Abstract
PURPOSE
The phase III PACIFIC trial compared durvalumab with placebo in patients with unresectable, stage III non–small-cell lung cancer and no disease progression after concurrent chemoradiotherapy. Consolidation durvalumab was associated with significant improvements in the primary end points of overall survival (OS; stratified hazard ratio [HR], 0.68; 95% CI, 0.53 to 0.87; P = .00251) and progression-free survival (PFS [blinded independent central review; RECIST v1.1]; stratified HR, 0.52; 95% CI, 0.42 to 0.65; P < .0001), with manageable safety. We report updated, exploratory analyses of survival, approximately 5 years after the last patient was randomly assigned.
METHODS
Patients with WHO performance status 0 or 1 (any tumor programmed cell death-ligand 1 status) were randomly assigned (2:1) to durvalumab (10 mg/kg intravenously; administered once every 2 weeks for 12 months) or placebo, stratified by age, sex, and smoking history. Time-to-event end point analyses were performed using stratified log-rank tests. Medians and landmark survival rates were estimated using the Kaplan-Meier method.
RESULTS
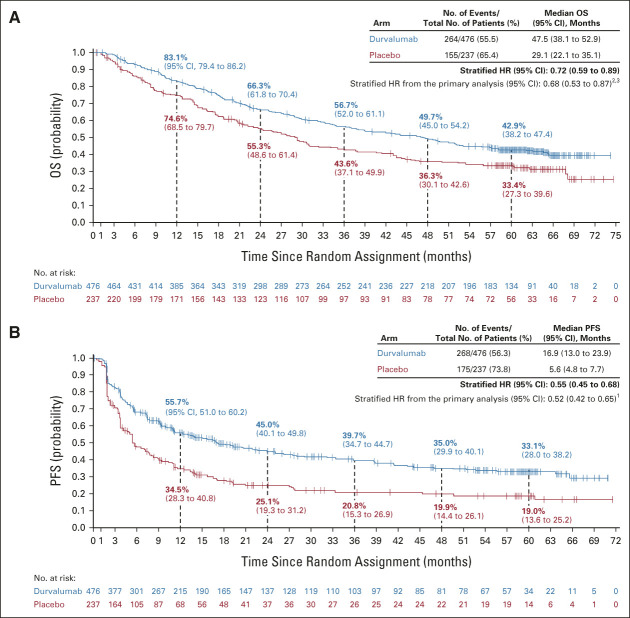
Seven hundred and nine of 713 randomly assigned patients received durvalumab (473 of 476) or placebo (236 of 237). As of January 11, 2021 (median follow-up, 34.2 months [all patients]; 61.6 months [censored patients]), updated OS (stratified HR, 0.72; 95% CI, 0.59 to 0.89; median, 47.5 v 29.1 months) and PFS (stratified HR, 0.55; 95% CI, 0.45 to 0.68; median, 16.9 v 5.6 months) remained consistent with the primary analyses. Estimated 5-year rates (95% CI) for durvalumab and placebo were 42.9% (38.2 to 47.4) versus 33.4% (27.3 to 39.6) for OS and 33.1% (28.0 to 38.2) versus 19.0% (13.6 to 25.2) for PFS.
CONCLUSION
These updated analyses demonstrate robust and sustained OS and durable PFS benefit with durvalumab after chemoradiotherapy. An estimated 42.9% of patients randomly assigned to durvalumab remain alive at 5 years and 33.1% of patients randomly assigned to durvalumab remain alive and free of disease progression, establishing a new benchmark for standard of care in this setting.
INTRODUCTION
In the phase III, placebo-controlled PACIFIC trial of patients with unresectable, stage III non–small-cell lung cancer (NSCLC) whose disease had not progressed after platinum-based concurrent chemoradiotherapy (CRT), administration of durvalumab (a programmed cell death-ligand 1 [PD-L1] inhibitor) for up to 12 months improved overall survival (OS; stratified hazard ratio [HR], 0.68; 95% CI, 0.53 to 0.87; P = .00251; March 22, 2018 data cutoff [DCO]) and progression-free survival (PFS; stratified HR, 0.52; 95% CI, 0.42 to 0.65; P < .0001; February 13, 2017 DCO).1-3 This degree of benefit with durvalumab versus placebo remained consistent at subsequent updates.4,5 Furthermore, durvalumab exhibited a manageable safety profile and did not detrimentally affect patient-reported outcomes compared with placebo.1,2,6 Durvalumab received global approvals on the basis of these findings,3,7,8 establishing consolidation durvalumab after CRT (the PACIFIC regimen) as standard of care (SoC) for patients with unresectable, stage III NSCLC.
CONTEXT
Key Objective
The phase III PACIFIC trial of patients with unresectable, stage III non–small-cell lung cancer whose disease had not progressed after chemoradiotherapy was, to our knowledge, the first study to demonstrate a survival advantage with immunotherapy in a curative-intent setting. Both primary end points of overall survival (OS) and progression-free survival (PFS) were improved with the programmed cell death-ligand 1 inhibitor durvalumab versus placebo. To provide insights into long-term outcomes, we report updated survival analyses, approximately 5 years after the last patient was randomly assigned.
Knowledge Generated
Updated OS and PFS remained consistent with the primary analyses; of patients randomly assigned to durvalumab, an estimated 42.9% remain alive at 5 years and 33.1% remain alive and progression-free. Consistent with prior reports, OS and PFS benefit continued to favor durvalumab over placebo in all prespecified patient subgroups.
Relevance
The findings support the continued use of consolidation durvalumab after chemoradiotherapy as the standard of care for patients with unresectable, stage III non–small-cell lung cancer.
Historically, SoC was CRT followed by observation alone; however, this was associated with poor long-term survival.9-12 There was no evidence that survival could be improved with induction or consolidation chemotherapy, consolidation therapy with other systemic anticancer agents, or by escalating the radiation dose.11,13-17 As the first study to demonstrate a survival advantage with immunotherapy in a curative-intent setting, PACIFIC represents a landmark advancement in the treatment of this population.
To provide insights into long-term outcomes from PACIFIC, we report updated, exploratory analyses on the basis of the January 11, 2021 DCO (approximately 5 years after the last patient was randomly assigned), including updates to the primary analyses of OS and PFS with durvalumab versus placebo as well as updates to key secondary end points. Furthermore, we report new exploratory analyses that examine the prognostic association of baseline factors (other than assigned study treatment) with OS and PFS.
METHODS
Study Design
The design of PACIFIC is published elsewhere.1,2 Patients with a WHO performance status (PS) of 0 or 1 and histologically or cytologically documented stage III (7th edition of the American Joint Committee on Cancer staging manual), unresectable NSCLC who had received concurrent CRT (≥ 2 cycles; total prescription radiation dose typically 60 to 66 Gy in 30 to 33 fractions)18 without disease progression were randomly assigned 1-42 days after CRT. Patients with unresolved grade > 2 toxicities (Common Terminology Criteria for Adverse Events [AEs] v4.03) or grade ≥ 2 pneumonitis and/or radiation pneumonitis from prior CRT were excluded. Tumor tissue collection was not required nor was enrollment restricted by PD-L1 expression level or oncogenic driver gene aberration status. Additional details of the work-up required to confirm diagnosis are provided in Appendix 1 (online only; also see the Data Supplement [online only]).
Patients were randomly assigned (2:1), stratified by age (< 65 v ≥ 65 years), sex, and smoking history (current or former smoker v never smoked), to durvalumab (10 mg/kg intravenously) or placebo, administered once every 2 weeks for up to 12 months; study treatment was discontinued if there was confirmed disease progression, initiation of alternative anticancer therapy, or the patient experienced unacceptable toxicity or withdrew consent. Patients were followed for survival after discontinuing study treatment. The study Protocol (online only) and amendments were approved by the relevant ethics committees. The study was performed in accordance with the International Conference on Harmonisation Guidelines on Good Clinical Practice and the Declaration of Helsinki.
End Points and Assessments
We updated the primary analyses of OS and PFS (RECIST v1.1; assessed by blinded independent central review [BICR]) with durvalumab versus placebo in the intent-to-treat (ITT) population. Updated analyses of OS and PFS in exploratory subgroups defined by prespecified baseline factors (demographics, clinicopathologic features, and prior CRT-related variables), including PD-L1 expression on tumor cells (TCs) on the basis of testing of archived (pre-CRT) tumor tissue scored at a prespecified (25%) threshold (Ventana SP263 Immunohistochemistry Assay), were also performed.1,2 We also updated analyses of additional PD-L1 subgroups defined by a post hoc (1%) threshold.3,19
Other updated end points include time to death or distant metastasis (TTDM; BICR), objective response rate (ORR; BICR), duration of response (BICR), incidence of new lesions (BICR), times to first (TFST) and second (TSST) subsequent therapy or death, and types of postdiscontinuation disease-related anticancer therapies administered.
In addition, we performed a new exploratory, post hoc analysis of time to second progression (ie, time from random assignment to the earliest of the progression events subsequent to that used for PFS analysis) in patients who received durvalumab retreatment. Time to second progression was investigator-assessed per local standard practice and could include objective progression (assessed radiologically), symptomatic progression, or death.
Finally, we performed new exploratory, post hoc analyses to examine the prognostic association of baseline factors (other than assigned study treatment) with OS and PFS (BICR) in the ITT population; this was to identify factors other than study treatment that may associate with better or worse survival in the PACIFIC trial cohort.
Statistical Analysis
For time-to-event end points, analyses comparing durvalumab with placebo (ITT population) were performed using log-rank tests stratified using the same factors used to stratify patients at random assignment; this was for consistency with the original analyses.1,2 Unstratified Cox regression models (with no adjustment for multiple comparisons) were used for subgroup analyses. Medians and landmark rates (eg, 5-year OS) were estimated by Kaplan-Meier method.
The prognostic association of baseline factors (other than assigned study treatment) with OS and PFS was analyzed using univariate and multivariable Cox regression models, as described in Appendix 1.
RESULTS
Patients
A total of 709 of 713 randomly assigned patients received study treatment in the durvalumab (473 of 476) and placebo arms (236 of 237). The last patient had completed protocol-defined study treatment in May 2017. Baseline characteristics were well balanced, as reported previously.1,2
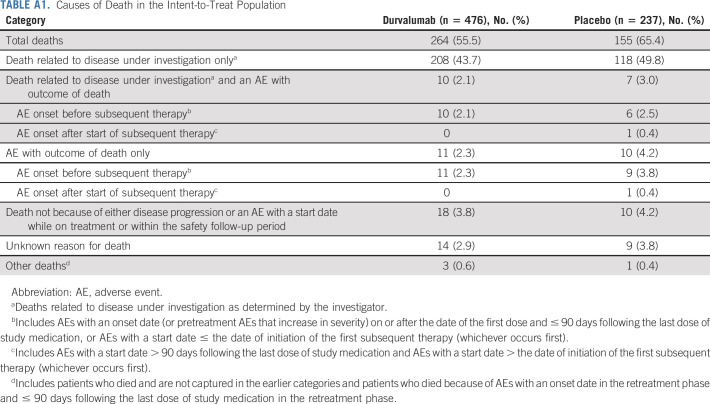
As of January 11, 2021, 419 of 713 (58.8%) patients had died, including 264 of 476 (55.5%) and 155 of 237 (65.4%) patients who were randomly assigned to durvalumab and placebo, respectively (Fig 1); a breakdown of the attribution of deaths to disease progression and/or AEs is provided (Appendix Table A1, online only). Median duration of follow-up was 34.2 months (range, 0.2-74.7 months) for all randomly assigned patients and 61.6 months (0.4-74.7 months) for censored patients (ie, patients last known to be alive). Overall, 49.0% and 34.7% of patients randomly assigned to durvalumab and placebo completed 12 months of study treatment, respectively; 31.3% versus 49.6% discontinued because of disease progression and 15.4% versus 9.7% discontinued because of AEs.
FIG 1.
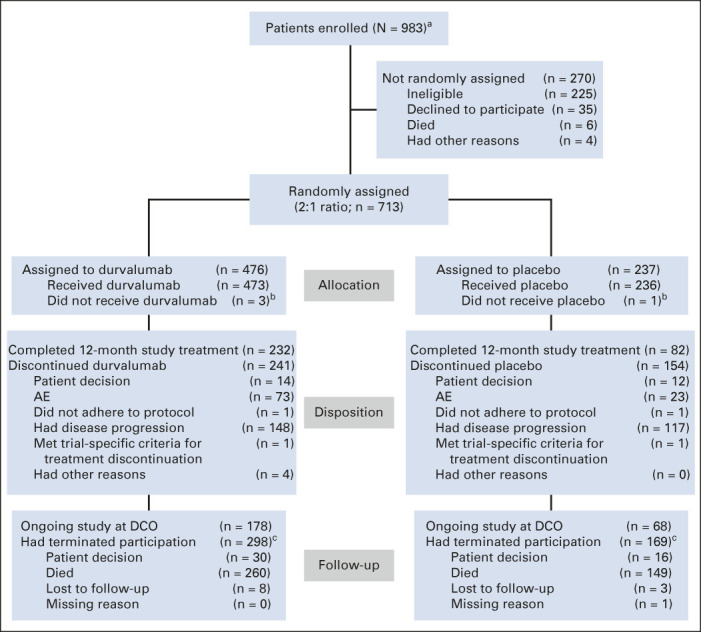
CONSORT diagram. Study data collected up to the DCO date of January 11, 2021. Patients who completed 12 months of study treatment are those for whom the electronic case report form showed that they had received the maximum number of cycles of study treatment. aInformed consent received. bFour patients did not receive their assigned study treatment because of neutropenia (n = 1), worsening chronic obstructive pulmonary disease (n = 1), and patient decision (n = 2). cNine patients (durvalumab, n = 4; placebo, n = 5) who terminated the study because of patient decision have subsequently died; one additional patient (placebo arm) with missing termination reason has subsequently died. AE, adverse event; DCO, data cutoff.
OS and PFS With Durvalumab Versus Placebo
In total, 120 additional deaths were reported since the primary OS analysis (March 22, 2018 DCO); 23 were reported since the last update of OS (March 20, 2020 DCO). Updated OS was consistent with the primary analysis, with a 28% reduction in the risk of death with durvalumab versus placebo (stratified HR, 0.72; 95% CI, 0.59 to 0.89; Fig 2A).2,3 Median OS was 47.5 months with durvalumab versus 29.1 months with placebo. The estimated 5-year OS rate was 42.9% with durvalumab versus 33.4% with placebo.
FIG 2.
Updated (A) OS and (B) PFS (blinded independent central review) in the intent-to-treat population. The vertical dashed lines indicate yearly landmarks; the associated numerical values represent the OS and PFS rates at the landmark. OS was defined as time from random assignment until death from any cause. PFS was defined as time from random assignment to the date of the first documented event of tumor progression or death in the absence of disease progression. For PFS, patients who had not progressed or died at the time of the data cutoff were censored at the time of their last evaluable RECIST assessment; however, if the patient progressed or died after ≥ 2 missed visits, they were censored at the time of the latest evaluable RECIST assessment before the two missed visits. HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
In total, 72 additional PFS events (BICR) were reported since the primary PFS analysis (February 13, 2017 DCO); three were reported since the last update of PFS (March 20, 2020 DCO). Updated PFS was consistent with the primary analysis, with a 45% reduction in the risk of disease progression or death with durvalumab versus placebo (stratified HR, 0.55; 95% CI, 0.45 to 0.68; Fig 2B).1 Median PFS was 16.9 months with durvalumab versus 5.6 months with placebo. The estimated 5-year PFS rate was 33.1% with durvalumab versus 19.0% with placebo.
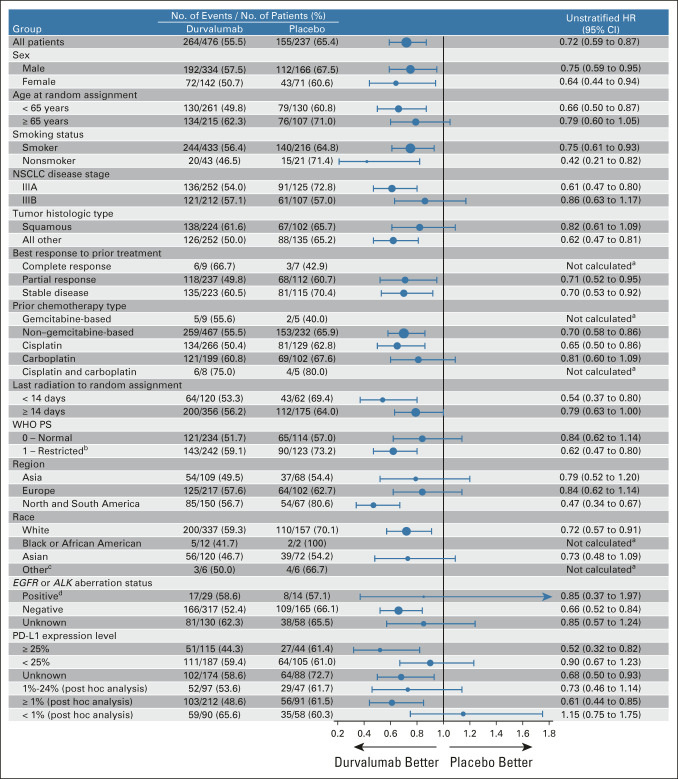
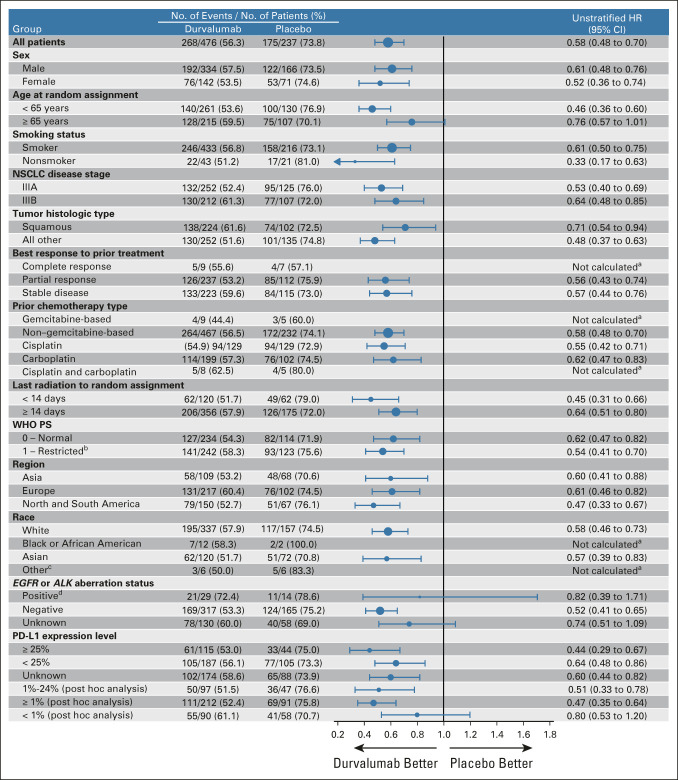
Updated OS and PFS (BICR) for patient subgroups were consistent with previous reports (Fig 3 and Appendix Fig A1, online only).1,2,5,19 OS and PFS benefit favored durvalumab versus placebo across all PD-L1 subgroups, with the exception of OS in patients with PD-L1 TC expression < 1% (HR, 1.15; 95% CI, 0.75 to 1.75). Kaplan-Meier curves for durvalumab versus placebo in PD-L1 subgroups are provided (Appendix Figs A2 and A3, online only).
FIG 3.
Updated OS by prespecified and exploratory, post hoc subgroups. aHRs and 95% CIs were not calculated if the subgroup had < 20 events. bThree patients with missing WHO PS were included in the PS 1 subgroup. cThe other race category includes American Indian or Alaskan Native (n = 9), Native Hawaiian or Other Pacific Islander (n = 2), and Other (n = 1). dThe subgroup includes 35 patients with tumors harboring EGFR mutations and, on the basis of local testing, eight patients with tumors harboring ALK alterations. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; HR, hazard ratio; NSCLC, non–small-cell lung cancer; OS, overall survival; PD-L1, programmed cell death-ligand 1; PS, performance status.
TTDM and the Incidence of New Lesions
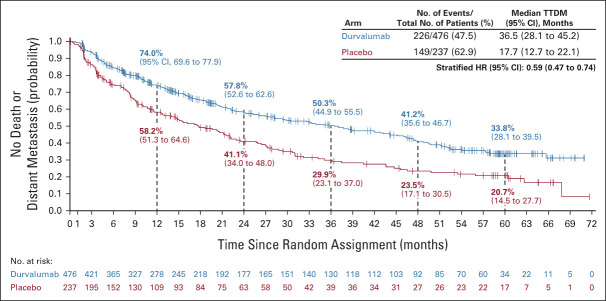
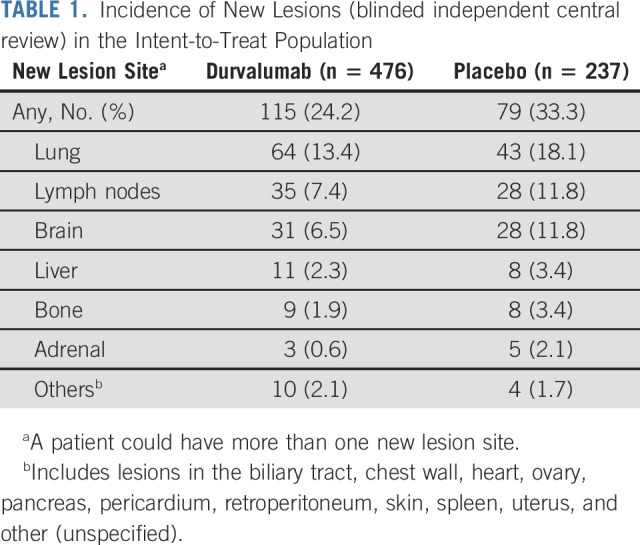
Updated TTDM (BICR) was consistent with previous analyses of this end point (on the basis of the February 13, 2017 and March 20, 2020 DCOs), with a 41% reduction in the risk of death or distant metastasis with durvalumab versus placebo (stratified HR, 0.59; 95% CI, 0.47 to 0.74; Fig 4).1,2 The incidence of new lesions (BICR) was proportionally lower with durvalumab (24.2%) versus placebo (33.3%); brain metastases were detected in 6.5% versus 11.8% of patients, respectively (imaging assessments of the CNS were performed at the investigator's discretion; Table 1).
FIG 4.
Updated TTDM (blinded independent central review) in the intent-to-treat population. The vertical dashed lines indicate yearly landmarks; the associated numerical values represent the TTDM rates at the landmark. TTDM was defined as time from random assignment until the first date of distant metastasis or death in the absence of distant metastasis. HR, hazard ratio; TTDM, time to death or distant metastasis.
TABLE 1.
Incidence of New Lesions (blinded independent central review) in the Intent-to-Treat Population
Antitumor Response
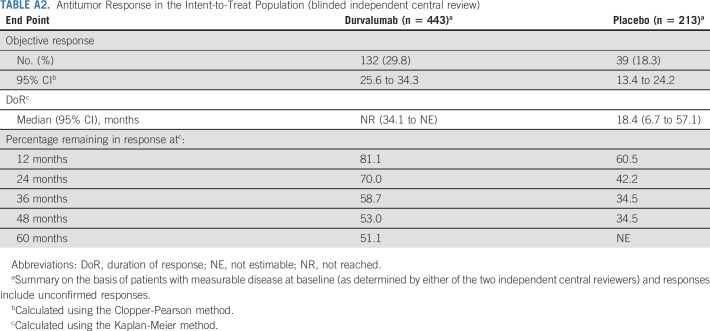
ORR (BICR) was proportionally higher with durvalumab (29.8%) versus placebo (18.3%); median duration of response was not reached with durvalumab versus 18.4 months with placebo (Appendix Table A2, online only). Among patients with an objective response, 81.1%, 58.7%, and 51.1% were estimated to have an ongoing response at 1, 3, and 5 years, respectively, with durvalumab, versus 60.5% and 34.5% at 1 and 3 years, respectively, with placebo (Appendix Table A2); the 5-year rate for placebo was not estimable as no patients with ongoing responses in the placebo arm had reached this landmark.
Durvalumab Retreatment
Durvalumab retreatment (at the investigator's discretion) was permitted for patients who completed the initial 12 months of treatment and had disease control at the end of the 12 months, provided their disease progressed during follow-up and they had not received another systemic anticancer therapy. Overall, 34 of 476 (7.1%) patients in the durvalumab arm received retreatment; 4 of 34 (11.8%) completed 12 months of retreatment and 23 of 34 (67.6%) discontinued (7 of 34 [20.6%] were ongoing retreatment at DCO). Median time to second progression (measured from random assignment) among retreated patients was 48.0 months (95% CI, 38.9 to 64.6); 100% (95% CI, 100 to 100), 50.9% (95% CI, 32.8 to 66.5), and 34.0% (95% CI, 18.0 to 50.6) of patients were estimated to be alive and without a second progression at 2, 4, and 5 years, respectively. Nevertheless, this post hoc analysis is difficult to interpret in the absence of a complementary subgroup against which to draw comparisons. Moreover, second progression was investigator-assessed per local practice and only a small number of patients received retreatment, further limiting interpretation.
Subsequent Anticancer Therapy
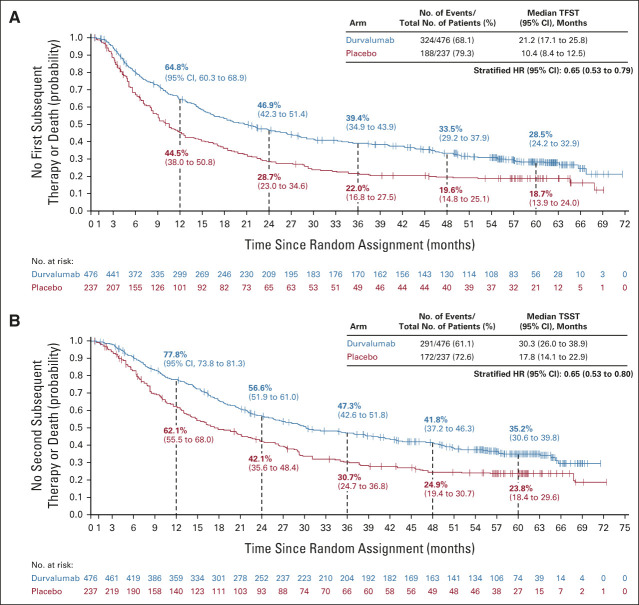
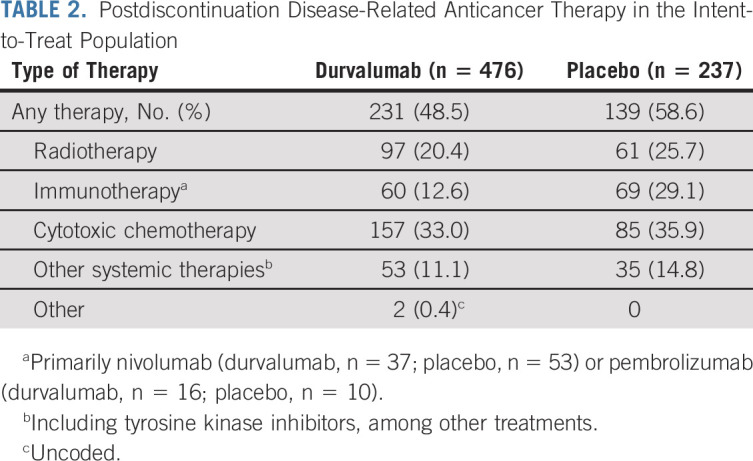
Overall, 48.5% and 58.6% of patients randomly assigned to durvalumab and placebo, respectively, received ≥ 1 subsequent, disease-related, anticancer therapy (after discontinuing study treatment), most commonly chemotherapy (durvalumab, 33.0%; placebo, 35.9%; Table 2). Subsequent immunotherapy was less commonly used among patients randomly assigned to durvalumab (12.6%) versus placebo (29.1%). TFST (stratified HR, 0.65; 95% CI, 0.53 to 0.79) and TSST (stratified HR, 0.65; 95% CI, 0.53 to 0.80) were improved with durvalumab versus placebo (Appendix Fig A4, online only), consistent with the previous analyses of these end points.2,4,5
TABLE 2.
Postdiscontinuation Disease-Related Anticancer Therapy in the Intent-to-Treat Population
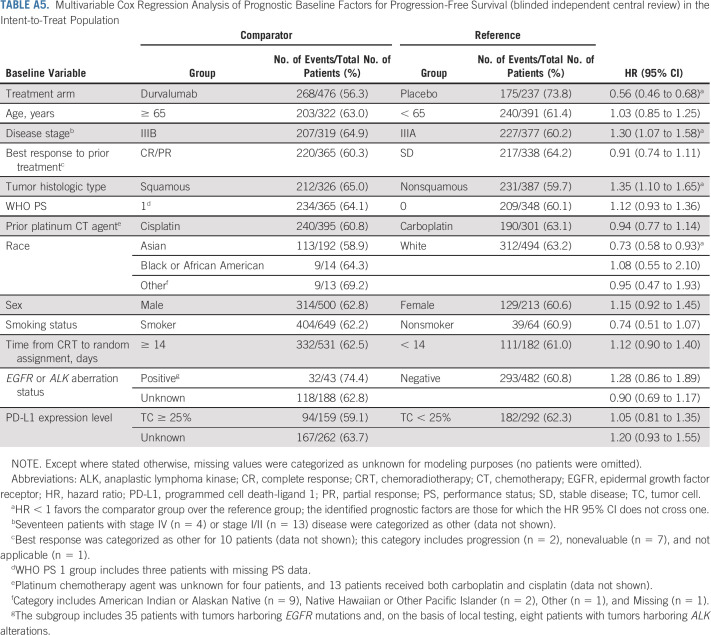
Prognostic Baseline Factors for OS and PFS
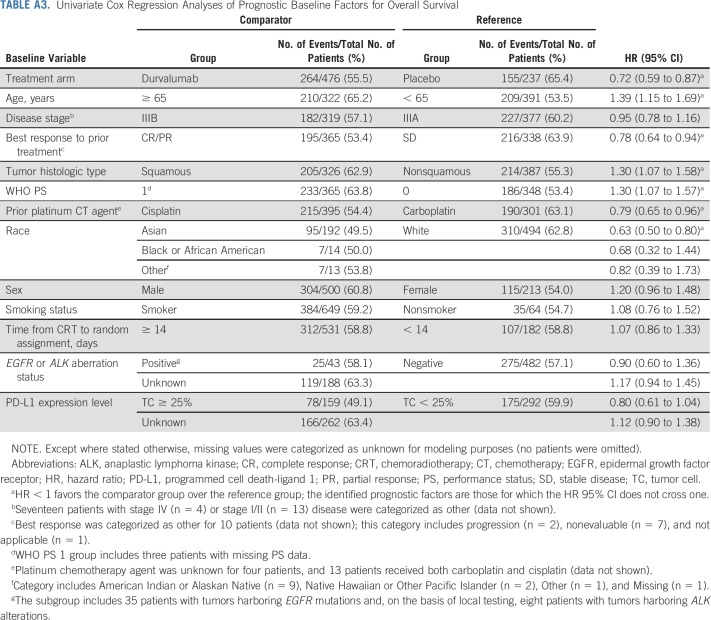
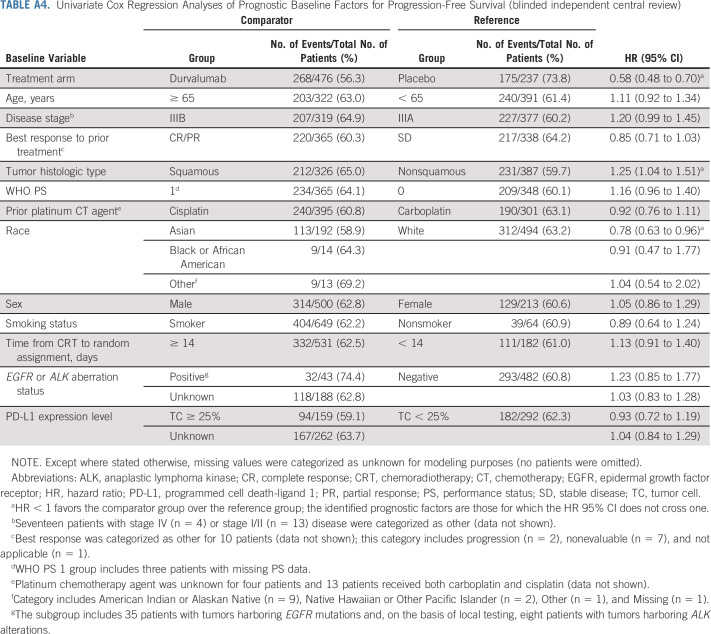
Univariate analyses identified younger age (v ≥ 65 years), objective tumor response during prior CRT (v stable disease), nonsquamous tumor histologic type (v squamous), WHO PS 0 (v 1), cisplatin use during prior CRT (v carboplatin), and Asian race (v White) as favorable prognostic factors for OS (Appendix Table A3, online only). Nonsquamous tumor histologic type and Asian race were also prognostic for better PFS in the univariate analyses (Appendix Table A4, online only).
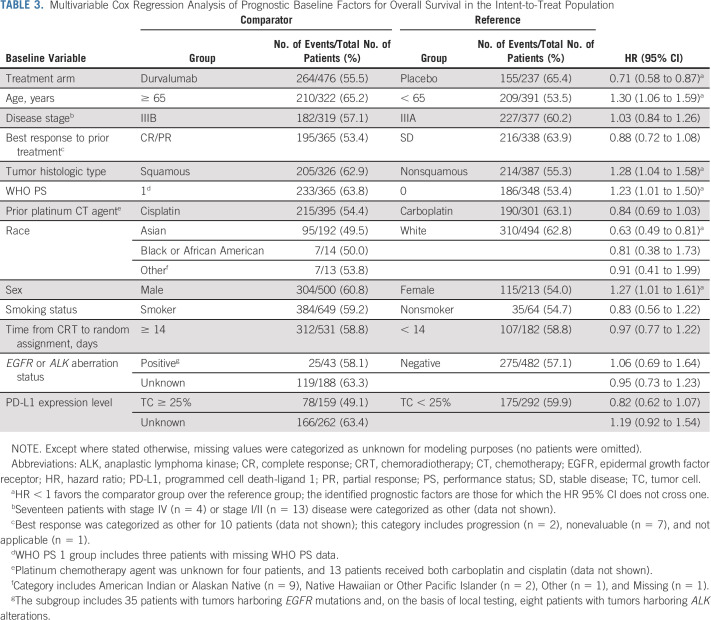
Multivariable analyses demonstrated that younger age, nonsquamous tumor histologic type, WHO PS 0, and Asian race remained favorable prognostic factors for OS (with female sex identified as an additional factor; Table 3), indicating that they are independent of one another and of the assigned study treatment. Nonsquamous tumor histologic type and Asian race also remained prognostic factors for improved PFS (with stage IIIA [v IIIB] disease identified as an additional factor; Appendix Table A5, online only).
TABLE 3.
Multivariable Cox Regression Analysis of Prognostic Baseline Factors for Overall Survival in the Intent-to-Treat Population
There was no change in the OS and PFS benefit observed with durvalumab versus placebo when accounting for differences in other baseline factors between treatment arms (Table 3 and Appendix Table A5): the treatment effects for OS (HR, 0.71; 95% CI, 0.58 to 0.87) and PFS (HR, 0.56; 95% CI, 0.46 to 0.68) were consistent with the main analyses of these end points (Fig 2).
DISCUSSION
The estimated 5-year OS and PFS rates were 42.9% and 33.1% for durvalumab and 33.4% and 19.0% for placebo, respectively. Together with the primary analyses,1,2 these updated results demonstrate robust and sustained survival benefit with durvalumab following CRT. Moreover, updates to secondary end points continue to demonstrate durable antitumor response and a reduced frequency of metastases with durvalumab. These findings support the continued use of the PACIFIC regimen as SoC for patients with unresectable, stage III NSCLC and are corroborated by the results of real-world studies.20-22
In PACIFIC, random assignment occurred after the completion of CRT and patients must have been free of disease progression, and have recovered from early CRT-related toxicities, as a condition of enrollment. Thus, the results reported here cannot be directly compared with the results of historic studies reporting long-term outcomes with CRT.
ORR (BICR) was approximately 10% higher with durvalumab versus placebo, and approximately half of the patients who responded to durvalumab had ongoing responses at 5 years. This biologically important and clinically relevant finding provides long-term evidence for a sustained improvement in local disease control with durvalumab. Furthermore, it supports a role for durvalumab in the treatment of patients with earlier-stage cancer.
Consistent with previous reports,1,2,4,5 OS and PFS benefit continued to favor durvalumab over placebo in all prespecified patient subgroups in the updated analyses, supporting the use of the PACIFIC regimen in a broad population. Previous exploratory analyses from PACIFIC also demonstrated consistent benefit with durvalumab across subgroups defined by additional (post hoc) CRT-related variables, including the nonplatinum chemotherapy agents used, the total radiation dose, and the use of induction chemotherapy before CRT.18 PACIFIC was designed to assess clinical outcomes with durvalumab in an all-comers population, preventing definitive conclusions for subgroups. These subgroup analyses are limited by small sample sizes and a resulting lack of statistical power; for example, survival benefit with durvalumab among patients with epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) aberration–positive tumors is uncertain, considering that the subgroup contained only 43 patients and EGFR and ALK status was unknown for 26.4% of all randomly assigned patients. Moreover, as random assignment was not stratified for most of the subgroup factors, the results may be affected by intersubgroup and intrasubgroup imbalances in other baseline factors. Indeed, the new multivariable analyses reported with this update identified several baseline factors that were prognostic for OS and/or PFS outcomes regardless of whether patients were assigned to receive durvalumab or placebo (including age, tumor histologic type, WHO PS, race, sex, and disease stage). The independent association of these factors with survival outcomes was not unexpected, and the factors that were identified are broadly aligned with the factors reported by other studies in the stage III NSCLC setting.23
Survival benefit favored durvalumab versus placebo across PD-L1 subgroups; the only exception was OS in the post hoc subgroup with PD-L1 TC expression < 1% (HR, 1.15; 95% CI, 0.75 to 1.75), although PFS still favored durvalumab in this subgroup (HR, 0.80; 95% CI, 0.53 to 1.20). Numerous limitations preclude definitive conclusions regarding the impact of tumoral PD-L1 expression on outcomes with the PACIFIC regimen (as described elsewhere).19,24 These include the use of tumor samples collected before CRT to determine PD-L1 expression (as CRT may upregulate PD-L1 expression), incomplete provision of tumor tissue (PD-L1–assessable samples were not available for 37% of randomly assigned patients), and the relatively small number of patients with PD-L1 TC expression < 1% (n = 148). Furthermore, the placebo arm appeared to overperform with respect to OS among patients with PD-L1 TC expression < 1% compared with the full PACIFIC ITT population (and with other trials of CRT for unresectable, stage III NSCLC),2,11,14,15 which may have been driven by imbalances in potentially prognostic baseline factors.24
Consistent with the considerable PFS benefit and fewer progression events observed with durvalumab, TFST was improved with durvalumab versus placebo, and fewer patients received subsequent anticancer treatment in the durvalumab arm. Durvalumab also improved TSST, and the treatment effect sizes for TFST and TSST were the same (HR, 0.65), suggesting that long-term survival benefit with durvalumab is largely driven by improvements in PFS and that between-arm differences in the use of salvage therapies did not meaningfully affect long-term survival benefit with durvalumab. Importantly, survival benefit was observed with durvalumab after CRT despite more patients receiving subsequent immunotherapy in the placebo arm.
Safety outcomes from PACIFIC were reported with the primary analyses and were not updated for this 5-year follow-up analysis as no patients remained on the 12-month study treatment beyond the time of the primary OS analysis (March 22, 2018 DCO).2 At the time of the primary OS analysis, all-causality AEs of maximum toxicity grade 3/4 occurred in 30.5% and 26.1% (and fatal AEs in 4.4% and 6.4%) of patients receiving durvalumab and placebo, respectively; 15.4% and 9.8% discontinued durvalumab and placebo because of AEs, mostly pneumonitis, radiation pneumonitis, and pneumonia.2 Analyses of patient-reported outcomes from PACIFIC found no evidence for a detrimental effect of up to 12 months of durvalumab treatment on symptoms, functioning, or global health status and quality of life, with the results being comparable to placebo.6 Taken together, these data suggest that clinical benefit with the PACIFIC regimen can be achieved without compromising safety or patient-reported outcomes. Subsequent, exploratory analyses from PACIFIC demonstrated broadly consistent results for safety and patient-reported outcomes irrespective of PD-L1 expression level and CRT-related variables, suggesting that durvalumab treatment is well managed regardless of these baseline factors.18,19,25
Further research is required to determine the optimum duration of durvalumab treatment following CRT. Use of a 12-month treatment duration in PACIFIC was an empiric decision made on the basis of the regimen used in a phase I/II first-in-human study of durvalumab (NCT01693562; the source of most of the available safety data for durvalumab at the time PACIFIC was designed and the results of which supported further development of the dose and duration).26
Given the unprecedented nature of the findings with the PACIFIC regimen, studies have been initiated to investigate the use of durvalumab after sequential CRT or radiotherapy alone (for patients who are chemotherapy-ineligible), and durvalumab in combination with novel anticancer agents post-CRT, with the aim of further extending clinical benefit to more patients in this setting.27 In addition, a placebo-controlled, phase III study is investigating durvalumab administered concurrently with CRT (followed by consolidative durvalumab).27 Finally, because PACIFIC was the first trial to show a survival advantage with an immunotherapy in a curative-intent setting, it established the rationale for further investigation of durvalumab in other curative-intent settings across other cancers.27
In conclusion, these updated survival analyses demonstrate robust and sustained survival benefit with durvalumab after CRT. An estimated 42.9% of patients randomly assigned to durvalumab remain alive at 5 years and 33.1% of patients randomly assigned to durvalumab remain alive and free of disease progression, establishing a new benchmark for SoC in this setting.
ACKNOWLEDGMENT
A complete list of the investigators in the PACIFIC trial is provided in Appendix 2 (online only).
APPENDIX 1. Supplementary Methods
Confirmation of Stage III Non–Small-Cell Lung Cancer Diagnosis
Imaging of the chest and abdomen was required at baseline (following chemoradiotherapy [CRT] and before random assignment) for assessing tumor burden at baseline and follow-up visits. Computed tomography examination of the chest and abdomen (including the liver and adrenal glands) with contrast media administration was the preferred method but was not compulsory. Use of magnetic resonance imaging (MRI) was recommended only where computed tomography was not feasible, or it was medically contraindicated. Imaging of the CNS was optional (ie, performed at the investigator’s discretion), and MRI was the preferred method. 18-fluorodeoxyglucose positron emission tomography (PET) was also optional, but was not recommended as the sole method for identifying lesions because of CRT-related inflammatory changes resulting in increased fluorodeoxyglucose uptake on a baseline scan performed within the screening period for the study (ie, within 1-42 days after CRT completion). Such scans would not have been very interpretable so closely following completion of CRT and, therefore, would not have typically met the image acquisition requirements for use of RECIST. However, use of baseline (post-CRT) PET imaging was distinct from, and should not be confused with, the standard diagnostic use of PET scans (and brain/CNS MRI) by investigators, before CRT, for purposes of disease staging (which was not collected in case report forms as part of the trial).
Analysis of the Prognostic Association of Baseline Factors With Overall Survival and Progression-Free Survival
The prognostic association of baseline factors (other than assigned study treatment) with overall survival and progression-free survival was analyzed using univariate and multivariable Cox regression models, with input variables aligned with the factors that were prespecified for comparing survival outcomes with durvalumab versus placebo in subgroups. Variable selection was not performed, and all variables were retained in the final models as there was no strong a priori rationale to exclude any specific prespecified variable (apart from region); moreover, data‐driven variable selection may produce biased regression coefficients. An additional benefit of including all prespecified variables in the models is that the impact of each variable on the outcome is adjusted for the effect of all other prespecified variables.
The prespecified variables were checked for the proportional hazards assumption using the Grambsch-Therneau statistical test (Grambsch PA, Therneau TM: Biometrika 81:515-526, 1994). In both multivariable and univariate analyses, most tests indicated proportional hazards with the only exceptions being for a few subgroups with small numbers of patients (eg, patients with epidermal growth factor receptor or anaplastic lymphoma kinase aberrations and patients in the other race category), which makes the results for these subgroups difficult to interpret. Thus, there was no clear evidence of nonproportional hazards.
FIG A1.
Updated PFS (blinded independent central review) by prespecified and exploratory, post hoc subgroups. aHRs and 95% CIs were not calculated if the subgroup had < 20 events. bThree patients with missing WHO PS were included in the PS 1 subgroup. cThe other race category includes American Indian or Alaskan Native (n = 9), Native Hawaiian or Other Pacific Islander (n = 2), and Other (n = 1). dThe subgroup includes 35 patients with tumors harboring EGFR mutations and, on the basis of local testing, eight patients with tumors harboring ALK alterations. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; HR, hazard ratio; NSCLC, non–small-cell lung cancer; PD-L1, programmed cell death-ligand 1; PFS, progression-free survival; PS, performance status.
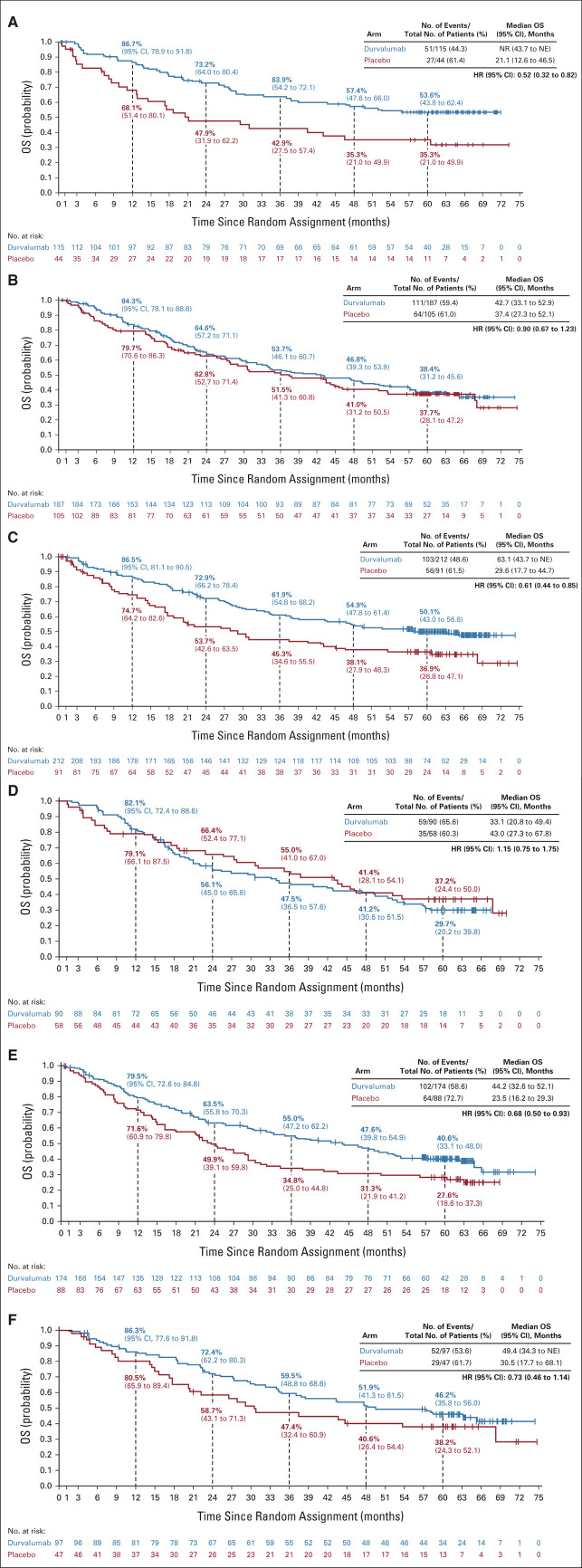
FIG A2.
Updated OS by tumor PD-L1 expression level: (A) PD-L1 TC ≥ 25%, (B) PD-L1 TC < 25%, (C) PD-L1 TC ≥ 1%, (D) PD-L1 TC < 1%, (E) unknown PD-L1 status, and (F) PD-L1 TC 1%-24%. The vertical dashed lines indicate yearly landmarks; the associated numerical values represent the OS rates at the landmark. HR, hazard ratio; NE, not estimable; NR, not reached; OS, overall survival; PD-L1, programmed cell death-ligand 1; TC, tumor cell.
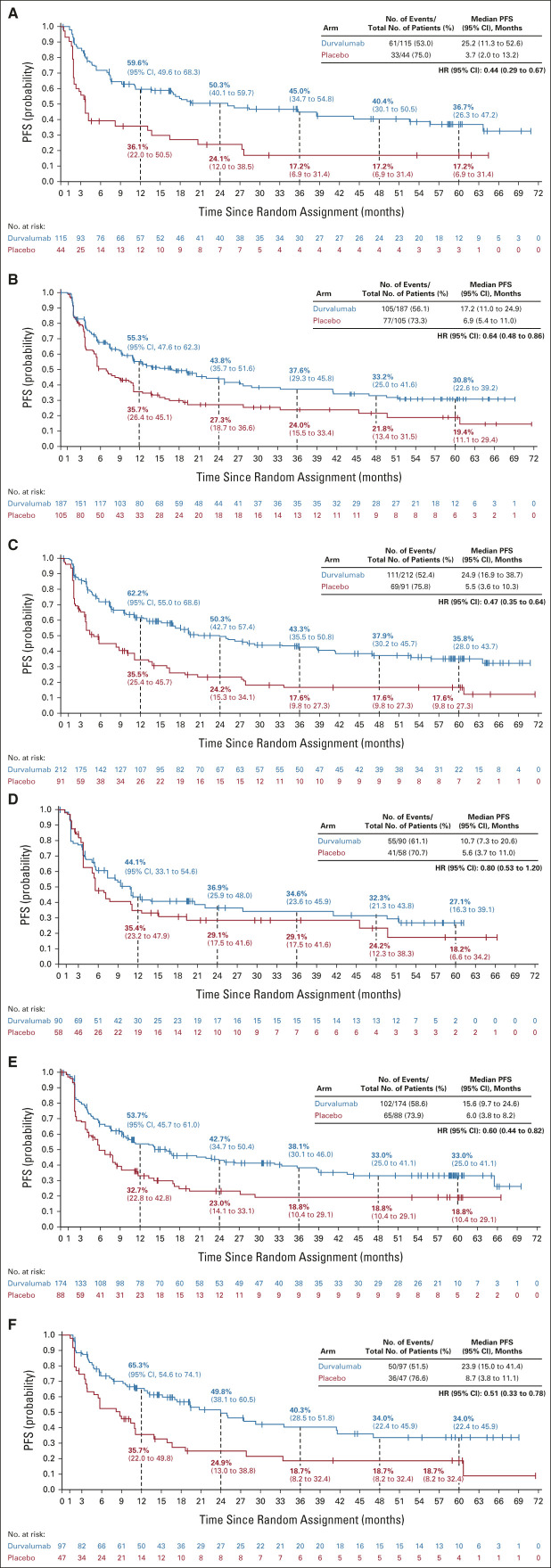
FIG A3.
Updated PFS (blinded independent central review) by tumor PD-L1 expression level: (A) PD-L1 TC ≥ 25%, (B) PD-L1 TC < 25%, (C) PD-L1 TC ≥ 1%, (D) PD-L1 TC < 1%, (E) unknown PD-L1 status, and (F) PD-L1 TC 1%-24%. The vertical dashed lines indicate yearly landmarks; the associated numerical values represent the PFS rates at the landmark. HR, hazard ratio; PD-L1, programmed cell death-ligand 1; PFS, progression-free survival; TC, tumor cell.
FIG A4.
Updated times to (A) first and (B) second subsequent therapy or death in the intent-to-treat population. The vertical dashed lines indicate yearly landmarks; the associated numerical values represent the TFST and TSST rates at the landmark. TFST was defined as time from random assignment to the start of the first subsequent anticancer therapy after discontinuation of study treatment, or death, whichever occurred earlier. TSST was defined as the time from random assignment to the start of the second subsequent anticancer therapy after discontinuation of study treatment, or death, whichever occurred earlier. HR, hazard ratio; TFST, time to first subsequent therapy or death; TSST, time to second subsequent therapy or death.
TABLE A1.
Causes of Death in the Intent-to-Treat Population
TABLE A2.
Antitumor Response in the Intent-to-Treat Population (blinded independent central review)
TABLE A3.
Univariate Cox Regression Analyses of Prognostic Baseline Factors for Overall Survival
TABLE A4.
Univariate Cox Regression Analyses of Prognostic Baseline Factors for Progression-Free Survival (blinded independent central review)
TABLE A5.
Multivariable Cox Regression Analysis of Prognostic Baseline Factors for Progression-Free Survival (blinded independent central review) in the Intent-to-Treat Population
APPENDIX 2. List of PACIFIC Investigators
Australia: Rina Hui, Christos S. Karapetis, Kevin Jasas, Kenneth Obyrne, Baerin Houghton, Brett Hughes, Craig Lewis, Matthew Links, Say Ng, Phillip Parente, Stanislaw Gauden, Chee Lee. Belgium: Maryam Bourhaba, Frédéric Forget, Piet Vercauter, Johan Vansteenkiste, Jean-Luc Canon, Astrid Paulus. Canada: Parnet Cheema, Mark Vincent, Nevin Murray, Jeffrey Rothenstein, Labib Zibdawi, Penelope Bradbury, Charles Butts, Robert El-Maraghi, Dafydd Bebb, Susanna Cheng, Janessa Laskin. Chile: Alejandro Acevedo Gaete, Eric Armando Orellana Ulunque, Osvaldo Rudy Aren Frontera. France: Xavier Quantin, Sandrine Hiret, David Planchard, Julien Mazières, Patrick Aldo Renault, Gilles Robinet, Alexis Cortot, Werner Hilgers, Michel Poudenx, Fabrice Barlesi, Claude El Kouri, Maurice Perol, Hervé Lena, Marielle Sabatini, Jean-Louis Pujol. Germany: Maike de Wit, Eckart Laack, Christian Scholz, Wolfram Brugger, Martin Faehling, Martin Reck, Thomas Wolff, Juergen Fischer, Till-Oliver Emde, Christian Schulz, Anja Rueckert. Greece: Haralabos Kalofonos, Athanasios Kotsakis, Konstantinos Syrigos, Konstantinos Papazisis, Kostantinos Zarogoulidis, Sofia Agelaki. Hungary: Gyula Ostoros, Zsuzsanna Sztancsik, Gyorgy Losonczy, Eszter Csanky. Israel: Hovav Nechushtan, Mirjana Wollner. Italy: Antonio Chella, Alessandra Bearz, Vincenzo Emanuele Chiuri, Marina Garassino, Luca Gianni, Matteo Brighenti, Fortunato Ciardiello, Michele Milella, Hector Soto Parra, Vanesa Gregorc. Japan: Shuji Murakami, Takashi Yokoi, Takaaki Tokito, Kaoru Kubota, Shunichi Sugawara, Shinji Atagi, Tomonori Hirashima, Fumio Imamura, Yasuo Iwamoto, Shintaro Kanda, Noriyuki Masuda, Koichi Minato, Kazuhiko Nakagawa, Seiji Niho, Hideo Saka, Toshiaki Takahashi, Yasuhito Fujisaka, Hiroshi Sakai, Kazuhisa Takahashi, Tomohisa Baba, Masao Harada, Kazuo Kasahara, Tadashi Maeda, Makoto Maemondo, Isamu Okamoto, Yuichiro Takeda, Kunihiko Kobayashi, Naoyuki Nogami, Katsuyuki Kiura, Terufumi Kato, Katsuyuki Hotta, Takayasu Kurata, Toru Kumagai, Tatsuya Yoshida, Masashi Kasajima, Tatsuro Fukuhara, Masahide Oki, Toshiyuki Kozuki, Kunihiko Kobayashi, Yasutaka Watanabe. Korea: Ki Hyeong Lee, Byoung Chul Cho, Young-Chul Kim, Keunchil Park, Eun Kyung Cho, Hoon Kyo Kim, Jong-Seok Lee, Choonhee Son, Sang-We Kim, Sang-Won Shin, Joo-Hang Kim. Ho Jung An, Hyung Soon Park, Insu Kim. Mexico: Miguel Pluma Jimenez, José David Gomez Rangel, Carlos Alberto Hernandez, Yamil Alonso Lopez Chuken, Osvaldo Hernandez Flores. The Netherlands: Adrianus Johannes de Langen, Ben van den Borne, Jeroen Steven Kloover, Michael van den Heuvel, Kornelis Harm van der Leest, Joachim G.J.V. Aerts, Sayed Hashemi. Peru: Wuilbert Rodriguez Pantigoso, Vanessa Bermudez Alfaro. Poland: Jacek Jassem, Slawomir Mandziuk, Dariusz Kowalski. Singapore: Akhil Chopra, Eng Huat Tan, Tan Min Chin, Ross Andrew Soo, Yee Hong Chia. Slovakia: Marian Stresko, Pavol Demo, Tibor Packan, Jozef Chovanec, Iveta Priatelova. South Africa: Graham Cohen, Conrad Jacobs, Daniel Rens. Spain: David Vicente Baz, Luis Paz-Ares Rodriguez, Javier de Castro Carpeño, Jesus Corral Jaime, Oscar Jose Juan Vidal, Alvaro Taus Garcia, Felipe Cardenal Alemany, Ramon Garcia Gomez, Esther Holgado Martin, Santiago Ponce Aix, Rut Porta Balanya, Dolores Isla Casado, Margarita Majem Tarruella, Diego Marquez Medina, Jesus Alfaro Lizaso, Ana Blasco Cordellat, Jose Miguel Sanchez Torres, Bartomeu Massuti Sureda, Amparo Sanchez, Cristobal Belda Iniesta, Rosa Alvarez, Joaquim Bosch-Barrera, Jenifer Gomez Mediavilla, Beatriz Jimenez Munarriz, Ramon Palmero Sanchez, Antonieta Salud Salvia. Taiwan: Te-Chun Hsia, Yuh-Min Chen, Kang-Yun Lee, Yung-Chang Lien, Chih-Hsin Yang, Chi-Li Chung. Thailand: Thanyanan Reungwetwattana, Busayamas Chewaskulyong, Sarayut Lucien Geater. Turkey: Mustafa Özgüroğlu, Tuncay Goksel, Hakan Harputluoglu, Mehmet Artac, Semra Paydas, Ozden Altundag, Orhan Sencan. United Kingdom: Jonathan Hicks, Corinne Faivre-Finn, Toby Talbot. United States: Davey Daniel, Augusto Villegas, Bijoy Telivala, Alberto Chiappori, Tarek Mekhail, Maen Hussein, Michael McCLeod, Dennis Slater, James V.H. Uyeki, David Waterhouse, Franklin L. Chen, Zhonglin Hao, Ronan Kelly, Daniel Morgensztern, Ray Page, Alexander I. Spira, Mark Awad, Joseph Thaddeus Beck, Alan Richard Berg, Janak Choksi, Scott Dorroh, Jonathan Goldman, Nicholas Iannotti, Kendra Kubiak, Suman Rao, Arvind Chaudhry, Chirag J. Dalsania, Nicholas J. Farrell, Robert Hermann, Charles S. Kuzma, Kristi J. McIntyre, William Mitchell, Estelamari Rodriguez, Ashish Sangal, David A. Smith, Kai Zu, Ian C. Anderson, Deepti Behl, William J. Edenfield, Hassan Ghazal, Giuseppe Giaccone, Christopher Thomas Hagenstad, Missak Haigentz Jr., Harry Harper, Charles Henderson, Borys Hrinczenko, Kartik Konduri, Marshall Levine, Danko Martincic, Rathi Narayana Pillai, Muhammad Salamat, Mikhail Shtivelband, Joginder Singh, Matei P. Socoteanu, David Spigel, Paul Zorsky, Renata Ferrarotto, Jorge Gomez, Leora Horn, Stephan DiSean Kendall, William E. Lawler, Leena Gandhi, Dylan Zylla, Vasileios Assikis, Steven McCune, Samuel Bailey, Anthony van Ho, Govinda Brahmanday, Jarushka Naidoo, Justinian Ngaiza, Nagla Abdel Karim, Ilicia Shugarman, Oleg Gligich, Balazs Halmos, Patrick Forde, Steven Dunder, Conor Steuer. Vietnam: Anh Tuan Le, Khoa Mai Trong, Luu Nguyen.
David R. Spigel
Leadership: ASCO (Inst)
Consulting or Advisory Role: Genentech/Roche (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Takeda (Inst), Evelo Therapeutics (Inst), Bayer (Inst), EMD Serono (Inst), Molecular Templates (Inst), Amgen (Inst), Curio Science (Inst), Intellisphere (Inst), Ipsen (Inst), Jazz Pharmaceuticals (Inst), Mirati Therapeutics (Inst), Puma Biotechnology (Inst), Sanofi/Aventis (Inst), Exelixis (Inst), Novocure (Inst), Regeneron (Inst), Lilly (Inst), Janssen (Inst), Evidera (Inst)
Research Funding: Genentech/Roche (Inst), Novartis (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), AstraZeneca (Inst), University of Texas Southwestern Medical Center—Simmons Cancer Center (Inst), Merck (Inst), G1 Therapeutics (Inst), Neon Therapeutics (Inst), Takeda (Inst), Nektar (Inst), Celldex (Inst), Clovis Oncology (Inst), Daiichi Sankyo (Inst), EMD Serono (Inst), Astellas Pharma (Inst), GRAIL (Inst), Transgene (Inst), Aeglea Biotherapeutics (Inst), Ipsen (Inst), BIND Therapeutics (Inst), Eisai (Inst), ImClone Systems (Inst), Immunogen (Inst), Janssen Oncology (Inst), MedImmune (Inst), Molecular Partners (Inst), Agios (Inst), GlaxoSmithKline (Inst), Tesaro (Inst), Cyteir (Inst), Apollomics (Inst), Novocure (Inst), Elevation Oncology (Inst), Calithera Biosciences (Inst), Arcus Biosciences (Inst), Arrys Therapeutics (Inst), Bayer (Inst), BeiGene (Inst), BioNTech (Inst), Blueprint Medicines (Inst), Boehringer Ingelheim (Inst), Denovo Biopharma (Inst), Hutchison MediPharma (Inst), Incyte (Inst), Kronos Bio (Inst), Loxo Oncology (Inst), Macrogenics (Inst), Molecular Templates (Inst), Oncologie (Inst), Pfizer (Inst), PTC Therapeutics (Inst), PureTech (Inst), Razor Genomics (Inst), Repare Therapeutics (Inst), Rgenix (Inst), Tizona Therapeutics Inc (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Genentech, Novartis
Corinne Faivre-Finn
Travel, Accommodations, Expenses: AstraZeneca
Jhanelle E. Gray
Honoraria: Merck Sharp & Dohme, Axiom Healthcare Strategies, Inivata
Consulting or Advisory Role: Novartis, AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, EMD Serono, Lilly, AstraZeneca, Sanofi, Merck Sharp & Dohme, Janssen Scientific Affairs
Research Funding: Array BioPharma (Inst), Merck (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Genentech/Roche (Inst), G1 Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Ludwig Institute for Cancer Research (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Inivata, Merck, EMD Serono, Novartis
David Vicente
Honoraria: AstraZeneca
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme Oncology, Roche/Genentech, Pfizer, AstraZeneca, Boehringer Ingelheim
Travel, Accommodations, Expenses: AstraZeneca
David Planchard
Honoraria: Prime Oncology, PeerVoice
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Roche, Pfizer, Merck Sharp & Dohme Oncology, Celgene, MedImmune, BeiGene, Samsung, AbbVie, Janssen, Daiichi Sankyo/Astra Zeneca
Research Funding: AstraZeneca/MedImmune (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Sanofi/Aventis (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), AbbVie (Inst), Janssen (Inst)
Johan F. Vansteenkiste
Honoraria: Bristol Myers Squibb (Inst), AstraZeneca (Inst), Merck Sharp & Dohme Oncology (Inst), Roche (Inst), Lilly (Inst), Daiichi-Sankyo (Inst)
Consulting or Advisory Role: Novartis (Inst), Boehringer Ingelheim (Inst), AstraZeneca (Inst), Merck Sharp & Dohme Oncology (Inst), Bristol Myers Squibb (Inst), Roche (Inst), Daiichi-Sankyo (Inst)
Research Funding: Merck Sharp & Dohme (Inst)
Marina C. Garassino
Honoraria: Merck Sharp & Dohme Oncology, AstraZeneca/MedImmune, GlaxoSmithKline, Takeda, Roche, Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Novartis, Takeda, Roche, Tiziana Life Sciences, Sanofi, Celgene, Daiiki Sankyo, Inivata, Incyte, Pfizer, Seattle Genetics, Lilly, GlaxoSmithKline, Bayer, Blueprint Medicines, Janssen, Regeneron
Speakers' Bureau: AstraZeneca, Takeda, Merck Sharp & Dohme Oncology, Celgene, Incyte, Roche, Bristol Myers Squibb, Otsuka, Lilly
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Incyte (Inst), Takeda (Inst), Spectrum Pharmaceuticals (Inst), Blueprint Medicines (Inst), Lilly (Inst), AstraZeneca (Inst), Ipsen (Inst), Janssen (Inst), Exelixis (Inst), MedImmune (Inst), Sanofi (Inst), Pfizer (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Rina Hui
Honoraria: Merck Sharp & Dohme, Novartis, Roche, AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, Seattle Genetics, Oncosec, Merck
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb, Novartis, Lilly, Pfizer, Seattle Genetics, Oncosec, Merck
Research Funding: AstraZeneca (Inst), Lilly (Inst), Merck Sharp & Dohme (Inst), Roche (Inst), Seattle Genetics (Inst), OncoSec (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Novartis
Xavier Quantin
Honoraria: Bristol Myers Squibb France, AstraZeneca, Amgen
Travel, Accommodations, Expenses: Pfizer, Boehringer Ingelheim, Merck Sharp & Dohme Oncology
Andreas Rimner
Honoraria: More Health
Consulting or Advisory Role: AstraZeneca, Merck, Boehringer Ingelheim
Research Funding: Varian Medical Systems (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), AstraZeneca (Inst), Merck (Inst)
Yi-Long Wu
Honoraria: AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, Merck Sharp & Dohme Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst)
Mustafa Özgüroğlu
Honoraria: Astellas Pharma, Novartis, Janssen Oncology (Inst)
Consulting or Advisory Role: Merck Sharp & Dohme Oncology, AstraZeneca
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Ki H. Lee
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Pfizer, Lilly
Terufumi Kato
Employment: Lilly (I)
Honoraria: Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Lilly, AstraZeneca, Taiho Pharmaceutical, Pfizer, Merck Sharp & Dohme, Novartis, Takeda, Daiichi Sankyo, Merck Serono
Consulting or Advisory Role: AstraZeneca, Merck Sharp & Dohme, Lilly, Pfizer, Merck Serono
Research Funding: Chugai Pharma (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Lilly (Inst), AbbVie (Inst), Ono Pharmaceutical (Inst), Regeneron (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Amgen (Inst), Merck Serono
Maike de Wit
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Ipsen, AbbVie, Jazz Pharmaceuticals
Speakers' Bureau: AstraZeneca, Ipsen, Janssen, Merck Sharp & Dohme, Sanofi
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Janssen (Inst), Merck Sharp & Dohme (Inst), Boehringer Ingelheim (Inst), Pierre Fabre (Inst), Ipsen (Inst), Amgen (Inst), Genzyme (Inst), Pfizer (Inst), Takeda (Inst), Noona Healthcare (Inst), AbbVie (Inst), Nucana (Inst), MorphoSys (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, AstraZeneca, Takeda, Pfizer
Takayasu Kurata
Honoraria: AstraZeneca, Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharma, Lilly, Boehringer Ingelheim, Merck Sharp & Dohme Oncology
Research Funding: Merck Sharp & Dohme Oncology (Inst), AstraZeneca (Inst), Lilly (Inst), Ono Pharmaceutical (Inst), Novartis (Inst), Takeda (Inst), Bristol Myers Squibb (Inst)
Martin Reck
Consulting or Advisory Role: Lilly, Merck Sharp & Dohme Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron
Speakers' Bureau: Roche/Genentech, Lilly, Merck Sharp & Dohme Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Byoung C. Cho
Leadership: Gencurix, Interpark Bio
Stock and Other Ownership Interests: Theravance, Gencurix, Bridgebio, Kanaph Therapeutics, Cyrus therapeutics, Interpark Bio
Consulting or Advisory Role: Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol Myers Squibb, Yuhan, Pfizer, Janssen, Takeda, Merck Sharp & Dohme, Ono Pharmaceutical, Lilly, Medpacto, Blueprint Medicines, Kanaph Therapeutics, Bridgebio, Cyrus Therapeutics, Guardant Health, Joseah Biopharma
Research Funding: Novartis, Bayer, AstraZeneca, Mogam Biotechnology Research Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, Merck Sharp & Dohme, AbbVie, Medpacto, GI Innovation, Lilly, Blueprint Medicines, Interpark Bio
Patents, Royalties, Other Intellectual Property: Champions Oncology
Other Relationship: DAAN Biotherapeutics
Suresh Senan
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca, Merck Sharp & Dohme Oncology, BeiGene, Pfizer
Research Funding: ViewRay (Inst), AstraZeneca (Inst), Varian Medical Systems (Inst), Bristol Myers Squibb (Inst)
Jarushka Naidoo
Honoraria: Bristol Myers Squibb, AstraZeneca/MedImmune, Merck, Daiichi Sankyo/Lilly, Takeda
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca/MedImmune, Roche/Genentech, Daiichi Sankyo/Lilly, Takeda, Pfizer, Kaleido Biosciences
Research Funding: Merck (Inst), AstraZeneca (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca/MedImmune
Helen Mann
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Michael Newton
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Piruntha Thiyagarajah
Employment: AstraZeneca/MedImmune, Reckitt Benckiser (I)
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Scott J. Antonia
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Memgen, Achilles Therapeutics, Amgen, Celsius Therapeutics, EMD Serono, G1 Therapeutics, GlaxoSmithKline, Glympse Bio, RAPT Therapeutics, Samyang, Venn Therapeutics, Tarus Therapeutics
Patents, Royalties, Other Intellectual Property: Oncolytic Virus
Travel, Accommodations, Expenses: RAPT Therapeutics, Celsius Therapeutics, Amgen, Achilles Therapeutics
No other potential conflicts of interest were reported.
See accompanying editorial on page 1271
SUPPORT
This study (NCT02125461) was sponsored by AstraZeneca. Medical writing support, under the direction of the authors, was provided by Aaron Korpal, PhD, of Ashfield MedComms (Manchester, United Kingdom), an Ashfield Health company, and was funded by AstraZeneca. Jolyon Faria of AstraZeneca (Cambridge, United Kingdom) carried out the technical modeling for the analyses of prognostic factors for survival. C.F.-F. is supported by a grant from the National Institute for Health Research Manchester Biomedical Research Centre. A.R. is supported, in part, by a grant from the National Institutes of Health/National Cancer Institute Cancer Center (support Grant No.: P30 CA008748).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy, which is described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
AUTHOR CONTRIBUTIONS
Conception and design: David R. Spigel, Corinne Faivre-Finn, Jhanelle E. Gray, David Vicente, Marina C. Garassino, Byoung C. Cho, Helen Mann, Michael Newton, Piruntha Thiyagarajah, Scott J. Antonia
Provision of study materials or patients: David R. Spigel, Corinne Faivre-Finn, David Vicente, David Planchard, Luis Paz-Ares, Johan F. Vansteenkiste, Marina C. Garassino, Rina Hui, Xavier Quantin, Mustafa Özgüroğlu, Ki H. Lee, Terufumi Kato, Maike de Wit, Takayasu Kurata, Martin Reck, Byoung C. Cho, Jarushka Naidoo
Collection and assembly of data: David R. Spigel, Luis Paz-Ares, Johan F. Vansteenkiste, Rina Hui, Xavier Quantin, Mustafa Özgüroğlu, Ki H. Lee, Terufumi Kato, Maike de Wit, Takayasu Kurata, Martin Reck, Byoung C. Cho, Jarushka Naidoo, Helen Mann, Michael Newton
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David R. Spigel
Leadership: ASCO (Inst)
Consulting or Advisory Role: Genentech/Roche (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Takeda (Inst), Evelo Therapeutics (Inst), Bayer (Inst), EMD Serono (Inst), Molecular Templates (Inst), Amgen (Inst), Curio Science (Inst), Intellisphere (Inst), Ipsen (Inst), Jazz Pharmaceuticals (Inst), Mirati Therapeutics (Inst), Puma Biotechnology (Inst), Sanofi/Aventis (Inst), Exelixis (Inst), Novocure (Inst), Regeneron (Inst), Lilly (Inst), Janssen (Inst), Evidera (Inst)
Research Funding: Genentech/Roche (Inst), Novartis (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), AstraZeneca (Inst), University of Texas Southwestern Medical Center—Simmons Cancer Center (Inst), Merck (Inst), G1 Therapeutics (Inst), Neon Therapeutics (Inst), Takeda (Inst), Nektar (Inst), Celldex (Inst), Clovis Oncology (Inst), Daiichi Sankyo (Inst), EMD Serono (Inst), Astellas Pharma (Inst), GRAIL (Inst), Transgene (Inst), Aeglea Biotherapeutics (Inst), Ipsen (Inst), BIND Therapeutics (Inst), Eisai (Inst), ImClone Systems (Inst), Immunogen (Inst), Janssen Oncology (Inst), MedImmune (Inst), Molecular Partners (Inst), Agios (Inst), GlaxoSmithKline (Inst), Tesaro (Inst), Cyteir (Inst), Apollomics (Inst), Novocure (Inst), Elevation Oncology (Inst), Calithera Biosciences (Inst), Arcus Biosciences (Inst), Arrys Therapeutics (Inst), Bayer (Inst), BeiGene (Inst), BioNTech (Inst), Blueprint Medicines (Inst), Boehringer Ingelheim (Inst), Denovo Biopharma (Inst), Hutchison MediPharma (Inst), Incyte (Inst), Kronos Bio (Inst), Loxo Oncology (Inst), Macrogenics (Inst), Molecular Templates (Inst), Oncologie (Inst), Pfizer (Inst), PTC Therapeutics (Inst), PureTech (Inst), Razor Genomics (Inst), Repare Therapeutics (Inst), Rgenix (Inst), Tizona Therapeutics Inc (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Genentech, Novartis
Corinne Faivre-Finn
Travel, Accommodations, Expenses: AstraZeneca
Jhanelle E. Gray
Honoraria: Merck Sharp & Dohme, Axiom Healthcare Strategies, Inivata
Consulting or Advisory Role: Novartis, AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, EMD Serono, Lilly, AstraZeneca, Sanofi, Merck Sharp & Dohme, Janssen Scientific Affairs
Research Funding: Array BioPharma (Inst), Merck (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Genentech/Roche (Inst), G1 Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Ludwig Institute for Cancer Research (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Inivata, Merck, EMD Serono, Novartis
David Vicente
Honoraria: AstraZeneca
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme Oncology, Roche/Genentech, Pfizer, AstraZeneca, Boehringer Ingelheim
Travel, Accommodations, Expenses: AstraZeneca
David Planchard
Honoraria: Prime Oncology, PeerVoice
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Roche, Pfizer, Merck Sharp & Dohme Oncology, Celgene, MedImmune, BeiGene, Samsung, AbbVie, Janssen, Daiichi Sankyo/Astra Zeneca
Research Funding: AstraZeneca/MedImmune (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Sanofi/Aventis (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), AbbVie (Inst), Janssen (Inst)
Johan F. Vansteenkiste
Honoraria: Bristol Myers Squibb (Inst), AstraZeneca (Inst), Merck Sharp & Dohme Oncology (Inst), Roche (Inst), Lilly (Inst), Daiichi-Sankyo (Inst)
Consulting or Advisory Role: Novartis (Inst), Boehringer Ingelheim (Inst), AstraZeneca (Inst), Merck Sharp & Dohme Oncology (Inst), Bristol Myers Squibb (Inst), Roche (Inst), Daiichi-Sankyo (Inst)
Research Funding: Merck Sharp & Dohme (Inst)
Marina C. Garassino
Honoraria: Merck Sharp & Dohme Oncology, AstraZeneca/MedImmune, GlaxoSmithKline, Takeda, Roche, Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Novartis, Takeda, Roche, Tiziana Life Sciences, Sanofi, Celgene, Daiiki Sankyo, Inivata, Incyte, Pfizer, Seattle Genetics, Lilly, GlaxoSmithKline, Bayer, Blueprint Medicines, Janssen, Regeneron
Speakers' Bureau: AstraZeneca, Takeda, Merck Sharp & Dohme Oncology, Celgene, Incyte, Roche, Bristol Myers Squibb, Otsuka, Lilly
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Incyte (Inst), Takeda (Inst), Spectrum Pharmaceuticals (Inst), Blueprint Medicines (Inst), Lilly (Inst), AstraZeneca (Inst), Ipsen (Inst), Janssen (Inst), Exelixis (Inst), MedImmune (Inst), Sanofi (Inst), Pfizer (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Rina Hui
Honoraria: Merck Sharp & Dohme, Novartis, Roche, AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, Seattle Genetics, Oncosec, Merck
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb, Novartis, Lilly, Pfizer, Seattle Genetics, Oncosec, Merck
Research Funding: AstraZeneca (Inst), Lilly (Inst), Merck Sharp & Dohme (Inst), Roche (Inst), Seattle Genetics (Inst), OncoSec (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Novartis
Xavier Quantin
Honoraria: Bristol Myers Squibb France, AstraZeneca, Amgen
Travel, Accommodations, Expenses: Pfizer, Boehringer Ingelheim, Merck Sharp & Dohme Oncology
Andreas Rimner
Honoraria: More Health
Consulting or Advisory Role: AstraZeneca, Merck, Boehringer Ingelheim
Research Funding: Varian Medical Systems (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), AstraZeneca (Inst), Merck (Inst)
Yi-Long Wu
Honoraria: AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, Merck Sharp & Dohme Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst)
Mustafa Özgüroğlu
Honoraria: Astellas Pharma, Novartis, Janssen Oncology (Inst)
Consulting or Advisory Role: Merck Sharp & Dohme Oncology, AstraZeneca
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Ki H. Lee
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Pfizer, Lilly
Terufumi Kato
Employment: Lilly (I)
Honoraria: Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Lilly, AstraZeneca, Taiho Pharmaceutical, Pfizer, Merck Sharp & Dohme, Novartis, Takeda, Daiichi Sankyo, Merck Serono
Consulting or Advisory Role: AstraZeneca, Merck Sharp & Dohme, Lilly, Pfizer, Merck Serono
Research Funding: Chugai Pharma (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Lilly (Inst), AbbVie (Inst), Ono Pharmaceutical (Inst), Regeneron (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Amgen (Inst), Merck Serono
Maike de Wit
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Ipsen, AbbVie, Jazz Pharmaceuticals
Speakers' Bureau: AstraZeneca, Ipsen, Janssen, Merck Sharp & Dohme, Sanofi
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Janssen (Inst), Merck Sharp & Dohme (Inst), Boehringer Ingelheim (Inst), Pierre Fabre (Inst), Ipsen (Inst), Amgen (Inst), Genzyme (Inst), Pfizer (Inst), Takeda (Inst), Noona Healthcare (Inst), AbbVie (Inst), Nucana (Inst), MorphoSys (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, AstraZeneca, Takeda, Pfizer
Takayasu Kurata
Honoraria: AstraZeneca, Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharma, Lilly, Boehringer Ingelheim, Merck Sharp & Dohme Oncology
Research Funding: Merck Sharp & Dohme Oncology (Inst), AstraZeneca (Inst), Lilly (Inst), Ono Pharmaceutical (Inst), Novartis (Inst), Takeda (Inst), Bristol Myers Squibb (Inst)
Martin Reck
Consulting or Advisory Role: Lilly, Merck Sharp & Dohme Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron
Speakers' Bureau: Roche/Genentech, Lilly, Merck Sharp & Dohme Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Byoung C. Cho
Leadership: Gencurix, Interpark Bio
Stock and Other Ownership Interests: Theravance, Gencurix, Bridgebio, Kanaph Therapeutics, Cyrus therapeutics, Interpark Bio
Consulting or Advisory Role: Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol Myers Squibb, Yuhan, Pfizer, Janssen, Takeda, Merck Sharp & Dohme, Ono Pharmaceutical, Lilly, Medpacto, Blueprint Medicines, Kanaph Therapeutics, Bridgebio, Cyrus Therapeutics, Guardant Health, Joseah Biopharma
Research Funding: Novartis, Bayer, AstraZeneca, Mogam Biotechnology Research Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, Merck Sharp & Dohme, AbbVie, Medpacto, GI Innovation, Lilly, Blueprint Medicines, Interpark Bio
Patents, Royalties, Other Intellectual Property: Champions Oncology
Other Relationship: DAAN Biotherapeutics
Suresh Senan
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca, Merck Sharp & Dohme Oncology, BeiGene, Pfizer
Research Funding: ViewRay (Inst), AstraZeneca (Inst), Varian Medical Systems (Inst), Bristol Myers Squibb (Inst)
Jarushka Naidoo
Honoraria: Bristol Myers Squibb, AstraZeneca/MedImmune, Merck, Daiichi Sankyo/Lilly, Takeda
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca/MedImmune, Roche/Genentech, Daiichi Sankyo/Lilly, Takeda, Pfizer, Kaleido Biosciences
Research Funding: Merck (Inst), AstraZeneca (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca/MedImmune
Helen Mann
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Michael Newton
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Piruntha Thiyagarajah
Employment: AstraZeneca/MedImmune, Reckitt Benckiser (I)
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Scott J. Antonia
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Memgen, Achilles Therapeutics, Amgen, Celsius Therapeutics, EMD Serono, G1 Therapeutics, GlaxoSmithKline, Glympse Bio, RAPT Therapeutics, Samyang, Venn Therapeutics, Tarus Therapeutics
Patents, Royalties, Other Intellectual Property: Oncolytic Virus
Travel, Accommodations, Expenses: RAPT Therapeutics, Celsius Therapeutics, Amgen, Achilles Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Antonia SJ, Villegas A, Daniel D, et al. : Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919-1929, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Antonia SJ, Villegas A, Daniel D, et al. : Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379:2342-2350, 2018 [DOI] [PubMed] [Google Scholar]
- 3.European Medicines Agency (EMA) : Imfinzi (Durvalumab) Product Information. 2021. https://www.ema.europa.eu/en/documents/product-information/imfizi-epar-product-information_en.pdf [Google Scholar]
- 4.Gray JE, Villegas A, Daniel D, et al. : Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC - update from PACIFIC. J Thorac Oncol 15:288-293, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faivre-Finn C, Vicente D, Kurata T, et al. : Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC - an update from the PACIFIC trial. J Thorac Oncol 16:860-867, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Hui R, Özgüroğlu M, Villegas A, et al. : Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): A randomised, controlled, phase 3 study. Lancet Oncol 20:1670-1680, 2019 [DOI] [PubMed] [Google Scholar]
- 7.FDA : Durvalumab Prescribing Information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf [Google Scholar]
- 8.Pmda : List of Approved Products: Financial Year 2018. https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html [Google Scholar]
- 9.Yoon SM, Shaikh T, Hallman M: Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol 8:1-20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auperin A, Le Pechoux C, Rolland E, et al. : Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 28:2181-2190, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Bradley JD, Hu C, Komaki RR, et al. : Long-term results of NRG oncology RTOG 0617: Standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol 38:706-714, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen RN, Zhang Y, Seal B, et al. : Long-term survival trends in patients with unresectable stage III non-small cell lung cancer receiving chemotherapy and radiation therapy: A SEER cancer registry analysis. BMC Cancer 20:276, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vokes EE, Herndon JE, II, Kelley MJ, et al. : Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 25:1698-1704, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hanna N, Neubauer M, Yiannoutsos C, et al. : Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 26:5755-5760, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Senan S, Brade A, Wang LH, et al. : PROCLAIM: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 34:953-962, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Ahn JS, Ahn YC, Kim JH, et al. : Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol 33:2660-2666, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Bradley JD, Paulus R, Komaki R, et al. : Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol 16:187-199, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faivre-Finn C, Spigel DR, Senan S, et al. : Impact of prior chemoradiotherapy-related variables on outcomes with durvalumab in unresectable stage III NSCLC (PACIFIC). Lung Cancer 151:30-38, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L, Spira A, Raben D, et al. : Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol 31:798-806, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faehling M, Schumann C, Christopoulos P, et al. : Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non-small cell lung cancer (NSCLC): Real-world data on survival and safety from the German expanded-access program (EAP). Lung Cancer 150:114-122, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Taugner J, Käsmann L, Eze C, et al. : Real-world prospective analysis of treatment patterns in durvalumab maintenance after chemoradiotherapy in unresectable, locally advanced NSCLC patients. Invest New Drugs 39:1189-1196, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung HA, Noh JM, Sun JM, et al. : Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer 146:23-29, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Berghmans T, Paesmans M, Sculier JP: Prognostic factors in stage III non-small cell lung cancer: A review of conventional, metabolic and new biological variables. Ther Adv Med Oncol 3:127-138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters S, Dafni U, Boyer M, et al. : Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann Oncol 30:161-165, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Garassino MC, Paz-Ares L, Hui R, et al. : Patient-reported outcomes with durvalumab by PD-L1 expression and prior chemoradiotherapy-related variables in unresectable stage III non-small-cell lung cancer. Future Oncol 17:1165-1184, 2021 [DOI] [PubMed] [Google Scholar]
- 26.Antonia SJ, Balmanoukian A, Brahmer J, et al. : Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol 14:1794-1806, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Melillo G, Chand V, Yovine A, et al. : Curative-intent treatment with durvalumab in early-stage cancers. Adv Ther 38:2759-2778, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy, which is described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.