Abstract
Many Rahnella strains have been widely described as plant growth-promoting rhizobacteria with the potential to benefit plant growth and protect plants from pathogens. R. aceris ZF458 is a beneficial plant bacterium isolated from swamp soil with the potential for biocontrol. Strain ZF458 has shown broad-spectrum antagonistic activities against a variety of plant pathogens and exhibited a dramatic effect on controlling Agrobacterium tumefaciens in sunflowers. The R. aceris ZF458 genome sequence contained a 4,861,340-bp circular chromosome and two plasmids, with an average G + C content of 52.20%. Phylogenetic analysis demonstrated that R. aceris ZF458 was closely related to R. aceris SAP-19. Genome annotation and comparative genomics identified the conservation and specificity of large numbers of genes associated with nitrogen fixation, plant growth hormone production, organic acid biosynthesis and pyrroloquinoline quinone production that specific to benefiting plants in strain ZF458. In addition, numerous conserved genes associated with environmental adaption, including the bacterial secretion system, selenium metabolism, two-component system, flagella biosynthesis, chemotaxis, and acid resistance, were also identified in the ZF458 genome. Overall, this was the first study to systematically analyze the genes linked with plant growth promotion and environmental adaption in R. aceris. The aim of this study was to derive genomic information that would provide an in-depth insight of the mechanisms of plant growth-promoting rhizobacteria, and could be further exploited to improve the application of R. aceris ZF458 in the agriculture field.
Keywords: Rahnella aceris ZF458, comparative genomic analysis, biological control, plant growth promotion, environmental adaptation
Introduction
Rahnella was a Gram-negative, rod-shaped, facultatively anaerobic bacterium belonging to the family Yersiniaceae (Adeolu et al., 2016), and was widely distributed in a variety of environments, including soil, phyllosphere, water, seeds, food, some clinical samples, and even in American mastodon remains (Berge et al., 1991; Rhodes et al., 1998). Rahnella was first proposed in 1979, and normally consisted of six species including R. aquatilis (which was described as the type species of Rahnella), R. variigena, R. inusitata, R. bruchi, R. woolbedingensis, and R. victoriana by multilocus sequence analysis (Brady et al., 2014). Recently, a new species named R. aceris was proposed in 2020, which shared a high similarity with the R. aquatilis (Lee et al., 2020). To date, only one R. aceris strain SAP-19 has been reported for general physiological and biochemical characteristics, shotgun genome information and phylogenetic analyses (Lee et al., 2020). Since there have been very few studies on R. aceris, its genetic background, taxonomic classification and potential biological function were still unclear.
Decades of research have proven that Rahnella spp. could be applied to preventing and controlling plant disease or promoting plant growth for their beneficial properties. Many Rahnella strains have been described to protect plants from a wide range of phytopathogenic organisms in different ways. R. aquatilis JZ-GX1 secreted volatile organic compounds (VOCs) that destroyed the mycelial growth of Colletotrichum gloeosporioides, and thus restrained the infection and expansion of anthracnose disease in leaves (Kong et al., 2020a). R. aquatilis Ra36 could exploit fungal chemotropism to efficiently colonize the roots of host plants, resulting in efficient protection from vascular wilt disease in tomatoes by interfering with the alkaline-triggered infection of Fusarium oxysporum via gluconic acid secretion (Palmieri et al., 2020). R. aquatilis HX2 was proven to decrease the disease incidence of crown gall disease caused by Agrobacterium vitis in grapevines by producing antibacterial substances (Chen et al., 2009). Besides, R. aquatilis Ra39 exhibited a significant effect on controlling Erwinia amylovora in apple, and the control efficiency was close to the efficacy of streptomycin when the strain was mixed with the Acibenzolar-S-methyl (ASM) (Abo-Elyousr et al., 2010). Application of R. aquatilis Ra to bean plants increased the content of phenolic compounds and the activity of peroxidase (PO) enzyme than untreated plants, and resulted in a marked disease suppression against Xanthomonas axonopodis pv. phaseoli under greenhouse and field conditions (Sallam, 2011). R. aquatilis 17 and 55 exhibited a marked siderophore production and a significant effect on controlling the bacterial spot of cucumber (el-Hendawy et al., 2003). In addition, many Rahnella strains have been demonstrated to benefit plant growth through various mechanisms, such as nitrogen fixation, phytohormone production, phosphate solubilization, biosynthesis of organic acids and pyrroloquinoline quinone. For example, R. aquatilis ZF7 significantly promoted the weight of aboveground parts and the root length in Chinese cabbage due to its high Indole-3-acetic acid (IAA) biosynthetic capacity (Yuan et al., 2020). R. aquatilis HX2 exhibited a significant promotion of the growth of corn, due to its ability to solubilize mineral phosphate, produce pyrroloquinoline quinine and IAA (Guo et al., 2009). R. aquatilis JZ-GX1 exhibited a significant greening effect on Cinnamomum camphora by producing organic acids (Kong et al., 2020b), and was also reported as a phytate-degrading rhizobacteria (PDRB) that could improve the growth of poplar and Masson pine (Li et al., 2013). To date, the majority of studies on plant growth promotion in Rahnella spp. focused on R. aquatilis strains. Given the close relationship between R. aceris and R. aquatilis, the R. aceris strains may possess similar mechanisms of biocontrol and plant growth promotion.
Previous studies have demonstrated that Rahnella strains were widely distributed and adapted to diverse ecological environments, which might be related to their resistance to acids, salts, selenium, antibiotics and heavy metals. For example, R. aquatilis HX2 exhibited a tolerance to high salt, strong acids, antibiotics, and stress tolerance (Li et al., 2019, 2021). In addition, R. aquatilis HX2 could grow in high selenium concentrations and could reduce selenate and selenite to BioSeNPs (Zhu et al., 2018). R. aquatilis JZ-GX1 could stimulate the production of exopolysaccharides, and resulting in high salt tolerance to 0–9% NaCl (Li et al., 2021). R. aquatilis ZF7 harbored 24 genes associated with the biosynthesis and metabolism of β-lactamase, and exhibited tolerance to a variety of antibiotics including ampicillin, carbenicillin and vancomycin (Yuan et al., 2020). Rahnella sp. SMO-1 was reported to encode extended-spectrum β-lactamase which could efficiently hydrolyze penicillins and cefotaxime, and the strain was also showed resistance to amoxicillin, ticarcillin, cefalotin and cefuroxime (Lartigue et al., 2013). In addition, Rahnella sp. JN6 showed a high resistance to Cd, Pb, and Zn and could improve the efficiency of remediation in heavy metal contaminated soils (He et al., 2013). Previous studies have indicated that a range of regulatory mechanisms including secretory system, two-component regulatory system (TCS) and acid-resistance genes played important roles in the environmental adaption of bacteria (Liu and Ochman, 2007). For instance, multiple secretion systems were found in Yersinia enterocolitica and were essential for virulence, the biosynthesis of antibiotics and the life activities (Heermann and Fuchs, 2008). Photorhabdus luminescens harbored 18 TCSs which had been found to be involved in metabolite utilization and adaptation to various stress factors (Heermann and Fuchs, 2008). Nevertheless, relatively few studies have focused on adapting to environmental variations in Rahnella strains. Therefore, to better promote the application prospects of Rahnella spp., studying the environmental adaption of Rahnella strains, especially at the molecular level was very necessary.
In this study, R. aceris ZF458 with broad spectrum of antagonistic activities against many plant pathogens was isolated from swamp soil. Phylogenetic analysis, ANI and DDH analysis were accomplished to definite the taxonomic position of ZF458 and the relationship with R. aceris SAP-19 and other R. aquatilis strains. The whole genome of ZF458 was sequenced, annotated and compared with the genomes of other typical and widely reported Rahnella strains HX2, ZF7, Y9602 and ATCC 33071T. Comparative genome analysis was used to reveal various genes involved in biocontrol factors and environmental adaption. These data would provide an in-depth insight into the mechanisms of plant growth promotion, and improve the bio-control application of R. aceris ZF458 in the future.
Materials and Methods
Strain Isolation, Antagonistic Assays and Biocontrol Assays
Stain ZF458 was isolated from swamp soil in Sichuan, China, in August, 2019, according to a standard 10-fold dilution plating assay as described by previous study (Wang B. et al., 2013). The antagonistic activities of R. aceris ZF458 against plant pathogens were performed by plate tests, and the control effect of ZF458 against Agrobacterium tumefaciens was tested on sunflowers. The growth curve of strain ZF458 was measured according to the following steps: strain ZF458 was incubated overnight at 28°C, and the suspension was diluted to an OD600 = 0.6 with sterile Nutrient Broth (NB) medium. Subsequently, 1 μL of the suspension was added to 999 μL NB culture via vortexing. Then, 200 μL of the diluent was dripped to a 96 well plate. The microtiter plates were kept in a stable shaking speed (180 rpm⋅min–1), and the OD600 of ZF458 culture was measured at 4 h intervals over 40 h. In addition, plate assays were conducted to assess siderophore and phosphatase activities based on reported approaches (Milagres et al., 1999; Luis et al., 2014). If siderophores were produced, the chrome azurol S (CAS) agar would change from blue to orange, while if phosphatases were produced, the Pikovskaya’s (PVK) agar plate would change from white to transparent.
Growth Conditions, Microscopic Analysis and Genomic DNA Extraction
Strain ZF458 was incubated in NB medium at 28°C with shaking for 24 h. The morphology of strain ZF458 was observed by scanning electron microscope (SEM) Hitachi-S3400N and transmission electron microscope (TEM) Hitachi-7700. The genomic DNA of strain ZF458 was extracted from cultured cells (OD600 = 0.8) by TIANamp Bacteria DNA kit (Tiangen Biotech (Beijing) Co., Ltd.).
Genome Sequencing and Annotation
The whole genome of R. aceris ZF458 was sequenced in Allwegene Technologies Corporation, China. The pacific Biosciences (PacBio) RS II platform was used for the whole-genome sequencing of strain ZF458, and a 10-kb SMRT Bell was used for template library construction. The sequence reads were assembled de novo using SMRT Link v.5.1.0.1 CGView was used to generate the circular genome visualization (Stothard and Wishart, 2005). GeneMarkS (version 4.17) software was used to predict the coding genes of the sequenced genome.2 Transfer RNA (tRNA), ribosomal RNA (rRNA) and small nuclear RNAs (snRNAs) were predicted using tRNAscan-SE version 1.3.1 (Lowe and Eddy, 1997), rRNAmmer version 1.2 (Lagesen et al., 2007) and cmsearch version 1.1 (Nawrocki et al., 2009), respectively. Functional gene annotation was carried out through multiple general databases, including Non-Redundant protein databases (NR) (Li et al., 2002), Gene Ontology database (GO) (Ashburner et al., 2000), Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa et al., 2006), Cluster of Orthologous Groups of proteins (COG) (Galperin et al., 2015), Transporter Classification Database (TCDB) (Saier et al., 2009), Pfam,3 Swiss-Prot4 (Amos and Rolf, 2000), Carbohydrate-Active enZTmes Database (CAZy) (Cantarel et al., 2009). Furthermore, signal peptides and transmembrane structure were predicted using SignalP 4.1 and TMHMM 2.0c (Petersen et al., 2011). In addition, prophages were predicted by using phiSpy 2.3 (You et al., 2011), and CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat Sequences) was identified via CRISPRdigger 1.0 (Ibtissem et al., 2007).
Phylogenetic Analysis and Comparative Genomic Analysis
Multilocus gene sequence analysis (MLSA) based on the four housekeeping genes (16S rRNA, gyrB, atpD, and rpoB) was used to determine the taxonomic position of R. aceris ZF458. The housekeeping gene sequences of diverse strains (Supplementary Table 1) were aligned using MUSCLE and the phylogenetic tree was generated using the maximum likelihood method by MEGA 6.0 (Tamura et al., 2013). Average nucleotide identities (ANI) and in silico DNA-DNA hybridization (DDH) analysis were performed using the OrthoANIu algorithm and the Genome-to-Genome Distance Calculator (GGDC), respectively (Meier-Kolthoff et al., 2013). Based on the phylogenetic analysis, four closely related Rahnella strains including R. aquatilis ZF7 (CP032296.1), R. aquatilis HX2 (CP003403.1), Rahnella sp. Y9602 (CP002505.1) and R. aquatilis ATCC 33071T (CP003244.1) were selected for comparative genomic analysis. Moreover, pairwise alignment of genomes was generated using the Mauve 2.3.1 comparison software, and Venn diagrams were conducted using R package (Richter and Rossello-Mora, 2009).
Functional Gene Analysis Linked With Plant Growth Promotion and Environmental Adaption
Functional genes beneficial to plants such as IAA production, nitrogen fixation, phosphate solubilization, organic acid and pyrroloquinoline quinone (PQQ) biosynthesis, were searched for in the NCBI database. In addition, functional genes involved in environmental adaption including bacteria secretion system, flagella biosynthesis and chemotaxis, two-component system, quorum sensing, SeNPs production, acid-resistance were detected using the NCBI database. All the functional genes retrieved from the ZF458 genome were compared at the amino acid level with four closely related Rahnella strains ZF7, HX2, Y9602 and ATCC 33071T by KEGG database.
Results
Antagonistic Activity and Biological Characteristics of Strain ZF458
Biocontrol Traits of Strain ZF458
Strain ZF458 which was isolated from swamp soil exhibited broad, obvious antagonistic activities against diverse plant-pathogenic fungi and bacteria, including Stemphylium solani, Corynespora cassiicola, Ascochyta citrulline, Colletotrichum sp., Phytophthora capsici, Fusarium oxysporum, Pectobacterium brasiliense, Pseudomonas amygdali pv. lachrymans, Pseudomonas syringae pv. tomato, Ralstonia solanacearum, Xanthomonas campestris pv. campestris, Clavibacter michiganensis subsp. michiganensis, Agrobacterium tumefaciens, and Acidovorax citrulli (Figure 1). Importantly, strain ZF458 presented a dramatic effect in controlling Agrobacterium tumefaciens on sunflowers (Supplementary Figure 1), with a control efficiency of 80.20%. Moreover, orange halos were appeared around colonies of strain ZF458 on CAS agar plates, revealing that strain ZF458 produced siderophores (Supplementary Figure 2A). Similarly, transparent halos were observed around ZF458 colonies on PVK agar plates, indicating that strain ZF458 produced phosphatases (Supplementary Figure 2B).
FIGURE 1.
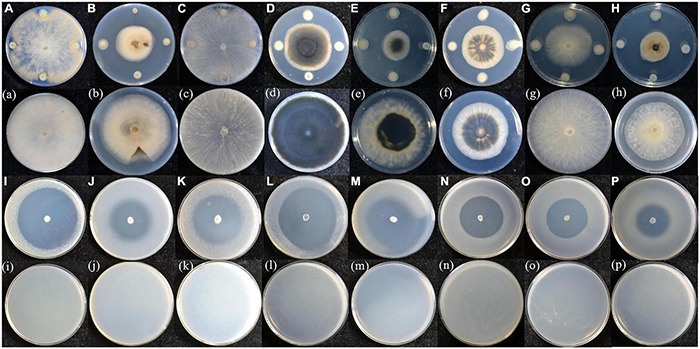
Antagonistic assays of R. aceris ZF458 against various plant fungal and bacterial pathogens. (A,a) Botrytis cinerea, (B,b) Stemphylium solani, (C,c) Rhizoctonia solani, (D,d) Corynespora cassiicola, (E,e) Ascochyta citrulline, (F,f) Colletotrichum sp., (G,g) Fusarium oxysporum, (H,h) Phytophthora capsici, (I,i) Pectobacterium brasiliense, (J,j) Pseudomonas amygdali pv. lachrymans, (K,k) Pseudomonas syringae pv. tomato, (L,l) Ralstonia solanacearum, (M,m) Xanthomonas campestris pv. campestris, (N,n) Clavibacter michiganensis subsp. michiganensis, (O,o) Agrobacterium tumefaciens, (P,p) Acidovorax citrulli. The capital letters represent plate containing strain ZF458, the small letters represent control.
Organism Information
Strain ZF458 was determined to be a Gram-negative, facultatively anaerobic bacterium belonging to the Yersiniaceae family. The strain produced white to light yellow-colored, circular, translucent, convex with margin colonies and reached 0.1–5.0 mm in a diameter on 1-day culture on NA plate at 28°C (Supplementary Figure 3A). Strain ZF458 displayed shot rod-shaped cells with lengths of 1.5–3.0 μm and diameters of 0.7–1.3 μm (Supplementary Figures 3B,C). The growth curve showed that strain ZF458 was in exponential growth phase between 4–20 h after inoculation, and attained the stationary phase at 20 h of incubation (Supplementary Figure 4A). Furthermore, the pH of strain ZF458 culture declined rapidly from approximately 7.0 to 3.2 within 12 h of incubation, and remained stable after 12 h (Supplementary Figure 4B).
Genome Structure and Genome Comparison Between R. aceris ZF458 and Other Completely Sequenced Rahnella Strains
General Genomic Features of R. aceris ZF458
The whole genome of R. aceris ZF458 was 5.60 Mb with an overall G + C content of 52.20% (Supplementary Table 2). The entire genome comprised a circular 4,861,340-bp chromosome (CP067057.1) and two plasmids with sizes of 188,256-bp (CP067058.1) and 553,387-bp (CP067059.1). The general genome structure and functions of strain ZF458 was represented by graphical circular genome map (Figure 2). In total 5,248 predicted genes were identified in the genome, including 4,988 protein coding genes, 109 RNA genes and 88 pseudogenes. According to KEGG database, a total of 4,954 genes of ZF458 were annotated to 40 different pathways which were associated with metabolism, cellular processes, environmental information processing, genetic information processing and organismal systems. By comparing the Swiss-Prot, Pfam, TCDB, SinalIP and CAZy databases, 3,316 (63.19%), 3,533 (67.32%), 939 (17.89%), 428 (8.16%), and 210 (4.00%) of the ORFs were annotated into different groups, respectively. Besides, the ZF458 genome contained 342 secreted proteins, 10 genomic islands and 7 prophage regions (Supplementary Table 2).
FIGURE 2.
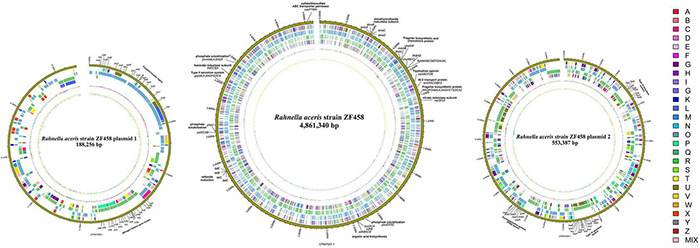
Graphical circular maps of the R. aceris ZF458 chromosome and plasmids pPlas1 and pPlas2 genome generated using the CGview server. From outside to center, ring 1 showed the genome sequence position, rings 2 and 5 showed protein-coding genes oriented in the forward (colored by COG categories) and reverse (colored by COG categories) directions, respectively. Rings 3 and 4 showed coding genes in the forward (blue) and reverse (green) directions, respectively. Ring 6 showed the G + C% content and the inner most ring showed the GC skews, where blue indicated positive values and yellow indicated negative values.
Moreover, a total of 4,237 functional genes of ZF458 were further annotated using COG database. Most of the genes were related to amino acid transport and metabolism (10.57%), followed by carbohydrate transport and metabolism (9.86%), transcription (8.68%), inorganic ion transport and metabolism (6.66%), cell wall/membrane biogenesis (6.21%) and translation, ribosomal structure and biogenesis (5.17%) (Table 1). However, 83 genes were not annotated by COG database, whose specific functions need to be further verified.
TABLE 1.
Number of genes associated with general COG functional categories.
| Code | Value | % age | Description |
| J | 258 | 5.17 | Translation, ribosomal structure and biogenesis |
| A | 1 | 0.02 | RNA processing and modification |
| K | 433 | 8.68 | Transcription |
| L | 134 | 2.69 | Replication, recombination and repair |
| D | 47 | 0.94 | Cell cycle control, Cell division, chromosome partitioning |
| V | 125 | 2.51 | Defense mechanisms |
| T | 243 | 4.87 | Signal transduction mechanisms |
| M | 310 | 6.21 | Cell wall/membrane biogenesis |
| N | 115 | 2.31 | Cell motility |
| W | 43 | 0.86 | Extracellular structures |
| U | 117 | 2.35 | Intracellular trafficking and secretion |
| O | 170 | 3.41 | Posttranslational modification, protein turnover, chaperones |
| C | 235 | 4.71 | Energy production and conversion |
| G | 492 | 9.86 | Carbohydrate transport and metabolism |
| E | 527 | 10.57 | Amino acid transport and metabolism |
| F | 110 | 2.21 | Nucleotide transport and metabolism |
| H | 208 | 4.17 | Coenzyme transport and metabolism |
| I | 162 | 3.25 | Lipid transport and metabolism |
| P | 332 | 6.66 | Inorganic ion transport and metabolism |
| Q | 116 | 2.33 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 395 | 7.92 | General function prediction only |
| S | 232 | 4.65 | Function unknown |
| X | 100 | 2.00 | Mobilome; prophages, transposons |
| − | 83 | 1.66 | Not in COGs |
The total % age is based on the total number of protein coding genes in the annotated genome.
Phylogenetic Tree
To understand the genetic relationships of R. aceris ZF458 with other related strains especially the Rahnella strains, a phylogenetic tree was established based on 16S rRNA and three other housekeeping genes (gyrB, atpD and rpoB). As expected, seventeen Rahnella strains were clustered into one major clade, and three Rouxiella strains, two Yersinia strains, two Serratia strains were in other clades (Supplementary Figure 5). The phylogenetic analysis indicated that 17 Rahnella strains including ZF458 were in seven Rahnella MLSA groups. Based on the observed genetic distance relationships, strain ZF458 was closely clustered together with R. aceris SAP-19, R. aquatilis ZF7, R. aquatilis HX2, Rahnella sp. Y9602 and the type strain R. aquatilis ATCC 33071T, successively followed by R. victoriana strains, R. variigena strains, R. bruchi strains, R. woolbedingensis strains, and R. inusitata strains.
Average Nucleotide Identities and DNA-DNA Hybridization Analysis
Average nucleotide identities and DDH were widely used to certify the genetic distance of bacteria at the genomic level, and strains revealing ANI values ≥96% and DDH values ≥70% were typically considered as the same species (Zhang et al., 2016). In the study, ANI and DDH calculations among Rahnella strains were performed. The results showed that the ANI values between ZF458 and SAP-19, ZF7, HX2, Y9602, KM05, KM12, MEM40 were both >98%, and the DDH values among them were both >80% (Supplementary Figure 6). These findings demonstrated that the eight R. aceris strains or R. aquatilis strains (ZF458, SAP-19, ZF7, HX2, Y9602, KM05, KM12, and MEM40) were closely clustered with one another and occupied a close genetic distance relationship. However, the ANI and DDH values between strains ZF458 and ATCC 33071T were 92.75 and 49.4% respectively, although the two strains were clustered closely in the phylogenetic tree (Supplementary Figure 5). Smaller ANI and DDH values were calculated when other Rahnella strains (except the R. aceris and R. aquatilis species) were used as reference genomes.
Genomic Features of Different Rahnella Strains
In the study, four Rahnella strains that have completed whole genome sequencing including R. aquatilis ZF7, R. aquatilis HX2, Rahnella sp. Y9602, and R. aquatilis ATCC 33071T were selected for comparative genomic analysis (Table 2). In comparison, the entire genome size of the five Rahnella strains ranged from 5.45 to 5.66 Mb, the G + C content ranged from 52.08 to 52.36%, and the predicted coding genes ranged from 4,804 to 5,065. The results illustrated that the genome size of strain ZF458 was larger than that of ZF7 and ATCC 33071T, but smaller than that of HX2. Furthermore, the genomes of strains ZF458 and Y9602 contained one circular chromosome and two plasmids, while HX2 and ATCC 33071T contained one circular chromosome and three plasmids, and the sequenced strain ZF7 harbored a circular chromosome and only one plasmid (Table 2).
TABLE 2.
Genomic features of Rahnella aceris ZF458 and other Rahnella spp.
| Features | Rahnella aceris ZF458 | Rahnella aquatilis ZF7 | Rahnella aquatilis HX2 | Rahnella sp. Y9602 | Rahnella aquatilis ATCC 33071 |
| Size (Mb) | 5.60 | 5.54 | 5,66 | 5.61 | 5.45 |
| G + C content (%) | 52.20 | 52.36 | 52.15 | 52.18 | 52.08 |
| Replicons | One chromosome Two plasmids |
One chromosome One plasmid |
One chromosome Three plasmids |
One chromosome Two plasmids |
One chromosome Three plasmids |
| Total genes | 5,185 | 5,115 | 5,208 | 5,217 | 4,989 |
| Predicted no. of CDS | 4,988 | 4,936 | 4,991 | 5,065 | 4,804 |
| Ribosomal RNA | 22 | 22 | 22 | 22 | 22 |
| Transfer RNA | 78 | 77 | 76 | 76 | 76 |
| Other RNA | 9 | 10 | 8 | 8 | 10 |
| Pseudogene | 88 | 60 | 111 | 46 | 77 |
| GenBank sequence | CP067057.1 | CP067057.1 | CP003403.1 | CP002505.1 | CP003244.1 |
Comparison of R. aceris ZF458 With the Four Typical Rahnella Strains
In addition, the entire genome of ZF458, SAP-19, ZF7, HX2, Y9602 and ATCC 33071T were compared using Mauve to assess the homology among these Rahnella strains. The results showed that more local collinear blocks (LCB) inversion was obviously observed between strains ZF458 and ATCC 33071T than that among strains ZF458, ZF7, HX2 and Y9602 (Figure 3A). This indicated that the ZF458 genome was highly syntenic with R. aquatilis ZF7, R. aquatilis HX2 and Rahnella sp. Y9602. At the species level, less local collinear blocks inversion was found between strains ZF458 and SAP-19, but compared with the ZF458 genome, large local collinear blocks (LCB) translocation occurred in the genome of SAP-19 (Figure 3B).
FIGURE 3.
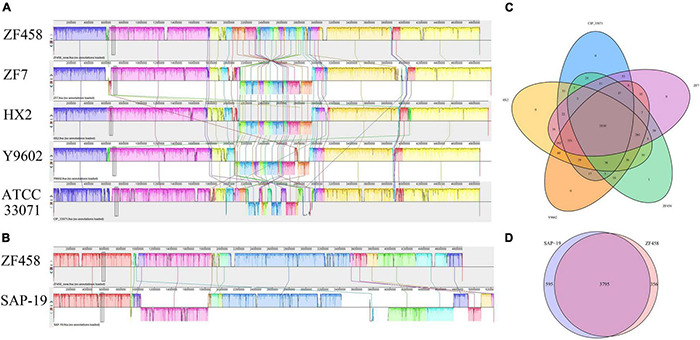
Global alignment of the genome sequence of completely sequenced R. aceris ZF458 against five other Rahnella genome sequences. (A) Mauve progressive alignment of the ZF458, ZF7, HX2, Y9602, and ATCC 33071 genomes. (B) Mauve progressive alignment of the ZF458 and SAP-19 genomes. ZF458 genome was used as the reference genome. Boxes with the same color indicate syntenic regions. Boxes below the horizontal strain line indicate inverted regions. Rearrangements are indicated with the colored lines. The scale is in nucleotides. (C) Venn diagram showing the numbers of shared and unique Clusters of Orthologous Genes among ZF458, ZF7, HX2, Y9602, and ATCC 33071 genomes. (D) Venn diagram showing the numbers of shared and unique Clusters of Orthologous Genes between the ZF458 and SAP-19 genomes.
As shown in Figure 3C, 3,530 conserved genes were shared by strains ZF458, ZF7, HX2, Y9602 and ATCC 33071T. ZF458 shared 3,893 genes with ZF7, 3,884 genes with HX2, and 3,928 genes with counterparts in the Y9602 genome, while only 3,647 orthologous genes existed in strains ZF458 and ATCC 33071. Interestingly, no unique genes were found in the genome of R. aquatilis strains ZF7, HX2, Y9602 and ATCC 33071T, while one unique gene was found in ZF458 genome (Figure 3C). In addition, 3,795 orthologous genes were found between the ZF458 and SAP-19 genomes, and 356 unique genes existed in the ZF458 genome were not retrieved in the genome of SAP-19 (Figure 3D).
Synergistic Interactions With the Host Plant: Benefiting Plants
Nitrogen Fixation and Metabolism
To compare the key genes associated with the plant growth promotion in R. aceris ZF458, four strains including ZF7, HX2, Y9602 and ATCC 33071T were selected. The results showed that a relatively whole nif gene cluster with 21 genes related to nitrogen fixation was retrieved in the genomes of strains ZF458, HX2 and ATCC 33071T (Table 3). All the genes were assigned to Mo nitrogenase structural genes, FeMo-co synthesis, maturation of nitrogenase, electron transport, regulation of nif gene expression and three unknown functional genes, and shared high similarities among the three strains. However, only one gene nifJ encoding pyruvate ferredoxin/flavodoxin oxidoreductase was present in the ZF7 genome, and indeed, the nif gene cluster which existed in strains ZF458, HX2 and ATCC 33071T was found in the plasmid genome, except the gene nifJ, which was retrieved from the chromosome genome. In addition, 7 highly homologous nitrogen regulation related genes (glnABDGO, ntrB, and nac) were found in the genomes of R. aceris ZF458 and four other Rahnella strains (Table 3).
TABLE 3.
Homolog analysis of nitrogen fixing gene cluster in R. aceris ZF458 and other Rahnella strains.
| Strain |
Rahnella aceris ZF458 |
R. aquatilis ZF7 |
R. aquatilis HX2 |
Rahnella sp. Y9602 |
R. aquatilis ATCC 33071 |
||||||
| Genes | Product definition | Locus Tag | Protein ID | Protein ID | Homology (%) | Protein ID | Homology (%) | Protein ID | Homology (%) | Protein ID | Homology (%) |
| Mo nitrogenase structural genes | |||||||||||
| nifH | Nitrogenase iron protein NifH | JHW33_RS22855 | WP_014333921.1 | NA | NA | WP_014333921.1 | 100 | NA | NA | WP_014333921.1 | 100 |
| nifD | Nitrogenase molybdenum-iron protein alpha chain | JHW33_RS22850 | WP_200227561.1 | NA | NA | WP_014683022.1 | 99 | NA | NA | WP_014333920.1 | 99 |
| nifK | nitrogenase molybdenum-iron protein subunit beta | JHW33_RS22845 | WP_014683023.1 | NA | NA | WP_014683023.1 | 100 | NA | NA | WP_014333919.1 | 99 |
| FeMo-co Synthesis | |||||||||||
| nifY | Nitrogen fixation protein NifY | JHW33_RS22835 | WP_014683024.1 | NA | NA | WP_014683024.1 | 100 | NA | NA | WP_014333917.1 | 97 |
| nifE | Nitrogenase molybdenum-cofactor synthesis protein NifE | JHW33_RS22830 | WP_014683025.1 | NA | NA | WP_014683025.1 | 100 | NA | NA | WP_014333916.1 | 99 |
| nifN | Nitrogenase molybdenum-cofactor biosynthesis protein NifN | JHW33_RS22825 | WP_014683026.1 | NA | NA | WP_014683026.1 | 100 | NA | NA | WP_014333915.1 | 99 |
| nifX | nitrogen fixation protein NifX | JHW33_RS22820 | WP_014683027.1 | NA | NA | WP_014683027.1 | 100 | NA | NA | WP_014333914.1 | 98 |
| nifU | Fe-S cluster assembly protein NifU | JHW33_RS22810 | WP_014683029.1 | NA | NA | WP_014683029.1 | 100 | NA | NA | WP_014333912.1 | 97 |
| nifS | Cysteine desulfurase | JHW33_RS22805 | WP_200227559.1 | NA | NA | WP_014683030.1 | 99 | NA | NA | WP_014333911.1 | 99 |
| nifV | Homocitrate synthase NifV | JHW33_RS22800 | WP_014683031.1 | NA | NA | WP_014683031.1 | 100 | NA | NA | WP_014333910.1 | 98 |
| nifQ | Nitrogen fixation protein NifQ | JHW33_RS22765 | WP_014683038.1 | NA | NA | WP_014683038.1 | 100 | NA | NA | WP_014333903.1 | 98 |
| nifB | FeMo cofactor biosynthesis protein NifB | JHW33_RS22770 | WP_200227556.1 | NA | NA | WP_014683037.1 | 99 | NA | NA | WP_014333904.1 | 98 |
| Maturation of Nitrogenase | |||||||||||
| nifM | Nitrogen fixation protein NifM | JHW33_RS22785 | WP_014683034.1 | NA | NA | WP_014683034.1 | 100 | NA | NA | WP_014333907.1 | 97 |
| nifZ | Nitrogen fixation protein NifZ | JHW33_RS22790 | WP_014683033.1 | NA | NA | WP_014683033.1 | 100 | NA | NA | WP_014333908.1 | 97 |
| Electron Transport | |||||||||||
| nifF | Flavodoxin I | JHW33_RS22760 | WP_014683039.1 | NA | NA | WP_014683039.1 | 100 | NA | NA | WP_014333902.1 | 96 |
| nifJ | Pyruvate ferredoxin/flavodoxin oxidoreductase | JHW33_RS06430 | WP_200225589.1 | WP_119261595.1 | 99 | WP_013575392.1 | 99 | WP_013575392.1 | 99 | WP_015697263.1 | 98 |
| Regulation of nif Gene Expression | |||||||||||
| nifL | Nitrogen fixation regulatory protein NifL | JHW33_RS22780 | WP_200227570.1 | NA | NA | WP_014683035.1 | 99 | NA | NA | WP_014333906.1 | 98 |
| nifA | nif-specific transcriptional activator NifA | JHW33_RS22775 | WP_014683036.1 | NA | NA | WP_014683036.1 | 100 | NA | NA | WP_014333905.1 | 99 |
| Unknown Function | |||||||||||
| nifT | Nitrogen fixation protein NifT | JHW33_RS22840 | WP_014333918.1 | NA | NA | WP_014333918.1 | 100 | NA | NA | WP_014333918.1 | 100 |
| nifW | Nif-specific regulatory protein NifW | JHW33_RS22795 | WP_014683032.1 | NA | NA | WP_014683032.1 | 100 | NA | NA | WP_014333909.1 | 97 |
| nifI | Hypothetical protein | JHW33_RS22815 | WP_014683028.1 | NA | NA | WP_014683028.1 | 100 | NA | NA | WP_014333913.1 | 97 |
| Nitrogen Regulation Related Genes | |||||||||||
| glnQ | Glutamine ABC transporter ATP-binding protein | JHW33_RS01275 | WP_013574681.1 | WP_013574681.1 | 100 | WP_013574681.1 | 100 | WP_013574681.1 | 100 | WP_015696540.1 | 99 |
| ntrB | Nitrogen regulation protein NR(II) | JHW33_RS04965 | WP_156106968.1 | WP_112152400.1 | 99 | WP_013577653.1 | 99 | WP_013575697.1 | 99 | WP_015697258.1 | 96 |
| nac | Nitrogen assimilation transcriptional regulator | JHW33_RS02570 | WP_013574993.1 | WP_119261788.1 | 100 | WP_015689657.1 | 99 | WP_013574993.1 | 99 | WP_037038858.1 | 95 |
| glnB | nitrogen regulatory protein P-II | JHW33_RS21825 | WP_013574351.1 | WP_013574351.1 | 100 | WP_013573506.1 | 100 | WP_013574351.1 | 100 | WP_013574351.1 | 99 |
| glnD | PII uridylyl-transferase | JHW33_RS20990 | WP_013574191.1 | WP_013574191.1 | 100 | WP_013574191.1 | 100 | WP_013574191.1 | 100 | WP_015696089.1 | 99 |
| glnA | Glutamine synthetase | JHW33_RS16330 | WP_013577652.1 | WP_013577652.1 | 100 | WP_015690611.1 | 100 | WP_013577652.1 | 99 | WP_013577652.1 | 98 |
| glnG | Nitrogen regulation protein NR(I) | JHW33_RS16340 | WP_013577654.1 | WP_013577654.1 | 100 | WP_013577654.1 | 100 | WP_013577654.1 | 100 | WP_015699252.1 | 99 |
ND, not determined; NA, not available.
Production of the Plant Growth Hormone Indole-3-Acetic Acid
The comparative genome analysis indicated that eleven key genes responsible for IAA production were found in the ZF458 genome, which contained eight genes related to tryptophan operon (trpG, trpE, trpD, trpCF, trpB, trpA, trpS, and trpR), mtr gene encoding tryptophan permease, ipdC gene encoding indolepyruvate decarboxylase, and acdS gene encoding 1-aminocyclopropane-1-carboxylate (ACC) deaminase. Moreover, all of these genes were found in the genomes of ZF458, ZF7, HX2, Y9602 and ATCC 33071T with sequence identities exceeding 93% at the amino acid level (Supplementary Table 3).
Phosphate Solubilization
According to the comparative genomic analysis, 26 genes involved in phosphate solubilization were found in the ZF458 genome. Among these genes, six genes (aphA, phoN, iap, phoA, phoB, and phoR) were related to organic phosphate acquisition, and twenty genes were associated with carbon-phosphorus lyase, which contained the whole carbon-phosphorus lyase operon (phnNMLKJIHGFAEDC) and phosphate transporter (pstSCAB) (Supplementary Table 4). All these genes retrieved from the ZF458 genome shared high similarities (greater than 94%) with those of four other Rahnella strains ZF7, HX2, Y9602 and ATCC 33071, except the gene aphA, which was absent in the ATCC 33071 genome (Supplementary Table 4).
Biosynthesis of Organic Acid
Furthermore, 44 genes linked with organic acid biosynthesis were found in the genome of strains ZF458, ZF7, HX2, and Y9602, while three genes eda, idn, and ldh were absent in the genome of ATCC 33071 (Table 4). The 44 genes were assigned for diverse pathways, including 3 genes for Glycolytic pathway (EMP), 24 genes for Tricarboxylic Acid Cycle pathway (TCA), 4 genes for Entner-Doudoroff pathway (ED), 3 genes for direct oxidation pathway, and 10 genes for another undefined pathway. The identities of these genes between ZF458 and ZF7, HX2, Y9602 were higher than those between ZF458 and ATCC 33071T strains, especially for the gene gntK, which only showed 52% sequence identities between ZF458 and ATCC 33071T (Table 4). The results demonstrated that these strains may play important roles in inorganic phosphorus dissolution.
TABLE 4.
Homolog analysis of organic acid biosynthesis genes in R. aceris ZF458 and other Rahnella strains.
| Strain |
Rahnella aceris ZF458 |
R. aquatilis ZF7 |
R. aquatilis HX2 |
Rahnella sp. Y9602 |
R. aquatilis ATCC 33071 |
||||||
| Genes | Product definition | Locus Tag | Protein ID | Protein ID | Homology (%) | Protein ID | Homology (%) | Protein ID | Homology (%) | Protein ID | Homology (%) |
| EMP Pathway | |||||||||||
| glk | Glucokinase | JHW33_RS00015 | WP_200224559.1 | WP_013574458.1 | 100 | WP_013574458.1 | 99 | WP_013574458.1 | 99 | WP_015696339.1 | 97 |
| zwf | Glucose-6-phosphate dehydrogenase | JHW33_RS06770 | WP_200225846.1 | WP_013575315.1 | 100 | WP_013575315.1 | 99 | WP_013575315.1 | 99 | WP_015697179.1 | 99 |
| ppc | Phosphoenolpyruvate carboxylase | JHW33_RS15915 | WP_013577573.1 | WP_013577573.1 | 100 | WP_013577573.1 | 100 | WP_013577573.1 | 100 | WP_015699176.1 | 99 |
| TCA Pathway | |||||||||||
| gltA | Citrate synthase | JHW33_RS09960 | WP_013576444.1 | WP_013576444.1 | 100 | WP_013576444.1 | 100 | WP_013576444.1 | 100 | WP_015698117.1 | 99 |
| acnB | Aconitate hydratase 2 | JHW33_RS12660 | WP_013576973.1 | WP_013576973.1 | 100 | WP_013576973.1 | 100 | WP_013576973.1 | 100 | WP_015698635.1 | 99 |
| acnA | Aconitate hydratase | JHW33_RS04170 | WP_037034468.1 | WP_013575910.1 | 100 | WP_015690067.1 | 99 | WP_013575910.1 | 99 | WP_015697115.1 | 98 |
| icd | NADP-dependent Isocitrate dehydrogenase | JHW33_RS08805 | WP_013576210.1 | WP_013576210.1 | 100 | WP_013576210.1 | 100 | WP_013576210.1 | 100 | WP_015697904.1 | 99 |
| aceK | bifunctional isocitrate dehydrogenase | JHW33_RS14895 | WP_200223135.1 | WP_112197688.1 | 99 | WP_015690563.1 | 99 | WP_013577389.1 | 98 | WP_015699020.1 | 95 |
| icl | isocitrate lyase/phosphoenolpyruvate mutase family protein | JHW33_RS17355 | WP_037033767.1 | WP_119261086.1 | 99 | WP_013573492.1 | 99 | WP_013573492.1 | 99 | WP_014333435.1 | 98 |
| sucA | 2-ketoglutarate dehydrogenase E1 component | JHW33_RS09935 | WP_013576439.1 | WP_119261848.1 | 100 | WP_013576439.1 | 100 | WP_013576439.1 | 100 | WP_015698113.1 | 99 |
| odhB | 2-oxoglutarate dehydrogenase complex | JHW33_RS09930 | WP_013576438.1 | WP_013576438.1 | 100 | WP_013576438.1 | 100 | WP_013576438.1 | 100 | WP_015698112.1 | 99 |
| sucC | Succinyl-CoA synthetase beta subunit | JHW33_RS09925 | WP_200227035.1 | WP_013576437.1 | 99 | WP_013576437.1 | 99 | WP_013576437.1 | 99 | WP_013576437.1 | 99 |
| sucD | Succinyl-CoA synthetase alpha subunit | JHW33_RS09920 | WP_013576436.1 | WP_013576436.1 | 100 | WP_013576436.1 | 100 | WP_013576436.1 | 100 | WP_013576436.1 | 100 |
| sdhA | Succinate dehydrogenase flavoprotein subunit | JHW33_RS09945 | WP_013576441.1 | WP_013576441.1 | 100 | WP_013576441.1 | 100 | WP_013576441.1 | 100 | WP_015698115.1 | 99 |
| sdhB | succinate dehydrogenase iron-sulfur subunit | JHW33_RS09940 | WP_013576440.1 | WP_013576440.1 | 100 | WP_013576440.1 | 100 | WP_013576440.1 | 100 | WP_015698114.1 | 99 |
| sdhC | Succinate dehydrogenase cytochrome b556 large membrane subunit | JHW33_RS09955 | WP_013576443.1 | WP_013576443.1 | 100 | WP_013576443.1 | 100 | WP_013576443.1 | 100 | WP_015698116.1 | 99 |
| sdhD | succinate dehydrogenase membrane anchor subunit | JHW33_RS09950 | WP_013576442.1 | WP_013576442.1 | 100 | WP_013576442.1 | 100 | WP_013576442.1 | 100 | WP_013576442.1 | 100 |
| fumA/B | Fumarate hydratase, class I | JHW33_RS24430 | WP_013578285.1 | WP_013578285.1 | 100 | WP_013578285.1 | 100 | WP_013578285.1 | 100 | WP_014341906.1 | 99 |
| fumC | Fumarate hydratase, class II | JHW33_RS05805 | WP_200225335.1 | WP_112152260.1 | 99 | WP_013575516.1 | 99 | WP_013575516.1 | 99 | WP_015697345.1 | 99 |
| frdA | Fumarate reductase flavoprotein subunit | JHW33_RS18705 | WP_014411553.1 | WP_119261153.1 | 100 | WP_014411553.1 | 100 | WP_013573749.1 | 100 | WP_014333648.1 | 99 |
| frdB | succinate dehydrogenase/fumarate reductase iron-sulfur subunit | JHW33_RS18700 | WP_013573748.1 | WP_119261152.1 | 100 | WP_013573748.1 | 100 | WP_013573748.1 | 100 | WP_014333647.1 | 97 |
| frdC | Fumarate reductase subunit C | JHW33_RS18695 | WP_200223783.1 | WP_119261151.1 | 99 | WP_013573747.1 | 99 | WP_013573747.1 | 99 | WP_014333646.1 | 97 |
| frdD | Fumarate reductase subunit D | JHW33_RS18690 | WP_014333645.1 | WP_013573746.1 | 100 | WP_013573746.1 | 100 | WP_013573746.1 | 99 | WP_014333645.1 | 99 |
| mdh | Malate dehydrogenase | JHW33_RS18970 | WP_013573800.1 | WP_037034246.1 | 100 | WP_013573800.1 | 100 | WP_013573800.1 | 100 | WP_014333686.1 | 97 |
| maeB | NADP-dependent oxaloacetate-decarboxylating malate dehydrogenase | JHW33_RS22145 | WP_013574414.1 | WP_013574414.1 | 100 | WP_013574414.1 | 100 | WP_013574414.1 | 100 | WP_015696301.1 | 99 |
| aceA | Isocitrate lyase | JHW33_RS14900 | WP_013577390.1 | WP_013577390.1 | 100 | WP_013577390.1 | 100 | WP_013577390.1 | 100 | WP_015699021.1 | 98 |
| aceB/glcB | Malate synthase | JHW33_RS14905 | WP_200223138.1 | WP_119262163.1 | 99 | WP_013577391.1 | 99 | WP_013577391.1 | 99 | WP_015699022.1 | 96 |
| ED Pathway | |||||||||||
| gntk | gluconokinase | JHW33_RS05130 | WP_200225227.1 | WP_015689980.1 | 99 | WP_015689980.1 | 99 | WP_015689980.1 | 98 | WP_013573544.1 | 52 |
| edd | phosphogluconate dehydratase | JHW33_RS05140 | WP_200225228.1 | WP_119261688.1 | 99 | WP_015689978.1 | 99 | WP_013575660.1 | 99 | WP_015697147.1 | 95 |
| eda | bifunctional 4-hydroxy-2-oxoglutarate aldolase/2-dehydro-3-deoxy-phosphogluconate aldolase | JHW33_RS05145 | WP_037035690.1 | WP_013575316.1 | 99 | WP_013575316.1 | 99 | WP_013575316.1 | 99 | WP_015697180.1 | 91 |
| eda | bifunctional 4-hydroxy-2-oxoglutarate aldolase/2-dehydro-3-deoxy-phosphogluconate aldolase | JHW33_RS06765 | WP_013575316.1 | WP_112198389.1 | 100 | WP_013575659.1 | 100 | WP_013575659.1 | 100 | NA | NA |
| Direct Oxidation Pathway | |||||||||||
| gcd | glucose/quinate/shikimate family membrane-bound PQQ-dependent dehydrogenase | JHW33_RS25310 | WP_013578038.1 | WP_112197657.1 | 100 | WP_013578038.1 | 100 | WP_013578038.1 | 100 | WP_014341759.1 | 97 |
| idn | gluconate 5-dehydrogenase | JHW33_RS05495 | WP_200225254.1 | WP_119261658.1 | 87 | WP_013575578.1 | 87 | WP_013575578.1 | 87 | NA | NA |
| idnO | Gluconate-5-dehydrogenase | JHW33_RS01075 | WP_037036159.1 | WP_112151190.1 | 99 | WP_013574641.1 | 99 | WP_013574641.1 | 99 | WP_015696505.1 | 99 |
| Other Pathway | |||||||||||
| ldh | L-lactate dehydrogenase | JHW33_RS10455 | WP_200227073.1 | WP_112151693.1 | 99 | WP_013576533.1 | 99 | WP_013576533.1 | 99 | NA | NA |
| aceE | pyruvate dehydrogenase (acetyl-transferring), homodimeric type | JHW33_RS12730 | WP_013576985.1 | WP_013576985.1 | 100 | WP_013576985.1 | 100 | WP_013576985.1 | 100 | WP_015698648.1 | 99 |
| aceF | Pyruvate dehydrogenase E2 component | JHW33_RS12725 | WP_200222913.1 | WP_015690459.1 | 99 | WP_015690459.1 | 99 | WP_013576984.1 | 99 | WP_015698647.1 | 99 |
| pta | Phosphate acetyltransferase | JHW33_RS00765 | WP_153374992.1 | WP_153374992.1 | 100 | WP_153374992.1 | 100 | WP_153374992.1 | 100 | WP_193785502.1 | 99 |
| ackA | Acetate kinase A | JHW33_RS00770 | WP_013574580.1 | WP_013574580.1 | 100 | WP_015689524.1 | 100 | WP_013574580.1 | 99 | WP_015696452.1 | 99 |
| poxB | Pyruvate dehydrogenase | JHW33_RS01625 | WP_200224987.1 | WP_112198008.1 | 99 | WP_013574762.1 | 99 | WP_013574762.1 | 99 | WP_015696595.1 | 99 |
| pflA | Pyruvate formate lyase activating enzyme I | JHW33_RS01755 | WP_015689580.1 | WP_015689580.1 | 100 | WP_015689580.1 | 100 | WP_015689580.1 | 100 | WP_015689580.1 | 100 |
| pflC | formate-C-acetyltransferase-activating enzyme | JHW33_RS15250 | WP_200223220.1 | WP_037035395.1 | 99 | WP_015690573.1 | 99 | WP_013577447.1 | 99 | WP_015699070.1 | 89 |
| pflB | formate C-acetyltransferase | JHW33_RS01760 | WP_013574828.1 | WP_013574828.1 | 100 | WP_013574828.1 | 100 | WP_013574828.1 | 100 | WP_013574828.1 | 100 |
| pflE | glycyl-radical enzyme activating protein | JHW33_RS04230 | WP_200225174.1 | WP_015690059.1 | 99 | WP_015690059.1 | 99 | WP_013575898.1 | 99 | WP_015697125.1 | 94 |
ND, not determined; NA, not available.
Production of Plant Promotion Hormone Pyrroloquinoline Quinone
According to the comparative analysis, the R. aceris ZF458 plasmid genome contained a highly conserved pyrroloquinoline quinone gene cluster (pqqABCDEF) and upstream promoter orfX, covering 6,631 bp with 7 ORFs (Supplementary Figure 7), and the gcd gene encoding PQQ-dependent dehydrogenase. The pqq gene cluster of ZF458 shared a high homology with those in other four Rahnella strains (>95% identity), except that the sequence identity of pqqF between ZF458 and ATCC 33071T was only 80% (Supplementary Table 5).
Survival and Adapting to Environmental Variations
Bacterial Secretion System
Type II Secretion System
In order to explore the different secretion systems, five released complete genome sequences of Rahnella strains namely ZF458, ZF7, HX2, Y9602, ATCC 33071T were compared. The genome of ZF458 contained a variety of secretion systems, which were closely associated with the life activities of bacteria. The comparative analysis showed that the R. aceris ZF458 chromosome contained a highly conserved T2SS (type II secretion system) gene cluster (gspACDEFGHIJKLM and pilD) (Figure 4D), covering 12,797 bp with 13 ORFs. All 13 genes existed in strains ZF458, HX2, Y9602 and ATCC 33071T, while only four genes (gspE, gspL, gspM, and pilD) were found in ZF7 genome. The gsp gene cluster from the ZF458 genome shared a high consistency with strains HX2 and Y9602 (exceeding 97%) at the amino acid level, whereas lower sequence identities for genes gspA, gspC, gspI, gspJ, gspL, and gspM (under 80%) were found between ZF458 and ATCC 33071T (Supplementary Table 6).
FIGURE 4.
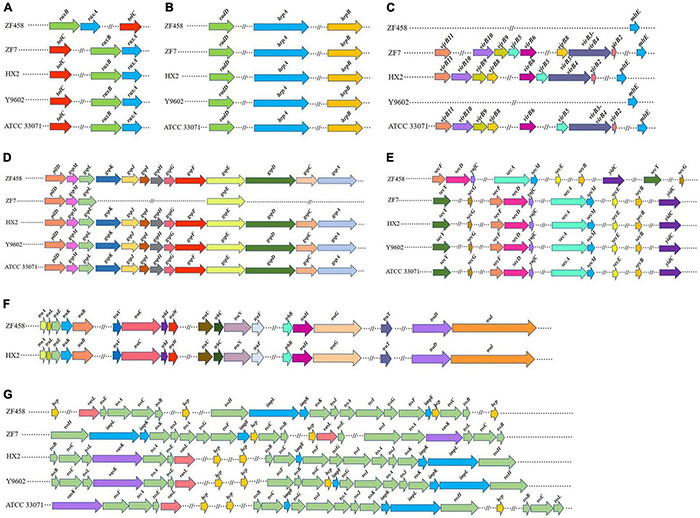
Comparison of the bacterial secretion system gene clusters of R. aceris ZF458 against four previously fully sequenced Rahnella genomes. (A) Type I secretion system. (B) Type III secretion system. (C) Type IV secretion system. (D) Type II secretion system. (E) Sec (secretion) system. (F) Conjugal transfer region of type IV secretion. (G) Type VI secretion system. The same color represented genes with the same or similar biological function. Arrows denoted putative transcriptional units. The length of blocks represented the size of genes (1 cm = 1000 bp).
Type IV Secretion System
In addition, nine key genes related to T4SS (virB2,3-4,5,6,8,9,10,11) were found in strains ZF7, HX2, and ATCC 33071T, but not in ZF458 and Y9602 (Figure 4C), and all the genes were retrieved from the chromosome genome. However, another gene mltE associated with T4SS was found in the five Rahnella strains and shared a 99% similarity at the amino acid level (Supplementary Table 6). Furthermore, the tra and trb gene clusters with 19 ORFs associated with the conjugal transfer region of the type IV secretion system were discovered in the plasmid genomes of ZF458 and HX2 (Figure 4F). Most of these genes were highly conserved with sequence identities exceeding 90%, except that traG and traI with sequence identities 87 and 89%, respectively (Supplementary Table 6). Interestingly, none of these genes existed in the genome of strains ZF7, Y9602 and ATCC 33071T.
Type VI Secretion System
In this study, 27 genes related to T6SS were found in the genome of R. aceris ZF458, and 13 genes were regarded as core genes (Figure 4G). The 13 core T6SS genes were highly conserved in the five Rahnella strains with a consistency of amino acid sequences over 90%, although the genes were in different encoding orders. However, 14 core T6SS genes existed in the genome of strains ZF7, HX2, Y9602, and ATCC 33071T, for the core gene tssL absent in the ZF458 genome (Supplementary Table 6). Moreover, other function genes involved in inner membrane proteins (ImpL and ImpK), extracellular structural components (vgrG and hcp) and regulatory proteins or structural proteins (VasL and ImpF) were present in the ZF458 genome, and shared a high similarity with those of the other 4 Rahnella strains at the amino acid level (Supplementary Table 6).
Other Secretion Systems
In addition, three genes related to T1SS (raxA, raxB, and tolC) (Figure 4A), three genes associated with T3SS (hrpA, hrpB, and radD) (Figure 4B), ten genes associated with Sec system (Figure 4E), four genes connected with twin arginine targeting (tatB, tatC, and tatE) and two genes related to SRP components (ffh and ftsY) were all present in the genomes of ZF458, ZF7, HX2, Y9602 and ATCC 33071T, most of which were highly conserved (Supplementary Table 6).
Two-Component System
Moreover, 19 TCSs (two-component system) were found in the genomes of strains ZF458, ZF7, HX2, Y9602 and ATCC 33071T, most of which were highly conserved (Supplementary Table 7). According to the homology, the topological characteristics of sensor histidine kinase (HK) and response regulator (RR) (Lavín et al., 2007), the 19 TCSs were divided into diverse subfamilies. For insurance, the motility TCS (CheA/Y) existed in ZF458 with a high identity (more than 98%) at the amino acid level among of the five Rahnella strains. The nitrogen regulation TCS (glnL/G) was searched for in ZF458 and shared a high homology with that in ZF7, HX2, Y9602 and ATCC 33071T (>99% identity). Phosphate regulation TCS (phoR/B) was observed in the genomes of strains ZF458, ZF7, HX2, Y9602 and ATCC 33071T, with sequence identities exceeding 98% (Supplementary Table 7). All these genes might play important roles in R. aceris ZF458 in terms of physiological metabolism and environmental adaptation.
Selenium Metabolism
In addition, many genes associated with selenium metabolism were found in the genome of R. aceris ZF458, which were similar to strains ZF7, HX2, Y9602 and ATCC 33071T (identities >90%) (Table 5). Among these genes, 8 genes including cysA, cysW, cysT, cysP, cysJ, cysI, tsgA, and hfq were associated with selenium transportation and regulation, 19 genes containing nar gene cluster and tat gene cluster were associated with selenate reduction, 15 genes were related to selenite reduction, and 8 genes were involved in selenium association (Table 5).
TABLE 5.
Genes related to selenium metabolism in R. aceris ZF458 and other Rahnella strains.
| Strain |
Rahnella aceris ZF458 |
R. aquatilis ZF7 |
R. aquatilis HX2 |
Rahnella sp. Y9602 |
R. aquatilis ATCC 33071 |
||||||
| Genes | Product definition | Locus Tag | Protein ID | Protein ID | Homology (%) | Protein ID | Homology (%) | Protein ID | Homology (%) | Protein ID | Homology (%) |
| Selenium transportation | |||||||||||
| cysA | sulfate/thiosulfate ABC transporter ATP-binding protein CysA | JHW33_RS22210 | WP_013574427.1 | WP_013574427.1 | 100 | WP_013574427.1 | 100 | WP_013574427.1 | 100 | WP_015696312.1 | 98 |
| cysW | sulfate/thiosulfate ABC transporter permease CysW | JHW33_RS22205 | WP_013574426.1 | WP_013574426.1 | 100 | WP_013574426.1 | 100 | WP_013574426.1 | 100 | WP_015696311.1 | 97 |
| cysT | sulfate/thiosulfate ABC transporter permease CysT | JHW33_RS22200 | WP_013574425.1 | WP_013574425.1 | 100 | WP_013574425.1 | 100 | WP_013574425.1 | 100 | WP_013574425.1 | 100 |
| cysP | thiosulfate-binding protein | JHW33_RS22195 | WP_200224495.1 | WP_112151264.1 | 99 | WP_013574424.1 | 99 | WP_013574424.1 | 99 | WP_015696310.1 | 97 |
| cysJ | NADPH-dependent assimilatory sulfite reductase flavoprotein subunit | JHW33_RS19955 | WP_037034152.1 | WP_119261245.1 | 99 | WP_013574003.1 | 99 | WP_013574003.1 | 99 | WP_015695893.1 | 98 |
| cysI | assimilatory sulfite reductase (NADPH) hemoprotein subunit | JHW33_RS19960 | WP_200227283.1 | WP_119262218.1 | 99 | WP_013574004.1 | 99 | WP_013574004.1 | 99 | WP_015695894.1 | 98 |
| tsgA | MFS transporter TsgA | JHW33_RS01475 | WP_200224981.1 | WP_013574731.1 | 99 | WP_015689560.1 | 99 | WP_013574731.1 | 99 | WP_015696567.1 | 97 |
| hfq | RNA chaperone Hfq | JHW33_RS18790 | WP_013573763.1 | WP_013573763.1 | 100 | WP_013573763.1 | 100 | WP_013573763.1 | 100 | WP_013573763.1 | 100 |
| Selenate reduction | |||||||||||
| narG | nitrate reductase subunit alpha | JHW33_RS04765 | WP_200225200.1 | WP_112152550.1 | 99 | WP_013575749.1 | 99 | WP_013575749.1 | 99 | WP_015697516.1 | 99 |
| narH | nitrate reductase subunit beta | JHW33_RS04770 | WP_200225201.1 | WP_037034411.1 | 99 | WP_013575748.1 | 99 | WP_013575748.1 | 99 | WP_015697515.1 | 99 |
| narJ | nitrate reductase molybdenum cofactor assembly chaperone | JHW33_RS04775 | WP_013575747.1 | WP_013575747.1 | 100 | WP_013575747.1 | 100 | WP_013575747.1 | 100 | WP_015697514.1 | 94 |
| narI | respiratory nitrate reductase subunit gamma | JHW33_RS04780 | WP_013575746.1 | WP_013575746.1 | 100 | WP_013575746.1 | 100 | WP_013575746.1 | 100 | WP_015697514.1 | 99 |
| narX | nitrate/nitrite two-component system sensor histidine kinase NarX | JHW33_RS06285 | WP_131637636.1 | WP_013575417.1 | 99 | WP_015689909.1 | 99 | WP_013575417.1 | 99 | WP_015697474.1 | 96 |
| narL | two-component system response regulator NarL | JHW33_RS06290 | WP_013575416.1 | WP_013575416.1 | 100 | WP_013575416.1 | 100 | WP_013575416.1 | 100 | WP_015697475.1 | 99 |
| fdnG | formate dehydrogenase-N subunit alpha | JHW33_RS16845 | WP_200223551.1 | WP_119261065.1 | 99 | WP_013573395.1 | 99 | WP_013573395.1 | 99 | WP_014333323.1 | 99 |
| dmsB | dimethylsulfoxide reductase subunit B | JHW33_RS01730 | WP_013574782.1 | WP_013574782.1 | 100 | WP_013574782.1 | 100 | WP_013574782.1 | 100 | WP_015696614.1 | 99 |
| dmsA | dimethylsulfoxide reductase subunit A | JHW33_RS01725 | WP_037036060.1 | WP_112198016.1 | 99 | WP_013574781.1 | 99 | WP_013574781.1 | 99 | WP_015696613.1 | 98 |
| dmsD | Tat proofreading chaperone DmsD | JHW33_RS01740 | WP_037036059.1 | WP_112151145.1 | 99 | WP_013574784.1 | 99 | WP_013574784.1 | 99 | WP_015696616.1 | 95 |
| dmsC | dimethyl sulfoxide reductase anchor subunit family protein | JHW33_RS01735 | WP_013574783.1 | WP_013574783.1 | 100 | WP_013574783.1 | 100 | WP_013574783.1 | 100 | WP_015696615.1 | 94 |
| ynfE | dimethyl sulfoxide reductase subunit A | NA | NA | WP_119261427.1 | NA | WP_013574780.1 | NA | WP_013574780.1 | NA | WP_015696612.1 | NA |
| arrA | molybdopterin-dependent oxidoreductase | JHW33_RS25040 | WP_013578099.1 | WP_119262333.1 | 100 | WP_013578099.1 | 100 | WP_013578099.1 | 100 | WP_014341807.1 | 94 |
| tatE | twin-arginine translocase subunit TatE | JHW33_RS10290 | WP_013576504.1 | WP_013576504.1 | 100 | WP_013576504.1 | 100 | WP_013576504.1 | 100 | WP_013576504.1 | 100 |
| tatE | twin-arginine translocase subunit TatE | JHW33_RS15180 | WP_013577433.1 | WP_013577433.1 | 100 | WP_013577433.1 | 100 | WP_013577433.1 | 100 | WP_013577433.1 | 100 |
| tatB | Sec-independent protein translocase subunit TatB | JHW33_RS15175 | WP_013577432.1 | WP_013577432.1 | 100 | WP_013577432.1 | 100 | WP_013577432.1 | 100 | WP_015699057.1 | 90 |
| tatC | Sec-independent protein translocase subunit TatC | JHW33_RS15170 | WP_013577431.1 | WP_013577431.1 | 100 | WP_013577431.1 | 100 | WP_013577431.1 | 100 | WP_013577431.1 | 100 |
| tatD | 3′-5′ ssDNA/RNA exonuclease TatD | JHW33_RS15165 | WP_037035383.1 | WP_119262167.1 | 100 | WP_015690569.1 | 100 | WP_013577430.1 | 100 | WP_015699056.1 | 100 |
| fnr | FNR family transcription factor | JHW33_RS05565 | WP_013575564.1 | WP_013575564.1 | 100 | WP_013575564.1 | 100 | WP_013575564.1 | 100 | WP_013575564.1 | 100 |
| Selenite reduction | |||||||||||
| nirB | nitrite reductase large subunit NirB | JHW33_RS04975 | WP_200225216.1 | WP_119262240.1 | 99 | WP_013575696.1 | 99 | WP_013575695.1 | 99 | WP_015697260.1 | 96 |
| nirD | nitrite reductase large subunit NirD | JHW33_RS17985 | WP_200223720.1 | WP_119261122.1 | 97 | WP_013573623.1 | 97 | WP_013573623.1 | 97 | WP_037040691.1 | 96 |
| frdA | flavocytochrome c | JHW33_RS05300 | WP_134705689.1 | WP_013575619.1 | 99 | WP_013575619.1 | 99 | WP_013575619.1 | 99 | WP_015697429.1 | 99 |
| frdD | fumarate reductase subunit FrdD | JHW33_RS18690 | WP_014333645.1 | WP_013573746.1 | 100 | WP_013573746.1 | 100 | WP_013573746.1 | 99 | WP_014333645.1 | 99 |
| frdC | fumarate reductase subunit FrdC | JHW33_RS18695 | WP_200223783.1 | WP_119261151.1 | 99 | WP_013573747.1 | 99 | WP_013573747.1 | 99 | WP_014333646.1 | 97 |
| frdA | fumarate reductase (quinol) flavoprotein subunit | JHW33_RS18705 | WP_014411553.1 | WP_119261153.1 | 100 | WP_014411553.1 | 99 | WP_013573749.1 | 99 | WP_014333648.1 | 99 |
| iscR | Fe-S cluster assembly transcriptional regulator IscR | JHW33_RS21885 | WP_013574363.1 | WP_013574363.1 | 100 | WP_013574363.1 | 100 | WP_013574363.1 | 100 | WP_015696248.1 | 99 |
| gorA | glutathione-disulfide reductase | JHW33_RS17480 | WP_037033742.1 | WP_119261551.1 | 99 | WP_013573520.1 | 99 | WP_013573520.1 | 99 | WP_014333456.1 | 99 |
| trxA | thioredoxin TrxA | JHW33_RS15705 | WP_013577537.1 | WP_013577537.1 | 100 | WP_013577537.1 | 100 | WP_013577537.1 | 100 | WP_013577537.1 | 100 |
| trxB | thioredoxin-disulfide reductase | JHW33_RS01690 | WP_015689578.1 | WP_015689578.1 | 100 | WP_015689578.1 | 100 | WP_013574774.1 | 99 | WP_015696607.1 | 99 |
| trxC | thioredoxin TrxC | JHW33_RS20360 | WP_013574074.1 | WP_013574074.1 | 100 | WP_013574074.1 | 100 | WP_013574074.1 | 100 | WP_015695963.1 | 99 |
| grxA | GrxA family glutaredoxin | JHW33_RS04425 | WP_013575818.1 | WP_013575818.1 | 100 | WP_013575818.1 | 100 | WP_013575818.1 | 100 | WP_013575818.1 | 100 |
| grxB | glutaredoxin 2 | JHW33_RS02700 | WP_013575015.1 | WP_013575015.1 | 100 | WP_013575015.1 | 100 | WP_013575015.1 | 100 | WP_015696848.1 | 95 |
| grxC | glutaredoxin 3 | JHW33_RS16070 | WP_013577603.1 | WP_013577603.1 | 100 | WP_013577603.1 | 100 | WP_013577603.1 | 100 | WP_015699202.1 | 99 |
| sodA | superoxide dismutase, Mn | JHW33_RS16835 | WP_013573393.1 | WP_013573393.1 | 100 | WP_013573393.1 | 100 | WP_013573393.1 | 100 | WP_014333320.1 | 98 |
| Selenium association | |||||||||||
| selA | DgaE family pyridoxal phosphate-dependent ammonia lyase | JHW33_RS14260 | WP_200223032.1 | WP_013577265.1 | 99 | WP_013577265.1 | 99 | WP_013577265.1 | 99 | WP_015698901.1 | 97 |
| cysK | cysteine synthase A | JHW33_RS22245 | WP_013574434.1 | WP_013574434.1 | 100 | WP_013574434.1 | 100 | WP_013574434.1 | 100 | WP_015696317.1 | 99 |
| metB | cystathionine gamma-synthase | JHW33_RS15930 | WP_013577576.1 | WP_013577576.1 | 100 | WP_013577576.1 | 100 | WP_013577576.1 | 100 | WP_015699179.1 | 99 |
| metC | cystathionine beta-lyase | JHW33_RS19835 | WP_200224006.1 | WP_119261241.1 | 99 | WP_013573978.1 | 99 | WP_013573978.1 | 99 | WP_015695874.1 | 96 |
| metE | 5-methyltetrahydropteroyl- triglutamate-homocysteine S-methyltransferase | JHW33_RS15280 | WP_200223241.1 | WP_119262170.1 | 99 | WP_013577450.1 | 99 | WP_013577450.1 | 99 | WP_015699072.1 | 97 |
| metH | methionine synthase | JHW33_RS14885 | WP_200223128.1 | WP_013577387.1 | 99 | WP_013577387.1 | 99 | WP_013577387.1 | 99 | WP_015699018.1 | 99 |
| sufS | cysteine desulfurase SufS | JHW33_RS07945 | WP_200226532.1 | WP_112197126.1 | 99 | WP_013576097.1 | 99 | WP_013576097.1 | 99 | WP_015697804.1 | 97 |
| sufE | cysteine desulfuration protein SufE | JHW33_RS07940 | WP_134705911.1 | WP_112152177.1 | 98 | WP_013576096.1 | 98 | WP_013576096.1 | 98 | WP_015697803.1 | 96 |
ND, not determined; NA, not available.
Acid Resistance
Rahnella strains adapted to diverse ecological environments, including water, soil and plants, which may be attributed to their stress tolerance traits. In this study, 28 genes associated with acid resistance were present in ZF458, and were grouped into different systems according to the enzyme activity. All the genes shared high similarities (greater than 90%) with those of other Rahnella strains, except for the gene ompF (encoding porin), which showed a lower homology with ZF7, HX2, Y9602 and ATCC 33071T (identity <90%) (Supplementary Table 8).
Motility, Chemotaxis and Quorum Sensing
A group of tightly gene clusters (flh, flg, and fli) related to flagella biosynthesis encoding 41 flagella-associated proteins (FlhDC, MotAB, FlhBAE, FlgA-N, FliC-T, FliA, and FliZ) were found in the genome of R. aceris ZF458, and had high amino acid similarities among the five Rahnella strains, except for the gene fliC, and fliD, which displayed low similarities between ZF458 and ATCC 33071T (49% identities) (Supplementary Table 9). In addition, 8 genes (cheA, cheW, mcp, tar, cheR, cheB, cheY, and cheZ) associated with chemotaxis were found in the genome of ZF458, with sequence similarities exceeding 97% between ZF458 and other four Rahnella strains (Supplementary Table 9). 12 key genes related to the autoinducer-2 (AI-2)- dependent signaling systems were detected in the five Rahnella strains, with sequences identities exceeding 94% between ZF458 and four other strains ZF7, HX2, Y9602 and ATCC 33071 (Supplementary Table 10).
Discussion
Rahnella was widely distributed in a variety of environments, and had been reported as PGPR that benefited plant growth (Sallam, 2011; Li et al., 2014). However, their specific mechanisms of plant-growth promotion and environmental adaption at the molecular level especially for R. aceris were still unclear. In this study, R. aceris ZF458 which was isolated from swamp soil was displayed. Strain ZF458 produced siderophores and phosphatases, which were key factors to promote plant growth (Li et al., 2013). ZF458 presented broad spectrum antagonistic activities against various plant pathogens and exhibited a significant effect in controlling Agrobacterium tumefaciens on sunflowers. All of these characteristics indicated that ZF458 might be a plant growth-promoting rhizobacteria. The complete genome of R. aceris ZF458 was sequenced and compared with other Rahnella strains to better understand the molecular mechanisms for plant-growth promotion and environmental adaption.
The phylogenetic trees showed that ZF458 clustered together with SAP-19, and the two strains both belonged to the R. aceris species, forming a tight cluster with R. aquatilis, which was consistent with a previous study (Lee et al., 2020). A previous study also indicated that R. aceris SAP-19 shared an ANI value of 92.7% and a DDH value under 48.6% with the genome sequence of R. aquatilis (Lee et al., 2020), however, our study showed that higher ANI and DDH values (>96and >70%, respectively) were calculated between R. aceris ZF458 and R. aquatilis strains. The results also indicated that strain ZF458 was closely related to the R. aquatilis strains, although it was classified as a new species of Rahnella genus by the phylogenetic analysis. According to the phylogenetic tree, strain ATCC 33071T was in a farther clade. Interestingly, the analysis of ANI and DDH revealed the same result, as the ANI and DDH values between ZF458 and ATCC 33071T were 92.75 and 49.4%. The results also indicated that the ANI and DDH values between ATCC 33071T and other R. aquatilis strains (including HX2, ZF7, Y9602) were below 95 and 70%, respectively, and this was consistent with the previous report showing that the ANI value between R. aquatilis strains ZF7 and ATCC 33071T was only 92.9% (Yuan et al., 2020). Previous studies indicated that strain ZF7, HX2 and Y9602 were both isolated from soil (Guo et al., 2012; Martinez et al., 2012a), while ATCC 33071T was isolated from drinking water (Martinez et al., 2012b), so, it may suggest that the genetic evolutionary distance could be associated with the habitat and adaptation. All five Rahnella strains harbored one or more plasmids, which could be related to genetic evolution, based on the previous report that 19% of strains in the genus Rahnella were plasmid-containing and had highly homologous regions for the same species (Rozhon et al., 2010).
In the study, an obvious gene transfer between ZF458 and other R. aquatilis strains indicated that strain ZF458 was another species not belonging to R. aquatilis. Furthermore, the nucleotide level similarities between ZF458 and ZF7, HX2, Y9602 were markedly higher than the similarity between ZF458 and ATCC 33071T, demonstrating the far phylogenetic distance between ZF458 and ATCC 33071T and supporting the phylogenetic result that ZF458 and ATCC 33071T were in different subclades. Low similarity regions were found between ZF458 and SAP-19, although the two strains both belonged to the R. aceris species, for the reason of strain SAP-19 was just whole genome shotgun sequence, not completed genome sequence. Based on the comparative analysis, only one unique gene existed in the ZF458 genome, while no unique genes existed in ZF7, HX2, Y9602 and ATCC 33071T, which confirmed the phylogenetic analysis described above that ZF458 belonged to R. aceris but was closely clustered with R. aquatilis.
Nitrogen was an important element for plant growth since plants only absorbed reduced forms of nitrogen, such as ammonia and nitrates. Biological nitrogen fixation (BNF), a microbiological process which converted atmospheric nitrogen into a plant usable form, offered an alternative to N fertilizers (Brewin and Legocki, 1992). This capability presented an opportunity to improve the nitrogen source utilization and crop yields, through the introduction of nitrogen fixing bacteria into crops, or the nitrogenase enzyme responsible for nitrogen fixation (Oldroyd and Dixon, 2014). Recently, a limited number of archaea and bacteria were found to fix nitrogen, such as Proteobacteria, Firmicutes, Cyanobacteria, and Actinobacteria (Santos et al., 2012). Rahnella spp. were also reported to possess the capacity for nitrogen fixation (Berge et al., 1991). For, insurance, R. aquatilis HX2 was reported as a PGPR and could promote the growth of corn for the ability to fix nitrogen (Guo et al., 2012). Previous studies indicated that the nifHDK gene cluster encoding Mo-nitrogenase played important role in fixing nitrogen, and nifBENXV was important for the synthesis and maturation of the FeMo cofactor (Wang L. et al., 2013). In this study, 21 nif genes were detected in the genome of ZF458, which predicted that ZF458 had a strong nitrogen fixation ability.
Auxin IAA was a primary plant hormone synthesized by plant growth-promoting bacteria that had a profound influence on benefiting plant (Bal et al., 2013). Currently, Rahnella spp. isolated from different environments were reported to have a strong capacity to produce IAA. Such as R. aquatilis ZF7 exhibited a high IAA biosynthesis capacity of 30.86 μg mL–1 and had a significant growth promoting effect on Chinese cabbage (Yuan et al., 2020). R. aquatilis HX2 could biosynthesize IAA and significantly promote maize growth (Guo et al., 2012). The endophyte Rahnella strains isolated from sweet potato plants, showed the ability to produce IAA and revealed obvious growth promotion in hybrid poplar (Khan and Doty, 2009). In this study, 11 genes associated with IAA biosynthesis were found in the ZF458 genome, including 9 genes (trp gene cluster) linked with tryptophan, ipdC gene encoding indole-3-pyruvate decarboxylase, and acdS gene encoding ACC deaminase, which revealed high similarities to R. aquatilis strains HX2 and ZF7. Previous study showed that acdS gene played a major role in PGPR activities. For example, the disruption of acdS gene in R. aquatilis HX2 reduced IAA production and decreased the growth promotion activity on corn (Peng et al., 2019). Previous studies demonstrated that the IPyA pathway was the main IAA synthetic pathway in a broad range of bacteria (Eastman et al., 2014), and the ipdC gene played an important role in regulating the IPyA pathway (Spaepen et al., 2007). So, the IPyA pathway may play an important role in the biosynthesis of IAA for R. aceris ZF458.
Phosphorus was considered as an important macronutrient for the growth and development of plants. Microorganisms were reported to play an important role in dissolving the insoluble phosphates in the rhizosphere (Passariello et al., 2006). Currently, phosphate solubilization of microorganisms was usually divided into two aspects, inorganic phosphorus solubilization and organic phosphorus solubilization, and organic phosphate in soil was degraded mainly through enzymes such as phosphatase, phytase and carbon-phosphorus lyase (White and Metcalf, 2007). Previous study showed that the carbon-phosphorus lyase system which encoded by phn gene cluster played important roles in organic phosphate dissolution (Jochimsen et al., 2011). In this study, the entire phn gene cluster was observed in the ZF458 genome, which was highly conserved in Rahnella strains, thus indicating that strain ZF458 may possess the strong ability to dissolve organic phosphorus. In addition, mineral phosphate solubilization could be improved by the production of organic acids, including gluconic, formic, citric, acetic, lactic, and acetic (Vassilev et al., 2006). According to the comparative genomic analysis, 44 genes related to organic acid biosynthesis were retrieved in the ZF458 genome, which were highly similar to other Rahnella strains. Thus, the organic acids generated by these genes played an important role in inorganic phosphorus solubilization for R. aceris ZF458. Many Rahnella strains have been reported to have a strong phosphate-solubilizing activity or organic acid synthesis ability. For example, R. aquatilis JZ-GX1 was able to secrete organic acids in an iron-deficient environment, which facilitated the production of phosphatases and siderophores, and thus promoted plant growth (Kong et al., 2020b). Rahnella sp. W25 isolated from the crop rhizosphere of calcareous soil, showed the maximum phosphate-solubilizing capability on tricalcium phosphate, aluminum phosphate, and ferric phosphate of 385.5, 110.4, and 216.6 mg⋅L–1 respectively (Qiao et al., 2013). In addition, the phosphorous solubilized by Rahnella strains W25 was significantly negatively correlated with the pH of the culture medium (Qiao et al., 2013). The aphA gene encoded a molecular class B bacterial phosphatase was reported to exhibit a better activity at acidic pH values (Passariello et al., 2006). Importantly, the pH value of the liquid culture of R. aceris ZF458 reduced to 3.24 from the original value of 7.0 with the growth of culture time, which might indicate that the AphA (protein id, WP_134705824.1) enzyme generated strong activity when solubilizing organic phosphate.
Gluconic acid was reported as a crucial antibacterial substance produced by R. aquatilis, and pyrroloquinoline quinone (PQQ) was necessary for the production of gluconic acid (Choi et al., 2008). Previous studies had demonstrated that the GDH (glucose dehydrogenase)-PQQ holoenzyme was important for the biosynthesis of an antimicrobial substance (Matsushita et al., 2002; Werra et al., 2009), and PQQ was reported as a plant growth promotion factor, which in addition to gluconic acid production, has also been associated with its antioxidant properties (Choi et al., 2008). Bacterial genes associated with PQQ biosynthesis have been identified in various species were clustered in pqqABCDEF operons (Guo et al., 2009). Previous studies revealed that pqqA gene encoded a small peptide that contained tyrosine and glutamate and served as the precursor and rate-determining step for PQQ production (Goosen et al., 1992). Also, pqqB was reported as a carrier that facilitated the secretion of PQQ across the plasma-membrane into the periplasm in K. pneumoniae (Velterop et al., 1995). For example, the disruption of the pqqA or pqqB gene in R. aquatilis HX2 reduced the plant growth promotion activity, and eliminated the ability to produce antibacterial substances thus decreasing the control effect against grapevine crown gall (Li et al., 2014). In this study, the whole pqq operons were observed in the ZF458 genome, and shared a high similarity with those in HX2, which indicated that R. aceris ZF458 could produce PQQ normally, although accurate biochemical functions remain to be confirmed.
PGPR exerted a series of regulatory mechanisms to adapt to the various complicated environments, among them, the bacterial secretion system, two-component system were well studied regulatory mechanisms associated with environmental adaption. Previous studies had indicated that large numbers of bacterial genomes (up to 17% in Proteobacteria genomes) encoded proteins in the general secretory pathway (GSP) (Bendtsen et al., 2005). Bacteria exported different kinds of substrates, including small molecules, extracellular enzymes, effector proteins and DNA via several sophisticated secretion systems, which were important for bacteria to adapt and survive the diverse ecological environments (Gerlach and Hensel, 2007).
Type II secretion system depended on the sec machinery and secreted folded proteins including various hydrolyzing enzymes such as the pseudolysin from the periplasm across the extracellular environment, which was important for bacterial survival in host or environment (Bendtsen et al., 2005). Many T2SS components had been well characterized, such as pectinases and cellulases associated with pathogenicity were secreted by T2SS in Pectobacterium (Filloux, 2004). T2SS was well-conserved and composed of 12–15 components, which were called Gsp proteins (Cianciotto, 2005). GspD formed a multimeric pore for transportation of secreted proteins and interacted with GspC, which played an important role in V. cholerae (Korotkov et al., 2011). The GspC, GspF, GspL, and GspM constituted the inner membrane platform and were associated with ATPase secretion in the cytoplasm (Johnson et al., 2006). In this study, ZF458 harbored 12 gsp genes which were highly conserved in the Rahnella strains, and the Gsps might be associated with the protein secretion and transportation, but the role of T2SS in R. aceris ZF458 needs to be determined in the future.
T4SS was characterized by the ability to regulate the translocation of single-stranded DNA or complex proteins, and existed in both Gram-negative and Gram-positive bacteria (Alvarez-Martinez and Christie, 2009). The T-DNA transfer system of A. tumefaciens was the prototypical type A T4SS, and together with the T4SS in E. coli was the best-characterized T4SS, which consisted of 12 proteins including VirB1-VirB11 and VirD4 (Christie et al., 2014). T4SS was recognized as the most ubiquitous secretion system in nature, for the ability to conjugate which was a common bacteria trait. However, no vir genes was found in the ZF458 genome, except for the conjugal transfer region (including tra and trb gene clusters) which existed in the plasmid genome. Previous studies revealed that T4SS evolved from bacterial conjugation machinery according to the sequence similarities (Christie and Cascales, 2003). Such as the octopine-type Ti-plasmids which were recognized as the conjugal transfer system in Agrobacterium tumefaciens was closely related to Ti plasmid vir genes (Alt-Mörbe et al., 1996). In addition, the tra genes related to conjugal transfer system were reported to be involved in the synthesis and possible retraction of the sex pilus (Winans and Walker, 1985), while the vir genes were also proven to be required for pilus biogenesis (Trokter et al., 2014). Thus, the gene clusters in the conjugal transfer region of ZF458 might possess the same function as T4SS, which needs to be further verified.
T6SS was reported to transfer effector proteins into eukaryotic and prokaryotic cells and had a crucial role in bacterial competition (Ho et al., 2014). T6SS was composed of 13 conserved core genes and few accessory genes which were necessary for function though the genes varied in composition among species (Chang et al., 2014). ImpL, an integral polytopic inner membrane protein, interacted with the essential T6SS component, and ImpK was proved to be necessary for its function of mediating the Hcp secretion in A. tumefaciens (Ma et al., 2009). TssL, TssM, and TssJ composed a membrane complex in the T6SS, and played an important role in protein secretion and the assembly of cell surface appendages in E. coli (Felisberto-Rodrigues et al., 2011). Notably, the comparative genomic analysis indicated the variation in the gene composition between Rahnella strains, but contained the 13 conserved core genes, which might reveal that Rahnella perform the normal function of T6SS. To date, T6SS had been proved to be associated with the symbiotic interactions with hosts in many bacteria, but more studies need to explore the function of T6SS in the Rahnella strains.
Two-component regulatory system was an essential mechanism for signal transduction, generally consisting of a sensor histidine kinase (HK) and a response regulator (RR) (Lavín et al., 2007). Previous studies showed that GacS/GacA system controlled the biosynthesis of secondary metabolites and extracellular enzymes that were linked with the ecological fitness and tolerance to stress (Heeb and Haas, 2001). PhoB/R TCS played a pivotal role in regulating the inorganic phosphate regulatory system (Yamada et al., 1989). The CheA/CheW TCS regulated chemotaxis and RcsC/RcsB were involved in the motility of bacteria (Majdalani and Gottesman, 2005). Overall, TCS played an essential role in the life activity of bacteria, for the diverse functional TCSs that could adapt to various environments. Recently, it was reported that TCS had a close relationship with biocontrol factors in bacteria, however, the functions of TCSs in ZF458 need to be elucidated.
Selenium (Se) was an important trace element for humans and animals and existed in four states with chemical forms of selenide, elemental selenium, selenite and selenate. Microorganism was reported to play a key role in the biogeochemical cycle of Se in the ecological environment (Skalickova et al., 2017). Many bacteria have been reported to reduce selenate and/or selenite to elemental Se nanoparticles (SeNPs) (Stolz et al., 2006). SeNPs could be used for medicine, therapeutics and environmental remediation due to a variety of advantages including biocompatibility and low toxicity (Wang et al., 2007). Recently, the SeNPs synthesized by bacteria had drawn extensive attention due to their great potential for the bioremediation of polluted environments. R. aquatilis HX2 was reported to show a strong tolerance to Se and reduce selenate and selenite to BioSeNPs (Zhu et al., 2018). R. aquatilis ZF7 could produce SeNPs from Na2SeO3, and possessed the most production of SeNPs at 0.85 mM (Yuan et al., 2020). Previous studies proved that the small RNA chaperone Hfq was a key regulator for the reduction of selenium nanoparticles in R. aquatilis HX2 (Xu et al., 2020). In this study, many genes linked with selenium metabolism were found in the ZF458 genome, and shared a high homology with those in the genomes of R. aquatilis strains ZF7 and HX2, and the results indicated that R. aceris ZF458 might synthesize SeNPs and had a widely applications in nano-agriculture.
Acid resistance genes were essential for successful colonization in acidic environments however, most studies on acid resistance genes had been conducted in E. coli. For instance, the alternative stress sigma factor (RpoS), was reported to be associated with the oxidative AR system, which allowed E. coli O157:H7 to survive in acidic environments (Masuda and Church, 2010). In addition, the glutamate and arginine decarboxylase systems in E. coli were also revealed as part of its acid resistance mechanism (Kim et al., 2016). Based on the comparative genomic analysis, many acid resistance genes were observed in the ZF458 genome, but their function still needs to be confirmed.
Conclusion
Rahnella aceris ZF458, which was isolated from the swamp soil, exhibited broad-spectrum antagonistic activities against 14 diverse plant pathogens, and displayed a significant effect in controlling Agrobacterium tumefaciens on sunflowers. A whole genomic analysis and a phylogenetic analysis showed that strain ZF458 belonged to R. aceris, and was closely related to R. aquatilis. A comparative genomic analysis indicated that R. aceris ZF458 harbored many genes related to nitrogen fixation, IAA production, organic phosphate dissolution, organic acid biosynthesis and PQQ production, which had been proved to be beneficial to plant growth. In addition, large numbers of genes associated with environmental adaption, such as the secretion system, two-component system, selenium metabolism and acid-resistance were found in the ZF458 genome and shared a high homology with Rahnella strains ZF7, HX2, Y9602 and ATCC 33071T. As far as we know, this was the first study to systematically analyze the genes associated with plant growth promotion and environmental adaption in R. aceris. Overall, these features of R. aceris ZF458 provided new insight into the comprehensive metabolic pathways (Figure 5), and would assist in studying plant growth promotion and biocontrol application in R. aceris.
FIGURE 5.
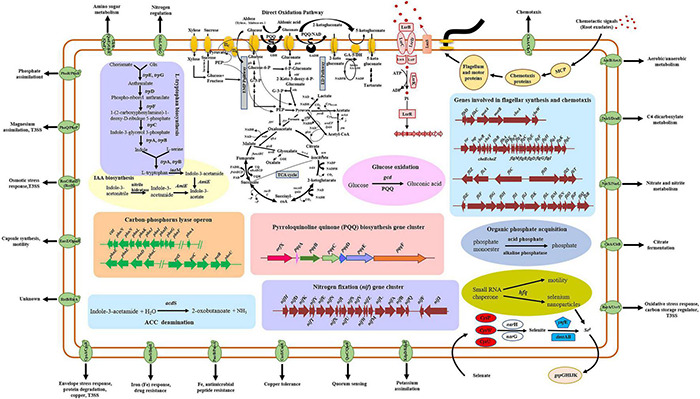
Metabolic pathway map of R. aceris ZF458 related to plant growth promotion and environmental adaption.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and access ion number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA796123.
Author Contributions
SX, LL, and BL conceived and designed the experiments. SX, YZ, and YP performed the experiments and analyzed the data. SX and LL wrote the manuscript. LL, XX, YS, and AC revised the manuscript. All authors have read and approved of the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge Yang Cao (Allwegene Technologies Corporation) for the genome data analysis. Finally, we thank the editor and reviewers for their kind suggestions.
Footnotes
Funding
This work was performed with the support of the China Agriculture Research System of MOF and MARA (CARS-25); the Science and Technology Innovation Program if the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-IVFCAAS); and the Key Laboratory of Horticultural Crops Genetic Improvement, Ministry of Agriculture in China (IVF2020).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.850084/full#supplementary-material
Control efficiency of R. aceris ZF458 against Agrobacterium tumefaciens on the stem of sunflowers. (A) Agrobacterium tumefaciens ACCC 19185, (B) Agrobacterium tumefaciens ACCC 19185 and R. aceris ZF458 (V:V = 1:1), (C) ZF458, (D) control of sterilized water.
Production of extracellular enzymes and siderophores by R. aceris ZF458. Plate assays for the production of siderophores (A) and phosphatases (B).
General characteristics of R. aceris ZF458. (A) Image of ZF458 colony morphology. (B) Image of ZF458 cells using transmission electron microscopy (Hitachi 7700, Japan). (C) Image of ZF458 cells using scanning electron microscope (Hitachi S3400N, Japan).
Growth curve aND pH curve of R. aceris ZF458 for different culture times. (A) Optical density of ZF458 at different culture times, (B) pH of ZF458 at different culture times.
Phylogenetic tree highlighting the relative positions of R. aceris ZF458 among other Rahnella strains. The phylogenetic tree was constructed based on four housekeeping genes (16S rRNA, gyrB, atpD, and rpoB) according to the aligned gene sequences using maximum likelihoods derived from MEGA 6.0 software. Bootstrap values (1,000 replicates) were shown at the branch points. The scale bar indicates 0.05 nucleotide substitutions per nucleotide position.
Percentage of the average nucleotide identities (ANI) and in silico DNA-DNA hybridization (DDH) among the selected Rahnella strains. ANI values were computed for a pairwise genome comparison using the OrthoANIu algorithm. The percentage of ANI was shown on the top right. DDH values were calculated by using the Genome-to-Genome Distance Calculator (GGDC). The percentage of DDH was shown on the bottom left.
Comparisons of Pyrroloquinoline Quinone genes of R. aceris ZF458 against four other previously fully sequenced Rahnella genomes. The same color represented genes with the same biological function.
References
- Abo-Elyousr A. M. K., Sallam M., Hassan M., Zeller W. (2010). Effect of Acibenzolar-S-methyl and Rahnella aquatilis (Ra39) on Chitinase and β-1, 3-glucanase activities and disease resistance of apple plants. Plant Pathol. J. 26 63–69. [Google Scholar]
- Adeolu M., Alnajar S., Naushad S., Gupta S. (2016). Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 66 5575–5599. 10.1099/ijsem.0.001485 [DOI] [PubMed] [Google Scholar]
- Alt-Mörbe J., Stryker J. L., Fuqua C., Li P. L., Farrand S. K., Winans S. C. (1996). The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J. Bacteriol. 178 4248–4257. 10.1128/jb.178.14.4248-4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Martinez C. E., Christie P. J. (2009). Biological diversity of prokaryotic type IV Secretion. Microbiol. Mol. Biol. Rev. 73 775–808. 10.1371/10.1128/MMBR.00023-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos B., Rolf A. (2000). The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 28 45–48. 10.1093/nar/28.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., et al. (2000). Gene ontology: tool for the unification of biology. Gene Ontol. Consort. Nat. Genet. 25 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal H. B., Das S., Dangar T. K., Adhya T. K. (2013). ACC deaminase and IAA producing growth promoting bacteria from the rhizosphere soil of tropical rice plants. J. Basic Microbiol. 53 972–984. 10.1002/jobm.201200445 [DOI] [PubMed] [Google Scholar]
- Bendtsen J. D., Binnewies T. T., Hallin P. F., Sicheritz-Pontén T., Ussery D. W. (2005). Genome update: prediction of secreted proteins in 225 bacterial proteomes. Microbiology 151 1725–1727. 10.1099/mic.0.28029-0 [DOI] [PubMed] [Google Scholar]
- Berge O., Heulin T., Achouak W., Richard C., Bally R., Balandreau J. (1991). Rahnella aquatilis, a nitrogen-fixing enteric bacterium associated with the rhizosphere of wheat and maize. Can. J. Microbiol. 37 195–203. 10.1139/m91-030 [DOI] [Google Scholar]
- Brady C., Hunter G., Kirk S., Arnold D., Denman S. (2014). Rahnella rasiliensana sp. nov., Rahnella bruchi sp. nov., Rahnella woolbedingensis sp. nov., classification of Rahnella genomospecies 2 and 3 as Rahnella variigena sp. nov. and Rahnella inusitata sp. nov., respectively and emended description of the genus Ra. Syst. Appl. Microbiol. 37 545–552. 10.1016/j.syapm.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Brewin N. J., Legocki A. B. (1992). Biological nitrogen fixation for sustainable agriculture: A perspective. Plant Soil. 141 1–11. [DOI] [PubMed] [Google Scholar]
- Cantarel B. L., Coutinho P. M., Corinne R., Thomas B., Vincent L., Bernard H. (2009). The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 37 D233–D238. 10.1093/nar/gkn663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. H., Desveaux D., Creason A. L. (2014). The ABCs and 123s of Bacterial Secretion Systems in Plant Pathogenesis. Annu. Rev. Phytopathol. 52 317–345. 10.1146/annurev-phyto-011014-015624 [DOI] [PubMed] [Google Scholar]
- Chen F., Li J. Y., Guo Y. B., Hui J., Wang H. M. (2009). Biological control of grapevine crown gall: partial characterisation of an antibacterial substance produced by Rahnella aquatilis strain HX2. Eur. J. Plant Pathol. 124 427–437. 10.1007/s10658-009-9429-z [DOI] [Google Scholar]
- Choi O., Kim J., Kim J. G., Jeong Y., Moon J. S. (2008). Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol. 146 657–668. 10.1104/pp.107.112748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Cascales E. (2003). The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 1 137–149. 10.1038/nrmicro753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Whitaker N., González-Rivera C. (2014). Mechanism and structure of the bacterial type IV secretion systems. Biochim. Biophys. Acta. 1843 1578–1591. 10.1016/j.bbamcr.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciotto N. P. (2005). Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13 581–588. 10.1016/j.tim.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Eastman A. W., Heinrichs D. E., Yuan Z. C. (2014). Comparative and genetic analysis of the four sequenced Paenibacillus polymyxa genomes reveals a diverse metabolism and conservation of genes relevant to plant-growth promotion and competitiveness. BMC Genomics 15:851. 10.1186/1471-2164-15-851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Hendawy H. H., Osman M. E., Sorour N. M. (2003). Characterization of two antagonistic strains of Rahnella aquatilis isolated from soil in Egypt. Folia Microbiol. 48 799–804. 10.1007/BF02931517 [DOI] [PubMed] [Google Scholar]
- Felisberto-Rodrigues C., Durand E., Aschtgen M. S., Blangy S., Ortiz-Lombardia M., Douzi B., et al. (2011). Towards a structural comprehension of bacterial type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Escherichia coli Pathovar. PLoS Pathog. 7:e1002386. 10.1371/journal.ppat.1002386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A. (2004). The underlying mechanisms of type II protein secretion. Biochim. Biophys. Acta. 1694 163–179. 10.1016/j.bbamcr.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Galperin M. Y., Makarova K. S., Wolf Y. I., Koonin E. V. (2015). Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 43 D261–D269. 10.1093/nar/gku1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach R. G., Hensel M. (2007). Protein secretion systems and adhesins: The molecular armory of Gram-negative pathogens. Int. J. Med. Microbiol. 297 401–415. 10.1016/j.ijmm.2007.03.017 [DOI] [PubMed] [Google Scholar]
- Goosen N., Huinen R. G., van de Putte P. (1992). A 24-amino-acid polypeptide is essential for the biosynthesis of the coenzyme pyrrolo-quinoline-quinone. J. Bacteriol. 174 1426–1427. 10.1128/jb.174.4.1426-1427.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. B., Jiao Z., Li L., Wu D., Crowley D. E., Wang Y., et al. (2012). Draft Genome Sequence of Rahnella aquatilis Strain HX2, a plant growth-promoting rhizobacterium isolated from vineyard soil in beijing. China J. Bacteriol. 194 6646–6647. 10.1128/JB.01769-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. B., Li J. Y., Li L., Chen F., Wu W. L., Wang J. H., et al. (2009). Mutations That Disrupt Either the pqq or the gdh Gene of Rahnella aquatilis abolish the production of an antibacterial substance and result in reduced biological control of grapevine crown gall. Appl. Environ. Microbiol. 75 6792–6803. 10.1128/AEM.00902-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Ye Z., Yang D., Yan J., Xiao L., Zhong T., et al. (2013). Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere 90 1960–1965. 10.1016/j.chemosphere.2012.10.057 [DOI] [PubMed] [Google Scholar]
- Heeb S., Haas D. (2001). Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol. Plant Microbe. Interact. 14 1351–1363. 10.1094/MPMI.2001.14.12.1351 [DOI] [PubMed] [Google Scholar]
- Heermann R., Fuchs T. M. (2008). Comparative analysis of the Photorhabdus luminescens and the Yersinia enterocolitica genomes: uncovering candidate genes involved in insect pathogenicity. BMC Genomics 9:40. 10.1186/1471-2164-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho B. T., Dong T. G., Mekalanos J. J. (2014). A view to a kill: the bacterial type VI secretion system. Cell Host Microbe. 15, 9–21. 10.1016/j.chom.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibtissem G., Gilles V., Christine P. (2007). CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 35 W52–W57 10.1093/nar/gkm360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochimsen B., Lolle S., McSorley F. R., Nabi M., Stougaard J., Zechel D. L., et al. (2011). Five phosphonate operon gene products as components of a multi-subunit complex of the carbon-phosphorus lyase pathway. Proc. Natl. Acad. Sci. USA 108 11393–11398. 10.1073/pnas.1104922108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. L., Abendroth J., Hol W., Sandkvist M. (2006). Type II secretion: From structure to function. FEMS Microbiol. Lett. 255 175–186. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Goto S., Hattori M., Aoki-Kinoshita K. F., Hirakawa M. (2006). From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 34 D354–D357. 10.1093/nar/gkj102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z., Doty S. L. (2009). Characterization of bacterial endophytes of sweet potato plants. Plant Soil 322 197–207. [Google Scholar]
- Kim G. H., Fratamico P., Breidt F., Oh D. H. (2016). Survival and expression of acid resistance genes in Shiga toxin-producing Escherichia coli acid adapted in pineapple juice and exposed to synthetic gastric fluid. J. Appl. Microbiol. 121 1416–1426. 10.1111/jam.13223 [DOI] [PubMed] [Google Scholar]
- Kong W. L., Rui L., Ni H., Wu X. Q. (2020a). Antifungal Effects of Volatile Organic Compounds Produced by Rahnella aquatilis JZ-GX1 Against Colletotrichum gloeosporioides in Liriodendron chinense × tulipifera. Front. Microbiol. 11:1114. 10.3389/fmicb.2020.01114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W. L., Wu X. Q., Zhao Y. J. (2020b). Effects of Rahnella aquatilis JZ-GX1 on Treat Chlorosis Induced by Iron Deficiency in Cinnamomum camphora. J. Plant Growth Regual. 39 877–887 [Google Scholar]
- Korotkov K. V., Johnson T. L., Jobling M. G., Pruneda J., Pardon E., Héroux A., et al. (2011). Structural and functional studies on the interaction of GspC and GspD in the type II secretion system. PLoS Pathog. 7:e1002228. 10.1371/journal.ppat.1002228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagesen K., Hallin P., Rødland E. A., Staerfeldt H. H., Rognes T., Ussery D. W. (2007). RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35 3100–3108. 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartigue M. F., Nordmann P., Edelstein M. V., Cuzon G., Brisse S., Poirel L. (2013). Characterization of an extended-spectrum class A β-lactamase from a novel enterobacterial species taxonomically related to Rahnella spp./Ewingella spp. J. Antimicrob. Chemother. 68 1733–1736. 10.1093/jac/dkt122 [DOI] [PubMed] [Google Scholar]
- Lavín J., Kiil K., Resano O., Ussery D. W., Oguiza J. A. (2007). Comparative genomic analysis of two-component regulatory proteins in Pseudomonas syringae. BMC Genomics 8:397–390. 10.1186/1471-2164-8-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. D., Jeon D., Kim I. S., Choe H., Kim J. S. (2020). Rahnella aceris sp. nov., isolated from sap drawn from Acer pictum. Arch Microbiol. 202:9. 10.1007/s00203-020-01961-5 [DOI] [PubMed] [Google Scholar]
- Li G. E., Wu X. Q., Ye J. R., Hou L., Zhou A. D. (2013). Isolation and identification of phytate-degrading rhizobacteria with activity of improving growth of poplar and Masson pine. World J. Microbiol. Biotechnol. 29 2181–2193. 10.1007/s11274-013-1384-3 [DOI] [PubMed] [Google Scholar]
- Li L., Jiao Z., Hale L., Wu W. L., Guo Y. B. (2014). Disruption of Gene pqqA or pqqB Reduces plant growth promotion activity and biocontrol of crown gall disease by Rahnella aquatilis HX2. PLoS One 9:e115010. 10.1371/journal.pone.0115010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Li J., Peng J., Wu W., Guo Y. (2019). Identification of atpD as an optimal reference gene to explore antibiotic resistance and stress tolerance in Rahnella aquatilis. J. Appl. Microbiol. 126 1096–1107. 10.1111/jam.14215 [DOI] [PubMed] [Google Scholar]
- Li P. S., Kong W. L., Wu X. Q. (2021). Salt tolerance mechanism of the rhizosphere bacterium JZ-GX1 and its effects on tomato seed germination and seedling growth. Front. Microbiol. 12:657238. 10.3389/fmicb.2021.657238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Lukasz J., Adam G. (2002). Tolerating some redundancy significantly speeds up clustering of large protein databases. Bioinformatics 18 77–82. 10.1093/bioinformatics/18.1.77 [DOI] [PubMed] [Google Scholar]
- Liu R., Ochman H. (2007). Stepwise formation of the bacterial flagellar system. Proc. Natl. Acad. Sci. U.S.A. 104, 7116–7121. 10.1073/pnas.0700266104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe T. M., Eddy S. R. (1997). tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25 955–964. 10.1093/nar/25.5.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis S. D. P., Prieto C., Marcela C. F. (2014). Screening phosphate solubilizing actinobacteria isolated from the rhizosphere of wild plants from the Eastern Cordillera of the Colombian Andes. Afr. J. Microbiol. Res. 8 734–742. 10.5897/AJMR2013.5940 [DOI] [Google Scholar]
- Ma L. S., Lin J. S., Lai E. M. (2009). An IcmF Family Protein, ImpLM, is an integral inner membrane protein interacting with ImpKL, and its walker a motif is required for type VI secretion system-mediated Hcp Secretion in Agrobacterium tumefaciens. J. Bacteriol. 191 4316–4329. 10.1128/JB.00029-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N., Gottesman S. (2005). The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59 379–405. 10.1146/annurev.micro.59.050405.101230 [DOI] [PubMed] [Google Scholar]
- Martinez R. J., Bruce D., De Tter C., Goodwin L. A., Han J., Han C. S., et al. (2012a). Complete Genome Sequence of Rahnella sp. Strain Y9602, a gammaproteobacterium isolate from metal- and radionuclide-contaminated soil. J. Bacteriol. 194 2113–2114. 10.1128/JB.00095-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J., Bruce D., Detter C., Goodwin L. A., Han J., Han C. S., et al. (2012b). Complete Genome Sequence of Rahnella aquatilis CIP 78.65. J. Bacteriol. 194 3020–3021. 10.1128/JB.00380-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Church G. M. (2010). Regulatory network of acid resistance genes in Escherichia coli. Mol. Microbiol. 48 699–712. 10.1046/j.1365-2958.2003.03477.x [DOI] [PubMed] [Google Scholar]
- Matsushita K., Toyama H., Yamada M., Adachi O. J. (2002). Quinoproteins: structure, function, and biotechnological applications. Plant Physiol. 58 13–22. 10.1007/s00253-001-0851-1 [DOI] [PubMed] [Google Scholar]
- Meier-Kolthoff J. P., Auch A. F., Klenk H. P., Gker M. (2013). Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 14:60. 10.1186/1471-2105-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milagres A. M., Machuca A., Napoleão D. (1999). Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J. Microbiol. Methods. 37 1–6. 10.1016/s0167-7012(99)00028-7 [DOI] [PubMed] [Google Scholar]
- Nawrocki E. P., Kolbe D. L., Eddy S. R. (2009). Infernal 1.0: inference of RNA alignments. Bioinformatics 25 1335–1337. 10.1093/bioinformatics/btp157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd G. E., Dixon R. (2014). Biotechnological solutions to the nitrogen problem. Curr. Opin. Biotechnol. 26 19–24. [DOI] [PubMed] [Google Scholar]
- Palmieri D., Vitale S., Lima G., Di Pietro A., Turra D. (2020). A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat. Commun. 11 52–64. 10.1038/s41467-020-18994-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passariello C., Forleo C., Micheli V., Schippa S., Leone R., Mangani S., et al. (2006). Biochemical characterization of the class B acid phosphatase (AphA) of Escherichia coli MG1655. Biochim. Biophys. Acta 1764 13–19. 10.1016/j.bbapap.2005.08.028 [DOI] [PubMed] [Google Scholar]
- Peng J., Wu D., Liang Y., Li L., Guo Y. (2019). Disruption of acdS gene reduces plant growth promotion activity and maize saline stress resistance by Rahnella aquatilis HX2. J. Basic Microbiol. 59 402–411. 10.1002/jobm.201800510 [DOI] [PubMed] [Google Scholar]
- Petersen T. N., Brunak S., von Heijne G., Nielsen H. (2011). SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8 785–786. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- Qiao Z. W., Hong J. P., Xie Y. H., Xuan L. L. (2013). Screening, identification and phosphate-solubilizing characteristics of Rahnella sp. phosphate-solubilizing bacteria in calcareous soil. Ying Yong Sheng Tai Xue Bao 24 2294–2300. [PubMed] [Google Scholar]
- Rhodes A. N., Urbance J. W., Youga H., Corlew-Newman H., Reddy C. A., Klug M. J., et al. (1998). Identification of bacterial isolates obtained from intestinal contents associated with 12,000-year-old mastodon remains. Appl. Environ. Microbiol. 64, 651–658. 10.1089/oli.1.1998.8.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M., Rossello-Mora R. (2009). Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 106 19126–19131. 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhon W., Petutschnig E., Khan M., Summers D. K., Poppenberger B. (2010). Frequency and diversity of small cryptic plasmids in the genus Rahnella. BMC Microbiol. 10:56. 10.1186/1471-2180-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Reddy V. S., Tamang D. G., Research V. A. (2009). The Transporter Classification Database: recent advances. Nucleic Acids Res. 37 D274–D278. 10.1093/nar/gkn862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam N. M. (2011). Biological control of common blight of bean (Phaseolus vulgaris) caused by Xanthomonas axonopodis pv. phaseoli by using the bacterium Rahnella aquatilis. Arch. Phytopathol. Plant Protec. 44 1966–1975. [Google Scholar]
- Santos P. D., Fang Z., Mason S. W., Setubal J. O. C., Dixon R. (2012). Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genomics 13:162. 10.1186/1471-2164-13-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalickova S., Milosavljevic V., Cihalova K., Horky P., Richtera L., Adam V. (2017). Selenium nanoparticles as a nutritional supplement. Nutrition 33 83–90. 10.1016/j.nut.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Spaepen S., Vanderleyden J., Remans R. (2007). Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 31, 425–448. 10.1111/j.1574-6976.2007.00072.x [DOI] [PubMed] [Google Scholar]
- Stolz J. F., Basu P., Santini J. M., Oremland R. S. (2006). Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol. 60 107–130. 10.1146/annurev.micro.60.080805.142053 [DOI] [PubMed] [Google Scholar]
- Stothard P., Wishart D. C. (2005). Circular genome visualization and exploration using CGView. Bioinformatics 21 537–539. 10.1093/bioinformatics/bti054 [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trokter M., Felisberto-Rodrigues C., Christie P. J., Waksman G. (2014). Recent advances in the structural and molecular biology of type IV secretion systems. Curr. Opin. Struct. Biol. 27 16–23. 10.1016/j.sbi.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev N., Vassileva M., Nikolaeva I. (2006). Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl. Microbiol. Biotechnol. 71 137–144. 10.1007/s00253-006-0380-z [DOI] [PubMed] [Google Scholar]
- Velterop J. S., Sellink E., Meulenberg J. J., David S., Bulder I., Postma P. W. (1995). Synthesis of pyrroloquinoline quinone in vivo and in vitro and detection of an intermediate in the biosynthetic pathway. J. Bacteriol. 177 5088–5098. 10.1128/jb.177.17.5088-5098.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Yuan J., Jian Z., Zhang M., Li R., Ruan Y., et al. (2013). Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol. Fert Soils. 49 435–446. 10.1007/s00374-012-0739-5 [DOI] [Google Scholar]
- Wang H., Zhang J., Yu H. (2007). Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic. Biol. Med. 42, 1524–1533. 10.1016/j.freeradbiomed.2007.02.013 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang L., Liu Z., Zhao D., Liu X., Zhang B., et al. (2013). A Minimal Nitrogen Fixation Gene Cluster from Paenibacillus sp. WLY78 Enables Expression of Active Nitrogenase in Escherichia coli. PLoS Genet. 9:e1003865. 10.1371/journal.pgen.1003865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werra P. D., Péchy-Tarr M., Keel C., Maurhofer M. (2009). Role of Gluconic Acid Production in the Regulation of Biocontrol Traits of Pseudomonas fluorescens CHA0. Appl. Environ. Microbiol. 75 4162–4174. 10.1128/AEM.00295-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. K., Metcalf W. W. (2007). Microbial metabolism of reduced phosphorus compounds. Annu. Rev. Microbiol. 61 379–400. 10.1146/annurev.micro.61.080706.093357 [DOI] [PubMed] [Google Scholar]
- Winans S. C., Walker G. C. (1985). Conjugal transfer system of the IncN plasmID pKM101. J. Bacteriol. 161 402–410. 10.1128/jb.161.1.402-410.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Song Y., Lin Z., Bauelos G., Guo Y. B. (2020). The small RNA chaperone Hfq is a critical regulator for bacterial biosynthesis of selenium nanoparticles and motility in Rahnella aquatilis. Appl. Microbiol. Biotechnol. 104 1721–1735. 10.1007/s00253-019-10231-4 [DOI] [PubMed] [Google Scholar]
- Yamada M., Makino K., Amemura M., Shinagawa H., Nakata A. (1989). Regulation of the phosphate regulon of Escherichia coli: analysis of mutant phoB and phoR genes causing different phenotypes. J. Bacteriol. 171 5601–5606. 10.1128/jb.171.10.5601-5606.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z., Liang Y., Lynch K. H., Dennis J. J., Wishart D. S. (2011). PHAST: A Fast Phage Search Tool. Nucleic Acids Res. 39 W347–W352. 10.1093/nar/gkr485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Li L., Zheng F., Shi Y., Xie X., Chai A., et al. (2020). The complete genome sequence of Rahnella aquatilis ZF7 reveals potential beneficial properties and stress tolerance capabilities. Arch Microbiol. 202 483–499. 10.1007/s00203-019-01758-1 [DOI] [PubMed] [Google Scholar]
- Zhang Y. C., Fan Q. R., Loria R. (2016). A re-evaluation of the taxonomy of phytopathogenic genera Dickeya and Pectobacterium using whole-genome sequencing data. Syst. Appl. Microbiol. 39 252–259. 10.1016/j.syapm.2016.04.001 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Ren B., Li H., Lin Z., Gary B., Li L., et al. (2018). Biosynthesis of selenium nanoparticles and effects of selenite, selenate, and selenomethionine on cell growth and morphology in Rahnella aquatilis HX2. Appl. Microbiol. Biotechnol. 102 6191–6205. 10.1007/s00253-018-9060-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Control efficiency of R. aceris ZF458 against Agrobacterium tumefaciens on the stem of sunflowers. (A) Agrobacterium tumefaciens ACCC 19185, (B) Agrobacterium tumefaciens ACCC 19185 and R. aceris ZF458 (V:V = 1:1), (C) ZF458, (D) control of sterilized water.
Production of extracellular enzymes and siderophores by R. aceris ZF458. Plate assays for the production of siderophores (A) and phosphatases (B).
General characteristics of R. aceris ZF458. (A) Image of ZF458 colony morphology. (B) Image of ZF458 cells using transmission electron microscopy (Hitachi 7700, Japan). (C) Image of ZF458 cells using scanning electron microscope (Hitachi S3400N, Japan).
Growth curve aND pH curve of R. aceris ZF458 for different culture times. (A) Optical density of ZF458 at different culture times, (B) pH of ZF458 at different culture times.
Phylogenetic tree highlighting the relative positions of R. aceris ZF458 among other Rahnella strains. The phylogenetic tree was constructed based on four housekeeping genes (16S rRNA, gyrB, atpD, and rpoB) according to the aligned gene sequences using maximum likelihoods derived from MEGA 6.0 software. Bootstrap values (1,000 replicates) were shown at the branch points. The scale bar indicates 0.05 nucleotide substitutions per nucleotide position.
Percentage of the average nucleotide identities (ANI) and in silico DNA-DNA hybridization (DDH) among the selected Rahnella strains. ANI values were computed for a pairwise genome comparison using the OrthoANIu algorithm. The percentage of ANI was shown on the top right. DDH values were calculated by using the Genome-to-Genome Distance Calculator (GGDC). The percentage of DDH was shown on the bottom left.
Comparisons of Pyrroloquinoline Quinone genes of R. aceris ZF458 against four other previously fully sequenced Rahnella genomes. The same color represented genes with the same biological function.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and access ion number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA796123.


