Abstract
Background:
Most botulinum toxin A (BoNT/A) products contain unnecessary bacterial components that increase the risk of developing neutralizing antibodies (nAbs). Reports of secondary nonresponse and treatment failures (STF) due to nAbs have accompanied a surge in new BoNT/A products.
Methods:
To formulate recommendations on managing toxin resistance, we reviewed the evidence on BoNT/A-associated immunogenicity and evaluated Asian physicians' current BoNT/A practices, knowledge, and real-world experiences, as provided by survey outcomes conducted with 128 Asian experts (regular botulinum toxin injectors).
Results:
Most doctors believe STF occurs, some patients exhibit partial symptoms, and impurities (eg, complexing proteins) in BoNT/A preparations risk STF. Bioassays that distinguish non-nAbs from nAbs that hinder toxin function remain unavailable to most doctors, though most would perform testing if given the option. Doctors in the Asia-Pacific region have differing strategies for managing STF, depending on the availability of alternatives or tests. They recommended switching to a highly-purified formulation free of complexing proteins and other impurities to lower the risk of immunogenicity, or offering treatment holidays of 2 -2.5 years. They suggested restarting treatment with the same highly purified formulation, especially for repeated treatments, large-dose injections, and younger patients who will accumulate higher lifetime doses, so as to minimize immunogenic risks and preserve long-term treatment outcomes. Importantly, doctors should always initiate patients on pure formulations rather than switching to these only after resistance develops.
Conclusion:
Choosing highly purified BoNT/A products at treatment initiation enhances long-term efficacy and patient satisfaction while minimizing the risk of immune activation and nAb formation.
Takeaways
Question: Reports of secondary nonresponse and treatment failures (STF) due to neutralising antibodies (nAbs) have prompted us to review the evidence on botulinumtoxinA (BoNT/A)-associated immunogenicity; survey Asian physicians' BoNT/A practices, knowledge, and real-world experiences; and provide recommendations on managing toxin resistance.
Findings: Most doctors believe STF occurs, some patients exhibit partial symptoms, and impurities (eg, complexing proteins) in BoNT/A preparations risk STF. Doctors in the Asia-Pacific region provided recommendations to overcome this, including always initiating patients on pure formulations rather than switching to these only after resistance develops.
Meaning: Choose a highly-purified BoNT/A product at treatment initiation to enhance long-term efficacy and patient satisfaction and minimize the risk of immune activation and nAb formation.
INTRODUCTION
An increase in new aesthetic botulinum toxin A (BoNT/A) products in the past year1–3 has coincided with anecdotal reports of BoNT/A-related side effects and negative outcomes. Established BoNT/A products include onabotulinumtoxinA (onaA; Botox, Allergan Inc, Irvine, Calif.), abobotulinumtoxinA (aboA; Dysport, Ipsen Ltd, Slough, Berkshire, UK), and incobotulinumtoxinA (incoA; Xeomin, Merz Pharmaceuticals GmbH, Frankfurt am Main, Hessen, Germany). Newer toxins in the Asia-Pacific (APAC) region include letibotulinumtoxinA (Botulax, Hugel Pharma, Seoul, Korea), prabotulinumtoxinA (Nabota, Daewoong Pharmaceuticals, Seoul, Korea), Innotox and Meditoxin (MedyTox Inc., Seoul, Korea), Hutox (Huons Co. Ltd., Gyeonggi-do, Korea), Relatox (Microgen, Russia), and Lantox (Lanzhou Institute of Biological Products, China). 4 Toxins awaiting approval or launch in Korea include Protox (PROTOX Inc., Seoul, Korea) and The Toxin (Jetema Inc., Seoul, Korea).
Increasingly, aesthetic physicians are encountering secondary nonresponse or “toxin resistance” due to neutralizing antibodies (nAbs) against BoNT/A (“anti-BoNT/A”) after treatment for cosmetic indications.5–12 Secondary treatment failure (STF) is defined as an initial response to BoNT/A followed by variable loss of clinical responsiveness13 over time with repeated injections.14 Partial STF (PSTF) is suspected when achieving the same clinical response requires higher doses of BoNT/A (“dose creep”), or the duration of effect is shorter when using the same dose (“interval creep”). A complete lack of clinical response to any amount of BoNT/A is a complete STF (CSTF).
The current testing benchmarks for detecting nAbs to BoNT/A include the mouse protection assay and mouse hemidiaphragm assay.14 Although standard structural immunological binding assays such as enzyme-linked immunosorbent and immunoprecipitation assays have the required sensitivity for BoNT/A antibody detection, they cannot distinguish nonneutralizing antibodies from the nAbs that hinder normal toxin function. We aimed to understand the situation in APAC by reviewing the published evidence on BoNT/A-associated immunogenicity and by surveying APAC physicians’ current practices with BoNT/A. We then convened an APAC virtual meeting on 24 July 2020 to identify knowledge gaps, share real-world experiences and perspectives, and formulate recommendations for the management of toxin resistance.
METHODS
Regular botulinum toxin providers in APAC (n = 128; “experts”), comprising dermatologists, plastic surgeons, and aesthetic physicians who were experienced users of BoNT/A and routinely used two or more BoNT/A brands, were surveyed. A SurveyMonkey questionnaire (SurveyMonkey Inc.; www.surveymonkey.com) was distributed by email and posed questions on their current practice with BoNT/A, their experiences and beliefs surrounding the issue of treatment failure secondary to nAb formation, their use of BoNT/A in body indications and intradermally (mesotoxin) for skin quality improvements, and their experiences with neurotoxin products from Asia (“Asian neurotoxins”). Thereafter, 38 experts willing to provide real-world insights were invited to a virtual discussion of their survey responses.
RESULTS
We emphasize that our survey was neither designed nor powered to form a consensus for specific APAC countries, regions, clinical practices, or preferences. Rather, the responses represent the broad perspectives, clinical issues, and practices related to immunogenicity experienced by the experts. Of the 128 experts surveyed, 38 attended a virtual meeting to discuss their results.
Secondary Treatment Failure and Neutralizing Antibodies
Over half (57%) of the experts believe that CSTF or PSTF can occur and suspect that some of their patients currently exhibit PSTF symptoms (Fig. 1). Importantly, 19% of experts who have encountered STF strongly believe that impurities (specifically, complexing proteins, inactivated neurotoxins, flagellins, and bacterial DNA contaminants)15 in BoNT/A preparations are a significant risk factor. While a quarter have not encountered STF, further work is needed to understand whether specific practices or BoNT/A products contributed to this result.
Fig. 1.
Perception of treatment failure secondary to nAb.
nAb Testing
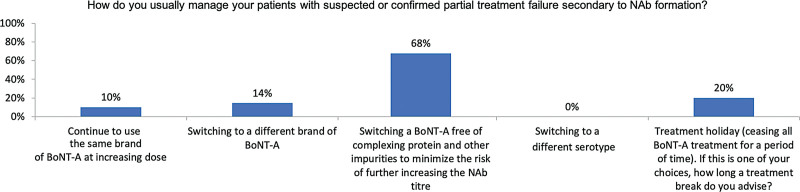
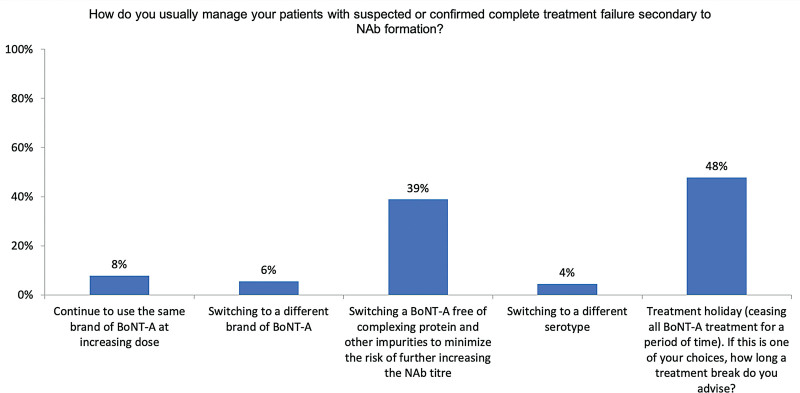
nAb testing is not locally available to most experts (68%; data not shown), yet 83.3% would test patients suspected of PSTF or CSTF (if available in their country). The remaining 16.7% who would not test for nAbs stated that test results would not change their management plans. To manage PSTF or CSTF, the experts may switch to a formulation free of complexing proteins and other impurities to lower the risk of increasing immunogenicity (68% for PSTF, Fig. 2; 39% for CSTF, Fig. 3) or offer treatment holidays (20% for PSTF, Fig. 2; 48% for CSTF, Fig. 3). Few would change to formulations other than incoA (14% for PSTF, Fig. 2; 6% for CSTF, Fig. 3) or increase the dose of their current brand (10% for PSTF, Fig. 2; 8% for CSTF, Fig. 3). This could reflect some patients’ persistent requests for continued treatments.
Fig. 2.
Management of patients with PSTF.
Fig. 3.
Management of patients with CSTF.
Mesotoxin for Reduction of Dynamic Wrinkles
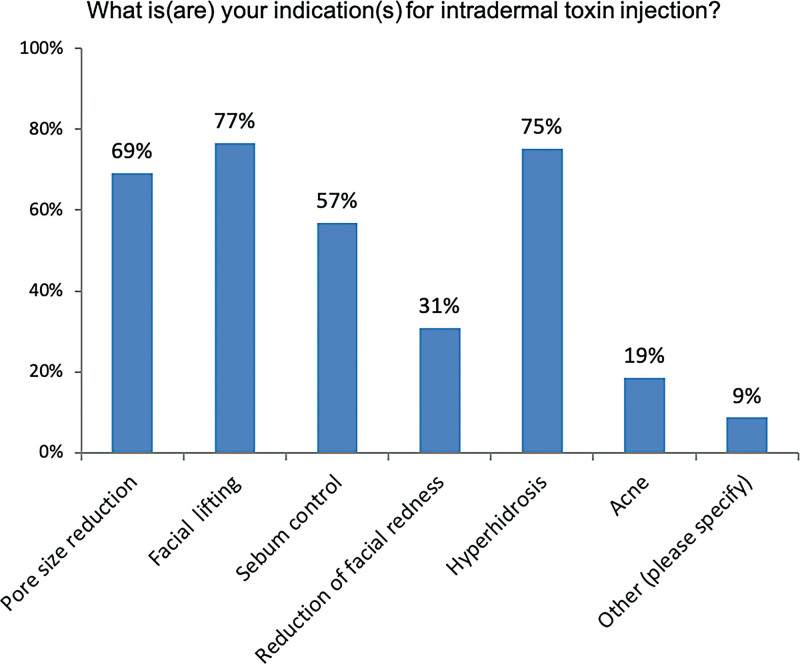
In descending frequency (Fig. 4), intradermal toxins are applied for facial lifting, hyperhidrosis, pore size reduction, sebum control, reduction of facial redness, acne, and other indications. Notably, 54% of experts believe that intradermal injections are more immunogenic than intramuscular injections (data not shown). This may reflect the understanding that dendritic cells that facilitate antigen capture are predominantly located in the dermis.16 Also, 94% think it is important to use a highly-purified, complexing protein-free BoNT/A with the lowest immunogenicity for intradermal injections.
Fig. 4.
Intradermal toxin injections are used for several facial indications. Other indications cited were fine wrinkles, a natural or softer muscle relaxation effect, and rosacea.
Body Indications
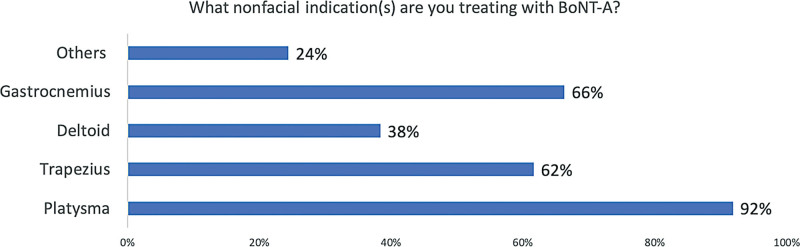
Nearly all experts (96%) inject BoNT/A into nonfacial areas, including the trapezius and gastrocnemius muscles (data not shown; Fig. 5). Unlike facial indications, body indications are considered more immunogenic (65%), mainly due to the use of larger doses (eg, >100U). Thus, 97.1% consider using a highly-purified preparation with the lowest immunogenicity to be important for nonfacial indications.
Fig. 5.
Intradermal toxin injections are used for several nonfacial indications. Other indications cited were hyperhidrosis in the palms, soles, and axillar areas.
APAC Physicians’ Experience with Asian BoNT/A Products
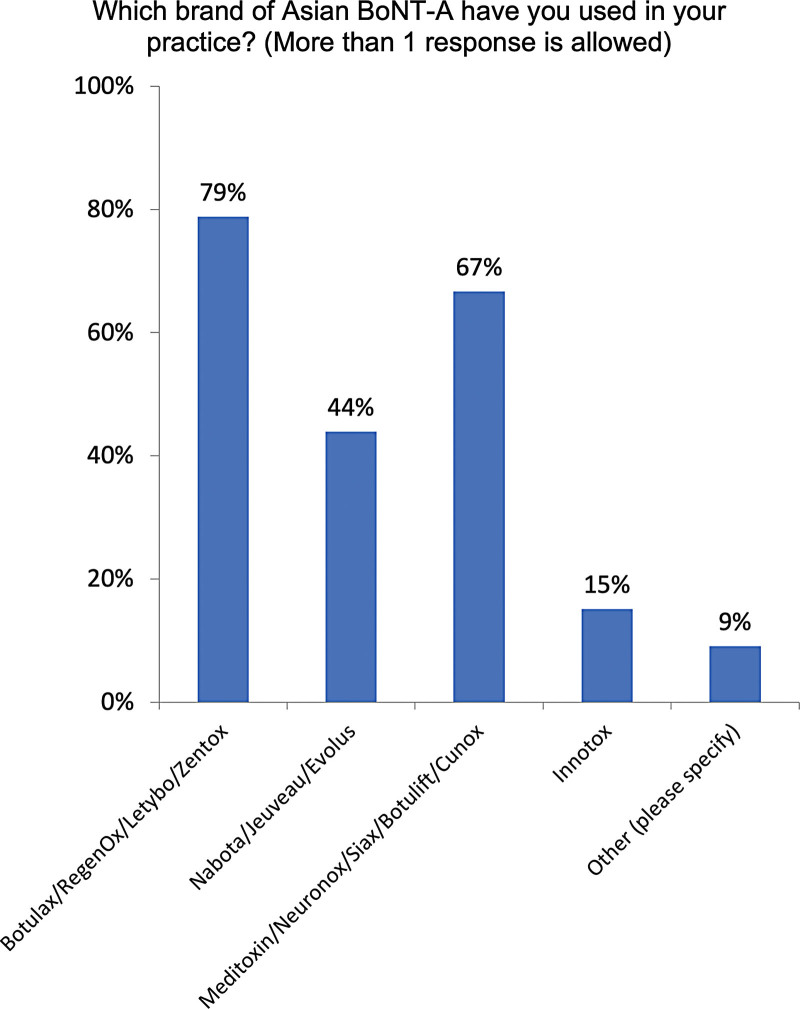
Seventy-three percent of experts have used Asian BoNT/A brands, including Botulax (also known as RegenOx, Letybo, or Zentox, 79%; Fig. 6), Meditoxin (also known as Neuronox, Botulift, Cunox, or Siax, 67%), and Nabota (also known as Jeuveau or Nucieva, 44%). Fewer than 20% have used Innotox. Importantly, 88% of experts believe that higher levels of impurities in Asian BoNT/A products increased the risk of STF due to nAb formation (data not shown).
Fig. 6.
Asian BoNT/A toxins used. Other brands cited were Hugel, Lanzox, and Hengli (lanbotulinumtoxinA).
DISCUSSION
Following the survey, the APAC experts discussed their interventions and perspectives on antibody testing, treatment holidays, switching products, intradermal and intramuscular injections, and experience with new Asian BoNT/A products. We stress that due to country-to-country differences, our survey responses are not a consensus of practices or viewpoints. We refrained from making conclusions on specific country practices to avoid misrepresenting our data. Rather, we aimed to provide an overview of BoNT/A-associated immunogenicity across APAC, and the real-world experiences of the Pan-Australasian medical aesthetics community.
As immunogenic consequences were similar across APAC, our findings are not insignificant. They underscore a need for careful toxin selection and physician guidance on strategies to address STF and/or patient insistence on treatment continuance. To identify variable practices, preferences, and specificities between individual regions (eg, East Asia versus Southeast Asia) or countries (eg, Korea versus Taiwan), additional investigations on toxin immunogenicity are needed. A more structured consensus (eg, Delphi consensus) would facilitate clinicians’ understanding of our survey findings and provide practical guidelines. Future studies, in particular longitudinal real-world studies and clinical registries, will complement our data to shed more light on the complex, dynamic and under-studied real-world experience with BoNT/A immunogenicity in aesthetic practice.
The subsequent sections summarize our clinical experiences of toxin immunogenicity, and may not relate directly to each survey question due to the informal nature of our virtual meeting.
CLINICAL PRACTICE WITH INCOBOTULINUMTOXINA
Although APAC experts have similar experiences, their management strategies diverge depending on the availability of alternative products or antibody testing facilities:
In Korea, antibody testing is not performed routinely; instead, suspected cases of STF are verified by frontalis testing. As continuous treatment in complete nonresponders is ineffective and may even be detrimental, Korean experts advise distinguishing CSTF from PSTF. However, treatment costs and patient insistence on treatment continuance (even if ineffective) often override physician advice or antibody testing results. In one expert’s practice (JY Park), 60%–70% of patients refuse treatment holidays, insist on continuing treatment, and are thus given incoA due to its purity and low immunogenic potential.
Malaysian physicians either switch to incoA after confirming that patients can afford treatment continuation, or were offered a 1-year holiday. If clinical response fails to recover, up to 2 additional years of treatment holiday may be recommended.
Australian physicians lack access to testing; so clinical management is determined by patient presentation. Some physicians (N Corduff) switch PSTF patients to incoA as nAb titres have been shown to decline despite continuous, three-monthly incoA treatments.17 For CSTF patients, a holiday is preferred over brand-switching. For those starting to develop PSTF or near-CSTF, advice on increased dose and costs, and reduced treatment efficacy or longevity, is necessary. A different treatment modality (eg, microfocused ultrasound) may be recommended.
Over the decade, the number of body treatments increased in some parts of East Asia (notably, Korea and Hong Kong) as patients sought slimmer legs and a “bridal shoulder” to elongate the neck.
Although some anti-BoNT/A antibody tests were recently published (R Wanitphakdeedecha)13,18 and other experts have developed their own assays (YY Chao), improvements in test accuracy or specificity are needed to differentiate nonneutralizing antibodies from nAbs. It is important to bear in mind that clinical nonresponse might also be due to suboptimal treatment, such as incorrect injection techniques or product reconstitution. Antibody testing is unhelpful in such situations. Nonetheless, if STF is due to nAb formation, nAb detection assays are useful for understanding patient antibody titres. Antibody titres can then be used to estimate the treatment holiday duration because higher titres will require longer treatment holidays. Importantly, doctors should switch to a highly-purified BoNT/A formulation that is free of complexing proteins and other impurities before patients develop signs of resistance. In fact, they should consider using a pure formulation with the lowest immunogenicity in treatment-naive patients so as to reduce the risk of STF from the beginning. Anecdotally, to treat PSTF, more experts would switch to incoA, whereas to treat CSTF, more experts advise treatment holidays of 2–2.5 years. To minimize the risk of re-activating the immune system, experts recommend restarting BoNT/A treatment using a pure product without unnecessary bacterial proteins and components.19
Mesotoxin or intradermal BoNT/A were regarded as more immunogenic than conventional intramuscular injections for dynamic wrinkle reduction, partly as relatively higher doses are used (up to 100 units per session). Also, the dermis is highly-populated with antigen presenting dendritic cells16 with key immunostimulatory functions like antigen capture and presentation to T-lymphocytes. These physiological functions are applied in vaccinology; some vaccines are delivered intradermally to elicit stronger immune responses.20 In body indications, higher doses of BoNT/A (several hundred units) are injected, thus increasing a patient’s exposure to foreign proteins and their risk of nAb formation. Consequently, it is prudent to use a highly-purified BoNT/A preparation containing only the 150-kDa neurotoxin when treating body indications. Doing so over the course of repeated injections minimizes immunogenic risks and ensures the durability and efficacy of long-term treatment outcomes.
Newer toxin formulations from Asian manufacturers are believed to contain higher levels of impurities. Studies on toxin purity levels4,15 correlate with Korean physicians’ anecdotal perceptions of heightened immunogenic risks associated with some toxins from Korea. Importantly, some BoNT/A manufacturers may be confusing and conflating product sterility with toxin purity when discussing these with physicians. Toxin purity should be defined as the absence of unnecessary bacterial components like complexing proteins, inactivated neurotoxin, flagellin, and DNA contaminants.15 Our survey found that, compared with incoA, aboA, and onaA, Botulax was most frequently used (58.82%), followed by Meditoxin (32.35%), Nabota (5.88%), and Innotox (2.94%) (data not shown). Most felt that muscle paralysis effects were similar (61.76%), 20.59% felt they were stronger, while 17.65% felt they were weaker. The majority (73.53%) also felt that the duration of action was shorter, while 23.53% felt it was similar and 2.94% felt it was longer.
Multiple studies have documented BoNT/A immunogenicity and its clinical implications.5,14,21–24 Fundamentally, BoNT/A is a foreign antigen capable of inducing nAbs25,26 that attenuate the toxin’s therapeutic action.24 This can lead to partial treatment failure (where the therapeutic effect is reduced in intensity and/or duration) or even complete treatment failure (where no therapeutic effect is detectable). Antibodies were observed to develop gradually in one case study,27 concurrent with a shorter duration of therapeutic effect (“interval creep”) and necessitating a higher dose to achieve the same results (“dose creep”). Most commercial BoNT/A formulations contain adjuvant substances such as haemagglutinins (in particular, HA3328), inactivated neurotoxin,1,4 flagellin,29 and clostridial DNA,30 which provide “danger” signals by binding to specific receptors on dendritic cells.15 Activated dendritic cells internalize and process foreign proteins, subsequently presenting parts of the foreign material to helper T-lymphocytes, which then become activated and stimulate B-lymphocyte maturation into plasma cells to produce antibodies. The pure, active, 150-kDa neurotoxin itself is a weak immunogen with no known associated pattern recognition receptors or toll-like receptors on dendritic cells.31 However, when adjuvants in the preparation are injected alongside the 150-kDa neurotoxin, they can activate dendritic cells that may accidentally internalize the 150-kDa neurotoxin. Activated dendritic cells may thereupon present parts to the 150-kDa molecule to activate helper T-lymphocytes, resulting in nAb production by B-lymphocytes. In APAC, Xeomin is the only commercially-available neurotoxin preparation, which is free of complexing proteins and other impurities of bacterial origin. As Xeomin only contains the active 150-kDa neurotoxin, it is unlikely to provide a “danger” signal to elicit an immune response and, therefore, presents a very low risk of nAb formation. To date, no incidence of Xeomin-related STF due to nAb formation has been observed in treatment-naive patients. As a detailed exploration is beyond the scope of this article, we direct readers to a recent review,32 which discusses immunogenicity due to BoNT/A formulations with or without accessory proteins, and the clinical and nonclinical evidence for this.
Although toxin purity is a critical risk factor in developing STF, other factors to consider include high treatment dosage, cumulative dosage, short inter-injection intervals, booster injections (re-injections) within 3 weeks of the initial injection, and the patient’s immune responsiveness. Globally, BoNT/A is also used for many off-label aesthetic indications such as muscle volume reduction,33 facial shaping,34 and body line contouring.35 Lower dosage (up to 50U) facial injections have been progressively replaced with high dosages (100U or more) for contouring and lifting, masseter and parotid gland reduction (in Korea and Thailand), platysmal band correction, jawline sharpening, and whole face intradermal lifting.36 In the body, BoNT/A is used for trapezius reduction37 and contouring of the upper arm,38 thigh,34 or calf.39 In the practice by one author (JY Park), calf size reduction is performed every 6 months through intramuscular injections, resulting in a cumulative toxin dose of approximately 640U over a 4-year period. However, extremely bulky calves will accumulate 2400U of toxins over just 3 years. Using such large doses may increase the incidence of secondary nonresponsiveness to BoNT/A; so a high index of suspicion for nAb-induced treatment failure must be maintained when there is nonresponsiveness or declining clinical response indicative of “interval creep” and “dose creep” after repetitive treatments.
Moreover, many presumed STF cases may actually be due to BoNT/A being injected at inadequate doses, using an incorrect technique, or being incorrectly stored, handled, or prepared, and not nAb formation. APAC physicians do not routinely test for nAbs to diagnose antibody-induced STF, but should proactively take measures to prevent immunoactivation and nAb formation. To do so will require a highly-purified toxin formulation, containing only the active 150-kDa molecule, to be chosen as soon as treatment initiation. This is especially important for patients who are toxin-naive, planning to receive large doses during a single session or more frequently than recommended, or for a long period of time. Young patients will also eventually receive a higher cumulative toxin dose over their lifetime. To ensure that only highly-purified BoNT/A formulations are used, clinicians should review and update their understanding of toxin immunogenicity, base their product selection on peer-reviewed scientific data and literature on specific evidence for purity, and refresh their awareness of clinical studies on immunogenicity in aesthetic medicine. Notably, the experts regarded the choice of highly-purified BoNT/A to be a preventative strategy, which preserves the option for the future use of BoNT/A in therapeutic or neurological interventions, if necessary.
CONCLUSIONS
APAC experts have seen an increase in the number of patients presenting with “toxin resistance” or STF due to nAb formation. This has coincided with wider clinical applications for BoNT/A, its use at higher doses, and the emergence of more commercial BoNT/A preparations. Based on their personal clinical observations and published studies on toxin purity levels,4,15 the experts agreed that most products contain, to varying degrees, unnecessary bacterial components with the potential to stimulate nAb formation. Thus, clinicians should avoid injecting extraneous components that increase the risk of STF, and consider selecting a highly-purified BoNT/A product that is supported by robust, peer-reviewed evidence, to facilitate long-term treatment efficacy and safeguard patient satisfaction.
Footnotes
Published online 18 April 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. All authors received an honorarium for attendance at a meeting. This study was supported by Merz Asia-Pacific Pte. Ltd.
REFERENCES
- 1.Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, et al. IncobotulinumtoxinA: a highly purified and precisely manufactured botulinum neurotoxin type A. J Drugs Dermatol. 2019;18:52–57. [PubMed] [Google Scholar]
- 2.Jeong S. 4 new botulinum toxins to undergo trials in crowded market. Available at https://www.koreabiomed.com/news/articleView.html?idxno=7021. Published 2020. Accessed September 28, 2020.
- 3.Lim J. Wrinkle treatment companies vie to fill hole left by Meditoxin. Available at http://www.koreaherald.com/view.php?ud=20200506000924. Published 2020. Accessed September 28, 2020.
- 4.Frevert J, Ahn KY, Park MY, et al. Comparison of botulinum neurotoxin type A formulations in Asia. Clin Cosmet Investig Dermatol. 2018;11:327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres S, Hamilton M, Sanches E, et al. Neutralizing antibodies to botulinum neurotoxin type A in aesthetic medicine: five case reports. Clin Cosmet Investig Dermatol. 2014;7:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borodic G. Immunologic resistance after repeated botulinum toxin type A injections for facial rhytides. Ophthalmic Plast Reconstr Surg. 2006;22:239–240. [DOI] [PubMed] [Google Scholar]
- 7.Dressler D, Wohlfahrt K, Meyer-Rogge E, et al. Antibody-induced failure of botulinum toxin A therapy in cosmetic indications. Dermatol Surg. 2010;36(Suppl 4):2182–2187. [DOI] [PubMed] [Google Scholar]
- 8.Dressler D. Clinical features of antibody-induced complete secondary failure of botulinum toxin therapy. Eur Neurol. 2002;48:26–29. [DOI] [PubMed] [Google Scholar]
- 9.Stengel G, Bee EK. Antibody-induced secondary treatment failure in a patient treated with botulinum toxin type A for glabellar frown lines. Clin Interv Aging. 2011;6:281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carruthers A, Kane MA, Flynn TC, et al. The convergence of medicine and neurotoxins: a focus on botulinum toxin type A and its application in aesthetic medicine—a global, evidence-based botulinum toxin consensus education initiative: part I: botulinum toxin in clinical and cosmetic practice. Dermatol Surg. 2013;39(3 Pt 2):493–509. [DOI] [PubMed] [Google Scholar]
- 11.Lee SK. Antibody-induced failure of botulinum toxin type A therapy in a patient with masseteric hypertrophy. Dermatol Surg. 2007;33(1 Spec No.):S105–S110. [DOI] [PubMed] [Google Scholar]
- 12.Stephan F, Habre M, Tomb R. Clinical resistance to three types of botulinum toxin type A in aesthetic medicine. J Cosmet Dermatol. 2014;13:346–348. [DOI] [PubMed] [Google Scholar]
- 13.Srinoulprasert Y, Wanitphakdeedecha R. Antibody-induced botulinum toxin treatment failure: A review and novel management approach. J Cosmet Dermatol. 2020;19:2491–2496. [DOI] [PubMed] [Google Scholar]
- 14.Bellows S, Jankovic J. Immunogenicity associated with botulinum toxin treatment. Toxins (Basel). 2019;11:E491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JY, Sunga O, Wanitphakdeedecha R, et al. Neurotoxin impurities: a review of threats to efficacy. Plast Reconstr Surg Glob Open. 2020;8:e2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417–428. [DOI] [PubMed] [Google Scholar]
- 17.Hefter H, Hartmann C, Kahlen U, et al. Prospective analysis of neutralising antibody titres in secondary non-responders under continuous treatment with a botulinumtoxin type A preparation free of complexing proteins—a single cohort 4-year follow-up study. BMJ Open. 2012;2:e000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinoulprasert Y, Kantaviro W, Nokdhes YN, et al. Development of inhibition ELISA to detect antibody-induced failure of botulinum toxin a therapy in cosmetic indications. J Immunol Methods. 2019;473:112635. [DOI] [PubMed] [Google Scholar]
- 19.Dressler D, Pan L, Adib Saberi F. Antibody-induced failure of botulinum toxin therapy: re-start with low-antigenicity drugs offers a new treatment opportunity. J Neural Transm (Vienna). 2018;125:1481–1486. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev Vaccines. 2015;14:1509–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brin MF, Comella CL, Jankovic J, et al.; CD-017 BoNTA Study Group. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord. 2008;23:1353–1360. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic J, Vuong KD, Ahsan J. Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology. 2003;60:1186–1188. [DOI] [PubMed] [Google Scholar]
- 23.Naumann M, Boo LM, Ackerman AH, et al. Immunogenicity of botulinum toxins. J Neural Transm (Vienna). 2013;120:275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benecke R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs. 2012;26:e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24:1720–1740; discussion 1719. [DOI] [PubMed] [Google Scholar]
- 26.Göschel H, Wohlfarth K, Frevert J, et al. Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies—therapeutic consequences. Exp Neurol. 1997;147:96–102. [DOI] [PubMed] [Google Scholar]
- 27.Dressler D, Bigalke H. Immunological aspects of botulinum toxin therapy. Expert Rev Neurother. 2017;17:487–494. [DOI] [PubMed] [Google Scholar]
- 28.Bryant AM, Cai S, Singh BR. Comparative immunochemical characteristics of botulinum neurotoxin type A and its associated proteins. Toxicon. 2013;72:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 2010;185:5677–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frevert J, Groenewald C. Presence of clostridial DNA in botulinum toxin products. Toxicon. 2015;93(suppl.):S28–S41. [Google Scholar]
- 31.Dey AK, Srivastava IK. Novel adjuvants and delivery systems for enhancing immune responses induced by immunogens. Expert Rev Vaccines. 2011;10:227–251. [DOI] [PubMed] [Google Scholar]
- 32.Carr WW, Jain N, Sublett JW. Immunogenicity of botulinum toxin formulations: potential therapeutic implications. Adv Ther. 2021;38:5046–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim NH, Park RH, Park JB. Botulinum toxin type A for the treatment of hypertrophy of the masseter muscle. Plast Reconstr Surg. 2010;125:1693–1705. [DOI] [PubMed] [Google Scholar]
- 34.Klein FH, Brenner FM, Sato MS, et al. Lower facial remodeling with botulinum toxin type A for the treatment of masseter hypertrophy. An Bras Dermatol. 2014;89:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo K. Body contouring with botulinum toxin. In: Seo KK, ed. Botulinum Toxin for Asians [Ebook]. 1st ed. Singapore: Springer; 2017:141–157. [Google Scholar]
- 36.Goldenberg G, Do T, Dong J, Lanoue J. An update on neurotoxin products and administration methods. Available at https://www.mdedge.com/dermatology/article/111411/aesthetic-dermatology/update-neurotoxin-products-and-administration. Published 2020. Accessed August 2, 2021. [PubMed]
- 37.Lee JH, Lee KY, Kim JY, et al. Botulinum toxin injection-site selection for a smooth shoulder line: an anatomical study. Biomed Res Int. 2017;2017:3092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo KK, Lee W. Medytoxin/Neuronox. In: Carruthers JCA, ed. Botulinum Toxin. Philadelphia, Pa.: Elsevier; 2012:52–58. [Google Scholar]
- 39.Lee HJ, Lee DW, Park YH, et al. Botulinum toxin A for aesthetic contouring of enlarged medial gastrocnemius muscle. Dermatol Surg. 2004;30:867–871; discussion 871. [DOI] [PubMed] [Google Scholar]