Abstract
The inflammatory/anti‐inflammatory balance has an important role in the clinical course of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) (coronavirus disease [COVID‐19]) infection, which has affected over 200 million people since it first appeared in China in December 2019. This study aimed to determine the effectiveness of montelukast, which has known anti‐inflammatory and bronchodilatory effects, in these patients. The prospective randomized controlled study included 180 patients who were hospitalized in the infectious diseases department of our hospital between May and July 2021 and were diagnosed with the delta variant of SARS‐CoV‐2 by real‐time polymerase chain reaction of nasopharyngeal swabs. The patients were divided into three groups and received only standard treatment according to national guidelines (Group 1) or standard treatment plus 10 mg/day montelukast (Group 2) or 20 mg/day montelukast (Group 3). Laboratory parameters and pulmonary function tests (PFTs) at admission and on Day 5 of treatment were compared. Comparison of laboratory parameters on Day 5 showed that Groups 2 and 3 had significantly lower levels of lactate dehydrogenase, fibrinogen, D‐dimer, C‐reactive protein, and procalcitonin compared with Group 1 (p = 0.04, 0.002, 0.05, 0.03, and 0.04, respectively). In the comparison between Groups 2 and 3, only fibrinogen was significantly lower in Group 3 (p = 0.02). PFT results did not differ between the groups at admission, while on Day 5, only Group 3 showed significant improvements in forced expiratory volume in 1 s, forced vital capacity, and peak expiratory flow 25–75 compared with admission (p = 0.001 for all). Montelukast may be beneficial in COVID‐19 patients to maintain the inflammatory/anti‐inflammatory balance, prevent respiratory failure through its bronchodilator activity, and reduce mortality.
Keywords: COVID‐19, montelukast, pulmonary function tests
Highlights
The study included 180 participants who were divided into three groups: Group 1 (n = 60) received standard treatment in accordance with our national COVID‐19 diagnosis and treatment guide, Group 2 (n = 60) received 10 mg/day oral montelukast in addition to standard treatment, and Group 3 (n = 60) received 20 mg/day oral montelukast in addition to standard treatment.
We aimed to investigate the effect of treatment with varying doses of montelukast as an adjunct to standard antiviral therapy on pulmonary function tests and clinical courses in patients with COVID‐19.
1. INTRODUCTION
“Pandemic” is a medical term that has become a ubiquitous part of the global vocabulary over the last year. Although pandemics have occurred throughout human history, their sociocultural, economic, and psychological impact can leave lasting damage. In the current coronavirus disease‐19 (COVID‐19) pandemic, more than 200 million confirmed cases of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection have been reported to date. While most people present with mild symptoms such as loss of taste and smell, sore throat, joint pain, and headache, it can cause serious morbidity and mortality, especially in individuals over 65 years of age and those with comorbidities. 1
Acute respiratory distress syndrome (ARDS) and macrophage activation syndrome (MAS) are among the main causes of morbidity and mortality in COVID‐19. A contributing factor in the development of these clinical conditions is the overproduction of proinflammatory cytokines, primarily tumor necrosis factor‐α (TNF‐α), interleukin‐6 (IL‐6), IL‐8, and IL‐1β. These cytokines cause increased leukocyte accumulation in the alveolar spaces and consequently an increase in reactive oxygen radicals and proteases, which inevitably leads to capillary endothelial damage and alveolar epithelial damage. 2 , 3
Montelukast is a potent cysteinyl leukotriene (cysLT) receptor antagonist with anti‐inflammatory activity and has been proven to significantly suppress oxidative stress. Moreover, cysLTs also have an important role in the regulation of cytokine production. Administration of high doses of montelukast reduces IL‐4, IL‐5, and IL‐13 production by T‐helper 2 cells. 4 This effect makes it an important anti‐inflammatory agent in the treatment of asthma. In addition, montelukast was shown to significantly inhibit bradykinin‐induced tracheal smooth muscle contraction, thus supporting an interaction between bradykinin and leukotriene mediators. 5
In studies investigating the efficacy of cysLT for ARDS and MAS, montelukast was found to increase interferon‐γ (IFN‐γ) production and significantly decrease the production of proinflammatory cytokines such as IL‐1β, IL‐6, and IL‐8 in mice infected with a respiratory syncytial virus. In another study, cysLT prevented neutrophil infiltration, lung inflammation, and oxidative stress and significantly decreased levels of TNF‐α and IL‐6 in both the lung parenchyma and bronchoalveolar lavage fluid in an animal model of ARDS induced by hemorrhagic shock.
In this study, we aimed to investigate the effect of treatment with varying doses of montelukast as an adjunct to standard antiviral therapy on pulmonary function tests and clinical courses in patients with COVID‐19.
2. MATERIALS AND METHODS
2.1. Study design and setting
This prospective, randomized, controlled, single‐blinded study included 180 participants who were divided into three groups: Group 1 (n = 60) received standard treatment in accordance with our national COVID‐19 diagnosis and treatment guide, Group 2 (n = 60) received 10 mg/day oral montelukast in addition to standard treatment, and Group 3 (n = 60) received 20 mg/day oral montelukast in addition to standard treatment (Figure 1).
Figure 1.
CONSORT diagram
As the primary outcome of the study, we evaluated the effect of treatment on progression to ARDS and MAS during follow‐up. Secondary outcomes were the effects of treatment on lung capacity in pulmonary function testing. In addition, the patients' hematological parameters, biochemical parameters including liver and kidney function tests, coagulation parameters, ferritin, D‐dimer, troponin‐I, and C‐reactive protein (CRP) levels were evaluated daily starting from the day of admission. Pulmonary function tests were performed at admission and repeated on Day 5 of treatment.
Based on the primary endpoint of the study, which was a 20% or greater reduction in the number of patients with clinical deterioration requiring admission to intensive care due to ARDS or MAS, we determined that 180 patients (60 in each group) were needed (α = 0.05, power = 95%, δ = −0.20).
Participants for this single‐center study were recruited from among patients hospitalized in the infectious diseases ward of Erzurum Regional Training and Research Hospital, which has been designated as a pandemic hospital in the city for approximately 2 years. Before starting the study, approval was obtained from the Atatürk University Faculty of Medicine Clinical Studies Ethics Committee.
The trial was registered on the ClinicalTrials. gov website with the official title “Effect of montelukast therapy on clinical course, pulmonary function, and mortality in patients with COVID‐19” (identifier code: NCT05094596).
2.2. Study sample
During the study period, patients who presented to the emergency department of Erzurum Regional Training and Research Hospital with a history of travel abroad within the last 14 days or contact with a confirmed or suspected COVID‐19 patient and had recent complaints of fever, cough, dyspnea, malaise, and sudden loss of taste and smell were evaluated for COVID‐19. Patients regarded as high risk for COVID‐19 underwent standard high‐resolution computed tomography (HRCT). Predominantly peripheral bilateral ground‐glass opacities, subsegmental consolidation or linear opacities, crazy‐paving pattern, and reverse halo sign were considered typical HRCT findings for COVID‐19. Patients with these findings and patients with radiologically atypical findings but consistent clinical symptoms were hospitalized with suspected COVID‐19. The diagnosis was confirmed by SARS‐CoV‐2 real‐time polymerase chain reaction (PCR) testing of nasopharyngeal swab samples.
The sample of this study consisted of 180 patients who were hospitalized in Erzurum Regional Training and Research Hospital between May 2021 and July 2021, had confirmed SARS‐CoV‐2 infection, did not have ARDS or MAS at admission, and had a PaO2/FiO2 ratio above 200 at admission and on Day 5 of treatment. Patients (or their relatives) were informed in detail about the aim of the study and all participants provided informed consent before inclusion.
Patients with any potential contraindications to pulmonary function testing (recent myocardial infarction, pulmonary embolism, cerebral aneurysm, active hemoptysis, pneumothorax, nausea/vomiting, recent thoracic, abdominal, or ocular surgery) were excluded before testing. In addition, patients who developed ARDS or MAS associated with secondary bacterial infection during the first week of treatment were also excluded.
2.3. Definitions and diagnosis
Fever was defined as an axillary temperature of 37.3°C or higher. Secondary bacterial infection was diagnosed based on signs and symptoms of bacteremia or pneumonia with the identification of a new bacterial pathogen in endotracheal aspirate or lower respiratory tract sputum culture. Treatment for patients diagnosed as having ventilator‐associated or hospital‐acquired pneumonia was planned according to available guidelines. Acute respiratory distress was diagnosed and graded according to the 2015 Berlin diagnostic criteria. Patients with daily cardiac‐specific troponin levels above the normal range underwent echocardiographic evaluation for new cardiac pathologies. Coagulopathy was defined as prothrombin time 3 s longer than normal and activated partial thromboplastin time 5 s longer than normal.
2.4. Pharmacological treatment
Treatment strategy was determined according to disease severity as per the COVID‐19 adult diagnosis and treatment guidelines issued by the Turkish Ministry of Health. Antiviral therapy consisted of an initial loading dose of 1600 mg favipiravir every 12 h, followed by a maintenance dose of 600 mg every 12 h for a total of 5 days. Patients with signs such as refractory fever, CRP, and ferritin levels that remained high or continued to rise, D‐dimer elevation, cytopenia manifesting as thrombocytopenia or lymphopenia, abnormal liver function tests, hypofibrinogenemia, or elevated triglyceride levels in spite of treatment were monitored for MAS. As changes in serial measures are more important than set threshold values for laboratory findings, we diagnosed MAS based on repeated follow‐up measurements of clinical and laboratory parameters. If these parameters continued to deteriorate during follow‐up with no apparent secondary bacterial infection, patients were treated with methylprednisolone at a dose of at least 250 mg/day for 3 days. If no response was obtained with this treatment, 8 mg/kg (max 800 mg) tocilizumab was administered for MAS unless contraindicated. Clinical and laboratory response was evaluated after 24 h. If an adequate response was not observed, treatment was repeated at the same dose.
2.5. Montelukast treatment
Patients in Group 1 were treated in accordance with the COVID‐19 adult diagnosis and treatment guidelines issued by the Turkish Ministry of Health as specified above. Patients in Group 2 received this standard treatment as well as 10 mg/day montelukast sodium (Zespira® 10 mg tablet) in addition to standard treatment, while patients in Group 3 received standard treatment and 20 mg/day montelukast sodium (Zespira® 10 mg tablet) once daily.
2.6. Pulmonary function testing
Pulmonary function tests were performed in a negative‐pressure room by a technician wearing protective equipment to prevent transmission. Before testing, patients were instructed to abstain from smoking (24 h), alcohol (4 h), strenuous exercise (30 min), and heavy meals (2 h). The patients' age, height, and weight were recorded. Tests were performed with the patients lightly dressed and BTPS (body temperature, pressure, water vapor saturated) correction was performed according to room air and barometric pressure. The technician explained the maneuver to the patients and three acceptable spirograms were obtained. Tests that met the 2019 ATS/ERS reproducibility and acceptability criteria for pulmonary function tests were included in the study. 6 The lower limits of the normal range determined for the healthy population according to the criteria specified in the same report were also calculated and presented by the spirometry device. All spirometry was performed by the same technician using a Plusmed MIR Spirolab III device.
2.7. Statistical analysis
Analyses were performed using IBM SPSS version 20.0 software (IBM Corp.). The data were presented as mean and SD or number and percentage. The normal distribution of continuous variables was evaluated using Shapiro–Wilk W test and Kolmogorov–Smirnov test. Comparisons of continuous variables between multiple independent groups were performed using analysis of variance (ANOVA) for normally distributed data and Kruskal–Wallis test for non‐normally distributed data. After ANOVA, post hoc analyses were performed using Tukey's test if variances were homogeneous and Tamhane's T2 test if nonhomogeneous. After the Kruskal–Wallis test, the Kruskal–Wallis one‐way ANOVA (k samples) test was used for post hoc analysis. Relationships between two quantitative variables were analyzed using Pearson's correlation analysis if they showed normal distribution and Spearman's correlation analysis if they did not. Binary logistic regression analysis was used to evaluate laboratory and pulmonary function parameters at different doses of montelukast. Results with p values < 0.05 were considered statistically significant.
3. RESULTS
The patients' mean age was 54.6 ± 15.3 years. The mean ages in Groups 1, 2, and 3 were 54.8 ± 14.8, 54.2 ± 16.5, and 52.8 ± 14.3 years, respectively, with no statistically significant difference between the groups (p = 0.7). Men accounted for 24 patients in Group 1, 22 in Group 2, and 30 in Group 3 (p = 0.28).
Comparison of comorbidity rates between the groups showed that comorbidities were present in 24 patients in Group 1, 28 patients in Group 2, and 26 patients in Group 3 (p = 0.16). Hypertension was observed in 20 patients in Group 1, 20 patients in Group 2, and 14 patients in Group 3 (p = 0.15), while diabetes was present in 11 patients in Group 1, 12 patients in Group 2, and in 11 patients in Group 3 (p = 0.45). Two patients in Group 3 had coronary artery disease, one patient in each group had asthma, and one patient in Group 2 had psychiatric disease.
3.1. Influence of montelukast treatment on inflammation markers and hematology profile
The comparison of the patients' laboratory parameters at admission is shown in Table 1. There was no statistically significant difference between the groups in any laboratory parameter at admission. The comparison of laboratory parameters on Day 5 of treatment is shown in Table 2. On Day 5, Groups 2 and 3 had significantly lower lactate dehydrogenase (LDH), fibrinogen, D‐dimer, CRP, and procalcitonin levels compared with Group 1 (p = 0.04, 0.002, 0.05, 0.03, and 0.04, respectively). In the comparison between Groups 2 and 3, only fibrinogen was significantly lower in Group 3 (p = 0.02).
Table 1.
Comparison of laboratory parameters at admission between the groups
| Group 1 (n = 60), mean ± SD | Group 2 (n = 60), mean ± SD | Group 3 (n = 60), mean ± SD | p | |
|---|---|---|---|---|
| WBC (/µl) | 6150.2 ± 2440.1 | 5979.7 ± 2448.7 | 6684.4 ± 2840.4 | 0.3 |
| Lymphocytes (/µl) | 1410.1 ± 612.9 | 1321.7 ± 531.5 | 1401.9 ± 566.4 | 0.56 |
| Neutrophils (/µl) | 4120.4 ± 1876.3 | 4035 ± 1985.5 | 4728.1 ± 2695.1 | 0.25 |
| Eosinophils (/µl) | 45.8 ± 40.1 | 87 ± 35.5 | 37.8 ± 56.8 | 0.45 |
| Hemoglobin (g/l) | 14.1 ± 1.1 | 13.9 ± 1.9 | 14.1 ± 1.9 | 0.63 |
| Platelets (/µl) | 211 560.1 ± 62 149.2 | 2 056 000 ± 55 720.7 | 222 115.6 ± 92 976.7 | 0.39 |
| MPV (fl) | 10.4 ± 1.3 | 10.6 ± 0.8 | 10.3 ± 1.1 | 0.3 |
| AST (U/L) | 41.4 ± 25.4 | 36.8 ± 19.6 | 52.4 ± 106.8 | 0.42 |
| ALT (U/L) | 41.9 ± 24.1 | 37.7 ± 19.9 | 38.3 ± 43.1 | 0.87 |
| GGT (U/L) | 51.6 ± 46.1 | 67.3 ± 92.7 | 45.7 ± 59.5 | 0.28 |
| ALP (U/L) | 80.5 ± 41.2 | 90.2 ± 72.7 | 76.9 ± 35.3 | 0.36 |
| LDH (U/L) | 276.1 ± 83.4 | 281.1 ± 82.3 | 293.1 ± 172.6 | 0.71 |
| Total protein (g/l) | 64.5 ± 6.1 | 61.7 ± 5.6 | 65.3 ± 4.6 | 0.56 |
| Albumin (g/l) | 44.1 ± 6.1 | 43.1 ± 5.5 | 43.1 ± 4.5 | 0.63 |
| Total bilirubin (mg/dl) | 0.7 ± 0.5 | 0.6 ± 0.5 | 1.2 ± 3.5 | 0.38 |
| Direct bilirubin (mg/dl) | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.33 |
| Ferritin (ng/ml) | 410.5 ± 356.8 | 442.3 ± 468.9 | 345.1 ± 421.1 | 0.39 |
| Fibrinogen (ng/ml) | 460.4 ± 113.4 | 509.9 ± 119.8 | 458.8 ± 143.7 | 0.14 |
| D‐dimer (ng/ml) | 743.4 ± 410.1 | 769 ± 684.7 | 570.6 ± 532.5 | 0.21 |
| Troponin‐ı (ng/dl) | 2.3 ± 2.1 | 4.7 ± 6.5 | 2.7 ± 0.8 | 0.09 |
| INR | 1.1 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.1 | 0.16 |
| PT (s) | 13.8 ± 2 | 14.2 ± 2.7 | 13.9 ± 1.6 | 0.63 |
| aPTT (s) | 30.4 ± 4.2 | 30 ± 4.1 | 30.8 ± 3.6 | 0.46 |
| CRP (mg/l) | 45.4 ± 39.8 | 46.4 ± 35.5 | 43.3 ± 55.3 | 0.78 |
| Procalcitonin (ng/ml) | 0.7 ± 0.7 | 0.7 ± 0.9 | 1 ± 0.6 | 0.12 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; GGT, γ‐glutamyl transferase; INR, international normalized ratio; LDH, lactate dehydrogenase; MPV, mean platelet volume; PT, prothrombin time; WBC, white blood cells.
Table 2.
Comparison of laboratory parameters on Day 5 of treatment between the groups
| Group 1 (n = 60), mean ± SD | Group 2 (n = 60), mean ± SD | Group 3 (n = 60), mean ± SD | p | |
|---|---|---|---|---|
| WBC (/µl) | 8602. 3 ± 2676.9 | 8352.7 ± 2538.2 | 8895.1 ± 3867.8 | 0.51 |
| Lymphocytes (/µl) | 1102. 4 ± 874.2 | 1283.7 ± 857.6 | 1305.9 ± 733.3 | 0.85 |
| Neutrophils (/µl) | 6643.1 ± 2403.4 | 6485.7 ± 2110.2 | 7063.8 ± 3786.8 | 0.46 |
| Eosinophils (/µl) | 12.4 ± 20.1 | 11.7 ± 28.8 | 13.8 ± 26.1 | 0.76 |
| Hemoglobin (g/L) | 13.4 ± 1.1 | 13.1 ± 1.8 | 13.6 ± 2.1 | 0.52 |
| Platelets (/µl) | 268 920.1 ± 101 000 | 2 783 66.7 ± 96 432.1 | 281 750 ± 119 759.7 | 0.85 |
| MPV (fl) | 10.7 ± 0.7 | 10.8 ± 1.2 | 10.3 ± 0.8 | 0.09 |
| AST (U/L) | 31.4 ± 30.1 | 33.8 ± 32.7 | 28.4 ± 12.7 | 0.38 |
| ALT (U/L) | 58.4 ± 40.4 | 60.2 ± 44.7 | 45.9 ± 28.5 | 0.15 |
| GGT (U/L) | 72.4 ± 60.2 | 80.6 ± 96.2 | 52.1 ± 52.3 | 0.21 |
| ALP (U/L) | 71.4 ± 60.4 | 85.9 ± 58.9 | 66.8 ± 23.6 | 0.1 |
| LDH (U/L) | 301.4 ± 120.4 | 227.9 ± 60.2 | 269.8 ± 106.9 | 0.04a |
| Total protein (g/l) | 58.9 ± 10.4 | 61.9 ± 7.4 | 59.9 ± 6.3 | 0.45 |
| Albumin (g/l) | 39.7 ± 9.5 | 40.8 ± 6.6 | 39.6 ± 4.9 | 0.36 |
| Total bilirubin (mg/dl) | 0.6 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.3 | 0.61 |
| Direct bilirubin (mg/dl) | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.78 |
| Ferritin (ng/ml) | 410.4 ± 214.6 | 345.2 ± 355.7 | 289.2 ± 292.8 | 0.5 |
| Fibrinogen (ng/ml) | 510.4 ± 110.1 | 405.4 ± 109.4 | 332.4 ± 72.1 | 0.002a |
| 0.02b | ||||
| D‐dimer (ng/ml) | 678.3 ± 300.4 | 589.7 ± 527.1 | 476.4 ± 205.7 | 0.05a |
| Troponin‐ı (ng/dl) | 4.1 ± 3.2 | 3.3 ± 1.9 | 4.4 ± 5.5 | 0.21 |
| INR | 1.2 ± 0.9 | 1.1 ± 0.1 | 1.1 ± 0.3 | 0.31 |
| PT (s) | 14.4 ± 1.7 | 13.8 ± 1.5 | 14.1 ± 2.1 | 0.44 |
| aPTT (s) | 28.4 ± 4.4 | 27.8 ± 2.8 | 28.9 ± 3.1 | 0.36 |
| CRP (mg/l) | 30.4 ± 20.4 | 12.6 ± 11.3 | 16.8 ± 18.3 | 0.03a |
| Procalcitonin (ng/ml) | 0.8 ± 0.5 | 0.3 ± 0.4 | 0.4 ± 0.4 | 0.04a |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; GGT, γ‐glutamyl transferase; INR, international normalized ratio; LDH, lactate dehydrogenase; MPV, mean platelet volume; PT, prothrombin time; WBC, white blood cells.
Groups 2 and 3 versus Group 1.
Group 2 versus Group 3.
Table 3 shows the results of logistic regression analysis of ferritin, fibrinogen, D‐dimer, CRP, and LDH, and pulmonary function parameters, which were found to have prognostic value for COVID‐19 among patients in Groups 2 and 3. Fibrinogen and peak expiratory flow 25–75 (PEF25–75) levels showed significant positive change in Group 3 (p = 0.05, 0.001).
Table 3.
Regression analysis of laboratory and pulmonary function parameters in Group 2 (10 mg montelukast) and Group 3 (20 mg montelukast)
| Unstandardized B | Coefficients std. error | Standardized coefficient β | t | p | 95% CI, lower bound | 95% CI, upper bound | |
|---|---|---|---|---|---|---|---|
| Δ Ferritin | 0.000 | 0.000 | 0.102 | 1.075 | 0.284 | 0.000 | 0.001 |
| Δ Fibrinogen | 0.001 | 0.000 | 0.189 | 1.963 | 0.05 | 0.000 | 0.001 |
| Δ D‐dimer | 1.926e−5 | 0.000 | 0.02 | 0.251 | 0.803 | 0.000 | 0.000 |
| Δ CRP | −0.002 | 0.001 | −0.177 | −1.572 | 0.119 | −0.004 | 0.000 |
| Δ LDH | 0.000 | 0.000 | −0.034 | −0.359 | 0.721 | −0.001 | 0.001 |
| Δ FVC | 0.006 | 0.099 | 0.008 | 0.061 | 0.952 | −0.190 | 0.202 |
| Δ FEV1 | −0.006 | 0.136 | −0.007 | −0.041 | 0.967 | −0.275 | 0.264 |
| Δ PEF25–75 | 0.264 | 0.064 | 0.495 | 4.092 | 0.001 | 0.136 | 0.392 |
Abbreviations: Δ, difference between admission and Day 5 of treatment; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LDH, lactate dehydrogenase; PEF, peak expiratory flow.
3.2. Influence of montelukast treatment on pulmonary function parameters
A comparison of the groups' pulmonary function test results at admission and on Day 5 is shown in Figure 2. There was no significant difference between the groups in terms of FEV1, FVC, and PEF25–75 levels at admission (p = 0.604, 0.77, and 0.84, respectively). However, on Day 5 of treatment, only Group 3 showed significant improvement in FEV1, FVC, and PEF25–75 values compared with admission (p = 0.001 for all).
Figure 2.
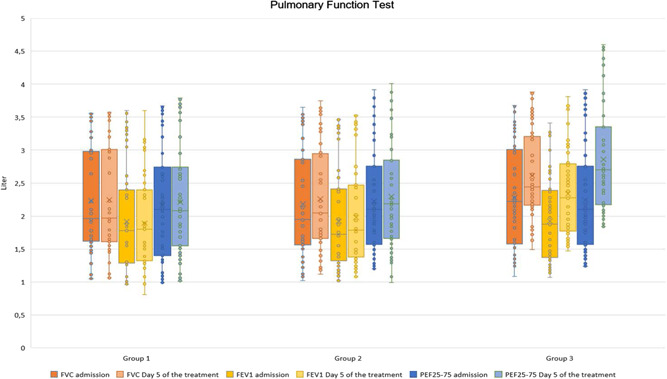
Comparison of pulmonary function parameters at admission and on Day 5 of treatment. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PEF25–75, peak expiratory flow 25–75
3.3. Influence of montelukast treatment on COVID‐19 severity
Evaluation of length of hospital stay and rates of MAS/respiratory failure and mortality in the study groups is shown in Table 4. The frequency of progression to MAS and respiratory failure was significantly lower in Groups 2 and 3 compared with Group 1 (p = 0.001). Four patients in Group 1 died (6.7%), while there was no mortality in Groups 2 and 3. Length of hospital stay did not differ significantly between Groups 2 and 3, but was significantly lower in both compared with Group 1 (p = 0.03).
Table 4.
Comparison of MAS/respiratory failure, mortality, and length of hospital stay between the groups
| Group 1 (n = 60) | Group 2 (n = 60) | Group 3 (n = 60) | p | |
|---|---|---|---|---|
| MAS or respiratory failure, n (%) | 8 (13.3) | 2 (3.3) | 1 (1.6) | 0.001a |
| Mortality, n (%) | 4 (6.7) | ‐ | ‐ | N/A |
| Length of hospital stay (days), mean ± SD | 11 ± 5.3 | 9.4 ± 2.1 | 9.3 ± 3.6 | 0.03 a |
Abbreviation: MAS, macrophage activation syndrome.
Groups 2 and 3 versus group 1
4. DISCUSSION
The results of our study showed that 10 and 20 mg montelukast used in addition to standard treatment caused a faster decrease in levels of LDH, fibrinogen, D‐dimer, CRP, and procalcitonin, parameters shown to have prognostic significance in COVID‐19. In addition, there was a greater decrease in fibrinogen level with 20 mg montelukast. Montelukast is known to have a bronchodilatory effect, and we also observed that 20 mg/day montelukast had a stronger bronchodilator effect than the 10 mg/day dosage used in daily practice. However, both doses of montelukast added to standard therapy had favorable effects on length of hospital stay, MAS, respiratory failure, and mortality.
SARS‐CoV‐2 is closely related to SARS‐CoV and MERS‐CoV, other coronaviruses that have caused epidemics with high morbidity and mortality in the past, and the number of people with confirmed COVID‐19 infections to date is reported to be over 230 million. 1 , 7
The observation of lymphopenia in the laboratory tests of most patients with COVID‐19 suggests that SARS‐CoV‐2 may primarily affect lymphocytes, especially T lymphocytes, as seen with SARS‐CoV. Virus particles that spread from the respiratory mucosa and infect other cells cause abnormal cytokine discharge in the body, which is referred to as a cytokine storm. 8 , 9 T lymphocyte damage also plays an important role in the development of cytokine storms. Nuclear factor‐κB (NF‐κB), which is overproduced in a cytokine storm, increases the synthesis of many proinflammatory cytokines, especially TNF‐α, IL‐1, IL‐2, IL‐6, and nitric oxide. 2 , 3 , 10 These cytokines increase vascular permeability, resulting in impaired tissue perfusion, endothelial damage, and microthrombus formation. Increased vascular permeability leads to fluid accumulation in the lung tissue and interstitial spaces, which manifests clinically as acute respiratory failure. Positive results suggesting that this clinical picture can be controlled with IL‐1 and IL‐6 antagonists have been reported.
Cellular SARS‐CoV‐2 infection/replication (inflammatory stimulation) leads to the release of arachidonic acid via the intracellular phospholipase A2 pathway and its subsequent conversion by 5‐lipoxygenase to leukotriene A4 (LTA4). 11 LTA4 leads to the production of leukotriene B4 and cysLTs (LTC4, LTD4, and LTE4), which bind to the respective leukotriene receptors and activate the NF‐κB pathway via the NF‐κB transcription factor. 12 Montelukast is a cysLT receptor 1 antagonist that blocks the binding of cysLTs, inhibits NF‐κB pathway activation, and reduces the production of proinflammatory mediators. 13
In addition, SARS‐CoV‐2 is known to enter cells by binding to human ACE2, an angiotensin‐converting enzyme (ACE) receptor, leading to severe pneumonia and thus increased mortality rates during infection. 14 The cough that may develop with ACE inhibition is caused by bradykinin elevation and its bronchoconstrictor effect, whereas the selective LTD4 antagonist montelukast has an inhibitory effect on bradykinin‐induced airway hypersensitivity. 15 Montelukast has been used in asthma patients for many years due to both its anti‐inflammatory and bronchodilatory properties. Due to its anti‐inflammatory effect on NF‐κB, its use has been found to have a neuroprotective effect in pregnant women with Zika virus. 16 , 17 In addition, in pediatric studies, positive results in exacerbations, cough, and disease remission were observed in children who developed bronchiolitis due to respiratory syncytial virus. 18
In our study, we detected no significant differences between the groups in laboratory and pulmonary function parameters on the day of admission, whereas LDH, fibrinogen, D‐dimer, CRP, and procalcitonin levels on Day 5 of treatment were significantly lower in patients who received montelukast compared with those who did not. This may be due to the anti‐inflammatory effect of montelukast sodium. In addition, the higher dose of montelukast only resulted in a greater decrease in fibrinogen, suggesting that the 10 mg/day dose may be as effective as 20 mg/day in terms of anti‐inflammatory effect. While there were no significant differences in the patients' pulmonary function tests at admission, only patients who received 20 mg/day of montelukast showed significant improvement in FEV1, FVC, and PEF25‐75 levels on Day 5 of treatment. This suggests that the increased bradykinin discharge in COVID‐19 patients may be more effectively antagonized at higher doses of montelukast, resulting in more efficient bronchodilator activity. Our logistic regression analysis between 10 and 20 mg/day montelukast sodium groups showed that patients who received 20 mg montelukast had higher PEF25–75, which more clearly reflects the situation in the small and middle airways, and a greater decrease in fibrinogen levels. In a previous study, we observed that fibrinogen was a better indicator of the condition of the airways than other proinflammatory cytokines. When our current findings are evaluated in correlation with our previous study, we conclude that high‐dose montelukast has better bronchodilator activity and that fibrinogen may be a useful guide in this respect. In the evaluation of hospital length of stay, MAS, and respiratory failure between the groups, we observed a lower frequency of MAS and respiratory failure and shorter hospital stays in both groups using montelukast, consistent with the previous data.
The main limitation of this study is the absence of long‐term data for the patients in our study groups and our inability to demonstrate the effectiveness of their treatments on laboratory parameters and pulmonary function tests in the longer term. However, we intend to also present the long‐term follow‐up of the patients in a later report. The limited number of patients was another limitation. When we started this study, a new SARS‐CoV‐2 variant still in the genetic sequencing stage emerged in place of the delta variant, which had been dominant in our region until that time. As different variants may have different effects on laboratory parameters and pulmonary function tests, we included only patients with the delta variant in our study, which limited our patient sample.
In conclusion, montelukast is known to have both anti‐inflammatory and bronchodilatory activity and is reported to be safe even at high doses. Its use as an adjunct therapy may prevent the development of MAS in COVID‐19 patients due to its anti‐inflammatory effects, while its bronchodilator effect at high doses may help minimize respiratory failure in these patients and reduce their length of hospital stay.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization, methodology, software, validation, and formal analysis: Buğra Kerget, Ferhan Kerget, Ömer Karaşahin. Investigation, resources, data curation: Ferhan Kerget, Buğra Kerget, Murat Aydın. Writing—original draft and writing—review and editing: Buğra Kerget. Visualization, supervision, and project administration: Buğra Kerget.
Kerget B, Kerget F, Aydın M, Karaşahin Ö. Effect of montelukast therapy on clinical course, pulmonary function, and mortality in patients with COVID‐19. J Med Virol. 2022;94:1950‐1958. 10.1002/jmv.27552
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Yuki K, Fujiogi M, Koutsogiannaki S. COVID‐19 pathophysiology: a review. Clin Immunol. 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kerget B, Kerget F, Aksakal A, Aşkın S, Sağlam L, Akgün M. Evaluation of alpha defensin, IL‐1 receptor antagonist, and IL‐18 levels in COVID‐19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J Med Virol. 2021;93(4):2090‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kerget B, Kerget F, Koçak AO, et al. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID‐19? Lung. 2020;198(5):777‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu A, Chik S, Chan A, Li Z, Tsang K, Li W. Anti‐inflammatory effects of high‐dose montelukast in an animal model of acute asthma. Clin Exp Allergy. 2003;33(3):359‐366. [DOI] [PubMed] [Google Scholar]
- 5. Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J. 1999;14(1):12‐18. [DOI] [PubMed] [Google Scholar]
- 6. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70‐e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brodeur A, Gray D, Islam A, Bhuiyan S. A literature review of the economics of COVID‐19. J Econ Surv. 2021;35(4):1007‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He R, Lu Z, Zhang L, et al. The clinical course and its correlated immune status in COVID‐19 pneumonia. J Clin Virol. 2020;127:104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaninov N. In the eye of the COVID‐19 cytokine storm. Nat Rev Immunol. 2020;20(5):277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kircheis R, Haasbach E, Lueftenegger D, Heyken WT, Ocker M, Planz O. NF‐κB pathway as a potential target for treatment of critical stage COVID‐19 patients. Front Immunol. 2020;11:3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanghai N, Tranmer GK. Taming the cytokine storm: repurposing montelukast for the attenuation and prophylaxis of severe COVID‐19 symptoms. Drug Discovery Today. 2020;25:2076‐2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wisastra R, Dekker FJ. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers. 2014;6(3):1500‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu G‐L, Wei E‐Q, Zhang S‐H, et al. Montelukast, a cysteinyl leukotriene receptor‐1 antagonist, dose‐and time‐dependently protects against focal cerebral ischemia in mice. Pharmacology. 2005;73(1):31‐40. [DOI] [PubMed] [Google Scholar]
- 14. Davidson AM, Wysocki J, Batlle D. Interaction of SARS‐CoV‐2 and other coronavirus with ACE (angiotensin‐converting enzyme)−2 as their main receptor: therapeutic implications. Hypertension. 2020;76(5):1339‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fidan C, Aydoğdu A. As a potential treatment of COVID‐19: montelukast. Med Hypotheses. 2020;142:109828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JY, Nguyen TTN, Myoung J. Zika virus‐encoded NS2A and NS4A strongly downregulate NF‐κB promoter activity. J Microbiol Biotechnol. 2020;30(11):1651‐1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Li Y, Wang X, Zou P. Montelukast, an anti‐asthmatic drug, inhibits zika virus infection by disrupting viral integrity. Front Microbiol. 2020;10:3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bisgaard H. A randomized trial of montelukast in respiratory syncytial virus postbronchiolitis. Am J Respir Crit Care Med. 2003;167(3):379‐383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


