Abstract
PURPOSE
CALGB 40603 (NCT00861705), a 2 × 2 randomized phase II trial, demonstrated that adding carboplatin or bevacizumab to weekly paclitaxel (wP) followed by doxorubicin and cyclophosphamide significantly increased the pathologic complete response (pCR) rate in stage II-III triple-negative breast cancer. We now report long-term outcomes (LTOs) and correlative science end points.
PATIENTS AND METHODS
The Kaplan-Meier method was used to estimate LTOs in 443 patients who initiated study treatment. Log-rank tests and Cox proportional hazards models evaluated the impact of clinical characteristics, pathologic response, calculated residual cancer burden (RCB) in patients with residual disease (RD), treatment assignment, and dose delivery during wP on LTOs, including event-free survival (EFS). Genomic predictors of treatment response and outcomes were assessed on pretreatment tumor samples by mRNA sequencing.
RESULTS
Among baseline characteristics, only the clinical stage was associated with LTOs. At a median follow-up of 7.9 years, LTOs were not significantly improved with either carboplatin or bevacizumab, overall or in patients with basal-like subtype cancers by genomic analysis. Patients with pCR (n = 205, 46.3%) had significantly higher 5-year EFS (85.5% v 56.6%, log-rank P < .0001) and overall survival (87.9% v 63.4%, P < .0001) rates compared with patients with RD, even those with RCB class I. Among clinical and genomic features, evidence of immune activation, including tumor-infiltrating lymphocytes and low B-cell receptor evenness, was associated with pCR and improved EFS.
CONCLUSION
Despite higher pCR rates, neither carboplatin nor bevacizumab appeared to improve LTOs although the study was not powered to assess these secondary end points. pCR was associated with superior LTOs even when compared with minimal RD. Markers of immune activation in pretreatment tumor biopsies were independently associated with higher pCR rates and improved survival.
INTRODUCTION
In triple-negative breast cancer (TNBC), pathologic response to neoadjuvant chemotherapy (NACT) is a powerful prognostic indicator, but on average, only one third of patients achieve pathologic complete response (pCR) with standard anthracycline- and taxane-based regimens.1 Cancer and Leukemia Group B (CALGB, now part of the Alliance for Clinical Trials in Oncology) 40603, a randomized phase II 2 × 2 factorial trial, investigated whether adding another chemotherapeutic agent, carboplatin or the vascular endothelial growth factor–targeted monoclonal antibody bevacizumab, to standard NACT would improve pCR rates in clinical stage II-III TNBC. The study met its primary objectives, demonstrating that pCR breast (ypT0 or Tis) was significantly increased with either carboplatin (60% v 46%) or bevacizumab (59% v 48%).2 This report focuses on the study's secondary end points, specifically the impact of these treatments on long-term outcomes (LTOs), including event-free survival (EFS), and the association between pCR and extent of residual disease (RD) and LTOs.
CONTEXT
Key Objective
This analysis assesses whether adding bevacizumab or carboplatin to anthracycline- and taxane-based neoadjuvant chemotherapy (NACT) in triple-negative breast cancer (TNBC) improves long-term outcomes (LTOs) and evaluates clinical and molecular features for predictors of response and survival.
Knowledge Generated
Achievement of pathologic complete response correlated with better event-free survival (EFS) and overall survival, with even minimal residual invasive disease associated with worse outcomes. Although both bevacizumab and carboplatin significantly increased pCRs, neither improved LTOs. From gene expression analyses, evidence of an active tumoral immune response correlated with increased pCRs and improved EFS.
Relevance
Addition of bevacizumab or carboplatin to TNBC NACT increased pCRs, but did not appear to improve LTOs. However, standard of care is now NACT including carboplatin with pembrolizumab; future studies should focus on optimizing chemotherapy plus immune checkpoint inhibition. Identification of immune activation as a predictive and prognostic biomarker may allow more tailored neoadjuvant approaches in nonmetastatic TNBC.
The study also had important correlative objectives, particularly to identify pretreatment clinical and genomic biomarkers predictive of achievement of pCR and/or prognostic of EFS and to evaluate the impact of study treatment on pCR rates in patients with genomically defined basal-like tumors, a coprimary end point of the study.
PATIENTS AND METHODS
Patients with clinical stage II-III TNBC (estrogen receptor and progesterone receptor ≤ 10% and human epidermal growth factor receptor 2–negative) received paclitaxel 80 mg/m2 once a week for 12 weeks (wP) and were randomly assigned to the control regimen (arm 1), with addition of bevacizumab 10 mg/kg once every 2 weeks for 9 doses (arm 2), carboplatin area under the curve 6 once every 3 weeks for four doses (arm 3), or both (arm 4), followed by dose-dense doxorubicin and cyclophosphamide (AC) and then surgery (Data Supplement 1, online only). Patients signed an institutional review board–approved Protocol (online only)–specific consent in accordance with federal and institutional guidelines. Pathologic response was assessed by institutional pathologists. pCR is defined as the absence of residual invasive disease in the breast and axilla (ypT0 or Tis N0), and patients with RD were stratified by residual cancer burden (RCB), as defined by Symmans et al.3 EFS is defined as time from random assignment to local, regional, or distant recurrence, any second invasive cancer, or death from any cause; patients not undergoing surgery were considered to have had an EFS event when they were removed from study treatment. Overall survival (OS) is defined as time from random assignment to death from any cause, and distant recurrence-free interval (DRFI) is defined as time from random assignment to detection of metastatic disease or death attributed to disease progression, with patients removed from follow-up for any other reason (including a second invasive cancer or death not attributed to breast cancer) censored as of their last disease assessment. The study database was frozen on April 2, 2020, and patients were censored as of their most recent follow-up data. Data collection, analysis, and quality review were conducted by the Alliance Statistics and Data Management Center and the study chair, following Alliance policies.
With support from the Breast Cancer Research Foundation, pretreatment tumor biopsies from all enrolled patients were required and submitted for genomic and other analyses. RNA sequencing (RNAseq) was performed as previously described,4 excluding those whose samples failed to meet RNA quality control metrics and those with estrogen receptor or progesterone receptor expression > 1% (to be consistent with the current clinical definition of TNBC5). RNA sequencing, clinical data, and patient outcomes are available through NCBI database of Genotypes and Phenotypes (dbGaP).6 The impact of adding bevacizumab or carboplatin on pCR and EFS was assessed in the subset of patients with basal-like tumors defined by PAM50 classification.7,8 We also assessed the ability of previously published TNBC molecular subtyping strategies to predict pCR and EFS. To investigate the entire genome for gene expression patterns correlated with response and survival, we evaluated hundreds of previously published gene expression signatures (n = 793) that have been extensively used to distill the expression of thousands of genes into biologically relevant patterns that comprehensively cover the biology of breast cancer9 and have been shown to outperform individual genes for providing prognostic value.10 Once we identified associations between the number of immune signatures and both pCR and EFS, we analyzed a subset of samples (n = 178) for tumor-infiltrating lymphocytes (TILs) according to international standards11 and evaluated the correlation with pCR and EFS. To further characterize the immune response and its prognostic significance, we analyzed B-cell receptor and T-cell receptor sequence repertoire abundance and diversity measures (which are derived from bulk RNAseq and described in Data Supplement 1). We used multivariable Cox proportional hazards (PH) models with baseline clinical features and several immune features, including TILs, to compare the prognostic value of these features in predicting EFS. Given the strong association between achievement of pCR and EFS (described below), we looked for clinical and genomic features predictive of EFS in patients who failed to achieve pCR. We created a Cox PH model to identify interactions between treatment variables (ie, with or without carboplatin and with or without bevacizumab) and genomic features to determine if this could improve our ability to predict EFS.
Statistical Considerations
The analysis was performed via a modified intent-to-treat (mITT) principle; patients who withdrew before starting protocol treatment were excluded. The Kaplan-Meier method was used to estimate LTOs. Log-rank tests and Cox PH models were used to evaluate the association between baseline characteristics, treatment assignment, pathologic response, treatment delivery, genomic features, and LTOs. A generalized linear model of binomial outcomes using logit link function was used to evaluate genomic and clinical features' association with probability of pCR. Consistent with the exploratory nature of many of the correlative science analyses, reported P values are from two-sided tests and have not been corrected for multiple comparisons.
Role of the Funding Sources
Representatives of the funding sources (the National Cancer Institute, Genentech, the Breast Cancer Research Foundation, and the American Recovery and Reconstruction Act of 2009) were not involved in data analysis or the preparation of this article.
RESULTS
Impact of Clinical Factors on Outcomes
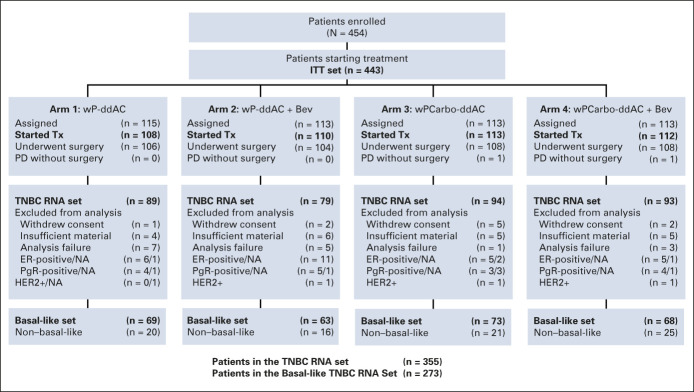
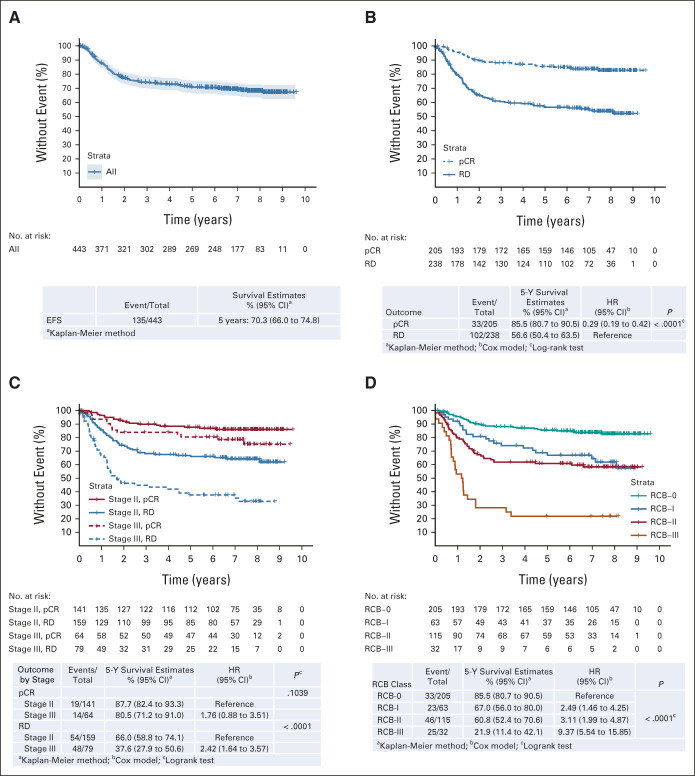
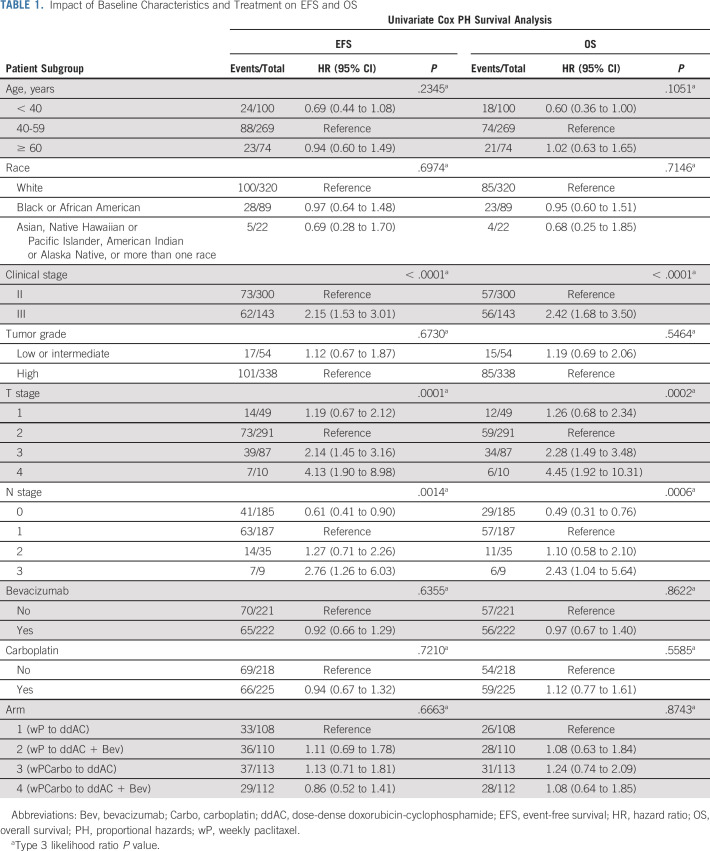
Of 443 patients in the mITT population, 426 underwent surgery (CONSORT diagram, Fig 1). The median follow-up is 7.9 years (95% CI, 7.6 to 8.1). The estimated 5-year EFS is 70.3%, OS 75.0%, and DRFI 76.1% (Fig 2A and Data Supplement). Among baseline characteristics, only the clinical stage (III v II) was significantly associated with EFS (hazard ratio [HR], 2.15; 95% CI, 1.53 to 3.01) and OS (HR, 2.42; 95% CI, 1.68 to 3.50), whereas age, race, and tumor grade were not (Table 1).
FIG 1.
CONSORT diagram (Alliance CALGB 40603 trial). Bev, bevacizumab; Carbo, carboplatin; ddAC, dose-dense doxorubicin and cyclophosphamide; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; ITT, intent-to-treat; NA, not available; PD, progressive disease; PgR, progesterone receptor; TNBC, triple-negative breast cancer; Tx, treatment; wP, weekly paclitaxel.
FIG 2.
Effect of the pretreatment clinical stage and response on EFS. (A) EFS in the mITT population. EFS stratified by (B) pCR versus RD, (C) pCR versus RD and stage, and (D) RCB. EFS, event-free survival; HR, hazard ratio; mITT, modified intent-to-treat; pCR, pathologic complete response; RCB, residual cancer burden; RD, residual disease.
TABLE 1.
Impact of Baseline Characteristics and Treatment on EFS and OS
In patients who achieved a pCR (205 of 443, 46.3%), the 5-year EFS is 85.5% versus 56.6% (HR, 0.29; 95% CI, 0.19 to 0.42; P < .0001; Fig 2B) and the 5-year OS is 87.9% versus 63.4% (HR, 0.28; 98% CI, 0.18 to 0.43; P < .0001; Data Supplement 1) compared with those with RD, and prognosis for baseline stage III versus II no longer differs significantly (Fig 2C) although this finding is based on a limited number of events. In patients with RD, the RCB class is prognostic for EFS (Fig 2D); however, even RCB-I is associated with a worse prognosis than RCB-0 (ie, pCR; HR, 2.49; 95% CI, 1.46 to 2.25; P < .0001). Similar results are seen for OS and DRFI (Data Supplement 1). In an exploratory analysis, baseline stage III versus II remains a significant prognostic variable in patients with RCB-I or RCB-II, although not in the smaller number of patients with RCB-III (Data Supplement 1). Significant improvements in EFS and OS with pCR over RD are seen across all arms of the study although differences vary in magnitude (Data Supplement 1).
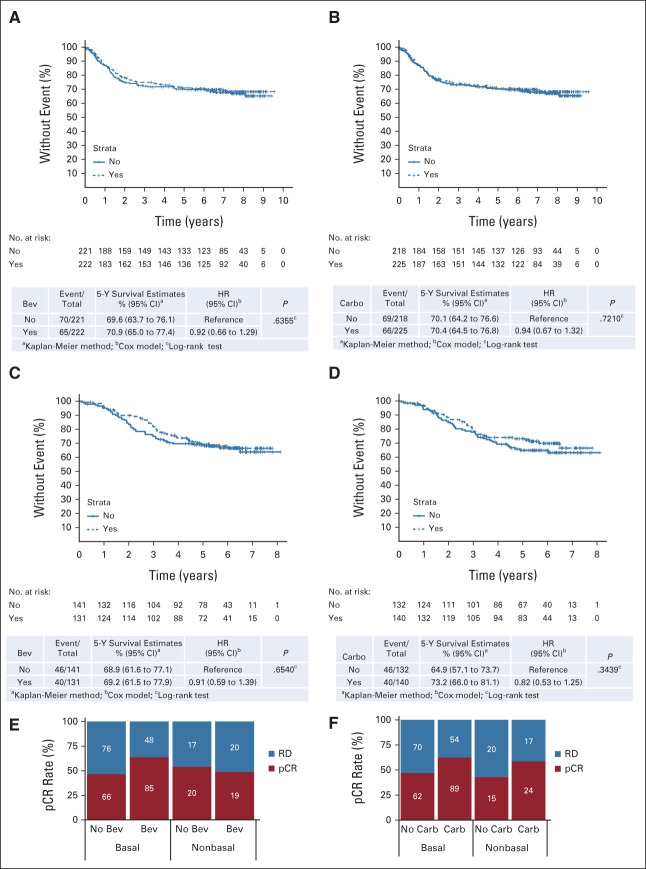
There is no improvement in EFS in the mITT population with the addition of either bevacizumab (HR, 0.92; 95% CI, 0.66 to 1.29; P = .64) or carboplatin (HR, 0.94; 95% CI, 0.67 to 1.32; P = .72; Figs 3A and 3B). Similar results are seen for OS and DRFI (Data Supplement 1). We identified no patient subset for which the addition of either agent improves EFS or OS (Data Supplement 1). Events by treatment arm are listed in Data Supplement 1.
FIG 3.
Effect of addition of bevacizumab and carboplatin to LTO in CALGB 40603. EFS stratified by (A and C) bevacizumab and (B and D) carboplatin treatment within the (A and B) mITT and (C and D) basal-like patient populations. pCR rate within the basal-like and non–basal-like subsets stratified by (E) bevacizumab and (F) carboplatin treatment. Bev, bevacizumab; Carbo, carboplatin; EFS, event-free survival; HR, hazard ratio; LTO, long-term outcome; mITT, modified intent-to-treat; pCR, pathologic complete response; RD, residual disease.
As noted in our previous publication,2 patients assigned to carboplatin were more likely to miss multiple doses of treatment during wP (35% v 15% not assigned to carboplatin). As very few patients discontinued treatment early, this discrepancy may be attributed to higher rates of hematologic toxicities with carboplatin and protocol dosing guidelines that required that treatment is skipped, rather than delayed, for these toxicities. In an exploratory analysis, we found a significant relationship between the number of wP doses received (stratified by ≥ 11, 9-10, 7-8, and ≤ 6) and EFS (P = .0025) in the overall study population (Data Supplement 1). Among all patients who received ≥ 11 doses of wP, the addition of carboplatin increased pCRs from 41% to 61%, with a trend for improved 5-year EFS (78.5% v 72%, HR, 0.68; 95% CI, 0.44 to 1.06; P = .089), which was not seen relative to bevacizumab assignment (Data Supplement 1).
Impact of Genomic Features on pCR and Outcomes
Within the subset of patients with genomically defined basal-like tumors, which comprised 77% of tumors tested by RNA-seq7 (Data Supplement 1), the addition of either bevacizumab or carboplatin to the control NACT regimen significantly increased the pCR rate (Figs 3E and 3F), but, as in the mITT population, failed to improve EFS (Figs 3C and 3D). Adding bevacizumab had a larger positive impact on the pCR rate in basal-like compared with non–basal-like tumors, whereas the increment in pCR with the addition of carboplatin was similar between the two cohorts (Figs 3E and 3F).
Of the published TNBC molecular subtyping approaches that we evaluated—TNBCtype,12 MD Anderson Cancer Center and Baylor College of Medicine subtype,13 and PAM50 + Claudin Low subtypes7,14—only tumors categorized as the basal-like immune-activated subtype by the MD Anderson Cancer Center and Baylor College of Medicine classification demonstrated a significantly higher pCR rate and none displayed significant prognostic differences for EFS (Data Supplement 1). Comparison between subtyping strategies demonstrated a moderate strength of association (0.40-0.46, Cramer's V test), but disagreements between classifications and a high proportion of unclassifiable specimens highlight a limitation of these strategies (Data Supplement 1).
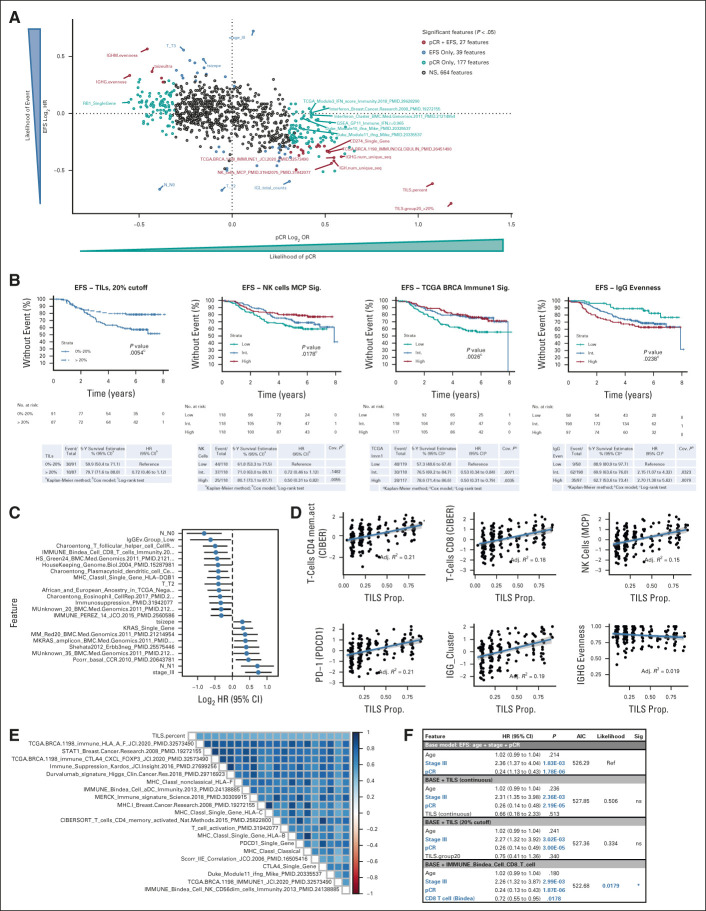
Of the > 850 clinical and genomic features that we analyzed for association with outcomes in these exploratory studies (Data Supplement 2, online only), a large number of features were associated with either pCR (n = 177) or EFS (n = 39), but only 27 were associated with both (Fig 4A and Data Supplement 2). Features associated with pCR but not EFS included all six signatures of interferon signaling, whereas clinical features such as the baseline tumor stage and nodal status were associated with EFS, but not pCR. Most (24 of 27) of the features associated with both pCR and EFS reflected the tumor's immune microenvironment (Fig 4B), including the presence of a variety of immune effector cells, including T and B lymphocytes and natural killer cells. Higher mRNA expression levels of immune checkpoint genes, including programmed cell death protein 1 (PDCD1) and programmed death-ligand 1 (CD274), were also associated with improvements in both pCR and EFS.
FIG 4.
Genomic correlates with response and survival in TNBC. (A) Clinical and genomic feature association with likelihood of pCR and EFS outcomes. Nonsignificant (P > .05) associations are given in gray, features associated with both pCR and EFS are given in red, features associated with just EFS are given in blue, and features associated with only pCR are given in light blue. A few selected significant features are labeled. (B) EFS Kaplan-Meier plots for patients with TNBC stratified by (left-to-right) TIL quantification (20% cutoff), NK_cells_MCP_PMID.31942075_PMID.31942077 signature tertiles, TCGA.BRCA.1198_IMMUNE1_JCI.2020_PMID.32573490 signature tertiles, and IgG evenness groups. (C) Features significantly associated with EFS in patients with residual disease (n = 191); negative log2 HR indicates lower risk of event. (D) Correlation of TILs with immune effector and checkpoint signatures: (left-to-right, top-first) CD4+ memory T cells, CD8+ T cells, NK cells, PD-1 expression, IgG cluster, and IgG evenness. (E) Spearman correlation matrix for continuous TIL quantification and top 20 most correlated signatures, ordered by correlation with TILs. (F) Comparison of multivariable Cox proportional hazards models for EFS within the set of TNBC with TIL quantification (n = 178). Features that are significant in the multivariate Cox model are in blue bold text, HR and 95% CI, AIC. AIC, Akaike information criteria; EFS, event-free survival; HR, hazard ratio; IgG, immunoglobulin G; MCP, Microenvironment Cell Populations-counter; NK, natural killer; ns, not significant; pCR, pathologic complete response; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TCGA, The Cancer Genome Atlas; TIL, tumor-infiltrating lymphocyte; TIL, tumor-infiltrating lymphocyte; TNBC, triple-negative breast cancer.
Analysis of B-cell receptor and T-cell receptor data demonstrated that low immunoglobulin G (IgG) evenness was associated with improvements in both pCR and EFS. IgG evenness is a measure of the uniformity of B-cell clonal abundance. Low IgG evenness may reflect oligoclonal B-cell expansion and immunoglobulin class switching caused by an antigen-driven immune response, in contrast to a nonspecific (polyclonal) immune response. In fact, there was a negative correlation between IgG evenness and IgG abundance (Data Supplement 1). Using IgG evenness cutoff values derived from recurrence-free survival data for patients with TNBC in The Cancer Genome Atlas (TCGA; Data Supplement 1), we found that patients with low IgG evenness have improved EFS (Fig 4B, right) and IgG evenness was an independent prognostic feature in a model including age, stage, and pCR status (Data Supplement 1). Among patients who failed to achieve pCR, only the tumor stage and node status were stronger prognostic features than low IgG evenness (Fig 4C). In addition, among patients who did not receive carboplatin (arms 1 and 2 of our study), only achievement of pCR was more strongly associated with EFS than low IgG evenness (Data Supplement 1).
As a continuous measure, TIL density, defined as the percentage of stromal area occupied by lymphocytes on a tumor biopsy,11 was strongly associated with both pCR and EFS. There was a correlation, albeit with a significant variation, between TIL density and mRNA signatures of many immune effector cells and immune checkpoints, but not with IgG evenness (Figs 4D and 4E). As there is no standard definition of low or high TILs, we sought to establish an optimal cutoff by constructing a series of Kaplan-Meier curves for each 10% increment in TILs between 10% and 50% and found that a level of > 20% led to optimal separation of EFS curves (Data Supplement 1). However, although TILs and other genomic features were prognostic for EFS in univariate analyses, once pCR status (yes or no) was included in a multivariable analysis, TILs, either as a continuous variable or with a > 20% cutoff, were no longer independently prognostic for EFS, whereas a genomic signature of CD8+ T cells, evaluated in the same model, within the same subset of patients, remained an independent prognostic variable that significantly improved the prognostic value of the model (Fig 4F and Data Supplement 2).
A Cox PH model on the basis of treatment (ie, plus carboplatin or plus bevacizumab), genomic variables, and the interaction between treatment and the genomic feature can identify potential features associated with treatment-specific sensitivity and resistance. We identified 11 features that had significant interaction with carboplatin treatment (Data Supplement 1), the most significant of which was RB1 mRNA expression, with low RB1 expression being associated with greater improvements in pCR and EFS with the addition of carboplatin (Data Supplement 1). There were 12 features that had significant interaction with bevacizumab (Data Supplement 1), including a signature of lung metastasizing breast cancer cells (Pcorr_Breast2Lung_LM2),15 for which higher expression correlated with worse survival in patients not receiving bevacizumab (Data Supplement 1), and menopausal status, with postmenopausal women demonstrating worse survival with bevacizumab (Data Supplement 1).
DISCUSSION
As expected,1,16 patients with TNBC in CALGB 40603 who achieved pCR with NACT had far superior LTOs compared with those with RD, in whom the baseline stage and extent of RD were prognostic. In this trial, patients with any RD, even RCB-I, had significantly worse outcomes than those with pCR, supporting consideration of adjuvant therapy even in the setting of minimal RD.
Despite the significant increase in pCR with both agents, there was no evidence that adding either bevacizumab or carboplatin improved EFS or other LTOs, although, like many neoadjuvant trials, CALGB 40603 was not powered to evaluate EFS. We did not collect data on whether study patients, particularly those who failed to achieve pCR, received additional chemotherapy or other systemic treatments after surgery, and thus, we cannot rule out the possibility that such treatments could have affected EFS and other LTOs and diminished the apparent benefit of achieving pCR although our study was completed before results of the CREATE-X trial were presented and made administration of postneoadjuvant therapy with capecitabine common.17 The absence of benefit from bevacizumab is not surprising, noting that in three other randomized trials—GeparQuinto, ARTemis, and NSABP B-40—the addition of bevacizumab to NACT did not improve disease-free survival (DFS) or OS in TNBC,18-23 despite significantly increasing pCR rates in the first two studies, nor has adding bevacizumab to adjuvant chemotherapy been shown to improve outcomes in TNBC.24,25 In both GeparQuinto and ARTemis, pCRs achieved with bevacizumab had higher rates of DFS events than those attained with NACT alone, leading the ARTemis investigators to hypothesize that although bevacizumab might enhance response to chemotherapy in an angiogenesis-driven breast tumor, it might not have the same effect on micrometastatic disease.21
Two other randomized trials—GeparSixto and BrighTNess—have demonstrated significant increases in pCR rates in TNBC with the addition of carboplatin to taxane- and anthracycline-containing NACT26,27; pCR rates of a similar magnitude have been reported in other multicenter studies (Data Supplement 1).28-30 In GeparSixto, the addition of weekly carboplatin to their control NACT regimen significantly improved DFS, along with a trend toward improvement in OS.28,29 LTOs from BrighTNess are of particular interest since this study used a control regimen identical to arm 1 of CALGB 40603. An intriguing but exploratory post hoc analysis of the two trials found that a higher proportion of patients in the carboplatin arm of BrighTNess (88%) received all 12 planned doses of the taxane than CALGB 40603 (65%). Patients assigned to carboplatin on BrighTNess had a larger absolute increase in pCR rate than on CALGB 40603 (27% v 13%)27 and in results presented at the 2021 ESMO Congress, had significantly better 4-year EFS and a trend toward improved OS31 although other factors might have contributed to these apparent discrepancies. Although EA1131 failed to demonstrate noninferiority of adjuvant platinum therapy compared with capecitabine in patients with TNBC with RD after (non–platinum-containing) NACT,32 no randomized trial has reported on the addition of carboplatin to adjuvant chemotherapy for TNBC; however, one is ongoing (NRG-BR003).
KEYNOTE-522 assessed the benefit of the addition of the programmed cell death protein 1–targeted monoclonal antibody pembrolizumab to a NACT regimen consisting of wP and carboplatin followed by AC or epirubicin-cyclophosphamide (EC) in TNBC, demonstrating improved pCR rates and 3-year EFS with the addition of the immune checkpoint inhibitor (ICI).33,34 This finding resulted in US Food and Drug Administration approval for the addition of pembrolizumab to NACT in TNBC. The chemotherapy backbone in KEYNOTE-522 included carboplatin, making a platinum-containing NACT regimen appropriate for patients with stage II and III TNBC being treated in this way. However, it should be noted that the design of KEYNOTE-522 does not allow assessment of the individual contribution of carboplatin to the EFS benefit observed with the addition of pembrolizumab.
From a correlative science perspective, the limited overlap between features associated with pCR and EFS suggests the need to be cautious in developing biomarkers for survival from studies for which pCR is the primary clinical end point and that are not powered to assess LTOs , even though pCR is the most powerful individual prognostic feature for EFS. Our results are consistent with previous observations that both increased TILs and immune-related gene expression signatures are associated with a higher likelihood of achieving pCR with NACT and improved survival in TNBC.35-37 Evaluating both on the same specimens demonstrates how inclusion of some of these genomic immune signatures, such as a CD8+ T-cell signature, may improve a multivariable prognostic model, whereas the abundance of TILs did not. In addition, we demonstrate that a more focused antigen-driven immune response, presumably in response to antigens expressed by the cancer, as reflected by lower IgG evenness, is associated with both better response to NACT and improved EFS and may help to identify patients with a good prognosis even in the absence of pCR. Low IgG evenness has also been associated with improved prognosis in cutaneous melanoma.38 Given the exploratory nature of these findings, we look forward to the presentation of correlative results from the BrighTNess trial, now that it has reported EFS and OS results, to see if we can validate these potential biomarkers. The finding that evidence of immune activation is associated with both pCR to NACT and improved survival heightens interest in the studying regimens that incorporate both effective NACT and ICIs. Recently reported trials have demonstrated that the addition of an ICI to NACT for TNBC not only increases pCR but can also improve EFS.30,39 Analyses of these studies failed to show that expression of a single marker of tumor-induced immune suppression, namely, programmed death-ligand 1, identified patients more likely to benefit from addition of immunotherapy, thus leaving open the possibility that a more detailed evaluation of immune activation as described herein may be necessary to identify biomarkers for ICI benefit in the neoadjuvant setting.
Our study has several important limitations. The sample size was calculated on the basis of analysis of our primary end point, pCR, which limits our ability to evaluate the impact of treatment assignment on EFS and other LTOs. Although the magnitude of the increment in pCR that would be expected to significantly improve EFS is not well defined, when presenting results of their meta-analysis of the impact of pCR on LTOs, Spring et al16 commented that to determine if the 13% absolute increase in pCRs observed with carboplatin in CALGB 40603 significantly affects EFS would require 1,381 events, a 10-fold increase over the 135 events reported herein. We did not require central pathologic review, relying on institutional pathologists to assess pCR and record the findings necessary to calculate RCB. In addition, we did not perform germline BRCA mutation testing; thus, we are unable to determine whether BRCA mutation status affects the impact of treatment assignment on pCR or EFS.
In conclusion, although adding either carboplatin or bevacizumab significantly increased pCR in our trial, neither appeared to improve LTO; however, CALGB 40603 was underpowered for these end points. It should be noted that although its impact on LTO remains unclear, adding carboplatin is consistently associated with a pCR advantage. Moreover, carboplatin is included in the NACT regimen given with pembrolizumab. For these reasons, inclusion of carboplatin in NACT is reasonable for patients with stage II and III TNBC, particularly if being given with an ICI. We found that TNBC patients with any amount of RD after NACT, even RCB-I, had inferior LTOs compared with patients with pCR. Immune activation as measured by TILs and gene expression signatures was associated with both higher pCR rates and improved EFS although only immune activation measured by multigene expression signatures was independently associated with EFS in multivariable analysis. These observations, from a study in which patients did not receive immune-targeted therapy, may provide an opportunity to test de-escalated or tailored chemotherapy in patients with markers of immune activation.
Karla Ballman
Consulting or Advisory Role: Medtronic, Takeda, Agenus
Patents, Royalties, Other Intellectual Property: Prostate cancer signature patent (Inst)
Expert Testimony: Janssen Oncology, Lilly, Sanofi
Sara Selitsky
Employment: QuantBio, Sash Biosciences
Consulting or Advisory Role: GeneCentric, C4 Therapeutics, Select ImmunoGenomics, FORMA Therapeutics, Atlas Venture, CytomX Therapeutics (Inst), Actym Therapeutics (Inst), Capulus Therapeutics (Inst), Codagenix (Inst)
Joel S. Parker
Stock and Other Ownership Interests: GeneCentric, Reveal Genomics
Consulting or Advisory Role: Bristol Myers Squibb/Celgene
Patents, Royalties, Other Intellectual Property: J.S.P. has authored patents related to the PAM50 algorithm, which are licensed to NanoString Technologies
Patricia A. Spears
Consulting or Advisory Role: Pfizer
Sara M. Tolaney
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech, Eisai, Sanofi, Celldex, Bristol Myers Squibb, Paxman, Seattle Genetics, Odonate Therapeutics, AbbVie, Silverback Therapeutics, G1 Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Immunomedics/Gilead, Mersana, Certara, 4D Pharma, Ellipses Pharma, OncoSec, Chugai Pharma, BeyondSpring Pharmaceuticals, OncXerna Therapeutics
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Cyclacel (Inst), Nektar (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Sanofi (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Eisai, NanoString Technologies, Puma Biotechnology, Celldex
Cynthia Ma
Consulting or Advisory Role: Novartis, Seattle Genetics, Agendia, AstraZeneca, Athenex, Bayer HealthCare Pharmaceuticals, Biovica Inc, Eisai, Olaris, Philips Electronics, Puma Biotechnology, Sanofi Genzyme, Jacobio, Natera, Inivata
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst)
Eleftherios Mamounas
Honoraria: Genentech/Roche, Genomic Health, Precisca
Consulting or Advisory Role: Genomic Health, BioTheranostics, Roche/Genentech, Merck, Puma Biotechnology, Precisca, Agendia
Speakers' Bureau: Genomic Health, Genentech/Roche
Mehra Golshan
Consulting or Advisory Role: AbbVie, Bertis
Research Funding: Breast Cancer Research Foundation
Jennifer R. Bellon
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: International Journal of Radiation Oncology Biology Physics
Honoraria: UpToDate, Accuray, Leidos Biomedical Research, Grupo Oncoclinicas
Research Funding: NanoString Technologies (Inst)
Patents, Royalties, Other Intellectual Property: Coeditor of breast cancer textbook (Radiation Therapy Techniques and Treatment Planning for Breast Cancer). Honorarium, Springer
Other Relationship: Varian Medical Systems
Deborah Collyar
Honoraria: Pfizer
Consulting or Advisory Role: Parexel, MaxisIT, Kinnate Biopharma
Travel, Accommodations, Expenses: Parexel
Olwen M. Hahn
Leadership: Via Oncology
Stock and Other Ownership Interests: Teleflex Medical
Honoraria: Cardinal Health (I)
Consulting or Advisory Role: Pfizer, hmpglobal.com
Travel, Accommodations, Expenses: Cardinal Health (I)
Clifford A. Hudis
Uncompensated Relationships: Alliance Foundation Trials, Columbia University, Memorial Sloan-Kettering Cancer Center
Open Payments Link: https://openpaymentsdata.cms.gov/physician/471974/summary
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks, Athenex
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Ann Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for coauthoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Terry Hyslop
Consulting or Advisory Role: AbbVie
Travel, Accommodations, Expenses: AbbVie
Lisa A. Carey
Research Funding: Syndax (Inst), Novartis (Inst), NanoString Technologies (Inst), AbbVie (Inst), Seattle Genetics (Inst), Veracyte (Inst)
Patents, Royalties, Other Intellectual Property: Royalty-sharing agreement, investorship interest in licensed IP to startup company, Falcon Therapeutics, that is designing neural stem-cell–based therapy for glioblastoma multiforme (I)
Uncompensated Relationships: Sanofi (Inst), Novartis (Inst), G1 Therapeutics (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), AstraZeneca/Daiichi Sankyo (Inst), Aptitude Health (Inst), Exact Sciences (Inst), Eisai
Open Payments Link: https://openpaymentsdata.cms.gov/physician/179671
Charles M. Perou
Leadership: GeneCentric
Stock and Other Ownership Interests: Bioclassifier, GeneCentric, Reveal Genomics
Consulting or Advisory Role: Bioclassifier, GeneCentric, NanoString Technologies, Veracyte, Reveal Genomics
Patents, Royalties, Other Intellectual Property: Royalties from PAM50 breast cancer gene patent application and from lung gene signature patent
Travel, Accommodations, Expenses: Takeda, Chugai Pharma
William M. Sikov
Honoraria: UpToDate
Speakers' Bureau: Lilly, Daiichi Sankyo, Seattle Genetics
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. https://acknowledgments.alliancefound.org. The results published here are in whole or part based upon data from the Cancer Genome Atlas managed by the NCI and NHGRI (dbGaP accession phs000178).
PRIOR PRESENTATION
Presented at ASCO, Chicago, IL, May 31-June 4, 2019, and SABCS, San Antonio, TX, December 9-13, 2014.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882, and U24CA196171 (to the Alliance for Clinical Trials in Oncology), UG1CA233180, UG1CA189858, UG1CA233180, UG1CA233253, UG1CA233290, UG1CA233327, UG1CA233339, UG1CA233373, P30CA033572, and P50CA58223 (L.A.C. and C.M.P.); the Breast Cancer Research Foundation (Alliance, L.A.C., and C.M.P.); and Susan G. Komen (L.A.C. and C.M.P.), the American Recovery and Reinvestment Act. Also supported in part by funds from Genentech.
CLINICAL TRIAL INFORMATION
NCT00861705 (ALLIANCE/CALGB 40603)
DATA SHARING STATEMENT
RNA-sequencing, clinical data, and patient outcomes are available through NCBI database of Genotypes and Phenotypes (dbGaP) (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001863.v1.p1).
AUTHOR CONTRIBUTIONS
Conception and design: Mei-Yin C. Polley, Joel S. Parker, Elisa R. Port, Mehra Golshan, Jennifer R. Bellon, Olwen M. Hahn, Clifford A. Hudis, Eric P. Winer, Lisa A. Carey, Charles M. Perou, William M. Sikov
Financial support: Charles M. Perou
Administrative support: Matthew G. Soloway, Mehra Golshan, Olwen M. Hahn, Clifford A. Hudis, Charles M. Perou
Provision of study materials or patients: George Somlo, Cynthia Ma, Deborah Collyar, Clifford A. Hudis, Charles M. Perou, William M. Sikov
Collection and assembly of data: Karla Ballman, Mei-Yin C. Polley, Cheng Fan, Joel S. Parker, Katherine A. Hoadley, Zhiyuan Hu, Matthew G. Soloway, Patricia A. Spears, Baljit Singh, Sara M. Tolaney, George Somlo, Elisa R. Port, Cynthia Ma, Charles Kuzma, Eleftherios Mamounas, Mehra Golshan, Olwen M. Hahn, Clifford A. Hudis, Terry Hyslop, Charles M. Perou
Data analysis and interpretation: Jonathan H. Shepherd, Karla Ballman, Mei-Yin C. Polley, Jordan D. Campbell, Cheng Fan, Sara Selitsky, Aranzazu Fernandez-Martinez, Joel S. Parker, Yan Li, George Somlo, Elisa R. Port, Mehra Golshan, Deborah Collyar, Clifford A. Hudis, Ann Partridge, Charles M. Perou, William M. Sikov
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
CALGB 40603 (Alliance): Long-Term Outcomes and Genomic Correlates of Response and Survival After Neoadjuvant Chemotherapy With or Without Carboplatin and Bevacizumab in Triple-Negative Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Karla Ballman
Consulting or Advisory Role: Medtronic, Takeda, Agenus
Patents, Royalties, Other Intellectual Property: Prostate cancer signature patent (Inst)
Expert Testimony: Janssen Oncology, Lilly, Sanofi
Sara Selitsky
Employment: QuantBio, Sash Biosciences
Consulting or Advisory Role: GeneCentric, C4 Therapeutics, Select ImmunoGenomics, FORMA Therapeutics, Atlas Venture, CytomX Therapeutics (Inst), Actym Therapeutics (Inst), Capulus Therapeutics (Inst), Codagenix (Inst)
Joel S. Parker
Stock and Other Ownership Interests: GeneCentric, Reveal Genomics
Consulting or Advisory Role: Bristol Myers Squibb/Celgene
Patents, Royalties, Other Intellectual Property: J.S.P. has authored patents related to the PAM50 algorithm, which are licensed to NanoString Technologies
Patricia A. Spears
Consulting or Advisory Role: Pfizer
Sara M. Tolaney
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech, Eisai, Sanofi, Celldex, Bristol Myers Squibb, Paxman, Seattle Genetics, Odonate Therapeutics, AbbVie, Silverback Therapeutics, G1 Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Immunomedics/Gilead, Mersana, Certara, 4D Pharma, Ellipses Pharma, OncoSec, Chugai Pharma, BeyondSpring Pharmaceuticals, OncXerna Therapeutics
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Cyclacel (Inst), Nektar (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Sanofi (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Eisai, NanoString Technologies, Puma Biotechnology, Celldex
Cynthia Ma
Consulting or Advisory Role: Novartis, Seattle Genetics, Agendia, AstraZeneca, Athenex, Bayer HealthCare Pharmaceuticals, Biovica Inc, Eisai, Olaris, Philips Electronics, Puma Biotechnology, Sanofi Genzyme, Jacobio, Natera, Inivata
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst)
Eleftherios Mamounas
Honoraria: Genentech/Roche, Genomic Health, Precisca
Consulting or Advisory Role: Genomic Health, BioTheranostics, Roche/Genentech, Merck, Puma Biotechnology, Precisca, Agendia
Speakers' Bureau: Genomic Health, Genentech/Roche
Mehra Golshan
Consulting or Advisory Role: AbbVie, Bertis
Research Funding: Breast Cancer Research Foundation
Jennifer R. Bellon
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: International Journal of Radiation Oncology Biology Physics
Honoraria: UpToDate, Accuray, Leidos Biomedical Research, Grupo Oncoclinicas
Research Funding: NanoString Technologies (Inst)
Patents, Royalties, Other Intellectual Property: Coeditor of breast cancer textbook (Radiation Therapy Techniques and Treatment Planning for Breast Cancer). Honorarium, Springer
Other Relationship: Varian Medical Systems
Deborah Collyar
Honoraria: Pfizer
Consulting or Advisory Role: Parexel, MaxisIT, Kinnate Biopharma
Travel, Accommodations, Expenses: Parexel
Olwen M. Hahn
Leadership: Via Oncology
Stock and Other Ownership Interests: Teleflex Medical
Honoraria: Cardinal Health (I)
Consulting or Advisory Role: Pfizer, hmpglobal.com
Travel, Accommodations, Expenses: Cardinal Health (I)
Clifford A. Hudis
Uncompensated Relationships: Alliance Foundation Trials, Columbia University, Memorial Sloan-Kettering Cancer Center
Open Payments Link: https://openpaymentsdata.cms.gov/physician/471974/summary
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks, Athenex
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Ann Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for coauthoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Terry Hyslop
Consulting or Advisory Role: AbbVie
Travel, Accommodations, Expenses: AbbVie
Lisa A. Carey
Research Funding: Syndax (Inst), Novartis (Inst), NanoString Technologies (Inst), AbbVie (Inst), Seattle Genetics (Inst), Veracyte (Inst)
Patents, Royalties, Other Intellectual Property: Royalty-sharing agreement, investorship interest in licensed IP to startup company, Falcon Therapeutics, that is designing neural stem-cell–based therapy for glioblastoma multiforme (I)
Uncompensated Relationships: Sanofi (Inst), Novartis (Inst), G1 Therapeutics (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), AstraZeneca/Daiichi Sankyo (Inst), Aptitude Health (Inst), Exact Sciences (Inst), Eisai
Open Payments Link: https://openpaymentsdata.cms.gov/physician/179671
Charles M. Perou
Leadership: GeneCentric
Stock and Other Ownership Interests: Bioclassifier, GeneCentric, Reveal Genomics
Consulting or Advisory Role: Bioclassifier, GeneCentric, NanoString Technologies, Veracyte, Reveal Genomics
Patents, Royalties, Other Intellectual Property: Royalties from PAM50 breast cancer gene patent application and from lung gene signature patent
Travel, Accommodations, Expenses: Takeda, Chugai Pharma
William M. Sikov
Honoraria: UpToDate
Speakers' Bureau: Lilly, Daiichi Sankyo, Seattle Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cortazar P, Zhang L, Untch M, et al. : Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164-172, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Sikov WM, Berry DA, Perou CM, et al. : Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33:13-21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Symmans WF, Peintinger F, Hatzis C, et al. : Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25:4414-4422, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Ciriello G, Gatza ML, Beck AH, et al. : Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163:506-519, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allison KH, Hammond MEH, Dowsett M, et al. : Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 38:1346-1366, 2020 [DOI] [PubMed] [Google Scholar]
- 6.https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001863.v1.p1
- 7.Parker JS, Mullins M, Cheang MC, et al. : Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27:1160-1167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Martinez A, Krop IE, Hillman DW, et al. : Survival, pathologic response, and genomics in CALGB 40601 (Alliance), a neoadjuvant phase III trial of paclitaxel-trastuzumab with or without lapatinib in HER2-positive breast cancer. J Clin Oncol 38:4184-4193, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoadley KA, Yau C, Wolf DM, et al. : Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 158:929-944, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong KY, Fan C, Tanioka M, et al. : I-boost: An integrative boosting approach for predicting survival time with multiple genomics platforms. Genome Biol 20:52, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salgado R, Denkert C, Demaria S, et al. : The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an international TILs Working Group 2014. Ann Oncol 26:259-271, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann BD, Bauer JA, Chen X, et al. : Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750-2767, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burstein MD, Tsimelzon A, Poage GM, et al. : Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 21:1688-1698, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prat A, Parker JS, Karginova O, et al. : Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12:R68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minn AJ, Gupta GP, Siegel PM, et al. : Genes that mediate breast cancer metastasis to lung. Nature 436:518-524, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spring LM, Fell G, Arfe A, et al. : Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin Cancer Res 26:2838-2848, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda N, Lee SJ, Ohtani S, et al. : Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 376:2147-2159, 2017 [DOI] [PubMed] [Google Scholar]
- 18.von Minckwitz G, Eidtmann H, Rezai M, et al. : Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med 366:299-309, 2012 [DOI] [PubMed] [Google Scholar]
- 19.von Minckwitz G, Loibl S, Untch M, et al. : Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44–GeparQuinto). Ann Oncol 25:2363-2372, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Earl HM, Hiller L, Dunn JA, et al. : Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): An open-label, randomised, phase 3 trial. Lancet Oncol 16:656-666, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Earl HM, Hiller L, Dunn JA, et al. : Disease-free and overall survival at 3.5 years for neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin and cyclophosphamide, for women with HER2 negative early breast cancer: ARTemis trial. Ann Oncol 28:1817-1824, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bear HD, Tang G, Rastogi P, et al. : Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med 366:310-320, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bear HD, Tang G, Rastogi P, et al. : Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): Secondary outcomes of a phase 3, randomised controlled trial. Lancet Oncol 16:1037-1048, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller KD, O'Neill A, Gradishar W, et al. : Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node–positive and high-risk lymph node–negative breast cancer (E5103). J Clin Oncol 36:2621-2629, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell R, Brown J, Parmar M, et al. : Final efficacy and updated safety results of the randomized phase III BEATRICE trial evaluating adjuvant bevacizumab-containing therapy in triple-negative early breast cancer. Ann Oncol 28:754-760, 2017 [DOI] [PubMed] [Google Scholar]
- 26.von Minckwitz G, Schneeweiss A, Loibl S, et al. : Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol 15:747-756, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Loibl S, O'Shaughnessy J, Untch M, et al. : Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol 19:497-509, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Rugo HS, Olopade OI, DeMichele A, et al. : Adaptive randomization of veliparib–carboplatin treatment in breast cancer. N Engl J Med 375:23-34, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanda R, Liu MC, Yau C, et al. : Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 6:676-684, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Untch M, Jackisch C, Schneeweiss A, et al. : Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto—GBG 69): A randomised, phase 3 trial. Lancet Oncol 17:345-356, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Loibl S, Sikov W, Huober J, et al. : Event-free survival (EFS), overall survival (OS), and safety of adding veliparib (V) plus carboplatin (Cb) or carboplatin alone to neoadjuvant chemotherapy in triple-negative breast cancer (TNBC) after ≥4 years of follow-up: BrighTNess, a randomized phase III trial. Ann Oncol 32:S408, 2021 [Google Scholar]
- 32.Mayer IA, Zhao F, Arteaga CL, et al. : Randomized phase III postoperative trial of platinum-based chemotherapy versus capecitabine in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: ECOG-ACRIN EA1131. J Clin Oncol 39:2539-2551, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid P, Cortes J, Pusztai L, et al. : Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810-821, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Schmid P, Cortes J, Dent R, et al. : VP7-2021: KEYNOTE-522: Phase III study of neoadjuvant pembrolizumab + chemotherapy vs. placebo + chemotherapy, followed by adjuvant pembrolizumab vs. placebo for early-stage TNBC. Ann Oncol 32:1198-1200, 2021 [Google Scholar]
- 35.Filho OM, Stover DG, Asad S, et al. : Association of immunophenotype with pathologic complete response to neoadjuvant chemotherapy for triple-negative breast cancer: A secondary analysis of the BrighTNess phase 3 randomized clinical trial. JAMA Oncol 7:603-608, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loi S, Drubay D, Adams S, et al. : Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient Analysis of early-stage triple-negative breast cancers. J Clin Oncol 37:559-569, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. : Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19:40-50, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Selitsky SR, Mose LE, Smith CC, et al. : Prognostic value of B cells in cutaneous melanoma. Genome Med 11:36, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loibl S, Weber KE, Timms KM, et al. : Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response—Final results from GeparSixto. Ann Oncol 29:2341-2347, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNA-sequencing, clinical data, and patient outcomes are available through NCBI database of Genotypes and Phenotypes (dbGaP) (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001863.v1.p1).