Abstract
Objective
To determine the long‐term impact of prior SARS‐CoV‐2 infection on immune responses after COVID‐19 vaccination.
Methods
Using longitudinally collected blood samples from the COMMUNITY study, we determined binding (WHO BAU mL−1) and neutralising antibody titres against ten SARS‐CoV‐2 variants over 7 months following BNT162b2 in SARS‐CoV‐2‐recovered (n = 118) and SARS‐CoV‐2‐naïve (n = 289) healthcare workers with confirmed prior SARS‐CoV‐2 infection. A smaller group with (n = 47) and without (n = 60) confirmed prior SARS‐CoV‐2 infection receiving ChAdOx1 nCoV‐19 was followed for 3 months. SARS‐CoV‐2‐specific memory T‐cell responses were investigated in a subset of SARS‐CoV‐2‐naïve and SARS‐CoV‐2‐recovered vaccinees.
Results
Vaccination with both vaccine platforms resulted in substantially enhanced T‐cell responses, anti‐spike IgG responses and neutralising antibodies effective against ten SARS‐CoV‐2 variants in SARS‐CoV‐2‐recovered participants as compared to SARS‐CoV‐2‐naïve participants. The enhanced immune responses sustained over 7 months following vaccination.
Conclusion
These findings imply that prior SARS‐CoV‐2 infection should be taken into consideration when planning booster doses and design of current and future COVID‐19 vaccine programmes.
Keywords: COVID‐19, hybrid immunity, immune responses, SARS‐CoV‐2, vaccination
The objective of this study was to determine the long‐term impact of prior SARS‐CoV‐2 infection on immune responses after COVID‐19 vaccination. SARS‐CoV‐2 infection should be taken into consideration when planning booster doses and design of current and future COVID‐19 vaccine programmes.
Introduction
Clinical trials and post‐marketing effectiveness data have shown that currently used COVID‐19 vaccines protect strongly against hospitalisation and death. 1 , 2 , 3 However, real‐world efficacy estimates are affected by population demographics, characteristics of circulating SARS‐CoV‐2 variants, vaccine protocols and time since vaccination. An increased risk of breakthrough infections is now observed, partly explained by immune waning, 4 , 5 , 6 , 7 , 8 and third vaccine doses are therefore being administered. A robust immune response after infection or vaccination is based on the induction of memory B‐ and T‐cells generating virus‐specific antibodies and T‐cell responses. 9 , 10 , 11 , 12 , 13 Antibody levels have been shown to correlate inversely with the risk of SARS‐CoV‐2 infection 14 , 15 and may, with standardised read‐outs, 16 be used as a marker for correlates of protection. The time between prime and boost, 17 the number of boosters administrated and infection prior to vaccination 18 impact the breadth and duration of immune responses. As an increasing number of persons become infected globally, vaccination post‐SARS‐CoV‐2 infection will become more frequent. Prior SARS‐CoV‐2 infection has been reported to positively impact vaccine responses, 9 , 19 , 20 , 21 , 22 , 23 , 24 but little is known regarding long‐term effects. To optimise immunisation programmes, it is therefore of importance to study the duration of immune responses including direct comparisons of vaccine platforms and the long‐term effect of prior SARS‐CoV‐2 infection on subsequent vaccine‐induced responses in real‐world evidence studies.
Using longitudinally collected blood samples from the COMMUNITY (COVID‐19 Immunity) study, 13 , 24 , 25 , 26 we herein report binding and pseudo‐neutralising antibody titres and memory T‐cell responses elicited over 7 months following mRNA BNT162b2 (Comirnaty, Pfizer‐BioNTech) and over 3 months following adenovirus‐vectored ChAdOx1 nCoV‐19 (Vaxzevria, AstraZeneca) vaccination in 514 healthcare workers (HCW) with and without confirmed SARS‐CoV‐2 infection prior to vaccination.
Results
The COMMUNITY study enrolled 2149 HCW at Danderyd Hospital, Stockholm, Sweden, between April and May 2020. Starting January 2021, all HCW at Danderyd Hospital were offered vaccination with either BNT162b2 or ChAdOx1 nCoV‐19, depending on availability. This substudy included a total of 514 HCW stratified into two groups depending on SARS‐CoV‐2 infection prior to vaccination. 335 HCW received BNT162b2 with a 3‐week dose interval (range 21–28 days), 72 HCW received BNT162b2 with a 6‐week dose interval (range 39–52 days), and 107 HCW received ChAdOx1 nCoV‐19 with a 12‐week dose interval (range 71–92 days; Figure 1). There was no difference between SARS‐CoV‐2‐naïve and SARS‐CoV‐2‐recovered HCW regarding concomitant chronic diseases (30.6% vs. 25.6%, P‐value = 0.3). Among 164 recovered HCW, 4 had been hospitalised because of COVID‐19, 153 had not been hospitalised, and 7 had a SARS‐CoV‐2 infection of unknown severity. Demographics, prior SARS‐CoV‐2 infection and vaccine status of the study population are presented in Table 1.
Figure 1.
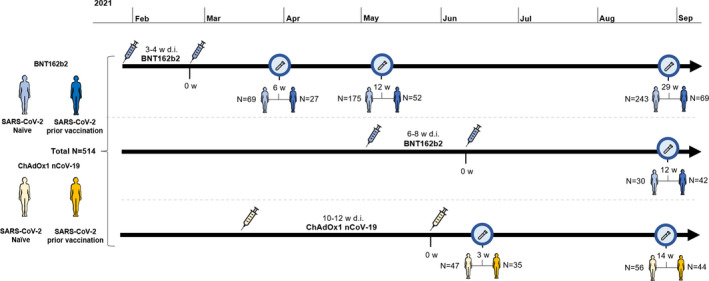
Timeline for vaccination and sample collection. The cohort (n = 514) is divided into participants receiving BNT162b2 with a 3‐ to 4‐week (n = 335) and 6‐ to 8‐week (n = 72) dose interval and ChAdOx1 nCoV‐19 (n = 107) with a 10‐ to 12‐week dose interval. Blue characters represent vaccinees who received BNT162b2, and yellow characters represent vaccinees who received ChAdOx1 nCoV‐19. Light‐coloured characters represent SARS‐CoV‐2 naïve, and dark‐coloured characters represent participants with SARS‐CoV‐2 infection prior to vaccination. Test tubes represent time for blood sampling, and syringes represent time for vaccination. W, weeks; d.i., dose interval.
Table 1.
Demographics of study participants
| SARS‐CoV‐2 naïve | SARS‐CoV‐2 recovered | |||||
|---|---|---|---|---|---|---|
| N | Age (IQR) | Female (%) | N | Age (IQR) | Female (%) | |
| BNT162b2 (3–4 weeks d.i.) | ||||||
| Total | 259 | 76 | ||||
| 6 weeks p.v. (5–8) | 69 | 50 (40–56) | 84 | 27 | 50 (41–55) | 81 |
| 12 weeks p.v. (11–14) | 175 | 51 (40–57) | 90 | 52 | 48 (39–56) | 81 |
| 29 weeks p.v. (27–30) | 243 | 50 (40–57) | 87 | 69 | 49 (39–57) | 81 |
| BNT162b2 (6–8 weeks d.i.) | ||||||
| Total | 30 | 42 | ||||
| 12 weeks p.v. (11–14) | 306 | 60 (5–61) | 93 | 42 | 55 (46–61) | 86 |
| ChAdOx1 nCoV‐19 (10–12 weeks d.i.) | ||||||
| Total | 60 | 47 | ||||
| 3 weeks p.v. (2–3) | 47 | 52 (42–57) | 90 | 35 | 52 (44–58) | 86 |
| 14 weeks p.v. (13–15) | 56 | 51 (38–56) | 89 | 44 | 52 (44–57) | 86 |
Age is presented as median with IQR, interquartile range. Weeks post‐second vaccine dose refers to point in time where sampling occurred and is presented as median together with the range. d.i., dose interval; p.v., post‐second vaccine dose.
Effect of previous SARS‐CoV‐2 infection on antibody responses over 7 months following BNT162b2 vaccination
Almost all (99.8%) participants had detectable levels of spike IgG antibodies after vaccination. Notably, at all sampling time points, spike IgG GMTs were markedly higher in previously SARS‐CoV‐2‐infected vaccinees than in SARS‐CoV‐2‐naïve vaccinees (all P‐values < 0.001; Table 2; Figure 2a). A twofold decrease in spike IgG GMTs between weeks 6 and 12 and a 6.6‐fold decrease between weeks 6 and 29 were observed in SARS‐CoV‐2‐naïve BNT162b2 vaccinees. For vaccinees with SARS‐CoV‐2 infection prior to vaccination, a 1.5‐fold decrease in GMTs was observed between weeks 6 and 12, and a 3.6‐fold decrease was observed between weeks 6 and 29 post‐vaccination.
Table 2.
Geometric mean titres (GMTs) of SARS‐CoV‐2 wild‐type spike IgG and pseudo‐neutralising antibodies in the different study groups
| SARS‐CoV‐2 naïve | SARS‐CoV‐2 recovered | |||
|---|---|---|---|---|
| GMTs | 95% CI | GMTs | 95% CI | |
| SARS‐CoV‐2 wild‐type IgG (BAU mL−1) | ||||
| BNT162b2 (3–4 weeks d.i.) | ||||
| 6 weeks p.v. (5–8) | 981 | (853–1128) | 1875 | (1222–2878) |
| 12 weeks p.v. (11–14) | 470 | (430–513) | 1278 | (911–1793) |
| 29 weeks p.v. (27–30) | 146 | (133–160) | 514 | (389–680) |
| BNT162b2 (6–8 weeks d.i.) | ||||
| 12 weeks p.v. (11–14) | 451 | (355–573) | 1333 | (1058–1680) |
| ChAdOx1 nCoV‐19 (10–12 weeks d.i.) | ||||
| 3 weeks p.v. (2–3) | 190 | (134–270) | 1350 | (954–1910) |
| 14 weeks p.v. (13–15) | 104 | (85–128) | 591 | (438–798) |
| Pseudo‐neutralising Ab (wild‐type variant; AU mL−1) | ||||
| BNT162b2 (3–4 weeks d.i.) | ||||
| 6 weeks p.v. (5–8) | 15.5 | (13.5–17.7) | 62.2 | (33.0–117.2) |
| 12 weeks p.v. (11–14) | 8.5 | (7.6–9.5) | 27.2 | (18.2–40.8) |
| 29 weeks p.v. (27–30) | 4.7 | (4.5–5.0) | 12.0 | (9.6–15.0) |
| BNT162b2 (6–8 weeks d.i.) | ||||
| 12 weeks p.v. (11–14) | 9.2 | (7.5–11.1) | 29.6 | (21.2–41.4) |
| ChAdOx1 nCoV‐19 (10–12 weeks d.i.) | ||||
| 3 weeks p.v. (2–3) | 5.6 | (5.0–6.3) | 16.7 | (11.9–23.4) |
| 14 weeks p.v. (13–15) | 4.0 | (3.5–4.4) | 12.7 | (9.5–16.9) |
| Pseudo‐neutralising Ab (Delta variant; AU mL−1) | ||||
| BNT162b2 (3–4 weeks d.i.) | ||||
| 6 weeks p.v. (5–8) | 21.9 | (18.1–26.5) | 62.6 | (30.8–127.0) |
| 12 weeks p.v. (11–14) | 8.4 | (7.1–9.9) | 30.1 | (20.4–44.5) |
| 29 weeks p.v. (27–30) | 4.7 | (4.0–5.6) | 17.3 | (13.6–21.9) |
| BNT162b2 (6–8 weeks d.i.) | ||||
| 12 weeks p.v. (11–14) | 12.2 | (9.9–15.1) | 39.4 | (29.7–52.2) |
| ChAdOx1 nCoV‐19 (10–12 weeks d.i.) | ||||
| 3 weeks p.v. (2–3) | 8.0 | (7.1–9.0) | 24.2 | (18.4–31.7) |
| 14 weeks p.v. (13–15) | 2.6 | (1.6–4.3) | 16.1 | (10.9–23.6) |
Weeks post‐second vaccine dose refers to point in time where sampling occurred and is presented as median together with the range. CI, confidence interval; Ab, antibodies; d.i., dose interval; p.v., post‐second vaccine dose; AU, arbitrary units; BAU, binding antibody units.
Figure 2.
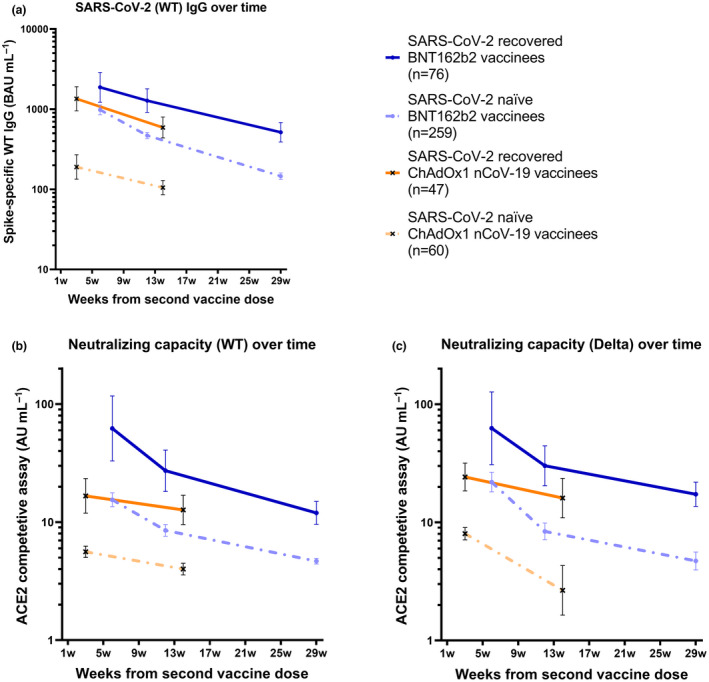
Binding and pseudo‐neutralising antibody titres over time following BNT162b2 and ChAdOx1 nCoV‐19 vaccination with and without prior SARS‐CoV‐2 infection. (a) Binding antibody titres against SARS‐CoV‐2 wild type over 7 months following the second BNT162b2 dose (n = 335) and 3 months following the second ChAdOx1 nCoV‐19 dose (n = 107) in SARS‐CoV‐2‐recovered and SARS‐CoV‐2‐naïve vaccinees, (b) pseudo‐neutralising antibodies against the wild type over 7 months following the second BNT162b2 dose (n = 312) and 3 months following the second ChAdOx1 nCoV‐19 dose (n = 100) in SARS‐CoV‐2‐recovered and SARS‐CoV‐2‐naïve vaccinees, and (c) pseudo‐neutralising antibodies against the Delta variant type over 7 months following the second BNT162b2 dose (n = 312) and 3 months following the second ChAdOx1 nCoV‐19 dose (n = 100) in SARS‐CoV‐2‐recovered and SARS‐CoV‐2‐naïve vaccinees. Dots and crosses represent GMTs, and bars represent 95% CI. Solid lines represent SARS‐CoV‐2‐recovered vaccinees, and dotted lines represent SARS‐CoV‐2‐naïve vaccinees. WT, wild type; BAU, binding antibody units; AU, arbitrary units.
Spike IgG titres correlated strongly to pseudo‐neutralising antibody titres against both the WT and the Delta variant (Spearman's rank correlation coefficient 0.92, and 0.83, respectively, both P‐values < 0.0001). Consistent with spike IgG, substantially higher GMTs of pseudo‐neutralising antibodies were observed in SARS‐CoV‐2‐recovered vaccinees than in SARS‐CoV‐2‐naïve vaccinees, at all sampling time points (all P‐values < 0.001; Table 2; Figure 2b and c). Among SARS‐CoV‐2‐naïve vaccinees, GMTs of pseudo‐neutralising antibodies against the WT and Delta variant decreased by 1.8‐fold and 2.6‐fold between weeks 6 and 12, and by 3.3‐fold and 4.6‐fold between weeks 6 and 29, respectively. For SARS‐CoV‐2‐recovered vaccinees, GMTs of pseudo‐neutralising antibodies against the WT and Delta variant decreased by 2.3‐fold and 2.1‐fold between weeks 6 and 12, and by 5.1‐fold and 3.6‐fold between weeks 6 and 29, respectively (Table 2; Figure 2b and c). Notably, pseudo‐neutralising GMTs against the Delta variant reached similar trajectories as pseudo‐neutralising GMTs against the WT, indicating a similar trend in response to the currently circulating Delta variant as to the initially circulating WT (Table 2; Figure 2b and c).
Effect of BNT162b2 dose interval on antibody levels
It has been reported that a longer interval between BNT162b2 doses can result in higher antibody titres. 27 In this cohort, BNT162b2 vaccinees were administered with a 3‐ to 4‐week dose interval in January to February 2021, and with a prolonged dose interval of 6–8 weeks in April to July 2021, allowing for a comparison in immune responses in these two groups.
Twelve weeks post‐second dose, there were no significant differences in spike IgG GMTs following BNT162b2 with the 3‐ to 4‐week and the 6‐ to 8‐week dose interval in either of the groups (with and without SARS‐CoV‐2 infection prior to vaccination; all P‐values > 0.05; Table 2). Pseudo‐neutralising antibody GMTs against both the WT and Delta variant were, however, increased 12 weeks following a second dose after the prolonged dose interval (P‐value = 0.016 and P‐value = 0.017, respectively) in SARS‐CoV‐2‐naïve vaccinees. A similar trend was seen in SARS‐CoV‐2‐recovered vaccinees, although the differences were not significant (all P‐values > 0.05; Table 2).
Effect of previous SARS‐CoV‐2 infection on antibody responses over 3 months following ChAdOx1 nCoV‐19 vaccination
We next analysed the effect of previous SARS‐CoV‐2 infection on immune responses after vaccination with the ChAdOx1 nCoV‐19 vaccine. At 12 weeks post‐vaccination, spike IgG GMTs in SARS‐CoV‐2‐naïve ChAdOx1 nCoV‐19 vaccinees were 4.5‐fold lower than those in SARS‐CoV‐2‐naïve BNT162b2 vaccinees (P‐value < 0.001). As observed after BNT162b2 vaccination, spike IgG GMTs were substantially increased in SARS‐CoV‐2‐recovered ChAdOx1 nCoV‐19 vaccinees compared with those in SARS‐CoV‐2‐naïve vaccinees at all sampling time points (all P‐values < 0.001; Table 2; Figure 2a–c). In SARS‐CoV‐2‐naïve vaccinees, spike IgG GMTs declined 1.9‐fold between weeks 3 and 14 post‐vaccination, whereas it declined 2.3‐fold between weeks 3 and 14 post‐vaccination in SARS‐CoV‐2‐recovered vaccinees (Table 2; Figure 2a–c).
Consistent with results for binding antibodies, substantially higher GMTs of pseudo‐neutralising antibodies were observed in SARS‐CoV‐2‐recovered ChAdOx1 nCoV‐19 vaccinees compared with those in SARS‐CoV‐2‐naïve vaccinees at all sampling time points (all P‐values < 0.001; Table 2; Figure 2b and c). In SARS‐CoV‐2‐naïve vaccinees, GMTs against the WT and Delta variant decreased by 1.4‐fold and threefold between weeks 3 and 14, respectively, whereas they decreased by 1.3‐fold and 1.5‐fold, respectively, in SARS‐CoV‐2‐recovered vaccinees. Similar to findings following BNT162b2 vaccination, GMTs against the Delta variant reached comparable levels and trajectories as GMTs against the WT (Table 2; Figure 2b and c).
Impact on antibody responses with increased time between SARS‐CoV‐2 infection and vaccination
Spike IgG and pseudo‐neutralising antibody titres against ten SARS‐CoV‐2 variants increased with time interval between confirmed seroconversion and vaccination (Figure 3). Notably, neutralising antibody titres against the Delta variant increased by a factor of 1.40 (95% CI 1.21–1.61) and 1.57 (95% CI 1.35–1.82) per 3 months’ increase in the time interval between confirmed seroconversion and vaccination following BNT162b2 and ChAdOx1 nCoV‐19, respectively.
Figure 3.
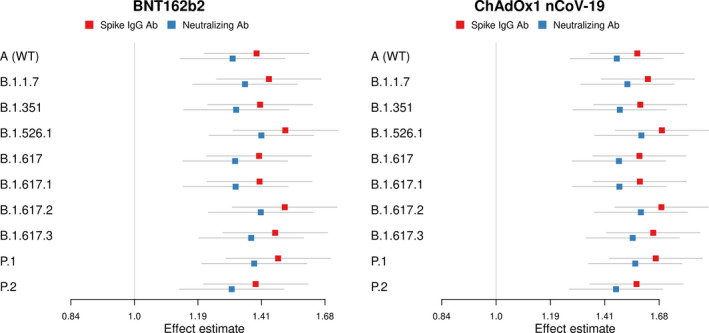
Impact of an additional 3‐month interval between infection (confirmed seroconversion) and vaccination on vaccine‐induced binding and neutralising antibody titres. Effect estimates on binding (blue squares) and neutralising (red squares) on antibody titres against ten SARS‐CoV‐2 variants including SARS‐CoV‐2 wild‐type (A (WT)) and B.1.1.7 (Alpha), B.1.351 (Beta), B.1.526.1 (New York), B.1.617 (India), B.1.617.1 (Kappa), B.1.617.2 (Delta), B.1.617.3 (India), P.1 (Gamma) and P.2 (Zeta) variants following BNT162b2 and ChAdOx1 nCoV‐19 vaccination. Red squares represent the factor increase in SARS‐CoV‐2 spike IgG, and blue squares represent the factor increase in SARS‐CoV‐2 pseudo‐neutralising antibodies per 3 months’ increase in the interval between infection (as defined by confirmed seroconversion) and vaccination.
Live microneutralisation compared with pseudo‐neutralisation
As a slightly higher capacity to inhibit binding of the Delta variant than the WT was found using the pseudo‐neutralising assay (Figure 2b and c), a live neutralisation assay was performed in a subset of 34 participants (17 SARS‐CoV‐2‐naïve BNT162b2 vaccinees and 17 SARS‐CoV‐2‐naïve ChAdOx1 nCoV‐19 vaccinees; Supplementary figure 1). While results from these two assays correlated with regard to the WT and the Delta variant (rS = 0.66 and 0.63, respectively, both P‐values < 0.0001), the live microneutralisation assay resulted in 25.2% lower titres against the Delta variant than the WT.
Neutralisation capacity against SARS‐CoV‐2 variants in vaccinees with and without SARS‐CoV‐2 infection prior to vaccination
In the light of the substantial increases in antibody titres observed in SARS‐CoV‐2‐recovered vaccinees as compared to SARS‐CoV‐2‐naïve vaccinees, we proceeded to determine whether it also had an effect on neutralising capacity against SARS‐CoV‐2 variants. Pseudo‐neutralising antibody titres were assessed against ten SARS‐CoV‐2 variants, including all variants of concern (VOC; B.1.1.7 (Alpha), B.135.1 (Beta), P.1 (Gamma) and B.1.617.2 (Delta)). Notably, pseudo‐neutralising antibody titres against all ten tested SARS‐CoV‐2 variants were at least twofold respectively threefold higher in SARS‐CoV‐2‐recovered than in SARS‐CoV‐2‐naïve vaccinees following BNT162b2 and ChAdOx1 nCoV‐19, respectively (all P‐values < 0.001; Figure 4a–c).
Figure 4.
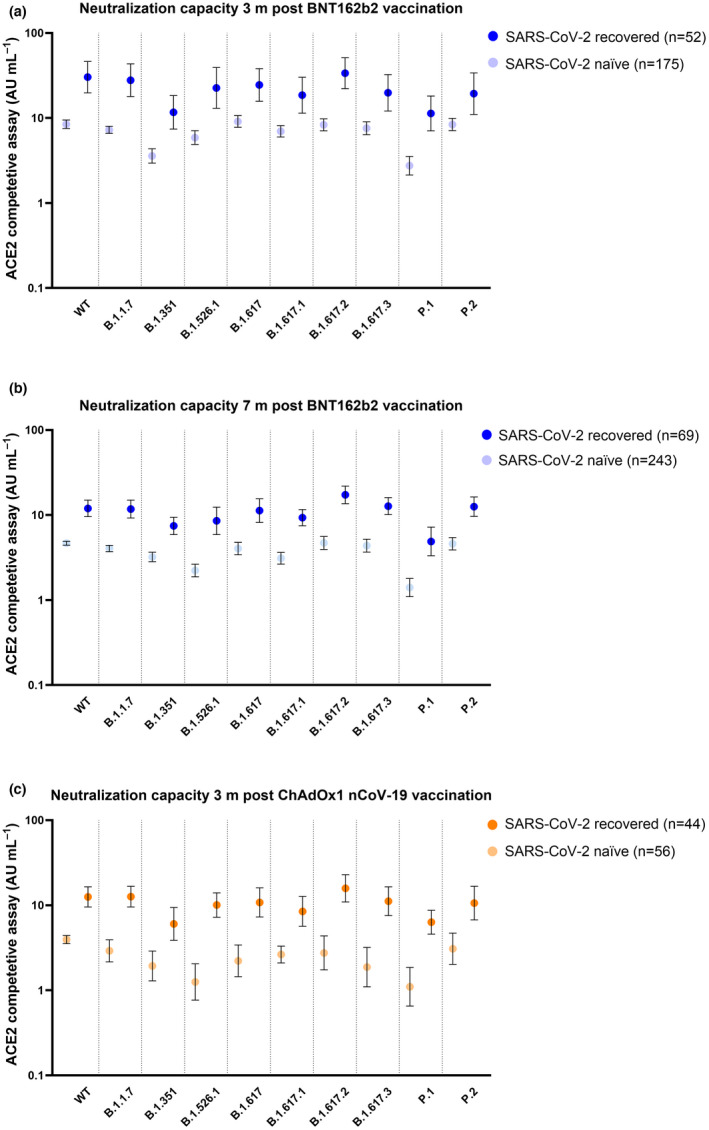
Neutralising capacity over time. Neutralising capacity (a) 3 months (n = 227) and (b) 7 months (n = 312) post‐BNT162b2 vaccination and (c) 3 months (n = 100) post‐ChAdOx1 nCoV‐19 vaccination against ten SARS‐CoV‐2 variants including the wild‐type (WT), B.1.1.7 (Alpha), B.1.351 (Beta), B.1.526.1 (New York), B.1.617 (India), B.1.617.1 (Kappa), B.1.617.2 (Delta), B.1.617.3 (India), P.1 (Gamma) and P.2 (Zeta) variants. Dots represent GMTs, and bars represent 95% CI. Dark‐coloured dots represent SARS‐CoV‐2‐recovered vaccinees, and light‐coloured dots represent SARS‐CoV‐2‐naïve vaccinees.
SARS‐CoV‐2‐specific memory T‐cell responses
We next proceeded to investigate SARS‐CoV‐2‐directed T‐cell responses in a subset of SARS‐CoV‐2‐naïve and SARS‐CoV‐2‐recovered vaccinees 3 months post‐ChAdOx1 nCoV‐19 and three and 7 months post‐BNT162b2. As expected, memory T‐cell responses were stronger in SARS‐CoV‐2‐recovered vaccinees than in SARS‐CoV‐2‐naïve vaccinees. We used a whole‐blood IGRA based on a pool of eight peptides derived from the spike protein (peptides that do not show any evidence of overlap with common cold coronaviruses and thus do not pose a risk of detecting T‐cell responses inflicted by a previous non‐SARS‐CoV‐related infection). 13 , 28 IFN‐γ GMTs were more than sixfold higher in SARS‐CoV‐2‐recovered BNT162b2 vaccinees during the whole follow‐up period of 7 months [166.4 pg mL−1 (95% CI 98.5–281.2) and 116.8 pg mL−1 (95% CI 34.6–394.7) in SARS‐CoV‐2‐recovered vaccinees three and 7 months post‐BNT162b2 vs. 26.8 pg mL−1 (95% CI 45.5–49.6) and 18.7 pg mL−1 (95% CI 9.5–36.6) in SARS‐CoV‐2‐naïve vaccinees; P‐value < 0.01; Figure 5a and b]. Similar differences were found 3 months post‐ChAdOx1 nCoV‐19, with a more than fourfold increase in IFN‐γ geometric mean levels in SARS‐CoV‐2‐recovered vaccinees (38.0 pg mL−1 (95% CI 20.4–70.9) in SARS‐CoV‐2‐recovered vaccinees vs. 8.0 pg mL−1 (95% CI 3.5–18.5) in SARS‐CoV‐2‐naïve vaccinees; P‐value < 0.01; Figure 5c).
Figure 5.
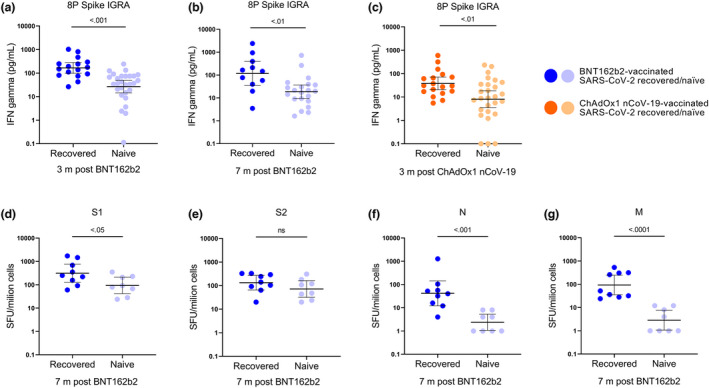
Memory T‐cell responses three and 7 months following BNT162b2 and ChAdOx1 nCoV‐19 vaccination in SARS‐CoV‐2‐recovered and SARS‐CoV‐2‐naïve participants. (a–c) The whole‐blood IGRA using a SARS‐CoV‐2‐specific peptide pool (8 SARS‐CoV‐2‐specific peptides covering the SARS‐CoV‐2 spike protein 13 , 28 ). (d–g) T‐cell responses against S1, S2, N and M analysed against a T‐SPOT® Discovery SARS‐CoV‐2 kit (Oxford Immunotec, Oxfordshire, UK). The cut‐off for positivity was set at t > 40 SFU mL−1. Blue dots represent BNT162b2 vaccinees (a, b, d–g), and orange dots represent ChAdOx1 nCoV‐19 vaccinees (c). Dark‐coloured dots represent SARS‐CoV‐2‐recovered (n = 9) participants, and light‐coloured dots represent SARS‐CoV‐2‐naïve (n = 8) participants. Lines represent geometric mean, and error bars represent 95% confidence interval.
These findings were corroborated using a commercial T‐spot assay, at 7 months post‐BNT162b2 vaccination, with a set of peptide pools that cover the S1, S2, N and M proteins. With this assay, we observed more than a fourfold increased response against the S1 protein in SARS‐CoV‐2‐recovered vaccinees than in SARS‐CoV‐2‐naïve vaccinees (556 SFU/million cells in SARS‐CoV‐2‐recovered vs. 135 SFU/million cells in SARS‐CoV‐2‐naïve vaccinees; P‐value < 0.05; Figure 5d–g). No significant difference in response against the S2 protein was observed between the two groups. Notably, responses against the N and M peptide pools remained enhanced in SARS‐CoV‐2‐recovered vaccinees, not only implying long‐lasting infection‐acquired immune responses against these proteins but also confirming the serological status prior to vaccination.
Discussion
Using a large cohort with longitudinally collected blood samples, we show that vaccination following SARS‐CoV‐2 infection resulted in remarkably and sustained enhancement of both humoral and cellular immune responses, with an increased neutralising potency and breadth against SARS‐CoV‐2 variants as compared to vaccination in SARS‐CoV‐2‐naïve individuals. Furthermore, we reveal a significant decline in BAU mL−1 and pseudo‐neutralising antibody titres over the first months following both ChAdOx1 nCoV‐19 and BNT162b2 vaccination. Direct comparisons showed substantially lower titres following immunisation with ChAdOx1 nCoV‐19 than with BNT162b2. These findings are important and can be of support in the decisions on booster doses and design of current and future SARS‐CoV‐2 vaccine programmes.
We and others have previously shown that COVID‐19‐recovered vaccinees mount strong immune responses following mRNA 9 , 19 , 20 , 21 , 22 , 23 and adenovirus‐vectored vaccines. 24 We here extend these findings by showing that both T‐cell responses (herein measured both by a traditional ELISpot assay using overlapping peptides spanning whole protein sequences and by a whole‐blood IGRA‐based method using carefully selected SARS‐CoV‐2 unique S‐derived peptides) and antibody titres remain substantially increased over 3 months following ChAdOx1 nCoV‐19 and 7 months following BNT162b2 in HCW with predominantly mild SARS‐CoV‐2 infection prior to vaccination. This suggests that the effect of a previous infection followed by vaccination on immune responses is not a temporary phenomenon. The memory compartment continues to evolve after both natural infection and vaccination, but with a lesser increase in breadth following vaccination than infection. 18 Importantly, we found that SARS‐CoV‐2‐recovered vaccinees developed higher neutralising antibody responses against SARS‐CoV‐2 variants than SARS‐CoV‐2‐naïve vaccinees throughout the study period. These findings suggest that vaccination of immune‐competent SARS‐CoV‐2‐recovered individuals evokes improved cellular responses and antibody levels compared with that of SARS‐CoV‐2‐naïve vaccinees.
Our findings of substantial reductions in antibody titres over the first 3 months following ChAdOx1 nCoV‐19 and 7 months following BNT162b2 are in line with several reports of waning vaccine efficacy from countries including Israel, 4 , 5 the UK 6 , 7 and the United States. 8 The comparatively lower titres following ChAdOx1 nCoV‐19 are also in line with prior data on lower vaccine efficacy of adenovirus‐vectored vaccines than that of mRNA vaccines. 2 , 29 Specific antibody levels are used as a correlate of protection against infection following several established vaccines, 30 and for SARS‐CoV‐2, binding and pseudo‐neutralising antibody titres correlate inversely with the risk of infection. 14 , 31 Recently, an 80% vaccine efficacy against symptomatic SARS‐CoV‐2 infection was observed at 264 BAU mL−1, declining to 60% vaccine efficacy at 54 BAU mL−1, 32 with similar results in another study. 33 Our findings suggest that antibody titres have declined below these thresholds in a portion of study participants within 3–7 months from the second vaccine dose, indicating a need for a third booster in a subpopulation of the vaccinated individuals. Importantly, the estimations were derived in eras with a dominance of the Alpha variant, and the estimated titres may provide lower efficacy against infection with the Delta variant, 34 , 35 , 36 which seems to have a higher transmissibility. 37 , 38
An optimal dose interval has been proposed to convey better vaccine efficacy via improved memory formation. 17 A longer interval may indeed generate an improved memory formation, 39 albeit at the price of a suboptimal protection during the longer interval between first and second doses in SARS‐CoV‐2‐naïve populations. Although no differences in binding antibody titres were observed, a significant difference in pseudo‐neutralisation titres was found between the 3‐ to 4‐week and 6‐ to 8‐week dose interval in SARS‐CoV‐2‐naïve participants, possibly indicating improved memory formation in the latter group. 39 Notably, the dose interval difference was not large in this study, and a longer dose interval may render greater enhancement, as recently demonstrated by Payne et al. In addition, we found that a longer time between SARS‐CoV‐2 infection and vaccination enhances neutralising antibody potency and breadth. Continued studies are needed to address potential differences in the longevity of the immunological memory induced by different prime‐boost intervals and combinations of various vaccine platforms, as well as the optimal timing of additional vaccine doses.
This study is limited by the observational and single‐centre nature. The study cohort, moreover, comprised HCW and a majority of women of general working age. Antibody trajectories may differ in older populations, and in settings without repeated viral encounters with potential boosting of the immune memory.
In summary, the striking and sustained enhanced cellular immune responses, antibody titres and neutralising breadth in previously SARS‐CoV‐2‐infected vaccinees as compared to SARS‐CoV‐2‐naïve vaccinees highlight the strong impact of infection prior to vaccination. These findings suggest that prior SARS‐CoV‐2 infection should be taken into consideration in vaccine policymaking, when planning booster doses, and in the design of current and future SARS‐CoV‐2 vaccine programmes.
Methods
Study population
The participants of the COMMUNITY study are followed every 4 months since inclusion in April 2020, and SARS‐CoV‐2 spike‐specific IgG antibodies are analysed by multiplex antigen bead array. 40 SARS‐CoV‐2 infection is confirmed by seroconversion at any of the follow‐up visits before vaccination and/or PCR‐confirmed infection obtained from the national registry holding all PCR‐verified SARS‐CoV‐2 infections in Sweden. 41 Prior to all visits, participants are asked to respond to a standardised questionnaire through a smartphone app system, including hospitalisation because of COVID‐19 and any predefined chronic diseases (hypertension, diabetes, and cardiovascular, pulmonary, renal, liver, neuropsychiatric/psychiatric, muscle/joint or thyroid disease). Participants with confirmed SARS‐CoV‐2 infection after vaccination (n = 3) were excluded from this study. Date and type of vaccine were obtained through the Swedish vaccination register (VAL Vaccinera). The study is approved by the Swedish Ethical Review Authority (dnr 2020‐01653), and written informed consent was obtained from all study participants.
Binding and pseudo‐neutralising antibodies
Binding antibodies (IgG) and pseudo‐neutralising antibodies against SARS‐CoV‐2 wild‐type (WT) and B.1.1.7 (Alpha), B.1.351 (Beta), B.1.526.1 (New York), B.1.617 (India), B.1.617.1 (Kappa), B.1.617.2 (Delta), B.1.617.3 (India), P.1 (Gamma) and P.2 (Zeta) variants were measured in post‐vaccination samples using the V‐PLEX SARS‐CoV‐2 Panel 13 (Meso Scale Diagnostics, Maryland, USA) for IgG and ACE2 (quantifying the ability to inhibit the binding between the ACE2 receptor and the spike protein), respectively, as previously described. 24 Binding antibody titres for WT were calibrated against the WHO standard 16 according to the manufacturer’s instructions and presented as binding antibody units (BAU mL−1). Pseudo‐neutralising antibodies are expressed as arbitrary units (AU mL−1).
Microneutralisation assay
Microneutralisation based on cytopathic effects (CPE) was performed essentially as previously described. 42 Briefly, serum was threefold serially diluted, mixed with virus, incubated for 1 h and finally added, in duplicates, to confluent Vero E6 cells in 96‐well plates. Original SARS‐CoV‐2 WT (isolated from a Swedish patient) and the Delta variant (from Statens Serum Institut, Copenhagen, Denmark) were used. After 5 days of incubation, the wells were inspected for signs of CPE by optical microscopy. Each well was scored as either neutralising (if no signs of CPE were observed) or non‐neutralising (if any CPE was observed). The arithmetic mean neutralisation titre of the reciprocals of the highest neutralising dilutions from the two duplicates for each sample was then calculated.
SARS‐CoV‐2‐specific memory T‐cell response
A whole‐blood interferon‐gamma (IFN‐γ) release assay (IGRA) was performed as previously described. 13 , 28 A SARS‐CoV‐2‐specific peptide pool was generated using 8 SARS‐CoV‐2‐specific peptides covering the SARS‐CoV‐2 spike. Peptides were synthesised with a purity of >95% and contained no more than 5‐mer length overlap with endemic coronaviruses. 13 , 28 Peripheral blood was collected in lithium heparin tubes, and 0.5 mL was added to glucose (2 mg mL−1 whole blood) and 0.9% NaCl with and without the stimulant. Samples were incubated at 37°C with 5% CO2 for 20 h. IFN‐γ was analysed in plasma using a Mesoscale Discovery V‐plex kit (Meso Scale Diagnostics, Maryland, USA). The whole‐blood IGRA was performed in a subset of 44 BNT162b2‐vaccinated participants 3 months post‐vaccination [28 SARS‐CoV‐2 naïve, age 53 (IQR 38–63), 79% women; and 16 SARS‐CoV‐2 recovered, age 47 (IQR 39–55), 69% women], 32 BNT162b2‐vaccinated participants 7 months post‐vaccination [21 SARS‐CoV‐2 naïve, age 53 (IQR 43–60), 95% women; and 11 SARS‐CoV‐2 recovered, age 51 (IQR 39–56), 82% women] and 45 ChAdOx1 nCoV‐19‐vaccinated participants 3 months post‐vaccination [27 SARS‐CoV‐2 naïve, age 55 (IQR 46–61), 85% women; and 18 recovered, age 52 (IQR 46–60), 83% women].
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using CPT tubes. After quantification and dilution of recovered cells, 250 000 PBMCs were plated into each well of a T‐SPOT® Discovery SARS‐CoV‐2 kit (Oxford Immunotec, Oxfordshire, UK), according to the manufacturer’s instructions. The kit contains overlapping peptide pools covering protein sequences of six SARS‐CoV‐2 antigens, without HLA restriction. Peptide sequences with high homology to endemic coronaviruses were removed, but sequences that may have homology to SARS‐CoV‐1 were retained. Cells were incubated for 20 h and interferon‐γ‐secreting T cells detected. The T‐spot analyses were performed in a subset of 17 BNT162b2 vaccinees 7 months post‐vaccination [8 SARS‐CoV‐2 naïve, age 58 (IQR 52–62), 100% women; and 9 recovered, age 51 (IQR 35–59), 67% women].
Statistical analyses
Spike IgG and pseudo‐neutralising antibody titres are presented as geometric mean titres (GMTs) with 95% confidence interval (CI). A linear regression analysis was used to compare continuous variables between the groups and was adjusted for sex and age. A t‐test on logarithmised values was used to compare differences between the T‐cell analysis groups, because of the small number of study participants. Multiple samples per subject were analysed using the linear mixed‐effects regression analysis with random intercepts per subject using the same adjustments as for the linear regression analysis. For the time since seroconversion to vaccination, we used similar mixed‐effects models but adjusted for sex, age, time since seroconversion to vaccine, side effects and time since vaccination. Interactions were added for sex and age and for side effect and prevalence of seroconversion. The correlation coefficient was calculated using Spearman’s correlation analysis. Linear regression and mixed‐effects regression analyses were performed in R (version 4.1.1) with nlme‐package version 3.1.152 and contrast‐package 0.22, while the remainder were done in GraphPad Prism (version 9.1.1).
Conflict of interest
SoH has participated in the AstraZeneca COVID‐19 SCG Virtual Advisory Board. Otherwise, the authors declare no competing interests.
Author contribution
Sebastian Havervall: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing. Ulrika Marking: Conceptualization; Investigation; Methodology; Writing – review & editing. Nina Greilert‐Norin: Investigation; Project administration; Writing – review & editing. Max Gordon: Data curation; Investigation; Methodology; Visualization; Writing – review & editing. Henry Ng: Formal analysis; Investigation; Writing – review & editing. Wanda Christ: Investigation; Methodology; Writing – review & editing. Mia Phillipson: Supervision; Writing – review & editing. Peter Nilsson: Funding acquisition; Investigation; Writing – review & editing. Sophia Hober: Funding acquisition; Investigation; Supervision; Writing – review & editing. Kim Blom: Formal analysis; Investigation; Methodology; Writing – review & editing. Jonas Klingström: Funding acquisition; Investigation; Methodology; Writing – review & editing. Sara Mangsbo: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Writing – review & editing. Mikael Åberg: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Writing – review & editing. Charlotte Thålin: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Supporting information
Supplementary figure 1. Live‐microneutralization titers in SARS‐CoV‐2 naïve healthcare workers vaccinated with either BNT162b2 or ChAdOx1 nCoV‐19.
Acknowledgments
This work has been funded by the Jonas & Christina af Jochnick Foundation (to CT); Lundblad Family Foundation (to CT); Region Stockholm (to CT); Knut and Alice Wallenberg Foundation (to CT and SM); Jonas Söderquist’s scholarship (to CT); Science for Life Laboratory (to PN); Erling‐Persson Family Foundation (to SoH); Center for Innovative Medicine (to JK); and Swedish Research Council (to JK). The funders above had no role in the design and conduction of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability statement
The anonymised data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voysey M, Clemens SAC, Madhi SA et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas SJ, Moreira ED, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine through 6 months. N Engl J Med 2021; 385: 1761–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldberg Y, Mandel M, Bar‐On YM et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med 2022; 385: e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bar‐On YM, Goldberg Y, Mandel M et al. Protection of BNT162b2 vaccine booster against covid‐19 in Israel. N Engl J Med 2021; 385: 1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pouwels KB, Pritchard E, Matthews PC et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS‐CoV‐2 infections in the UK. Nat Med 2021; 27: 2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shrotri M, Navaratnam AMD, Nguyen V et al. Spike‐antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021; 398: 385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tartof SY, Slezak JM, Fischer H et al. Effectiveness of mRNA BNT162b2 COVID‐19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021; 398: 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goel RR, Apostolidis SA, Painter MM et al. Distinct antibody and memory B cell responses in SARS‐CoV‐2 naïve and recovered individuals following mRNA vaccination. Sci Immunol 2021; 6: eabi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazzoni A, Di Lauria N, Maggi L et al. First‐dose mRNA vaccination is sufficient to reactivate immunological memory to SARS‐CoV‐2 in subjects who have recovered from COVID‐19. J Clin Invest 2021; 131: e149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Painter MM, Mathew D, Goel RR et al. Rapid induction of antigen‐specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS‐CoV‐2 mRNA vaccination. Immunity 2021; 54: 2133–2142.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oberhardt V, Luxenburger H, Kemming J et al. Rapid and stable mobilization of CD8+ T cells by SARS‐CoV‐2 mRNA vaccine. Nature 2021; 597: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Havervall S, Ng H, Falk AJ et al. Robust humoral and cellular immune responses and low risk for reinfection at least eight months following asymptomatic to mild COVID‐19. J Intern Med 2022; 291: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khoury DS, Cromer D, Reynaldi A et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med 2021; 27: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 15. Bergwerk M, Gonen T, Lustig Y et al. Covid‐19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021; 385: 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kristiansen PA, Page M, Bernasconi V et al. WHO International Standard for anti‐SARS‐CoV‐2 immunoglobulin. Lancet 2021; 397: 1347–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity 2010; 33: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cho A, Muecksch F, Schaefer‐Babajew D et al. Anti‐SARS‐CoV‐2 receptor binding domain antibody evolution after mRNA vaccination. Nature 2021; 600: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krammer F, Srivastava K, Alshammary H et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med 2021; 384: 1372–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stamatatos L, Czartoski J, Wan YH et al. mRNA vaccination boosts cross‐variant neutralizing antibodies elicited by SARS‐CoV‐2 infection. Science 2021; 372: 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Z, Schmidt F, Weisblum Y et al. mRNA vaccine‐elicited antibodies to SARS‐CoV‐2 and circulating variants. Nature 2021; 592: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lucas C, Vogels CBF, Yildirim I et al. Impact of circulating SARS‐CoV‐2 variants on mRNA vaccine‐induced immunity. Nature 2021; 600: 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ebinger JE, Fert‐Bober J, Printsev I et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nat Med 2021; 27: 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Havervall S, Marking U, Greilert‐Norin N et al. Antibody responses after a single dose of ChAdOx1 nCoV‐19 vaccine in healthcare workers previously infected with SARS‐CoV‐2. EBioMedicine 2021; 70: e103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rudberg A‐S, Havervall S, Månberg A et al. SARS‐CoV‐2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun 2020; 11: 5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Havervall S, Rosell A, Phillipson M et al. Symptoms and functional impairment assessed 8 months after mild COVID‐19 among health care workers. JAMA 2021; 325: 2015–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parry H, Bruton R, Stephens C et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines 2022; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mangsbo SM, Havervall S, Laurén I et al. An evaluation of a FluoroSpot assay as a diagnostic tool to determine SARS‐CoV‐2 specific T cell responses. PLoS One 2021; 16: e0258041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel MM, Thornburg NJ, Stubblefield WB et al. Change in antibodies to SARS‐CoV‐2 over 60 days among health care personnel in Nashville, Tennessee. JAMA 2020; 324: 1781–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plotkin SA. Correlates of protection induced by vaccination. Clinical Vaccine Immunol 2010; 17: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Earle KA, Ambrosino DM, Fiore‐Gartland A et al. Evidence for antibody as a protective correlate for COVID‐19 vaccines. Vaccine 2021; 39: 4423–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng S, Phillips DJ, White T et al. Correlates of protection against symptomatic and asymptomatic SARS‐CoV‐2 infection. Nature Med 2021; 27: 2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilbert PB, Montefiori DC, McDermott A et al. Immune correlates analysis of the mRNA‐1273 COVID‐19 vaccine efficacy clinical trial. Science 2022; 375: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheikh A, McMenamin J, Taylor B, Robertson C. SARS‐CoV‐2 delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021; 397: 2461–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez Bernal J, Andrews N, Gower C et al. Effectiveness of Covid‐19 vaccines against the B.1.617.2 (delta) variant. New England J Med 2021; 385: 585–594. 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wall EC, Wu M, Harvey R et al. Neutralising antibody activity against SARS‐CoV‐2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021; 397: 2331–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ong SWX, Chiew CJ, Ang LW et al. Clinical and virological features of SARS‐CoV‐2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin Infect Dis 2021; ciab721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teyssou E, Delagrèverie H, Visseaux B et al. The Delta SARS‐CoV‐2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID‐19 patients. J Infect 2021; 83: e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Payne RP, Longet S, Austin JA et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021; 184: 5699–5714.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hober S, Hellström C, Olofsson J et al. Systematic evaluation of SARS‐CoV‐2 antigens enables a highly specific and sensitive multiplex serological COVID‐19 assay. Clin Transl Immunol 2021; 10: e1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Public Health Agency of Sweden . SmiNet, 2022‐04‐07. https://www.folkhalsomyndigheten.se/sminet/
- 42. Varnaitė R, García M, Glans H et al. Expansion of SARS‐CoV‐2‐specific antibody‐secreting cells and generation of neutralizing antibodies in hospitalized COVID‐19 patients. J Immunol 2020; 205: 2437–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Live‐microneutralization titers in SARS‐CoV‐2 naïve healthcare workers vaccinated with either BNT162b2 or ChAdOx1 nCoV‐19.
Data Availability Statement
The anonymised data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


