Abstract
Background
Umbilical cord mesenchymal stem cells (UC-MSCs)-derived secretome is currently used in regenerative therapy. MSCs are believed to secrete a wide spectrum of bioactive molecules which give paracrine effects in immunomodulation and regenerative capacities. One group that was found in secretome is interleukins (ILs), a cytokine that plays an essential role in the process of proliferation, differentiation, maturation, migration, and adhesion of immune cells. However, as there are many types of ILs, the profile of ILs in the UC-MSCs-derived secretome has been limitedly reported. Therefore, in this study, we would like to profile and detect the interleukin concentration secreted by UC-MSCs.
Methods
UC-MSCs-derived secretome was collected from UC-MSCs passage 5 after 24- and 48-hour incubation (n=9). Secretome was filtered using 0.2 µm and stored at −80°C for further detection. All samples were normalized before the interleukin (IL-2, IL-4, IL-6, IL-9, IL-10, IL-12, IL-17A) detection using a MACSPlex Cytokine Kit.
Results
The IL-6 has the highest concentration among other interleukins in both groups and increases significantly (p<0.003) after incubation for 48 hours. The pro-inflammatory factors are decreasing while anti-inflammatory factors are increasing after 48-hour incubation.
Discussion
Our studies show that the UC-MSCs secrete pro- and anti-inflammatory interleukins. The concentration of anti-inflammatory interleukins shows to be increasing, while the pro-inflammatory interleukins are decreasing within the longer incubation time, but this not be applicable for IL-10 and IL-6. IL-6 has the highest concentration among other ILs. These results may provide important clues regarding when is the right time for secretome to be used in therapy patients, because all the molecules in the secretome can lead to many clinical manifestations.
Keywords: secretome, mesenchymal stem cell, interleukin, conditioned medium, umbilical cord
Introduction
In recent years, mesenchymal stem cells (MSCs) have gained much interest as therapeutic agents for immunomodulation and tissue regeneration in regenerative medicine.1 The MSCs are shown to have the ability to differentiate into various cell.2 While most of the sources of MSCs show the same characteristics as the minimal criteria release by the International Society of Cell and Gene Therapy (ISCT), the umbilical cord (UC) tissue is reported to be an eminent source of human MSCs (hMSCs) compared to other sources.3,4 The UC-MSCs are reported to have more immune privilege than the other sources because they show less immunogenicity, and also have a higher proliferation capacity. Besides that, when UC tissues are collected as the source of MSCs, the process is proved to be less invasive compared to the process of adipose tissue and bone marrow collection.5,6
Although there are limited reports that the transplanted MSCs in humans lead to graft rejection, the cell-free therapy using MSCs-derived secretome is currently in great demand for clinical application.7 The paracrine effects hypothesis becomes more consolidated because the MSCs secrete a wide spectrum of bioactive molecules that contains extracellular vesicles, proteins, growth factors, lipids, chemokines, and cytokines.8 These bioactive molecules are referred to as secretome, which holds capacity as immunomodulatory and regenerative agents to repair and maintain the homeostasis in injured tissues.9
The therapeutic effects of secretome rely on the factors inside the secretome. Many physiological processes such as cells apoptosis, proliferation, migration, and angiogenesis are regulated by the extracellular vesicles in the secretome.9 However, some difficulties arise in obtaining MSCs-derived secretome due to the complex mechanisms of the production process. Many factors could affect the secretion of MSCs’ secretome, such as the source of MSCs, the culturing method, the pre-conditioning treatment during in vitro culture, and others.10–12 Hence, there are many studies which have tried to explore the profile of the secretome. Currently, it has been reported that there are over 400 proteins detected in the secretome.13 However, there are still limited studies reporting on the profile of Interleukins (ILs). ILs are a type of cytokine that is found in the secretome. The ILs are produced by cells and play an essential role in the immune system. There are two types of ILs’ properties: pro-inflammatory and anti-inflammatory, which will activate the process of proliferation, differentiation, maturation, migration, and adhesion of immune cells during inflammation and immune responses. Understanding the profile and the concentration of ILs in the UC-MSCs-derived secretome will give more information of when is the right time for the secretome to be used in clinical applications. Therefore, in this study, we would like to profile and detect the interleukin concentration secreted by UC-MSCs. Another finding is the time collection of UC-MSCs-derived secretome will affect the interleukins concentration.
Method
This study had been approved by the ethical advisory board of the University of Padjadjaran with ethical number 672/UN6.KEP.EC/2020. All the studies were conducted at Prodia StemCell (ProSTEM) laboratory.
UC-MSCS Expansion
The UC-MSCs used in the secretome production were isolated after cesarean section and had already been screened for infectious diseases according to the Food and Drug Association. All the donors has signed the informed consent. The UC-MSCs were expanded until passage 5 using the complete growth medium. DMEM High Glucose (Lonza, Walkersville, MD) supplemented with 5% Human Platelet Lysate (AventaCell, Atlanta, GA) were used as the complete growth medium. The cultures were maintained at 37°C with 5% CO2 and the growth medium was changed every 3–4 days until it reaches the confluency.
Characteristic of UC-MSCs
The confirmation of UC-MSCs characteristics were conducted before the secretome production to confirm the minimal criteria of MSCs according to the International Society of Cell and Gene Therapy (ISCT) in 2006.14,15 The morphology of UC-MSCs were checked using an inverted microscope.
UC-MSCs Immunophenotyping
The identity of UC-MSCs was confirmed using flow cytometry to detect the phenotypic cell surface marker.16,17 Antibodies of CD73-PerCP.Cy5, CD90-FITC, and CD105-APC were used as positive markers, and Lineage marker (CD34-PE, CD45-PE, CD11b-PE, CD19-PE, HLA-DR-PE) (BD Bioscience, San Diego, CA) as negative markers. Antibodies were incubated with 1×106 UC-MSCs for 30 minutes and centrifuge 300 g for 5 minutes. The supernatant was discarded, and the samples were resuspended with PBS prior to detection with a BD FACSCanto II.
UC-MSCs Differentiation
The differentiation ability of UC-MSCs was tested for adipogenic, chondrogenic, and osteogenic according to ISCT requirements using a commercialized differentiation medium (Lonza, Walkersville, MD). The three differentiation abilities were verified using Oil Red O staining for adipogenic differentiation, Alcian Blue staining for chondrogenic differentiation, and Alizarin Red S staining for osteogenic differentiation.
UC-MSCs Secretome Production
The production of secretome was conducted in three batches and tripled. The seeding density of UC-MSCs was 5,000 cells/cm2 and the the cells were incubated at 37°C with 5% CO2. Upon the UC-MSCs reaching 80% confluency, the growth medium was discarded and changed into DMEM without supplementation for secretome production. The treatments were separated into two groups with 24- and 48-hours incubation time after medium changing. Each sample collected was filtered using 0.2 µm and stored at −80°C.18
Detection of Total Protein Concentration
The total protein concentration was calculated using the BCA Protein Assay (Abcam, New Territories, Hong Kong) before conducting the interleukin assay to normalize the samples concentration.19 Working solution was added to the samples in a clear bottom 96-well plate. Samples were incubated for 90 minutes at 37°C before measuring the optical density at 562 nm.
Interleukin Assay
Total protein concentration was detected to normalize the secretome before interleukin assay. Interleukin assay was detected using MACSPlex Cytokine Kit (Miltenyi GmbH, Bergisch, Germany). Samples were prepared according to manufacture kit instructions and detected using BD FACS Diva Software.20
Statistical
The data are presented as mean ± standard deviation (SD). Data analysis was conducted using SPSS 24. A Shapiro–Wilk test for normality was carried out for all datasets, and data were analyzed using either parametric or non-parametric tests accordingly. P-values <0.05 were considered significant.
Results
Characteristics of UC-MSCs
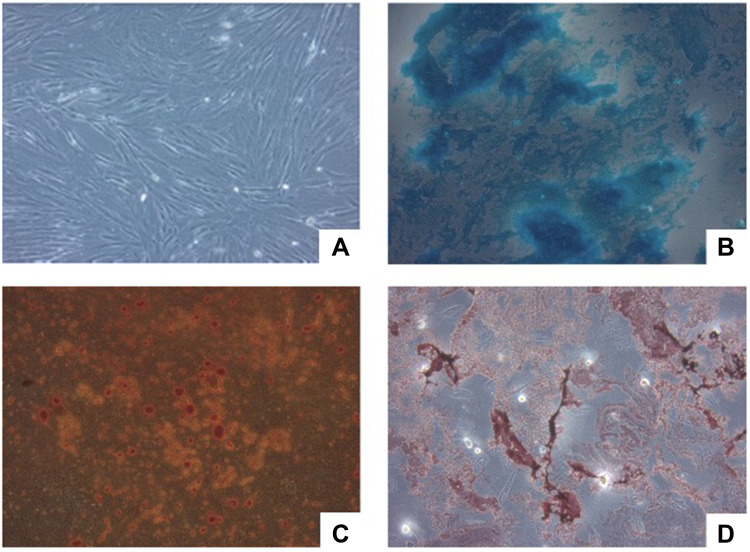
The morphological cell was observed before secretome production using inverted microscopy. The UC-MSCs were plastic-adherent cells which attach to the bottom of the culture dish. The shape of UC-MSCs were relatively homogenous with characteristic elongated, spindle-shaped, and fibroblast-like morphology (Figure 1A).
Figure 1.
Microscopic analysis of cell morphology and differentiation capacity. (A) UC-MSCs morphology under a 100x inverted microscope. (B) Positive result of UC-MSCs chondrogenic differentiation capacity. (C) Positive result of UC-MSCs osteogenic differentiation capacity. (D) Positive result of UC-MSCs adipogenic differentiation capacity.
Differentiation Capacity
The UC-MSCs used for secretome production show the ability to differentiate into three lineages (Figure 1B–D). This is consistent with the minimal criteria of MSCs released by ISCT in 2006.14 The adipogenic differentiation capacity was shown by the red color in Figure 1D. The Oil Red O stained the lipids that are produced by the adipocyte cells. In Figure 1B, the blue color represented a positive result of Alcian Blue that stains the acidic polysaccharides produced by chondrocytes cells. The UC-MSCs are also shown to have the capacity to differentiate into osteogenic cells by staining the calcium deposited using the Alizarin Red S (Figure 1C).
Immunophenotyping of UC-MSCs
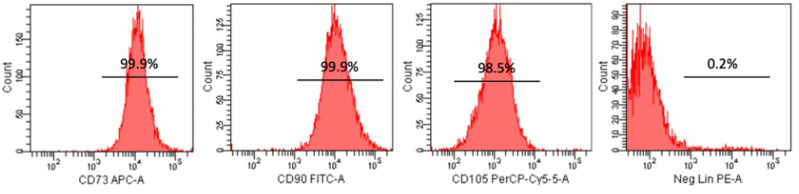
The profile of UC-MSCs was determined for the expression of cell surface marker proteins using flow cytometry (Figure 2). The results indicate that the UC-MSCs stained with CD73, CD90, and CD105 show positive expression with percentages of 99.9%, 99.9%, and 98.5%, respectively. In turn, the lineage marker which consists of an antibody cocktail (CD34, CD45, CD11b, CD19, and HLA-DR) was negative (0.2%). This result shows that the UC-MSCs represented the MSCs criteria.14
Figure 2.
Immunophenotyping of UC-MSC using CD73, CD90, and CD105 as positive marker and lineage as a negative marker.
Effect of Incubation Time in Total Protein Concentration
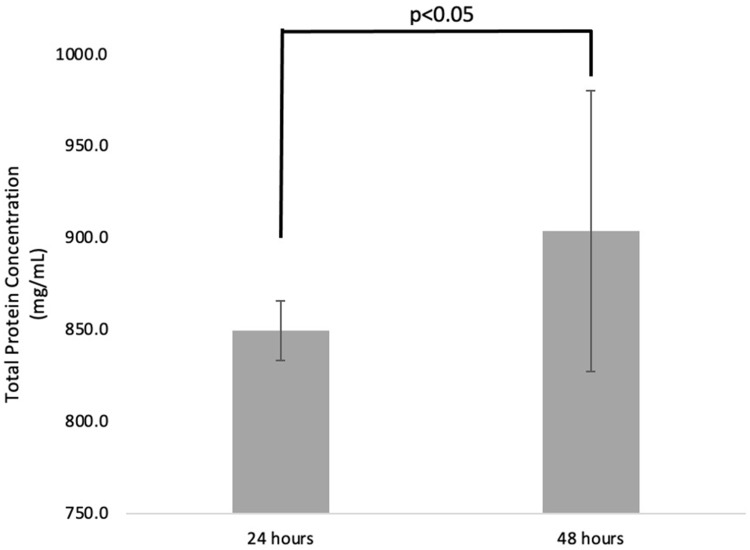
Nine samples of total protein secreted by the UC-MSCs from each group were detected after 24 hours and 48 hours post-treatment. The concentration of total protein (mean±SD) for 24 hours and 48 hours was 849.5±16.27 mg/mL and 903.8±76.46 mg/mL, respectively. The total proteins were increasing significantly (p<0.05) after 48 hours compared to 24 hours post-treatment (Figure 3).
Figure 3.
The concentration of total protein UC-MSCs-derived secretome after 24- and 48-hours post-treatment.
Effect of Incubation Time in Interleukin Concentration
Interleukin concentration was detected for pro- and anti-inflammatory factors, as shown in Table 1. The IL-6 has the highest concentration among other interleukins in both 24 and 48 hours post-treatment. As the pro-inflammatory cytokine, IL-6 is the only one that increased significantly (p<0.003) when incubated for 48 hours, about 3-times higher. The other pro-inflammatory cytokines (IL-9, IL-12, and IL-17A) are decreasing, but only IL-12 has a significant difference between both treatments (p<0.004).
Table 1.
Interleukin Concentration
| Treatment Time | p<0.05 | |||||
|---|---|---|---|---|---|---|
| 24 Hours (n=9) | 48 Hours (n=9) | |||||
| Mean | SD | Mean | SD | |||
| Anti-inflammatory | IL-2 (ng/mL) | 187 | 78 | 507 | 526 | 0.009* |
| IL-4 (ng/mL) | 493 | 27 | 505 | 4 | 0.206 | |
| IL-10 (ng/mL) | 1,069 | 55 | 1,026 | 15 | 0.036* | |
| Pro-inflammatory | IL-6 (ng/mL) | 5,061 | 2792 | 15,651 | 8,666 | 0.003* |
| IL-9 (ng/mL) | 299 | 71 | 276 | 8 | 0.363 | |
| IL-12 (ng/mL) | 476 | 8 | 467 | 4 | 0.004* | |
| IL-17A (ng/mL) | 3,031 | 29 | 3,005 | 33 | 0.095 | |
Note: *Significantly different (p<0.05).
Abbreviation: IL, interleukins.
The anti-inflammatory factor concentrations such as IL-2 and IL-4 are increasing after a 48 hours incubation period compared to a 24 hours period. However only the IL-2 concentration increased significantly (p<0.009). The mean concentration of IL-10 is significantly higher after 24 hours incubation compare to 48 hours (p<0.036).
Discussion
Umbilical cords have emerged as the source of MSCs because they can be harvested easily and non-invasively during the labor process.3 Many clinical trials are reported to use the UC-MSCs for treating various diseases due to their potency to self-renew and the ability to differentiate into multi-lineage cell types.1 The other benefits of using UC-MSCs are they could be cultured and expanded while retaining the stemness characteristic. MSCs also show low immune rejection when transplanted for allogenic. Those characteristics lead to a high number of research using the UC-MSCs for clinical applications.21
In 2006, Dominici et al14 released the minimal criteria for MSCs to address the issue of standardization of MSCs used for clinical applications. The first characteristic is that the MSCs must be plastic adherent when culture in a standard culture treated dish. Based on the observation of the UC-MSCs morphology characteristic under an inverted microscope used in this study, the first characteristic is present in the UC-MSCs. This becomes the basic standard characterization during the expansion of the UC-MSCs. The phenotypic marker of cluster differentiation (CD) is the second characteristic used to determine the identity of the MSCs. Phenotypically there is no specific marker for MSCs, however it is generally agreed that the human MSCs can express CD105 (SH2), CD73 (SH3/4), CD44, CD90 (Thy-1), CD71, and Stro-1 but not the hematopoietic markers such as CD45, CD34, CD14, or CD11.22–24 The UC-MSCs used in this study corresponded with all the criteria which represented over 98% for the positive marker and below 2% for the negative marker for the cell surface marker characteristic.
In addition to identification of UC-MSCs, the differentiation capacity into the three lineages, bone, fat, and cartilage, are carried out to convince the UC-MSCs prior to the secretome production. The dexamethasone, insulin, isobutyl methyl xanthine, and indomethacin are used in the culture medium for the adipogenic differentiation. The adipocyte capacity is characterized by the Oil Red s staining that stains the lipids vacuole produced by the adipocyte cells. The Alizarin Red are used to detect the calcium deposit from osteoblast when cultured with differentiation medium supplemented with ascorbic acid, B-glycerophosphate, and dexamethasone for 2 weeks.25 During the culture period, the MSCs will form aggregates that accumulate the calcium over the time. Last, the chondrogenic differentiation capacity is induced by the formation of proteoglycans and will present by color when stained with the Alcian Blue.26
UC-MSCs are reported to secrete biologically active substances that have the paracrine ability called secretome. The secretome composed by cytokines, chemokines, growth factors, proteins, and extracellular vesicles.10 Every active substance can produce different effects leading to the process of therapeutic action of MSCs-derived secretome when transplanted to a patient. As the culture time is increasing, the MSCs will secrete more secretome. This was proved by the increasing concentration of total protein concentration after 24 hours and 48 hours treatment.
One of the major groups of active substances is interleukin, a group of cytokine that act as anti- and pro-inflammatory factors. The interleukins were first detected in the leukocytes and have a major function related to immune systems. They play an essential role in the activation, proliferation, and differentiation of immune cells. It is known that the production of interleukins is a self-limited process.27 Therefore, it is important to understand the effect of culture time related to interleukins secreted by the UC-MSCs.
In this study, the IL-6 concentration was compared with other interleukins and reported to be the highest both in 24 hours and 48 hours secretome collection. It has been reported in other studies before that the IL-6 secreted predominantly in MSCs population. The role of IL-6 in the regulation of immune systems has been described as both pro- and anti-inflammatory. IL-6 induces the endothelial permeability and cell recruitment, B-cell maturation, and T-cell survival but also induces the secretion of anti-inflammatory molecule such as IL-10 and Tumor Necrosis Factor (TNF)-A. Dorronsoro et al reported in their study that the IL-6 is essential for proliferation of hMSCs, this correlate to our result that the concentration of IL-6 is increasing significantly within time-dependent.28,29
The IL-6 might affect the secretion of IL-10, an anti-inflammatory cytokine that inhibits the IL-2, IL-12, and Interferon (IFN)-G. The IL-10 act as an inducer for immune tolerance on the dendritic cells. The mechanism related to immunoregulation of IL-6 from MSCs has been discussed and some of the published data are quite contradictory. Our study shows, when the UC-MSCs culture in a longer incubation period, the concentration of IL-10 is decreased significantly. Some studies have reported a positive secretion of IL-10 by the MSCs. These differences might be due to the involvement of the microenvironments and the presence of other cytokines that affect the secretion of IL-10. A further study to determine the IL-10 secretion from MSCs should be conducted.30
Other anti-inflammatory cytokine that significantly increases between the time culture periods is IL-2. The IL-2 effects the T-cells proliferation and increases cytokine synthesis, Fas-mediated apoptosis, and promoting regulatory T-cell development and differentiation.31 However, there is no significant difference for the IL-4, although the concentration of IL-4 seems to be increasing within the incubation period. The IL-4 is reported to play a role in the inhibition of IFN-G secretion and maintaining the IgE and IgG level in the human body.32 The mechanism of action from UC-MSCs-derived secretomes are explored limitedly.
The other pro-inflammatory interleukin concentrations that also detected in this study are decreasing within the incubation, except for the IL-6. Only IL-12 is reported to decrease significantly. Each of the interleukins have their own function. The IL-12 induces the production of IFN-G and could cause allergic disorders. The IL-9 is used to enhance the T-cell survival along with erythropoietin (EPO) and the IL-17A is an inducer for IL-6 secretion.27,33
Based on the profile of the UC-MSCs-derived secretome, pro- and anti-inflammatory cytokines are found to be secreted by the MSCs. The concentration of anti-inflammatory interleukins is shown to be increasing, while the pro-inflammatory interleukins are decreasing within the longer incubation time, but this not applicable for IL-10 and IL-6. These results might be due to the role of MSCs in keeping the homeostasis in their niche. Further studies should be conducted to evaluate to mechanism of interleukin interaction during this process. While using the UC-MSCs derived secretome, it is important to have an interprofessional team that will monitor the patient because all the molecules in the secretome can lead to many clinical manifestations.
Conclusion
Pro- and anti-inflammatory interleukins are secreted by UC-MSCS. IL-6 has the highest concentration among all ILs. The culture time is significantly influencing the concentration of total protein and interleukin concentration secreted by the UC-MSCs. These results may provide important clues regarding when is the right time to used secretome during therapy. Taken together, further studies must be conducted to clarify the regulation and roles to understanding the biological function of secretome for patients’ treatment.
Funding Statement
No funding to declare.
Data Sharing Statement
The reference journals used to support this study are included within the article and cited at relevant places within the test as references.
Ethics Approval and Consent to Participate
This study had been approved by the ethical advisory board of University of Padjadjaran with ethical number 672/UN6.KEP.EC/2020. All procedures were performed in compliance with provisions from the Declaration of Helsinki regarding research on human participants and all the donor signed informed consent for donation of umbilical cord.
Consent for Publication
There is no consent for publication applicable for this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicting interest in this work.
References
- 1.Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886. doi: 10.3390/cells8080886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2):e00191. doi: 10.1042/BSR20150025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6:195–202. doi: 10.4252/wjsc.v6.i2.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013;2013:1–8. doi: 10.1155/2013/916136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016;2016:1–17. doi: 10.1155/2016/6901286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selich A, Zimmermann K, Tenspolde M, et al. Umbilical cord as a long-term source of activatable mesenchymal stromal cells for immunomodulation. Stem Cell Res Ther. 2019;10(1):285. doi: 10.1186/s13287-019-1376-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):1852. doi: 10.3390/ijms18091852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabrowska S, Andrzejewska A, Janowski M, Lukomska B. Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front Immunol. 2021;11:591065. doi: 10.3389/fimmu.2020.591065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merino-González C, Zuñiga FA, Escudero C, et al. Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front Physiol. 2016:7. doi: 10.3389/fphys.2016.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9:2837. doi: 10.3389/fimmu.2018.02837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phelps J, Sanati-Nezhad A, Ungrin M, Duncan NA, Sen A. Bioprocessing of mesenchymal stem cells and their derivatives: toward cell-free therapeutics. Stem Cells Int. 2018;2018:1–23. doi: 10.1155/2018/9415367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chouw A, Facicilia G, Sartika CR, Faried A, Milanda T. Factors influencing the therapeutic potential of the MSC-derived secretome. Regen Eng Transl Med. 2022. doi: 10.1007/s40883-021-00242-x [DOI] [Google Scholar]
- 13.Eleuteri S, Fierabracci A. Insights into the secretome of mesenchymal stem cells and its potential applications. IJMS. 2019;20(18):4597. doi: 10.3390/ijms20184597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 15.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234 [DOI] [PubMed] [Google Scholar]
- 16.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342 [DOI] [PubMed] [Google Scholar]
- 17.Mirzaee Khalilabadi R, Halabian R, Bahmani P, Roushandeh AM, Kuwahara Y, Fukumoto M. Blood transfusion research center, high institute for research and education in transfusion medicine, Tehran, Iran, Yari F, blood transfusion research center, high institute for research and education in transfusion medicine, Tehran, Iran, amirizadeh N, blood transfusion research center, high institute for research and education in transfusion medicine, Tehran, Iran, et al. Free Radic Res. 2017;4:50–57. doi: 10.18869/acadpub.jbrms.4.2.50 [DOI] [Google Scholar]
- 18.Smith JR, Pfeifer K, Petry F, Powell N, Delzeit J, Weiss ML. Standardizing umbilical cord mesenchymal stromal cells for translation to clinical use: SELECTION of GMP-compliant medium and a simplified isolation method. Stem Cells Int. 2016;2016:1–14. doi: 10.1155/2016/6810980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Shi H, Yin S, et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12(8):7613–7628. doi: 10.1021/acsnano.7b07643 [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Zhu S, Li Y, et al. Human umbilical cord mesenchymal stem cells ameliorate skin fibrosis development in a mouse model of bleomycin‑induced systemic sclerosis. Exp Ther Med. 2020;20(5):1. doi: 10.3892/etm.2020.9387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J-H, Jo CH, Kim H-R, Hwang Y. Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical cord, and adipose tissue. Stem Cells Int. 2018;2018:1–12. doi: 10.1155/2018/8429042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojewski MT, Weber BM, Schrezenmeier H. Phenotypic characterization of mesenchymal stem cells from various tissues. Transfus Med Hemother. 2008;35(3):168–184. doi: 10.1159/000129013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musiał-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28:801–812. doi: 10.1177/0963689719837897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maleki M, Ghanbarvand F, Reza Behvarz M, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7(2):118–126. doi: 10.15283/ijsc.2014.7.2.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Shou P, Zheng C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128–1139. doi: 10.1038/cdd.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert AW, Marcon BH, Dallagiovanna B, Shigunov P. Adipogenesis, osteogenesis, and chondrogenesis of human mesenchymal stem/stromal cells: a comparative transcriptome approach. Front Cell Dev Biol. 2020;8:561. doi: 10.3389/fcell.2020.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justiz Vaillant AA, Qurie A. Interleukin StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [Google Scholar]
- 28.Caseiro AR, Santos Pedrosa S, Ivanova G, et al. Mesenchymal stem/ stromal cells metabolomic and bioactive factors profiles: a comparative analysis on the umbilical cord and dental pulp derived stem/ stromal cells secretome. PLoS One. 2019;14(11):e0221378. doi: 10.1371/journal.pone.0221378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorronsoro A, Lang V, Ferrin I, et al. Intracellular role of IL-6 in mesenchymal stromal cell immunosuppression and proliferation. Sci Rep. 2020;10(1):21853. doi: 10.1038/s41598-020-78864-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyurkchiev D. Secretion of immunoregulatory cytokines by mesenchymal stem cells. WJSC. 2014;6(5):552. doi: 10.4252/wjsc.v6.i5.552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada A, Arakaki R, Saito M, Kudo Y, Ishimaru N. Dual role of fas/fasl-mediated signal in peripheral immune tolerance. Front Immunol. 2017;8:8. doi: 10.3389/fimmu.2017.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snapper CM, Finkelman FD, Paul WE. Regulation of IgG1 and IgE Production by Interleukin 4. Immunol Rev. 1988;102(1):51–75. doi: 10.1111/j.1600-065X.1988.tb00741.x [DOI] [PubMed] [Google Scholar]
- 33.Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984–1010. doi: 10.1016/j.jaci.2016.06.033 [DOI] [PubMed] [Google Scholar]