experience.14,16,22 Continued efforts to level these differences, which has the benefit of targeting all pregnancies and not only those with a higher risk, is more likely to be effective in increasing detection rates. An assessment of the variability in detection rates by region, type of institution, medical specialty and type of CHD will be the subject of a subsequent analysis of the FREQUENCY study data.
Strengths and Limitations
Given its populational nature, our study provides real-world evidence of SCHD screening in Quebec. This approach improves the external validity by including all institutions involved in prenatal detection of CHD, but it requires use of administrative health care databases, which are more prone to misclassification. True SCHD cases missed by our classification algorithm would artificially increase sensitivity, but this would likely not be related to FE indications. Clinical data for 2 of our participating centers were extracted from house registries not originally intended for research with a potential bias towards underrepresentation of mild CHD or normal studies, which could have biased our results towards the null hypothesis. This was mitigated by crosslinking clinical databases with administrative healthcare databases.
We inferred whether there was a suspicion of CHD at the second-trimester ultrasound from FE indications in FE reports, which could have artificially decreased sensitivity. Indications for FE were grouped in broad categories as detailed information on indication was often difficult to retrieve. We recognize that risk of CHD may vary within these categories (eg, family history of a single septal defect versus multiple cases of left heart obstruction),15,16,20 which could artificially increase the NNT for these indications. Because we included all those referred, and not only those who should have been referred, our results reflect the real-world referral pattern that occurred in the study period, during which clinicians adhered to the latest American Heart Association guidelines. Multiple pregnancies were excluded from this study and our results cannot be generalized to multiple pregnancies. Finally, we acknowledge that the trajectory of care for pregnant women is not static and the current situation may be different from that of the study period. Specifically, increasing use of level 2 morphology scans and FE in the first-trimester and early second-trimester may increase the overall detection rate, which would reduce the number of missed SCHD cases that could potential be detected by a screening second-trimester FE.
Conclusions
We showed that in the setting of a normal second-trimester ultrasound, adding a FE for common fetal and maternal risk factors offered low incremental value to the detection rate of SCHD in singleton pregnancies. Because performing these FE for risk factor was resource intensive, the ratio of clinical gains versus the FE resources needed to screen for SCHD in pregnancies with isolated risk factors may not be favorable. Further studies should evaluate whether these resources could be better allocated to increase SCHD sensitivity at the ultrasound level and to help decrease heterogeneity between regions, institutions, and operators.
Article Information
Acknowledgments
We would like to thank the Unité de recherche clinique et épidémiologique of the Centre de recherche du Centre hospitalier universtaire de Sherbrooke for providing logistical and biostatistical support. We would also like to acknowledge the hard work of interns and medical students who reviewed thousands of individual patient charts and contributed to cleaning the data: Alexandre Nadeau, Annie Chabot, Antoine Déry, Coby Rangsitratkul, Ève Pellerin, Garrett Newell, Jenna Donaldson, Kevin Bornais, Laurel Walfish, Laurence Gobeil, Laurence Proulx, Léanne Pilote, Olivier Guertin, Rayhaan Bassawon, Steeven Breton, Elizabeth Richard, and Vincent Hamilton.
Sources of Funding
This project was funded by an unrestricted governmental grant from the Canadian Institute of Health Research, Canada (Grant No. PJT 166022) and by an unrestricted institutional grant from the Centre de recherche du Centre hospitalier universitaire de Sherbrooke.
Disclosures
None.
Supplemental Materials
Supplemental Methods
Tables S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CHD
- congenital heart disease
- FE
- fetal echocardiogram
- NNT
- number needed to test
- SCHD
- severe CHD
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.121.013796
For Sources of Funding and Disclosures, see page 247.
Contributor Information
Mikhail-Paul Cardinal, Email: Mikhail-Paul.Cardinal@USherbrooke.ca.
Marie-Hélène Gagnon, Email: mh.gagnon@mail.mcgill.ca.
Cassandre Têtu, Email: cassandre.tetu@mail.mcgill.ca.
Francis-Olivier Beauchamp, Email: Francis-Olivier.Beauchamp@USherbrooke.ca.
Louis-Olivier Roy, Email: Louis-Olivier.Roy2@USherbrooke.ca.
Camille Noël, Email: camille.noel@usherbrooke.ca.
Laurence Vaujois, Email: laurence.vaujois.med@ssss.gouv.qc.ca.
Tiscar Cavallé-Garrido, Email: maria.cavalle-garrido@mcgill.ca.
Jean-Luc Bigras, Email: Jean-Luc.Bigras.med@ssss.gouv.qc.ca.
Marie-Ève Roy-Lacroix, Email: Marie-Eve.Roy-Lacroix@USherbrooke.ca.
References
- 1.Rocha LA, Araujo Júnior E, Rolo LC, Barros FS, da Silva KP, Leslie AT, Nardozza LM, Moron AF. Prenatal detection of congenital heart diseases: one-year survey performing a screening protocol in a single reference center in Brazil. Cardiol Res Pract. 2014;2014:175635. doi: 10.1155/2014/175635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rychik J, Ayres N, Cuneo B, Gotteiner N, Hornberger L, Spevak PJ, Van Der Veld M. American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. J Am Soc Echocardiogr. 2004;17:803–810. doi: 10.1016/j.echo.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 3.Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, et al. ; American Heart Association Adults With Congenital Heart Disease Joint Committee of the Council on Cardiovascular Disease in the Young and Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Council on Cardiovascular and Stroke Nursing. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d [DOI] [PubMed] [Google Scholar]
- 4.Finneran MM, Ware CA, Kiefer MK, Buschur EO, Foy PM, Thung SF, Landon MB, Gabbe SG. The accuracy and cost-effectiveness of selective fetal echocardiography for the diagnosis of congenital heart disease in patients with pregestational diabetes stratified by hemoglobin A1c. Am J Perinatol. 2019;36:1216–1222. doi: 10.1055/s-0039-1685490 [DOI] [PubMed] [Google Scholar]
- 5.Garg S, Sharma P, Sharma D, Behera V, Durairaj M, Dhall A. Use of fetal echocardiography for characterization of fetal cardiac structure in women with normal pregnancies and gestational diabetes mellitus. J Ultrasound Med. 2014;33:1365–1369. doi: 10.7863/ultra.33.8.1365 [DOI] [PubMed] [Google Scholar]
- 6.Nayak K, Chandra G S N, Shetty R, Narayan PK. Evaluation of fetal echocardiography as a routine antenatal screening tool for detection of congenital heart disease. Cardiovasc Diagn Ther. 2016;6:44–49. doi: 10.3978/j.issn.2223-3652.2015.12.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starikov RS, Bsat FA, Knee AB, Tsirka AE, Paris Y, Markenson GR. Utility of fetal echocardiography after normal cardiac imaging findings on detailed fetal anatomic ultrasonography. J Ultrasound Med. 2009;28:603–608. doi: 10.7863/jum.2009.28.5.603 [DOI] [PubMed] [Google Scholar]
- 8.Bellavance S, Cardinal MP, Gobeil L, Roy-Lacroix ME, Dallaire F. The mathematical limitations of fetal echocardiography as a screening tool in the setting of a normal second-trimester ultrasound. CJC Open. 2021;3:987–993. doi: 10.1016/j.cjco.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noël C, Gagnon MH, Cardinal MP, Guertin O, Déry A, Têtu C, Vanasse A, Roy-Lacroix MÈ, Poder TG, Marelli AJ, et al. Rationale and design of the FREQUENCY study: the fetal cardiac registry of québec to improve resource utilization in fetal cardiology. J Obstet Gynaecol Can. 2019;41:459–465.e12. doi: 10.1016/j.jogc.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 10.Béland MJ, Harris KC, Marelli AJ, Houyel L, Bailliard F, Dallaire F. Improving quality of congenital heart disease research in Canada: standardizing nomenclature across Canada. Can J Cardiol. 2018;34:1674–1676. doi: 10.1016/j.cjca.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 11.Cargill Y, Morin L; DIAGNOSTIC IMAGING COMMITTEE. Content of a complete routine second trimester obstetrical ultrasound examination and report. J Obstet Gynaecol Can. 2009;31:272–275. doi: 10.1016/S1701-2163(16)34127-5 [DOI] [PubMed] [Google Scholar]
- 12.Bernard LS, Ramos GA, Fines V, Hull AD. Reducing the cost of detection of congenital heart disease in fetuses of women with pregestational diabetes mellitus. Ultrasound Obstet Gynecol. 2009;33:676–682. doi: 10.1002/uog.6302 [DOI] [PubMed] [Google Scholar]
- 13.Wright L, Stauffer N, Samai C, Oster M. Who should be referred? An evaluation of referral indications for fetal echocardiography in the detection of structural congenital heart disease. Pediatr Cardiol. 2014;35:928–933. doi: 10.1007/s00246-014-0877-7 [DOI] [PubMed] [Google Scholar]
- 14.Nagata H, Glick L, Lougheed J, Grattan M, Mondal T, Thakur V, Schwartz SM, Jaeggi E. Prenatal diagnosis of transposition of the great arteries reduces postnatal mortality: a population-based study. Can J Cardiol. 2020;36:1592–1597. doi: 10.1016/j.cjca.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 15.Letourneau KM, Horne D, Soni RN, McDonald KR, Karlicki FC, Fransoo RR. Advancing prenatal detection of congenital heart disease: a novel screening protocol improves early diagnosis of complex congenital heart disease. J Ultrasound Med. 2018;37:1073–1079. doi: 10.1002/jum.14453 [DOI] [PubMed] [Google Scholar]
- 16.Bakker MK, Bergman JEH, Krikov S, Amar E, Cocchi G, Cragan J, de Walle HEK, Gatt M, Groisman B, Liu S, et al. Prenatal diagnosis and prevalence of critical congenital heart defects: an international retrospective cohort study. BMJ Open. 2019;9:e028139. doi: 10.1136/bmjopen-2018-028139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 18.Lytzen R, Vejlstrup N, Bjerre J, Petersen OB, Leenskjold S, Dodd JK, Jørgensen FS, Søndergaard L. Live-born major congenital heart disease in denmark: incidence, detection rate, and termination of pregnancy rate from 1996 to 2013. JAMA Cardiol. 2018;3:829–837. doi: 10.1001/jamacardio.2018.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756. doi: 10.1161/CIRCULATIONAHA.113.008396 [DOI] [PubMed] [Google Scholar]
- 20.Ravi P, Mills L, Fruitman D, Savard W, Colen T, Khoo N, Serrano-Lomelin J, Hornberger LK. Population trends in prenatal detection of transposition of great arteries: impact of obstetric screening ultrasound guidelines. Ultrasound Obstet Gynecol. 2018;51:659–664. doi: 10.1002/uog.17496 [DOI] [PubMed] [Google Scholar]
- 21.Hautala J, Gissler M, Ritvanen A, Tekay A, Pitkänen-Argillander O, Stefanovic V, Sarkola T, Helle E, Pihkala J, Pätilä T, et al. The implementation of a nationwide anomaly screening programme improves prenatal detection of major cardiac defects: an 11-year national population-based cohort study. BJOG. 2019;126:864–873. doi: 10.1111/1471-0528.15589 [DOI] [PubMed] [Google Scholar]
- 22.van Nisselrooij AEL, Teunissen AKK, Clur SA, Rozendaal L, Pajkrt E, Linskens IH, Rammeloo L, van Lith JMM, Blom NA, Haak MC. Why are congenital heart defects being missed? Ultrasound Obstet Gynecol. 2020;55:747–757. doi: 10.1002/uog.20358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellavance S, Cardinal MP, Gobeil L, Roy-Lacroix ME, Dallaire F. The mathematical limitations of fetal echocardiography as a screening tool in the setting of a normal second-trimester ultrasound. CJC Open. 2021;3:987–993. doi: 10.1016/j.cjco.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkola T, Ojala TH, Ulander VM, Jaeggi E, Pitkänen OM. Screening for congenital heart defects by transabdominal ultrasound - role of early gestational screening and importance of operator training. Acta Obstet Gynecol Scand. 2015;94:231–235. doi: 10.1111/aogs.12572 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.