Abstract
Development of new antimycobacterial agents for Mycobacterium avium complex (MAC) infections is important particularly for persons coinfected with human immunodeficiency virus. The objectives of this study were to evaluate the in vitro activity of 2,4-diamino-5-methyl-5-deazapteridines (DMDPs) against MAC and to assess their activities against MAC dihydrofolate reductase recombinant enzyme (rDHFR). Seventy-seven DMDP derivatives were evaluated initially for in vitro activity against one to three strains of MAC (NJ168, NJ211, and/or NJ3404). MICs were determined with 10-fold dilutions of drug and a colorimetric (Alamar Blue) microdilution broth assay. MAC rDHFR 50% inhibitory concentrations versus those of human rDHFR were also determined. Substitutions at position 5 of the pteridine moiety included CH3, CH2CH3, and CH2OCH3 groups. Additionally, different substituted and unsubstituted aryl groups were linked at position 6 through a two-atom bridge of either CH2NH, CH2N(CH3), CH2CH2, or CH2S. All but 4 of the 77 derivatives were active against MAC NJ168 at concentrations of ≤13 μg/ml. Depending on the MAC strain used, 81 to 87% had MICs of ≤1.3 μg/ml. Twenty-one derivatives were >100-fold more active against MAC rDHFR than against human rDHFR. In general, selectivity was dependent on the composition of the two-atom bridge at position 6 and the attached aryl group with substitutions at the 2′ and 5′ positions on the phenyl ring. Using this assessment, a rational synthetic approach was implemented that resulted in a DMDP derivative that had significant intracellular activity against a MAC-infected Mono Mac 6 monocytic cell line. These results demonstrate that it is possible to synthesize pteridine derivatives that have selective activity against MAC.
Dihydrofolate reductase (DHFR) is a key enzyme in the folate metabolic pathway that is necessary for the biosynthesis of RNA, DNA, and protein. The enzyme is an important target for medicinal chemistry (5), and inhibitors for it have been used in anticancer (methotrexate [4]), antibacterial (trimethoprim [9]), and antimalarial (pyrimethamine [24]) chemotherapy. DHFR is present in all cells and is necessary for the maintenance of intracellular folate pools in a biochemically active reduced state (18). Enzyme inhibition is effective because binding affinities for substrate analogs are so great that they are not readily displaced by the natural substrates (18). Inhibition results in depletion of intracellular reduced folates, which are necessary for one-carbon transfer reactions. One-carbon transfer reactions are important for the biosynthesis of thymidylate, purine nucleotides, methionine, serine, glycine, and many other compounds necessary for RNA, DNA, and protein synthesis (13). Some bacteria have an uptake system for folates, but most require de novo folate synthesis. Reduction of dihydrofolate to tetrahydrofolate is, however, a universal requirement (13).
Although DHFR does not represent a new target, there is still enthusiasm for the development of improved derivatives of this class of inhibitor (1, 5, 18, 22, 25, 26), particularly with regard to mycobacteria (7, 15, 16, 17, 19, 28, 29; W. J. Suling, R. C. Reynolds, J. R. Piper, V. Pathak, E. W. Barrow, L. E. Gundy, S. Z. V. Ginkel, L. Westbrook, and W. W. Barrow, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 339, 1999). A unique feature of DHFR is the selectivity that is possible in the design of inhibitors. This makes it an ideal “old” target for rational and effective drug design for antimycobacterial agents. The antifolate drug trimethoprim is perhaps the best example of selectivity. However, trimethoprim is a poor inhibitor of Mycobacterium avium complex (MAC; 28). Results reported here support the hypothesis that new antifolates can be developed for treatment of mycobacterial infections.
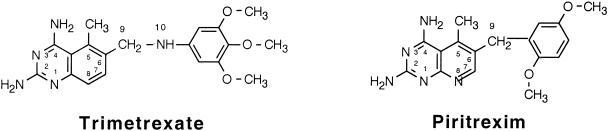
The antifolate compounds evaluated in this investigation are derivatives of 2,4-diamino-5-methyl-5-deazapteridine (DMDP), having structures similar to the trimetrexate/piritrexim class of antifolates (Fig. 1). Due to an interest in new antimycobacterial drugs, several DMDP inhibitors were chosen from the Southern Research Institute chemical repository for screening against MAC. We had previously reported on antimycobacterial activity by 12 of these derivatives for Mycobacterium tuberculosis and MAC (28). At the same time, DMDP derivatives were also reported active against MAC by Shoen et al. (27). As an extension of that work, we report on the results of further studies with other newly synthesized DMDP derivatives with regard to activity against MAC. Most of these derivatives showed good activity against three strains of MAC, with MICs ranging from <0.13 to 1.3 μg/ml. As a result, the derivatives were also evaluated for their ability to inhibit MAC recombinant DHFR (rDHFR) activity. This was compared to their inhibition of purified human rDHFR. Evaluation of selective enzyme inhibition by the 77 DMDP derivatives led to the synthesis of a 78th derivative that showed significant activity against intracellularly replicating MAC.
FIG. 1.
Structures of trimetrexate and piritrexim, with positions numbered.
(Some of this research was presented at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26 to 29 September 1999.)
MATERIALS AND METHODS
Synthesis of DMDP derivatives.
The structures of the compounds used in the present study are presented below (see Tables 2 through 5) and are numbered consecutively as 1 through 78. The compounds were synthesized according to the method of Piper et al. (22). The synthesis of each particular class of analogs was carried out by the exact procedures that are well described by Piper et al. (22), and these descriptions need not be duplicated here. In general, the appropriate 5-substituted 5-deaza-6-bromomethylpteridine was reacted with the desired, commercially available thiophenol or aniline to obtain the S- or N-linked analogs, respectively. The 10-CH2-linked analogs were prepared by Wittig coupling of the reaction product of triphenylphosphine and 5-methyl-5-deaza-6-bromomethylpteridine with the appropriately substituted and commercially available benzaldehyde derivative followed by catalytic hydrogenation (22). All compound structures were verified by mass and 1H-labeled nuclear magnetic resonance (NMR) spectroscopy. Sample purity was assessed by thin-layer chromatography (TLC) and elemental analysis; all compounds gave single spots by TLC and were within acceptable combustion parameters (0.4%). It is notable that these compounds do not have defined melting points, but generally decompose above 300°C. The full experimental details of these compounds are available in the reference of Piper et al. (22) or will be reported elsewhere (unpublished data). As examples of these preparations, both compound 1 (SRI-8686) and compound 78 (SRI-20094) are presented.
TABLE 2.
DMDP derivatives with modifications at the 5 position (R1) and substitutions on the phenyl group (R2 through R6)
| Derivative no.a | SRI no.c | Substitution at DMDP
position
|
IC50 (nM) for:
|
Selectivity ratiob | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | Human DHFR | MAC DHFR | |||
| 1 | 8686 | CH3 | OCH2CH3 | H | H | OCH2CH3 | H | 2,300 | 0.84 | 2,738 |
| 2 | 8117 | CH3 | OCH3 | H | H | OCH3 | H | 1,000 | 1.1 | 909 |
| 3 | 8687c | CH3 | OCH2CH3 | H | H | OCH2CH3 | H | 1,000 | 1.4 | 714 |
| 4 | 8202 | CH3 | CH3 | H | H | OCH3 | H | 150 | 0.40 | 375 |
| 5 | 9734 | CH3 | CH3 | H | Br | H | CH3 | 1,900 | 5.3 | 358 |
| 6 | 8922 | CH2CH3 | OCH3 | H | H | OCH3 | H | 1,000 | 2.8 | 357 |
| 7 | 8229 | CH3 | OCH3 | H | H | CH3 | H | 300 | 0.86 | 349 |
| 8 | 9786 | CH3 | OCHF2 | H | H | H | H | 277 | 0.87 | 318 |
| 9 | 9674 | CH3 | F | H | H | CH3 | H | 250 | 1.1 | 227 |
| 10 | 9717 | CH3 | Cl | H | CH3 | H | CH3 | 850 | 3.8 | 224 |
| 11 | 9643 | CH3 | Cl | H | H | H | H | 120 | 0.91 | 132 |
| 12 | 8911 | CH2CH3 | OCH3 | H | H | CH3 | H | 370 | 3.1 | 119 |
| 13 | 9672 | CH3 | F | H | H | F | H | 69 | 0.90 | 77 |
| 14 | 9826 | CH3 | OCH3 | H | C6H5 | H | H | 68 | 0.91 | 75 |
| 15 | 9018 | CH3 | CH3 | H | H | CH3 | H | 57 | 0.92 | 62 |
| 16 | 9647 | CH3 | Cl | H | H | CH3 | H | 44 | 0.95 | 46 |
| 17 | 9782 | CH3 | Cl | H | F | CH3 | H | 33 | 0.78 | 42.3 |
| 18 | 9645 | CH3 | H | H | Cl | H | H | 27 | 0.84 | 32.1 |
| 19 | 8574c | CH3 | OCH3 | H | H | CF3 | H | 36 | 1.2 | 30 |
| 20 | 8709 | CH3 | H | OCH3 | H | OCH3 | H | 15 | 0.64 | 23.4 |
| 21 | 9675 | CH3 | CH3 | H | H | F | H | 19 | 0.85 | 22 |
| 22 | 9644 | CH3 | H | Cl | H | H | H | 17 | 0.82 | 20.7 |
| 23 | 9787 | CH3 | H | H | OCHF2 | H | H | 20 | 1.1 | 18 |
| 24 | 9981 | CH3 | H | OCH3 | OCH2CH3 | H | H | 18 | 1.1 | 16.5 |
| 25 | 9980 | CH3 | H | OCH3 | O(CH2)2CH3 | H | H | 15 | 0.93 | 16.1 |
| 26 | 7746 | H | H | OCH3 | OCH3 | OCH3 | H | 3,900 | 240 | 16 |
| 27 | 9827 | CH3 | H | F | OCH3 | H | H | 14 | 0.91 | 15 |
| 28 | 9596 | CH3 | CH3 | H | Cl | H | H | 2.8 | 0.19 | 14.7 |
| 29 | 8758 | CH2OCH3 | OCH3 | H | H | OCH3 | H | 31,000 | 2,400 | 12.9 |
| 30 | 8710c | CH3 | H | OCH3 | H | OCH3 | H | 8.7 | 0.73 | 11.9 |
| 31 | 9733 | CH3 | OCH3 | H | Cl | CH3 | H | 10 | 0.94 | 10.6 |
| 32 | 8577 | H | H | H | Cl | H | H | 670 | 73 | 9.2 |
| 33 | 9646 | CH3 | Cl | H | H | Cl | H | 8.0 | 0.89 | 9.0 |
| 34 | 9595 | CH3 | CH3 | H | H | Cl | H | 6.1 | 0.79 | 7.7 |
| 35 | 9600 | CH3 | H | Cl | CH3 | H | H | 6.7 | 0.90 | 7.44 |
| 36 | 8692 | CH3 | H | OCH3 | OCH3 | H | H | 5.1 | 0.70 | 7.29 |
| 37 | 9614 | CH3 | H | CH3 | Br | H | H | 6.1 | 0.87 | 7.01 |
| 38 | 9613 | CH3 | CH3 | H | Br | H | H | 5.9 | 0.85 | 6.94 |
| 39 | 9676 | CH3 | Br | H | H | Br | H | 4.6 | 0.70 | 6.6 |
| 40 | 9599 | CH3 | CH3 | Cl | H | H | H | 5.2 | 0.82 | 6.34 |
| 41 | 9612 | CH3 | H | Br | CH3 | H | H | 3.7 | 0.60 | 6.17 |
| 42 | 8227 | CH3 | H | OCH3 | H | CF3 | H | 5.5 | 0.93 | 5.91 |
| 43 | 7714 | CH3 | H | OCH3 | OCH3 | OCH3 | H | 4.7 | 0.82 | 5.7 |
| 44 | 9632 | CH3 | H | Cl | Cl | H | H | 5.0 | 0.92 | 5.43 |
| 45 | 8766 | CH2OCH3 | H | OCH3 | OCH3 | H | H | 6,100 | 1,200 | 5.1 |
| 46 | 9684 | CH3 | F | H | H | CF3 | H | 4.1 | 0.86 | 4.8 |
| 47 | 8228 | CH3 | OCH3 | H | H | CF3 | H | 2.7 | 0.66 | 4.1 |
| 48 | 8923 | CH2CH3 | OCH3 | H | H | CF3 | H | 3.6 | 0.88 | 4.1 |
| 49 | 9683 | CH3 | Br | H | H | CF3 | H | 3.2 | 0.82 | 3.9 |
| 50 | 8765 | CH2OCH3 | H | OCH3 | OCH3 | OCH3 | H | 1,300 | 550 | 2.4 |
| 51 | 8759 | CH2OCH3 | H | H | Cl | H | H | 2,600 | 1,500 | 1.76 |
Derivatives are listed in descending order by selectivity ratio (see last column).
Selectivity ratio equals IC50 human DHFR/IC50 MAC DHFR.
H group on N10 link is replaced with CH3. SRI, Southern Research Institute.
TABLE 5.
DMDP derivatives in the series involving substitutions at the R2 and R5 positions
| Derivative no.a | SRI no.b | Substitution at
position
|
MICc of MAC (μg/ml) | IC50 (nM)
for:
|
Selectivity ratiod | ||
|---|---|---|---|---|---|---|---|
| R2 | R5 | Human DHFR | MAC DHFR | ||||
| 15 | 9018 | CH3 | CH3 | ++++ | 57 | 0.92 | 62 |
| 7 | 8229 | OCH3 | CH3 | ++++ | 300 | 0.86 | 349 |
| 4 | 8202 | CH3 | OCH3 | ++++ | 150 | 0.40 | 375 |
| 2 | 8117 | OCH3 | OCH3 | ++++ | 1,000 | 1.1 | 909 |
| 1 | 8686 | OCH2CH3 | OCH2CH3 | ++++ | 2,300 | 0.84 | 2,738 |
| 78 | 20094 | O(CH2)2CH3 | O(CH2)2CH3 | ++++ | 7,300 | 1.0 | 7,300 |
Derivatives are listed in ascending order by selectivity ratio (see last column).
SRI, Southern Research Institute.
++++, ≤0.13. MAC strain NJ3440 was used for comparison of MIC.
Selectivity ratio equals IC50 human DHFR/IC50 MAC DHFR.
Preparation of compound 1.
To dissolve as much material as possible, 2,4-diamino-5-methyl-5-deaza-6-cyanopteridine (3.0 g, 0.015 mmol) was stirred with glacial acetic acid (342 ml) with warming (<50°C). After a brief period, the suspension was cooled to ambient temperature and both 2,5-diethoxyaniline (2.98 g, 0.016 mmol) and Raney Nickel (4.995 g of a 50% slurry in water) were added, and the reaction was placed in a hydrogenation apparatus under H2 pressure (atmospheric) with vigorous stirring. After several hours, the reaction had absorbed the required H2, and stirring was halted. The reaction was left standing under H2 pressure overnight. After removal of remaining hydrogen, the reaction mixture was filtered through a celite pad, and the solid was rinsed with glacial acetic acid until the washings were clear. The filtrate was concentrated under vacuum and diluted with 342 ml of H2O, and the pH was adjusted to 9 to 10 with concentrated ammonia. The resulting suspension was placed in the refrigerator overnight, and the suspension was filtered the following morning to yield 3.312 g of crude material after drying under vacuum (P2O5, 25°C). This material was further purified by dissolving in 250 ml of hot dimethylformamide (DMF) drying onto 16.56 g of flash silica gel (220 to 400 mesh) under high vacuum, and then loading onto a flash chromatography column packed with 352 g of flash silica gel and 4:1 chloroform/methanol with 1% glacial acetic acid. Elution with the same solvent gave three separate pools of fractions containing the major product. After evaporation, the middle pool gave 0.1343 g of highly pure material showing a single spot by TLC (4:1 chloroform/methanol with 1% glacial acetic acid): FAB-MS m/z 369.0 (M+H)+, 1H-NMR (DMSO-d6) δ 1.24 (t, 3H 5′-OCH2CH3), 1.30 (t, 3H, 2′-OCH2CH3), 1.91 (s, CH3CO2H), 2.67 (s, 3H, 5-CH3), 3.86 (q, 2H, 5′-OCH2CH3), 3.94 (q, 2H, 2′-OCH2CH3), 4.28 (d, 2H, CH2NH ), 5.13 (t, 1H, CH2NH ), 6.05 (dd, 1H, 4′-H), 6.10 (d, 1H, 6′-H), 6.33 (bs, 2H, 2 or 4-NH2), 6.68 (d, 1H, 3′-H), 7.06 (bs, 2H, 2 or 4-NH2), 8.42 (s, 1H 7-H). Analytical calculated for C19H24N6O2 · 0.5H2O: C, 60.46; H, 6.68; N, 22.27. Found C, 60.48; H, 6.80; N, 22.05.
Preparation of compound 78.
As an alternative to the catalytic hydrogenation method described previously, the synthesis of SRI-20094 represents a direct coupling of an aniline derivative with 2,4-diamino-5-methyl-5-deaza-6-bromomethylpteridine. 2,5-Dipropoxyaniline (500 mg, 1.24 mmol) was dissolved in DMF, and the solution was flushed with dry nitrogen. 2,4-Diamino-5-methyl-5-deaza-6-bromomethylpteridine (500 mg, 2.38 mmol) was added, and the reaction was stirred for 24 h. Next, the reaction was diluted with water and neutralized with saturated NaHCO3. The suspension was filtered, and the solid was washed with water followed by diethylether. Drying under vacuum yielded 210 mg of the crude material, and this material was further purified by dissolving in a minimum of hot DMF and drying on 2.0 g of silica gel (60 to 120 mesh) followed by column chromatography. The powder was placed on a small column packed with silica gel (60 to 120 mesh) loaded with 7:1 chloroform/methanol and 1% concentrated NH4OH. Elution with the same solvent gave several pure fractions that were combined and dried to yield 35 mg of pure SRI-20094. Purity was confirmed by TLC with the aforementioned solvent system: FAB-MS m/z 397.0 (M+H)+, 1H-NMR (DMSO-d6) δ 0.93 (t, 3H, 2′-CH2CH2CH3), 0.94 (t, 3H, 5′- CH2CH2CH3), 1.63 (m, 2H, 5′-CH2CH2CH3), 1.69 (m, 2H, 2′-CH2CH2CH3), 2.67 (s, 3H, 5-CH3), 3.77 (t, 2H, 5′-CH2CH2CH3), 3.85 (t, 2H, 2′-CH2CH2CH3), 4.28 (d, 2H, CH2NH), 5.06 (t, 1H, CH2NH), 6.06 (dd, 1H, 4′-H), 6.13 (d, 1H, 6′-H), 6.25 (bs, 2H, 2 or 4-NH2), 6.67 (d, 1H, 3′-H), 7.01 (bs, 2H, 2 or 4-NH2), 8.42 (s, 1H, 7-H). Analytical calculated for C21H28N6O2 · 0.5H2O: C, 62.06; H, 7.21; N, 20.67. Found C, 62.05; H, 6.95; N, 20.50.
MIC.
MICs were determined against three strains of MAC (NJ168, serovar 1; NJ211, serovar 4/6; and NJ3404, serovar 4), using a colorimetric microdilution broth assay as reported previously (6, 28, 32; Suling et al., 39th ICAAC, abstr. 339). The MAC strains were kindly provided by L. Heifets, National Jewish Center for Immunology and Respiratory Diseases, Denver, Colo. A frozen culture in 7H9 broth (Difco Laboratories, Detroit, Mich.), supplemented with ADC enrichment (Difco) and 0.2% glycerol, was thawed and diluted in broth to about 2 × 105 CFU/ml and used as the inoculum. The assay used a 96-well (U-shaped) microtiter plate and a format designed to accommodate seven compounds in four log10 dilutions. The assay plates also contained uninoculated drug and medium controls and viability controls. Each test compound was dissolved in dimethyl sulfoxide (DMSO), then diluted in broth at twice the desired concentration, and 0.05 ml was added to duplicate assay wells. The highest concentration of DMSO in the assay medium was 1.3%. DMSO did not affect growth at this concentration. Each plate was then inoculated with 0.05 ml of standardized culture and the plates were incubated at 37°C for 6 or 13 days, depending on the assay strain. The REDOX indicator Alamar Blue (Acumed International, Inc., Westlake, Ohio) was then added to each well as a mixture with Tween 80, and the plates were incubated for an additional 18 to 22 h. The plates were read in an optical microtiter plate reader programmed to subtract the absorbance at 600 nm from that at 570 nm to blank out turbidity and absorbance due to oxidized dye. The MIC was reported as the lowest concentration of drug yielding a differential absorbance of zero or less. This approximated the color change of blue to red that was observed visually after metabolic reduction of dyes and represented the concentration at which no visible growth occurred. Trimethoprim or ethambutol was used as a positive control.
Human rDHFR.
Purified human rDHFR, which was generated using a recombinant system in Escherichia coli (23), was provided by Anatrace (Maumee, Ohio). According to the supplier, the enzyme has properties identical to those isolated from human cells as reported by Delcamp et al. (8), and it contained an equimolar concentration of enzyme and dihydrofolate.
Recombinant MAC DHFR.
The MAC DHFR gene was cloned into the vector pET15b at the NdeI, BamHI restriction sites and expressed in E. coli strain BL21(DE3)pLysS as a fusion protein with a His tag (34). The plasmid was grown in Luria-Bertani broth containing 34 μg of chloramphenicol per ml and 50 μg of ampicillin per ml at 28°C to an optical density of 0.6 at 600 nm, then was induced with 0.1 mM isopropyl β-d-thiogalactopyranoside for 20 h. The cells were harvested and lysed on ice in a buffer containing 5 mM imidazole, 500 mM NaCl, 20 mM Tris (pH 7.9), 20 μg of DNase per ml, 10 mM MgCl2, 22 μg of leupeptin per ml, 20 μg of pepstatin A per ml, 0.5 mM phenylmethylsulfonyl fluoride, and 0.1% Triton X-100. The insoluble proteins were removed by centrifugation, and the soluble proteins were purified by binding the His tag portion of the fusion protein to a His bind (Novagen, Inc., Madison, Wis.) resin column and elution with a 5 to 500 mM imidazole salt gradient in a buffer containing 500 mM NaCl and 20 mM Tris-NCl (pH 7.9). All steps were carried out at 4°C. Fractions with DHFR activity were pooled, and the buffer was exchanged to 5 mM imidazole-500 mM NaCl–20 mM Tris-HCl (pH 7.9) with a PD 10 column (Pharmacia), at 4°C. The His tag fusion protein was then removed by cleavage with 0.5 U of thrombin per mg of recombinant protein for 1 h on ice. The recombinant DHFR was stabilized throughout the purification by the addition of bovine serum albumin (1 mg/ml) and was stored at −70°C.
DHFR assay and enzyme inhibition studies.
DHFR activity of both human and MAC enzymes was measured at 30°C as the decrease in absorbance at 340 nm (34). The reaction mixture (1 ml) contained 10 mM 2-mercaptoethanol, 0.1 mM NADPH, 0.1 mM dihydrofolate, 1 mM EDTA, 55 mM potassium phosphate (pH 7), and enzyme. The reaction was initiated by the addition of dihydrofolate after preincubation of the other components for 3 min. For inhibition assays, various amounts of inhibitor were added to the mixture before the 3-min preincubation period. The 50% inhibitory concentration (IC50) was determined from a plot of the log10 of the drug concentration versus percent inhibition as the amount of inhibitor required to inhibit the reaction by 50%. Each reported IC50 is the average of two or more determinations. The amount of enzyme used for the inhibition assays was about 0.0024 U/ml, which yielded a mean rate plus or minus standard deviation of −0.036 ± 0.0046 per min for MAC DHFR and −0.029 ± 0.0026 for human DHFR. Linearity was maintained for >7 min. One unit of enzyme is defined as the amount which reduces 1 μmol of dihydrofolate per min using a molar extinction coefficient of 12,300 M−1 (14). The selectivity ratio was determined as the ratio of the human DHFR IC50 to the MAC DHFR IC50.
Intracellular efficacy.
Intracellular efficacy for compounds 1 (SRI-8686) and 78 (SRI-20094) was assessed by using a previously described method involving the Mono Mac 6 monocytic cell line (MM6; 2, 31). MM6 cells were obtained from the German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany. The MM6 was originally established by H. W. L. Ziegler-Heitbrock, Institute of Immunology, University of Munich, Germany (33), and it is a human acute monocytic leukemia cell line. This cell line has been previously used by us (2, 31) and others (30) to examine effectiveness of antimycobacterial drugs against intracellularly replicating M. tuberculosis. MM6 cells were maintained in RPMI 1640 containing 10% (vol/vol) fetal calf serum, 2 mM l-glutamine, nonessential amino acids, 1 mM sodium pyruvic acid, and 9 μg of bovine insulin (Sigma Chemical Co., St. Louis, Mo.) per ml (MM6 medium). Cells were routinely assayed to verify absence of mycoplasma contamination using the Gen-Probe Mycoplasma Rapid Detection System (Gen-Probe, San Diego, Calif.).
Preparation of mycobacteria for infection of monocytes.
Before infection of MM6, MAC strain NJ3404 was initially grown in Middlebrook 7H9 (Difco) containing 0.2% glycerol and 10% oleic acid albumin-dextrose-catalase (Difco). After the mycobacteria had reached exponential phase, they were dispersed by vortexing with glass beads, and clumps were allowed to settle for 30 min (20). The supernatant was removed, aliquoted, frozen at −70°C, and then thawed and used for infection by resuspension in appropriate cell culture medium. Actual CFUs per milliliter were determined by preparation of serial dilutions in Dulbecco's phosphate-buffered saline (DPBS; Mediatech, Inc., Herndon, Va.) and plating on 7H10 agar.
Infection of monocytes.
Before infection, MM6 cells were adjusted to 8 × 105 cells per ml, and 0.5 ml per well was dispensed in 12-well tissue culture dishes (Corning Costar Corp., Cambridge, Mass.). The MAC cells were then added to the MM6 cells to achieve a final ratio of 10 mycobacteria per macrophage, with a density of 4 × 105 MM6 cells per 1.0 ml per well. After infection for 24 h, the infected MM6 cells were collected by centrifugation (200 × g) and washed twice with DPBS to remove any unphagocytosed mycobacteria. The cells were then replated at a density of 4 × 105 cells per 1.0 ml per well, with appropriate wells containing drug preparations, and the plates were incubated at 37°C with 5% carbon dioxide. One milliliter of fresh medium was added at day 4, and infection continued until CFU assay at day 7.
Determination of CFU.
The determination of CFU at zero hour was conducted by first lysing the monocytes with 0.25% (wt/vol) sodium dodecyl sulfate in DPBS and then plating serial dilutions onto 7H10 agar plates (31). As a means of decreasing viscosity, 5 μl (5 U of activity) of RQ DNase (Promega Corp. Madison, Wis.), with MgSO4 (5 mM), was added to each well following addition of sodium dodecyl sulfate, and the plates were incubated at 37°C for 20 min (31). The plating procedure was repeated at 7 days, and CFU were enumerated after 10 to 14 days of incubation of cultures.
Macrophage viability assays.
Cell viability was determined by means of an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide [thiazolyl blue]) cytotoxicity assay (Sigma), modified in our laboratory as described previously (2, 31). Viability is reported as a percentage of the nontreated cells.
RESULTS
In vitro activity of DMDP derivatives against MAC strains.
In vitro inhibition was evaluated against three MAC strains (NJ168, NJ211, and NJ3404) for 50 of the DMDP derivatives (Table 1). MAC strain NJ168 was consistently the most resistant of the three strains tested. With this strain, four derivatives had MICs of >13 μg/ml (Table 1 [indicated by +, derivatives 29, 45, 50, and 51]), while MAC strains NJ211 and NJ3404 responded with MICs in this range for only one (derivative 29) and two (derivatives 29 and 50 [Table 1]) of the derivatives, respectively (+ [Table 1]). The MICs of most of the 50 compounds for all three strains were ≤1.3 μg/ml, with NJ168 having the least (38 derivatives [76%]). Both strains NJ211 and NJ3404 had 43 derivatives (86%) with MICs of ≤1.3 μg/ml. Strain NJ168 was used to screen an additional 27 derivatives. Of those 27 derivatives, 24 had MICs of ≤1.3 μg/ml (+++ and ++++ [Table 1]), for a total of 62 out of 77 (81%). More significant is the fact that, out of all the derivatives tested against each strain, 39% had MICs of ≤0.13 μg/ml for NJ168, and 64% and 71% had MICs of ≤0.13 μg/ml for NJ211 and NJ3404, respectively. The antibacterial DHFR inhibitor trimethoprim had an MIC of >13 μg/ml for all three strains (data not shown).
TABLE 1.
Numerical listing of DMDP derivatives, along with their Southern Research Institute (SRI) numbers
| DMDP derivative no. | SRI no. | MIC
rangesa for MAC strain
|
||
|---|---|---|---|---|
| NJ168 | NJ211 | NJ3404 | ||
| 1 | 8686 | +++ | ++++ | ++++ |
| 2 | 8117 | +++ | ++++ | ++++ |
| 3 | 8687 | +++ | ++++ | ++++ |
| 4 | 8202 | ++++ | ++++ | ++++ |
| 5 | 9734 | ++++ | ND | ND |
| 6 | 8922 | +++ | +++ | ++++ |
| 7 | 8229 | +++ | ++++ | ++++ |
| 8 | 9786 | +++ | ND | ++++ |
| 9 | 9674 | ++++ | ND | ND |
| 10 | 9717 | +++ | ND | ND |
| 11 | 9643 | ++++ | ND | ND |
| 12 | 8911 | ++++ | ++++ | ++++ |
| 13 | 9672 | ++++ | ND | ND |
| 14 | 9826 | +++ | ++++ | ++++ |
| 15 | 9018 | +++ | ++++ | ++++ |
| 16 | 9647 | ++++ | ND | ND |
| 17 | 9782 | +++ | ++++ | ++++ |
| 18 | 9645 | ++++ | ND | ND |
| 19 | 8574 | +++ | ++++ | ++++ |
| 20 | 8709 | +++ | ++++ | ++++ |
| 21 | 9675 | +++ | ND | ND |
| 22 | 9644 | ++++ | ND | ND |
| 23 | 9787 | +++ | ++++ | ++++ |
| 24 | 9981 | +++ | ++++ | ++++ |
| 25 | 9980 | +++ | ++++ | ++++ |
| 26 | 7746 | ++ | ++ | ++ |
| 27 | 9827 | +++ | ++++ | ++++ |
| 28 | 9596 | ++++ | ND | ND |
| 29 | 8758 | + | + | + |
| 30 | 8710 | +++ | ++++ | ++++ |
| 31 | 9733 | +++ | ND | ND |
| 32 | 8577 | ++ | +++ | +++ |
| 33 | 9646 | ++++ | ND | ND |
| 34 | 9595 | ++++ | ND | ND |
| 35 | 9600 | ++++ | ND | ND |
| 36 | 8692 | ++++ | ++++ | ++++ |
| 37 | 9614 | ++++ | ND | ND |
| 38 | 9613 | +++ | ND | ND |
| 39 | 9676 | +++ | ND | ND |
| 40 | 9599 | ++++ | ND | ND |
| 41 | 9612 | ++++ | ND | ND |
| 42 | 8227 | ++++ | ++++ | ++++ |
| 43 | 7714 | ++++ | ++++ | ++++ |
| 44 | 9632 | ++++ | ND | ND |
| 45 | 8766 | + | ++ | ++ |
| 46 | 9684 | ++++ | ND | ND |
| 47 | 8228 | ++++ | ++++ | ++++ |
| 48 | 8923 | +++ | ++++ | ++++ |
| 49 | 9683 | ++++ | ND | ND |
| 50 | 8765 | + | ++ | + |
| 51 | 8759 | + | ++ | ++ |
| 52 | 8708 | +++ | +++ | +++ |
| 53 | 8691 | +++ | ++++ | ++++ |
| 54 | 9735 | ++ | ND | ND |
| 55 | 9468 | ++++ | ++++ | ++++ |
| 56 | 9771 | ++ | ++ | ++ |
| 57 | 9449 | ++++ | ++++ | ++++ |
| 58 | 7747 | ++ | +++ | +++ |
| 59 | 9487 | ++++ | ++++ | ++++ |
| 60 | 9450 | ++++ | ++++ | ++++ |
| 61 | 9824 | +++ | ++++ | ++++ |
| 62 | 7711 | ++++ | ++++ | ++++ |
| 63 | 9825 | +++ | ++++ | ++++ |
| 64 | 9692 | ++++ | ND | ND |
| 65 | 9439 | ++++ | ++++ | ++++ |
| 66 | 9823 | +++ | ++++ | ++++ |
| 67 | 9673 | ++ | ND | ND |
| 68 | 9388 | ++ | ND | +++ |
| 69 | 8455 | +++ | +++ | ++++ |
| 70 | 9437 | ++ | +++ | +++ |
| 71 | 8451 | +++ | ++++ | ++++ |
| 72 | 8450 | +++ | +++ | +++ |
| 73 | 9490 | +++ | +++ | ++++ |
| 74 | 8573 | ++ | +++ | ++++ |
| 75 | 7896 | ++ | ++ | ++ |
| 76 | 9165 | ++ | +++ | +++ |
| 77 | 8453 | +++ | +++ | +++ |
+, >13 μg/ml; ++, >1.3 to ≤13 μg/ml; +++, >0.13 to ≤1.3 μg/ml; ++++, ≤0.13 μg/ml. ND, not done.
Enzyme inhibition assays with DMDP derivatives.
Using MAC and human rDHFR, 77 DMDP derivatives were evaluated initially for their ability to inhibit enzyme activity. In this assay, the IC50s of trimethoprim, trimetrexate, and piritrexim for the human rDHFR were >350,000, 4.7, and 4.4 nM, respectively. The IC50s of the same three drugs for the MAC rDHFR were 4,100, 1.0, and 1.4 nM, respectively (data not shown).
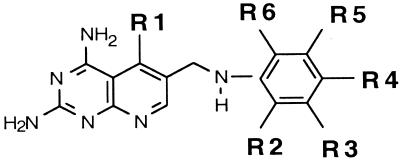
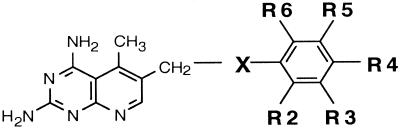
The DMDP analogs contained modifications in three areas, the 5 position, the linking atom in the 10 position of the bridge, and the aromatic nucleus attached to the 10 position. The results are reported in Tables 2 through 4 and are based on structural similarity of derivatives in each group. Table 2 contains the data obtained from those derivatives having a CN bridge and substitutions on the attached phenyl ring, denoted as R2, R3, R4, R5, and R6. The 5-substituent (R1) also had various modifications. Table 3 contains similar derivatives but having alternative aromatic-ring systems instead of a phenyl group attached to the bridge nitrogen. Table 4 contains derivatives similar to those in Table 2 except that the bridge nitrogen was replaced with either a CH2 or S .
TABLE 4.
DMDP derivatives involving modification of the bridge molecules ( X )
| Derivative no.a | SRI no.b | Modification at
positionc
|
IC50 (nM)
for:
|
Selectivity ratiod | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X | R2 | R3 | R4 | R5 | R6 | Human DHFR | MAC DHFR | |||
| 68 | 9388 | CH2 | H | OCH3 | H | OCH3 | H | 990 | 1.5 | 660 |
| 69 | 8455 | S | H | H | OCH3 | OCH3 | H | 440 | 1.5 | 293 |
| 70 | 9437 | S | H | OCH3 | OCH3 | OCH3 | H | 1,200 | 4.5 | 267 |
| 71 | 8451 | S | H | H | H | OCH3 | H | 500 | 2.7 | 185 |
| 72 | 8450 | S | H | H | Cl | H | H | 590 | 3.6 | 164 |
| 73 | 9490 | S | OCH3 | H | H | OCH3 | H | 2,500 | 23 | 109 |
| 74 | 8573 | S | H | H | H | Cl | H | 420 | 3.9 | 108 |
| 75 | 7896 | S | 1-Naphthyl | 440 | 4.6 | 95.7 | ||||
| 76 | 9165 | CH2 | OCH3 | H | H | OCH3 | H | 2,700 | 47 | 57 |
| 77 | 8453 | S | H | H | H | CH3 | H | 850 | 16 | 53 |
Derivatives are listed in decreasing order of selectivity ratio (see last column).
SRI, Southern Research Institute.
Substitutions on the phenyl group are designated as R2 through R6.
Selectivity ratio equals IC50 human DHFR/IC50 MAC DHFR.
TABLE 3.
DMDP derivatives with complex ring substitutions attached to the CN bridge
| Derivative no.a | SRI no.b | X | IC50 (nM)
for:
|
Selectivity ratioc | |
|---|---|---|---|---|---|
| Human DHFR | MAC DHFR | ||||
| 52 | 8708d |  |
710 | 1.9 | 374 |
| 53 | 8691 | 142 | 0.93 | 153 | |
| 54 | 9735 | 5,300 | 320 | 16.6 | |
| 55 | 9468 | 10 | 0.76 | 13.2 | |
| 56 | 9771 | 3,400 | 280 | 12.1 | |
| 57 | 9449 | 12 | 1.2 | 10.0 | |
| 58 | 7747e | 820 | 86 | 9.5 | |
| 59 | 9487 | 6.7 | 0.86 | 7.79 | |
| 60 | 9450 | 7.1 | 0.95 | 7.47 | |
| 61 | 9824 | 6.2 | 1.0 | 6.2 | |
| 62 | 7711 | 4.3 | 0.81 | 5.31 | |
| 63 | 9825 | 2.0 | 0.43 | 4.65 | |
| 64 | 9692 | 4.0 | 0.87 | 4.6 | |
| 65 | 9439 | 4.3 | 0.98 | 4.39 | |
| 66 | 9823 | 3.8 | 0.88 | 4.3 | |
| 67 | 9673 | >25,300 | 620 | ||
Derivatives are listed in descending order by selectivity ratio (see last column).
SRI, Southern Research Institute.
Selectivity ratio = IC50 human DHFR/IC50 MAC DHFR.
H group on N link is replaced with a CH3 group.
CH3 group in position 5 is replaced with an H.
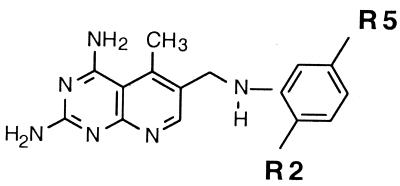
Derivatives with modifications at the 5-position and substitutions on the phenyl group.
In the case of the 5-substituent (R1), we evaluated 5-H, 5-CH3, 5-CH2CH3, and 5-CH2OCH3. Only two compounds were tested that had the 5-H substitution (compounds 26 and 32 [Table 2]); therefore, it was not possible to make any conclusive evaluation regarding selectivity. It appeared that the 5-H substitution may have modestly improved selectivity; the selectivity ratio was increased only by about threefold (compounds 26 versus 43 [Table 2]). The 5-H substitution, however, lowered the activity for both human and MAC rDHFR when compared to the 5-CH3 substitution (compound 26 versus 43 and compound 32 versus 18 [Table 2]). Several direct comparisons were available in the 5-CH2CH3 and 5-CH2OCH3 substitutions. These substitutions in general resulted in decreased activity for both enzymes versus their 5-CH3 counterparts, and in contrast to the 5-H substitution, they did not improve the selectivity ratio. For example, a comparison of compound 2 with compounds 6 and 29 (Table 2) showed that substitution on the 5-position of a CH2CH3 or CH2OCH3 yielded a reduction in the selectivity ratio from 909 to 357 and 12.9, respectively. Thus, the optimum substitution at the 5-position was CH3.
An overall evaluation of the data presented in Table 2 suggested that the 5-CH3 substitution and substitutions at the 2′ (R2) and 5′ (R5) positions on the phenyl ring resulted in an optimal selectivity ratio. The best examples for this comparison are compounds 15, 7, 4, 2, and 1 (Table 2), which demonstrated an increase in the selectivity ratio from 62 to 2,738, respectively. This 44-fold improvement was obtained by extended modifications at the 2′ and 5′ positions.
Derivatives with complex ring substituents attached to the CN bridge.
Sixteen derivatives were evaluated with modifications involving heterocyclic ring substitutions attached to the CN bridge. Although many of the compounds had good activity versus MAC DHFR, selectivity for the MAC compared to the human enzyme was poor except for tetrahydronaphthyl-linked compounds 52 and 53 (Table 3). The selectivity ratio for these compounds was 374 and 153, respectively.
Derivatives involving modification of the bridge molecules.
We also evaluated modifications of the 10-position bridging atom from a nitrogen to a CH2 or S . Although only 10 derivatives in this classification were examined, results suggested that modifications at this position might result in improved selectivity. For example, a comparison of compound 68 (Table 4) with 20 (Table 2) revealed that substitution of the CH2NH bridge with CH2CH2 caused a 66-fold decrease in activity for the human rDHFR, resulting in an increase in the selectivity ratio from 23.4 to 660 (28-fold). Interestingly, the alkyl substitutions on the phenyl ring of the above compounds are at the R3 and R5 positions. In contrast, the same does not apply for those derivatives with substitutions on the R2 and R5 positions. For example, a comparison of compounds 76 (Table 4) and 2 (Table 2), in which the CH2NH bridge is substituted with CH2CH2 , showed that this modest alteration resulted in such a decrease in activity against the MAC rDHFR that the selectivity ratio was reduced from 909 to 57 (16-fold).
A small number of compounds were evaluated wherein the X- linkage was replaced by S- (eight compounds [Table 4]). It is interesting to note that all of the S-substituted linkages were selective for the MAC enzyme. It is also notable that in every comparison of pairs except one (compounds 70, 72, 73, and 75 in Table 4 versus 43, 18, and 2, in Table 2 and 62 in Table 3, respectively) the S-linkage was significantly more selective than the 10-aza counterpart. Unfortunately, the one exception is a critical case; the 2′,5′-dialkoxy (R2, R5) substitution yielded the greatest selectivity in the 10-aza linkage, and the 2′,5′-dimethoxy analog (compound 2 [Table 2]) is about ninefold more selective than its S-linked counterpart (compound 73 [Table 4]).
A small sample set (Table 2, compounds 3, 19, and 30 versus 1, 47, and 20, respectively) was screened in which both the NH and NCH3 were available with the same substitution pattern for comparison. In two instances, the substitution of a CH3 for a H at the 10-aza group reduced selectivity by increasing inhibition of the human enzyme with little or no change in activity against MAC rDHFR. However, in one instance (compound 19 versus 47), the activity against the human enzyme was decreased by over 10-fold with a small effect on the MAC enzyme inhibition, giving an almost 8-fold increase in selectivity.
Intracellular efficacy of compound 1 (SRI-8686).
Based on the data presented in Tables 2 through 4, we chose the derivative with the highest selectivity ratio for intracellular efficacy evaluation. Compound 1 was evaluated in the MM6 cells by using concentrations of 0.1 and 1.0 μg/ml. Unfortunately, excessive loss of MM6 viability at the end of the 7-day experimental period for the highest concentration (i.e., 1.0 μg) prevented proper assessment of data and indicated that use of higher concentrations would not be successful for the same reason. Because of these results, we evaluated the enzyme data (Table 2) in an attempt to rationalize improvements that could be made to reduce toxicity to the human cell line.
Based on our results with regard to substitutions at the R2 and R5 positions (Table 2), we hypothesized that further alkyl extension at these positions would improve the selectivity ratio by decreasing the activity for the human DHFR. If such a modification could be made while maintaining activity for the MAC DHFR, then it should be possible to demonstrate significant intracellular efficacy because toxicity to the cell lines would be reduced. As a result, compound 78 (SRI-20094) was synthesized with O(CH2)2CH3 substitutions at the R2 and R5 positions. To demonstrate the rationale for our experimental approach, this derivative is given in Table 5, along with similar data already reported for other DMDP derivatives in the same series (Tables 1 and 2). As the data suggest, further extension at the R2 and R5 positions resulted in decreased activity (threefold) for the human rDHFR with continued maintenance of activity for the MAC DHFR (Table 5). This yielded a selectivity ratio of 7,300.
Intracellular efficacy of compound 78 (SRI-20094).
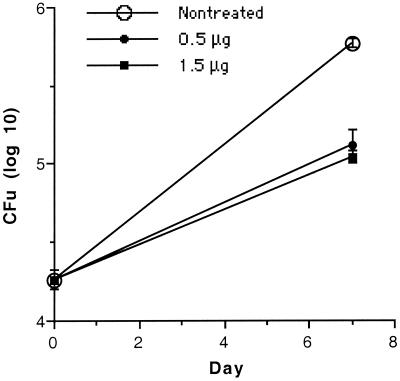
Intracellular efficacy of compound 78 was evaluated in MM6 cells using concentrations of 0.5 and 1.5 μg/ml. Results revealed that compound 78 was able to reduce MAC CFUs at 0.5 and 1.5 μg/ml in the MM6 cell model (Fig. 2). Significant CFU reductions of 0.65 and 0.73 log, respectively, were observed for the 0.5- and 1.5-μg/ml concentrations (P < 0.001) (Fig. 2). At the end of the 7-day experimental period, viability of the MM6 cells was determined by the MTT assay (see above). Viability of the cells treated with 0.5 and 1.5 μg/ml was 79 and 76% of the nontreated control, respectively.
FIG. 2.
Treatment of MAC-infected MM6 cells with compound 78 (SRI-20094). MM6 cells were infected with MAC strain NJ3404 as described in Materials and Methods. CFUs were determined in triplicate at 0 and 7 days for nontreated (○) cells and those treated with 0.5 μg (●) of compound 78 per ml and 1.5 μg (▪) of compound 78 per ml. Values represent the mean plus or minus standard error of the mean for triplicate assays. The differences between the mean values for the cells treated with 0.5 μg and 1.5 μg of compound 78 per ml versus the nontreated control cells were both significant at a P value of <0.001. Significance was determined by one-way analysis of variance, and a correction for multiple comparisons (posttest) was performed by the Tukey-Kramer multiple-comparisons test using InStat (GraphPad Software).
DISCUSSION
Previous reports suggest that DMDP derivatives might be developed into clinically useful antimycobacterial drugs (27, 28). We therefore conducted this study to determine the structure-activity relationships of several DMDP derivatives as antimycobacterial agents against MAC and as selective inhibitors of MAC DHFR. All derivatives were modified to test the impact of key substitutions on binding and selectivity of the compounds for mycobacterial DHFR versus the human enzyme, while retaining the critical 2,4-diaminopyrimidine portion of the deazapteridine nucleus that is so critical for protonation in the active site of DHFR enzymes (3). The resulting salt bridge between the inhibitor and enzyme forms the basis for the potent binding and inhibition of these antifolate drugs. Selectivity for eukaryotic versus prokaryotic DHFRs is generally driven through alterations outside of this critical binding function (3). Therefore, while most of the DMDP derivatives had good antimycobacterial activity against MAC, we found that enzyme inhibition and selectivity for the MAC rDHFR could be dramatically improved by making certain chemical substitutions at strategic positions on the molecule.
In the case of the 5-substituent (R1 in Table 2), four groups were evaluated, 5-H, 5-CH3, 5-CH2CH3, and 5-CH2OCH3. It has been reported in other systems that the 5-H versus 5-CH3 substitution confers a greater decrease in activity against human DHFR than other DHFRs, resulting in higher selectivity (12). In this study, only a modest increase in selectivity was observed but always at the expense of enzyme inhibition. It is clear from X-ray data in other systems that the 5-CH3 substitution optimally occupies a hydrophobic pocket in the DHFR enzyme. This observation is true for the human enzyme as well as homology models derived for the mycobacterial enzymes (R. C. Reynolds, unpublished results). The larger 5-substituent CH2CH3 generally had only a modest effect on inhibition of the human enzyme while slightly increasing the IC50 against the MAC enzyme. Thus, the selectivity ratios generally dropped slightly. When a larger substituent such as a 5-CH2OCH3 was tested, it was clear that enzyme inhibition was significantly and negatively impacted, confirming that the 5-CH3 substitution optimally fills this hydrophobic cleft within the DHFR enzyme.
In the bridge linker region ( X ), a variety of linkers were evaluated (X = NH or NCH3 [Table 2]; X = CH2 and S [Table 4]). However, the majority of derivatives contained a 10-aza linkage. Our results with a small sample set (compounds 3/1, 19/47, 30/20 [Table 2]), in which CH3 replaced the H on the bridge nitrogen, indicated generally that this substitution enhanced the inhibition of human DHFR while activity decreased for MAC DHFR. Others have reported a mixed trend in activity for other DHFRs with this substitution on DMDP derivatives (10, 11). It is difficult to argue a trend with such a small data set. It will be necessary to investigate a small number of NCH3 derivatives of other highly active, selective N-linked analogs to further investigate the effect of this substitution.
When we assayed a limited number of derivatives in which the bridge nitrogen was replaced with a sulfur atom, we found that the S-linked derivatives were more active against the MAC DHFR than the human DHFR. Also, except for the 2′,5′-dimethoxy-substituted derivative 73 (Table 4), all of the S-linked derivatives yielded a higher selectivity ratio than their N-linked counterparts. However, as mentioned previously, the 2′,5′-dialkoxy, 10-aza-linked derivatives had the highest selectivity ratios. Thus, it may not be true that the selectivity of any highly active sample can be improved by changing the 10-aza linkage to a 10-thia linkage. A different mode of binding for the S-linked compounds versus the highly selective —N-linked-2′,5′-dialkoxy compounds in the MAC enzyme may explain why the selectivity of some samples is improved while the highly potent and selective 2′,5′-dialkoxy compounds actually decrease. It may be possible, however, to enhance the selectivity of other less selective but potent inhibitors by altering the linkage from nitrogen to sulfur. For example, some of the halogen- and trifluoromethyl-substituted compounds were active against both the MAC and human enzymes. It will be worthwhile to make the equivalent S-linked analogs to test this hypothesis (compare compound 72 [Table 4] versus 18 [Table 2]).
There are only two examples of a CH2-substituted bridge linkage in our data set (Table 4). With such a limited sample, the results are equivocal in terms of a clear trend in enzyme inhibition with this linkage alteration. Again, it would appear that for the potent and selective 2,5-dimethoxy analog, this alteration (compound 2 [Table 2] versus 76 [Table 4]) reduced selectivity although this result came by decreasing activity against both enzymes; human inhibition was decreased 2.7-fold whereas MAC inhibition was reduced about 40-fold. In the case of the 3,5-dimethoxy substitution (compound 20 [Table 2] versus 68 [Table 4]), selectivity was enhanced from 23.4 to 660 by altering the NH linkage to a CH2 linkage. Enzyme inhibition was again lessened, but only slightly for MAC (0.64 nM versus 1.5 nM) versus about 66-fold for human enzyme (15 nM versus 990 nM). Therefore, substitution at the 3,5-position of the C-linked deazapteridine system may be fruitful for developing other active, selective DHFR inhibitors.
The remaining portion of the DMDP analogs that requires discussion is the substituted aromatic ring (R2 through R6) attached through the linkage to the 6 position of the deazapteridine ring system (Table 2). In certain cases, these substitutions have been discussed above, relative to other changes in the molecule. In general, we next discuss the 5-CH3 10-aza system, since more predictive data are available. Several substitution patterns stand out. The 2′,4′,6′-trisubstituted analogs appeared to show good selectivity and activity (compounds 5 and 10 [Table 2]). No 2′,6′- or 4′,6′-disubstituted analogs were evaluated in this study. Based on this small data set of compounds, it will be worthwhile to pursue a modest number of analogs to probe the 2′,4′-, 2′,6′-, 4′,6′-, and 2′,4′,6′-substitution patterns in order to enhance selectivity and potency. It was the 2′,5′-dialkoxy substitution that showed a clear trend towards higher selectivity while retaining nanomolar potency against the MAC enzyme. The 2′,5′-dimethoxy analog (compound 2 [Table 2]) was quite active against MAC (MIC ≤ 0.13 μg/ml). This analog inhibited MAC rDHFR at 1.1 nM versus 1,000 nM for the human enzyme, giving a good selectivity ratio of 909. Extension of the methoxy moiety at the R2 and R5 positions (i.e., diethoxy analog 1 [Table 2]) was similarly more active against the MAC rDHFR (0.84 nM), while the activity against the human enzyme decreased to 2,300 nM for a selectivity ratio of 2,378. Further extension at the R2 and R5 positions resulted in a derivative with decreased potency for the human rDHFR but continued activity for the MAC DHFR (compound 78 [Table 5]). This improvement reduced the toxicity of the series further and allowed for the demonstration of intracellular efficacy in the MM6 cells. Clearly, other substitutions at the 2′,5′ position of the phenyl ring would be fruitful in order to develop more selective and potent inhibitors of MAC DHFR. Studies are in progress to improve the potency of the DMDP derivatives. It is interesting to note that Piper et al. (22) previously investigated the activity of several of the DMDP derivatives reported here, but against Pneumocystis carinii and Toxoplasma gondii DHFR. These investigators concluded that, in general, compounds with a 2′,5′-disubstituted phenyl group are better in potency and selectivity than compounds substituted on other positions of the phenyl group.
In summary, our results support the conclusion that DMDP derivatives can be developed which are highly active against MAC and which have minimum toxicity to the host. However, a comparison of our results with those reported by others for trimethoprim (21) suggests that further improvement of the selectivity ratio by about 10-fold is needed to achieve this goal. Trimethoprim, a clinically effective antibacterial agent and DHFR inhibitor, has reported IC50s for E. coli DHFR of 5 to 8 nM while the IC50s for rat liver DHFR are 2.6 × 105 to 3.7 × 105 nM (21). These data give a selectivity ratio of 32,500 to 74,000. The selectivity ratio for compound 78 was 7,300. Further studies are in progress to improve the activity of DMDP derivatives against MAC and to assess their efficacy in an animal model. Providing that structural modifications can be made to increase the IC50 for the human DHFR, while maintaining selective potency against the MAC DHFR, we feel that it will be possible to increase the selectivity ratio to levels comparable to those observed in other bacterial systems with trimethoprim. This should result in a DHFR inhibitor that can be used effectively to treat MAC infections, providing that other factors such as cell uptake of drug and pharmacologic parameters are favorable.
It is important to note that, although initial in vitro screening of these DMDP derivatives with MAC demonstrated good activity (27, 28), no clear rational synthetic approach was possible until enzyme inhibition data were obtained. Preliminary studies with M. tuberculosis have revealed that most of the DMDP derivatives presented here show less in vitro activity against whole cells (Suling et al., 39th ICAAC, abstr. 339). The results of subsequent preliminary studies with M. tuberculosis rDHFR have suggested that the lower activity is due to differences in inhibitor binding with the enzyme (W. J. Suling, unpublished data). Thus, additional studies utilizing recombinant M. tuberculosis DHFR will be useful in organizing a rational synthetic approach for improved activity against M. tuberculosis. Those studies are also in progress.
ACKNOWLEDGMENTS
This research was primarily funded by National Institutes of Health (NIH) grant AI41348 (W.W.B., Principal Investigator). Earlier work on some of the DMDP derivatives was funded by NIH grants AI38706 and AI30279 (J.R.P., Principal Investigator).
The M. avium strains were kindly provided by L. Heifets, National Jewish Center for Immunology and Respiratory Diseases, Denver, Colo.
REFERENCES
- 1.Baccanari D P, Kuyper L F. Basis of selectivity of antibacterial diaminopyrimidines. J Chemother. 1993;5:393–399. [PubMed] [Google Scholar]
- 2.Barrow E L W, Winchester G A, Staas J K, Quenelle D C, Barrow W W. Use of microsphere technology for sustained and targeted delivery of rifampin to Mycobacterium tuberculosis-infected macrophages. Antimicrob Agents Chemother. 1998;42:2682–2689. doi: 10.1128/aac.42.10.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaney J M, Hansch C, Silipo C, Vittoria A. Structure-activity relationships of dihydrofolate inhibitors. Chem Rev. 1984;84:333–407. [Google Scholar]
- 4.Bleyer W A. The clinical pharmacology of methotrexate. New applications of an old drug. Cancer Treat Rev. 1978;41:36–51. doi: 10.1002/1097-0142(197801)41:1<36::aid-cncr2820410108>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Bowden K, Harris N V, Watson C A. Structure-activity relationships of dihydrofolate reductase inhibitors. J Chemother. 1993;5:377–388. [PubMed] [Google Scholar]
- 6.Collins L A, Franzblau S G. Microplate Alamar Blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czaplinski K-H, Hänsel W, Wiese M, Seydel J K. New benzylpyrimidines: inhibition of DHFR from various species. QSAR, CoMFA and PC analysis. Eur J Med Chem. 1995;30:779–787. [Google Scholar]
- 8.Delcamp T J, Susten S S, Blankenship D T, Freisheim J H. Purification and characterization of dihydrofolate reductase from methotrexate-resistant human lymphoblastoid cells. Biochemistry. 1983;22:633–639. doi: 10.1021/bi00272a017. [DOI] [PubMed] [Google Scholar]
- 9.Finland M, Kass E H. Symposium on trimethoprim-sulfamethoxazole. J Infect Dis. 1973;128:S425–S816. doi: 10.1093/infdis/128.supplement_3.s792. [DOI] [PubMed] [Google Scholar]
- 10.Gangjee A, Adair O, Queener S F. Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase inhibitors and antitumor agents: synthesis and biological activities of 2,4-diamino-5-methyl-6-[(monosubstituted anilino) methyl]-pyrido [2,3-d] pyrimidines. J Med Chem. 1999;42:2447–2455. doi: 10.1021/jm990079m. [DOI] [PubMed] [Google Scholar]
- 11.Gangjee A, Shi J, Queener S F, Barrows L R, Kisliuk R L. Synthesis of 5-methyl-5-deaza nonclassical antifolates as inhibitors of dihydrofolate reductases and potential antipneumocystis, antitoxoplasma, and antitumor agents. J Med Chem. 1993;36:3437–3443. doi: 10.1021/jm00074a026. [DOI] [PubMed] [Google Scholar]
- 12.Gangjee A, Vasudevan A, Queener S F, Kisliuk R L. 2,4-Diamino-5-deaza-6-substituted pyrido[2,3-d]pyrimidine antifolates as potent and selective nonclassical inhibitors of dihydrofolate reductases. J Med Chem. 1996;39:1438–1446. doi: 10.1021/jm950786p. [DOI] [PubMed] [Google Scholar]
- 13.Hartman P G. Molecular aspects and mechanism of action of dihydrofolate reductase inhibitors. J Chemother. 1993;5:369–376. [PubMed] [Google Scholar]
- 14.Hillcoat B L, Dixon P F, Blakley R L. Effect of substrate decomposition on the spectrophotometric assay of dihydrofolate reductase. Anal Biochem. 1967;21:178–189. doi: 10.1016/0003-2697(67)90179-0. [DOI] [PubMed] [Google Scholar]
- 15.Kansy M, Seydel J K, Wiese M, Haller R. Synthesis of new 2,4-diamino-5-benzylpyrimidines active against various bacterial species. Eur J Med Chem. 1992;27:237–244. [Google Scholar]
- 16.Li R, Sirawaraporn R, Chitnumsub P, Sirawaraporn W, Wooden J, Athappilly F, Turley S, Hol W G J. Three-dimensional structure of M. tuberculosisdihydrofolate reductase reveals opportunities for the design of novel tuberculosis drugs. J Mol Biol. 2000;295:307–323. doi: 10.1006/jmbi.1999.3328. [DOI] [PubMed] [Google Scholar]
- 17.Locher H H, Schlunegger H, Hartman P G, Angehrn P, Then R L. Antibacterial activities of epiroprim, a new dihydrofolate reductase inhibitor, alone and in combination with dapsone. Antimicrob Agents Chemother. 1996;40:1376–1381. doi: 10.1128/aac.40.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCourt M, Cody V. Conformational analysis of lipophilic antifolates: crystal structure of 2-amino-4-oxo-6-adamantylpteridine and a comparison of its binding to bacterial and avian dihydrofolate reductase. J Am Chem Soc. 1991;113:6634–6639. [Google Scholar]
- 19.Meyer S C C, Majumder S K, Cynamon M H. In vitro activities of PS-15, a new dihydrofolate reductase inhibitor, and its cyclic metabolite against Mycobacterium aviumcomplex. Antimicrob Agents Chemother. 1995;39:1862–1863. doi: 10.1128/aac.39.8.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Tentative standard M24-T. Antimycobacterial susceptibility testing for Mycobacterium tuberculosis. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 21.Ohemeng K A, Roth B. Receptor-based design of novel dihydrofolate reductase inhibitors: benzimidazole and indole derivatives. J Med Chem. 1991;34:1383–1394. doi: 10.1021/jm00108a022. [DOI] [PubMed] [Google Scholar]
- 22.Piper J R, Johnson C A, Krauth C A, Carter R L, Hosmer C A, Queener S F, Borotz S E, Pfefferkorn E R. Lipophilic antifolates as agents against opportunistic infections. 1. Agents superior to trimetrexate and piritrexim against Toxoplasma gondii and Pneumocystis carinii in in vitroevaluations. J Med Chem. 1996;39:1271–1280. doi: 10.1021/jm950760y. [DOI] [PubMed] [Google Scholar]
- 23.Prendergast N J, Delcamp T J, Smith P L, Freisheim J H. Expression and site-directed mutagenesis of human dihydrofolate reductase. Biochemistry. 1988;27:3664–3671. doi: 10.1021/bi00410a022. [DOI] [PubMed] [Google Scholar]
- 24.Saxena A K, Saxena M. Advances in chemotherapy of malaria. Prog Drug Res. 1986;30:221–280. doi: 10.1007/978-3-0348-9311-4_8. [DOI] [PubMed] [Google Scholar]
- 25.Schweitzer B I, Dicker A P, Bertino J R. Dihydrofolate reductase as a therapeutic target. FASEB J. 1990;4:2441–2452. doi: 10.1096/fasebj.4.8.2185970. [DOI] [PubMed] [Google Scholar]
- 26.Seydel J K. In vitro and in vivo results of brodimoprim and analogues alone and in combination against E. coliand mycobacteria. J Chemother. 1993;5:422–429. [PubMed] [Google Scholar]
- 27.Shoen C, Choromanska O, Reynolds R C, Piper J R, Cynamon M. In vitro activities of several diaminomethylpyridopyrimidines against Mycobacterium aviumcomplex. Antimicrob Agents Chemother. 1998;42:3315–3316. doi: 10.1128/aac.42.12.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suling W J, Reynolds R C, Barrow E W, Wilson L N, Piper J R, Barrow W W. Susceptibilities of Mycobacterium tuberculosis and Mycobacterium aviumcomplex to lipophilic deazapteridine derivatives, inhibitors of dihydrofolate reductase. J Antimicrob Chemother. 1998;42:811–815. doi: 10.1093/jac/42.6.811. [DOI] [PubMed] [Google Scholar]
- 29.Then R L. History and future of antimicrobial diaminopyrimidines. J Chemother. 1993;5:361–368. [PubMed] [Google Scholar]
- 30.Tomioka H, Sato K, Kajitani H, Akaki T, Shishido S. Comparative antimicrobial activities of the newly synthesized quinolone WQ-3034, levofloxacin, sparfloxacin, and ciprofloxacin, against Mycobacterium tuberculosis and Mycobacterium aviumcomplex. Antimicrob Agents Chemother. 2000;44:283–286. doi: 10.1128/aac.44.2.283-286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright E L, Quenelle D C, Suling W J, Barrow W W. Use of Mono Mac 6 human monocytic cell line and J774 murine macrophage cell line in parallel antimycobacterial drug studies. Antimicrob Agents Chemother. 1996;40:2206–2208. doi: 10.1128/aac.40.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yajko D M, Madej J J, Lancaster M V, Sanders C A, Cawthon V L, Gee B, Babst A, Hadley W K. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microbiol. 1995;33:2324–2327. doi: 10.1128/jcm.33.9.2324-2327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler-Heitbrock H W L, Thiel E, Fütterer A, Herzog V, Wirtz A. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988;41:456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]
- 34.Zywno-vanGinkel S, Dooley T P, Suling W J, Barrow W W. Identification and cloning of the Mycobacterium avium folA gene, required for dihydrofolate reductase activity. FEMS Microbiol Lett. 1997;156:69–78. doi: 10.1111/j.1574-6968.1997.tb12707.x. [DOI] [PubMed] [Google Scholar]