Abstract
Background and Aims
Patients develop breakthrough COVID‐19 infection despite vaccination. The aim of this study was to identify outcomes in patients with cirrhosis who developed postvaccination COVID‐19.
Methods
We performed a retrospective cohort study among US veterans with cirrhosis and postvaccination or unvaccinated COVID‐19. Patients were considered fully vaccinated if COVID‐19 was diagnosed 14 days after the second dose of either the Pfizer BNT162b2, the Moderna 1273‐mRNA, or the single‐dose Janssen Ad.26.COV2.S vaccines and partially vaccinated if COVID‐19 was diagnosed 7 days after the first dose of any vaccine but prior to full vaccination. We investigated the association of postvaccination COVID‐19 with mortality.
Results
We identified 3242 unvaccinated and 254 postvaccination COVID‐19 patients with cirrhosis (82 after full and 172 after partial vaccination). In a multivariable analysis of a 1:2 propensity‐matched cohort including vaccinated (n = 254) and unvaccinated (n = 508) participants, postvaccination COVID‐19 was associated with reduced risk of death (adjusted HR [aHR], 0.21; 95% CI, 0.11–0.42).
The reduction was observed after both full (aHR, 0.22; 95% CI, 0.08–0.63) and partial (aHR, 0.19; 95% CI, 0.07–0.54) vaccination, following the 1273‐mRNA (aHR, 0.12; 95% CI 0.04–0.37) and BNT162b2 (aHR, 0.27; 95% CI, 0.10–0.71) vaccines and among patients with compensated (aHR, 0.19; 95% CI, 0.08–0.45) and decompensated (aHR, 0.27; 95% CI, 0.08–0.90) cirrhosis. Findings were consistent in a sensitivity analysis restricted to participants who developed COVID‐19 after vaccine availability.
Conclusions
Though patients with cirrhosis can develop breakthrough COVID‐19 after full or partial vaccination, these infections are associated with reduced mortality.
Abbreviations
- aHR
adjusted HR
- AUDIT‐C
Alcohol Use Disorders Identification Test–Concise
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CirCom
Cirrhosis Comorbidity Index
- CTP
Child‐Turcotte‐Pugh
- eCTP
electronic CTP
- IQR
interquartile range
- MELD‐Na
Model for End‐Stage Liver Disease–Sodium
- PS
propensity score
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- VA
Veterans Administration
- VOCAL
Veterans Outcomes and Costs Associated with Liver Disease
INTRODUCTION
The availability of effective mRNA and viral vector–based vaccines for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has contributed to a reduction in the COVID‐19 pandemic in countries with a high uptake of vaccination. While receipt of an mRNA‐based COVID‐19 vaccine was associated with a 66.8% reduction in COVID‐19 infection after 28 days of the first dose of an mRNA vaccine in patients with cirrhosis, this was lower than observed in the general population.[ 1 ] Therefore, breakthrough infections are being reported, even among fully vaccinated persons with cirrhosis.
Most recently, the increase in variants, particularly the B.1.617.2 (delta) variant, has been described in patients with postvaccination breakthrough infections. While the clinical course and mortality of COVID‐19 in unvaccinated patients have been well described, there are limited data on postvaccination COVID‐19.[ 2 , 3 ]
The aim of this study was to better understand the clinical characteristics and mortality of patients with cirrhosis who develop breakthrough COVID‐19 after vaccination with either the Pfizer BNT162b2, the Moderna 1273‐mRNA, or the Janssen Ad.26.COV2.S vaccine compared to a patients with unvaccinated COVID‐19.
PARTICIPANTS AND METHODS
Study design
This study was conducted in the Veterans Health System using national data extracted from the Veterans Outcomes and Costs Associated with Liver Disease (VOCAL) cohort, which consists of over 120,000 veterans with cirrhosis. The assembly of this cohort has been described.[ 4 , 5 ] In response to the SARS‐CoV‐2 pandemic, the Veterans Administration (VA) created the national VA COVID‐19 Shared Data Resource using case definitions that were validated across the system and including all veterans with a confirmed laboratory diagnosis of SARS‐CoV‐2 infection, both those tested within the VA and outside the VA. Institutional review boards at all participating VA health systems approved the protocol and waived the need for informed consent.
Inclusion and exclusion criteria
Eligibility criteria included all patients with cirrhosis in the VOCAL cohort aged 18 years or older diagnosed with SARS‐CoV2 infection based on a positive polymerase chain reaction (PCR) between March 1, 2020, and June 1, 2021. Patients who received a liver transplant prior to SARS‐CoV‐2 infection were excluded.
Variables
Patients in the VOCAL cohort who received either the BNT162b2 mRNA, the 1273‐mRNA, or the Ad.26.COV2.S vaccine prior to SARS‐CoV‐2 infection were classified as postvaccination COVID‐19 (after full or partial vaccination) or unvaccinated COVID‐19, based on the following criteria.
Patients were considered fully vaccinated if COVID‐19 was diagnosed more than 14 days after the second dose of the BNT162b2 mRNA or the 1273‐mRNA vaccine or after the single dose of the Ad.26.COV2.S vaccine,[ 6 ] partially vaccinated if COVID‐19 was diagnosed 7 days after the first dose of a vaccine but prior to the date of full vaccination, and unvaccinated if COVID‐19 was diagnosed prior to or within 7 days of the first dose of a vaccine. The cutoff for partial vaccination was chosen because of data that suggest an antibody response and reduced SARS‐CoV‐2 infection as early as 7 days after the first dose.[ 7 ]
The baseline was defined as the date of the first positive COVID‐19 PCR obtained by nasal swab. Patients were considered to have a new infection if they developed a positive COVID‐19 PCR 90 days after the prior infection, with at least one intervening negative test. Each episode of SARS‐CoV‐2 infection was analyzed as a separate event.
Data on demographics (age, sex, race/ethnicity) were collected from the Corporate Data Warehouse (CDW). Participants were drawn from over 130 VA locations through the United States and categorized into one of six locations (Northeast, Southeast, Midwest, South, Northwest, and Southwest). Comorbidities were assessed using the previously validated Cirrhosis Comorbidity Index (CirCom),[ 8 ] and this score incorporates comorbidities that are associated with increased risk for COVID‐19, including chronic kidney disease and cancer. Severity of liver disease was assessed using the electronic Child‐Turcotte‐Pugh (eCTP) score and Model for End‐Stage Liver Disease–Sodium (MELD‐Na).[ 9 ] Alcohol‐associated cirrhosis was defined using International Classification of Diseases codes, as previously described and validated in veterans.[ 10 ]
We obtained body mass index (BMI), Alcohol Use Disorders Identification Test–Concise (AUDIT‐C) scores, and tobacco use, classified as current use, former use, or lifetime nonuse, from the CDW, closest to the baseline date.
Laboratory values were obtained closest to baseline (date of the positive COVID‐19 PCR). For hospitalized patients who did not have labs drawn between baseline and hospitalization, the labs on the date of hospital admission were used.
Outcomes
The primary outcome was death due to any cause within 60 days of diagnosis of SARS‐CoV‐2 infection. Secondary outcomes included COVID‐19‐related death, hospitalization for any reason (7 days before or up to 60 days after first positive COVID‐19 PCR), COVID‐19‐related hospitalization, and need for mechanical ventilation (7 days before or up to 60 days after first positive COVID‐19 PCR). We defined death within 60 days of COVID‐19 diagnosis to avoid inclusion of delayed and unrelated deaths, after recovery from COVID‐19.
Causes of death and hospitalization were abstracted from the inpatient medical records by two reviewers. The cause of death or hospitalization was defined as COVID‐19 if the patient was admitted with symptoms of SARS‐CoV‐2 infection and the hospital course and progression was consistent with a clinical course of COVID‐19, and it was defined as cirrhosis‐related if hospitalization or death was due to symptoms of hepatic decompensation or HCC and unrelated to symptomatic SARS‐CoV‐2 infection. Patients without symptomatic COVID‐19 or cirrhosis‐related complications, hospitalized for unrelated reasons such as congestive heart failure or stroke, were classified as unrelated to COVID‐19 or cirrhosis.
Vaccination status was not disclosed to the reviewers, and they were instructed to review the hospital discharge/death summary and clinical notes during the period of hospitalization. However, the process was not completely blinded, particularly for patients who developed COVID‐19 prior to vaccine availability. Hospitalization and mechanical ventilation from 7 days prior to the first positive PCR were captured as patients with COVID‐19 could be diagnosed shortly after hospitalization or initiation of mechanical ventilation.
Statistical analysis
We did propensity score (PS) matching to ensure comparability of the postvaccine and unvaccinated patients with COVID‐19.
The PSs were estimated using logistic regression as the predicted probability of developing postvaccination COVID‐19 by the patient’s baseline characteristics including age, sex, race, alcohol as etiology of liver disease, BMI at baseline, smoking history, AUDIT‐C, CirCom, eCTP, and MELD‐Na. Included in this PS matching were exact matching for age, sex, and race.
We then used the Greedy method to select the most closely matched neighbor among the possible matched patients. The two groups were evaluated before and after PS matching, and covariate balance was assessed using the standardized mean differences, with comparisons presented using Love plots. Standardized differences of 0.1 or less in variables between the two groups were considered acceptable matching.
Descriptive statistics were compared between postvaccinated and unvaccinated COVID‐19 groups for both matched and full samples, and p values were calculated using the Wilcoxon signed‐rank test comparing medians of continuous variables or chi‐squared tests for binary and categorical variables.
Cox proportional hazards models were fit for time to death after COVID‐19 and time to COVID‐19‐related death.
Unadjusted and adjusted HRs (aHRs) controlling for potential baseline confounders were estimated, and cumulative incidence curves were estimated for the two groups. Patients were censored at the end of the study (June 1, 2021).
A sensitivity analysis using different cutoff dates for partial vaccination, by changing the definition of onset of partial vaccination from 7 days after the first dose of mRNA vaccine to 14 and 21 days, was performed. We performed an additional sensitivity analysis that restricted analysis by excluding participants who developed COVID‐19 before vaccine availability. We also performed a sensitivity analysis by adjusting for prior COVID‐19 infection.
In addition, we performed a stratified analysis among patients who received an mRNA vaccine, to identify differences between the BNT162b2 mRNA and the 1273‐mRNA vaccines and their associations with overall and COVID‐19‐related mortality. A subgroup analysis for postvaccination COVID‐19 following the Ad.26.COV2.S vaccine was not performed due to a low number of events. Stratified analysis was also performed among patients with compensated and decompensated cirrhosis.
Statistical significance was defined as p < 0.05. Statistical analysis was performed using SAS 4.9 (SAS Inc.).
RESULTS
Baseline characteristics
A total of 3242 participants with unvaccinated and 254 with postvaccination COVID‐19 (82 after full and 172 after partial vaccination) were identified (Figure 1).
FIGURE 1.
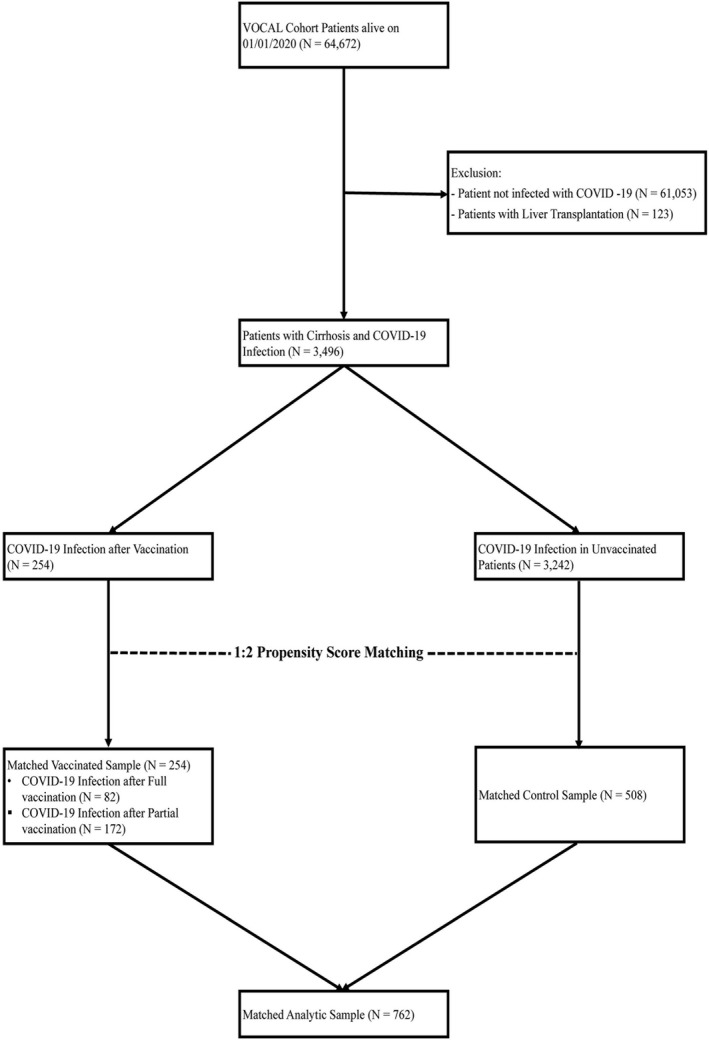
Study flowchart
The 254 participants with postvaccination COVID‐19 were matched to 508 with unvaccinated COVID‐19. The median age of the PS‐matched cohort was 63.8 years (interquartile range [IQR], 10.4) in the postvaccination and 64.2 years (IQR, 10.0) in the unvaccinated COVID‐19 cohort. Participants were predominantly male (n = 832, 96.1%) and White (n = 423, 55.5%), consistent with a veteran population. A higher proportion of patients with postvaccination COVID‐19 was from the Northeast (25.6 vs. 13.4%, p = 0.001), while a higher proportion of unvaccinated COVID‐19 was from the Southeast (17.9 vs. 13.0%, p = 0.001) and the Midwest (28.2 vs. 22.4%, p = 0.001). As expected, due to their later occurrence during the pandemic, a greater number of patients with postvaccination COVID‐19 had prior COVID‐19 (n = 38, 15%) compared to unvaccinated COVID‐19 (n = 11, 2.2%, p < 0.0001). Though patients were matched on the CirCom and not on individual comorbidities, the two groups were similar in terms of specific comorbidities such as HIV, cancer, and chronic kidney disease; however, patients who had a nonliver solid organ transplant were more likely to have postvaccination COVID‐19 (4.3 vs. 1.8%, p = 0.03).
Both groups were otherwise well balanced after PS matching, with no differences in median age, sex, race/ethnicity, BMI, AUDIT‐C scores, active tobacco use, CirCom, CTP scores, or baseline labs (Table 1; Figure S1).
TABLE 1.
Descriptive statistics for study patients
| Variable | Propensity‐matched sample | ||
|---|---|---|---|
| postvaccination COVID‐19 | Unvaccinated COVID‐19 | p | |
| (n = 254) | (n = 508) | ||
| Type of vaccination, n (%) | – | ||
| Janssen Ad.26.COV2.S vaccine | 7 (2.8%) | – | |
| Pfizer BNT162b2 mRNA vaccine | 126 (47.6%) | – | |
| Moderna 1273 mRNA vaccine | 121 (47.6%) | – | |
| Sex, n (%) | 1.0000 | ||
| Male | 244 (96.1%) | 488 (96.1%) | |
| Female | 10 (3.9%) | 20 (3.9%) | |
| Age (years), median (IQR) | 63.8 (10.4) | 64.2 (10.0) | 0.5899 |
| Age group, n (%) | 1.0000 | ||
| <50 | 21 (8.3%) | 42 (8.3%) | |
| 50–59.9 | 55 (21.7%) | 110 (21.7%) | |
| 60–69.9 | 126 (49.6%) | 252 (49.6%) | |
| 70–85 | 52 (20.5%) | 104 (20.5%) | |
| ≥ 85 | – | – | |
| Race/ethnicity, n (%) | 0.9177 | ||
| White | 141 (55.5%) | 282 (55.5%) | |
| Black | 68 (26.8%) | 148 (29.1%) | |
| Hispanic/Latino | 25 (9.8%) | 37 (7.3%) | |
| Other | 16 (6.3%) | 36 (7.1%) | |
| Unknown | 4 (1.6%) | 5 (1.0%) | |
| Location, n (%) | 0.0015 | ||
| Northeast | 65 (25.6%) | 68 (13.4%) | |
| Southeast | 33 (13.0%) | 91 (17.9%) | |
| Midwest | 57 (22.4%) | 143 (28.2%) | |
| South | 47 (18.5%) | 106 (20.9%) | |
| Northwest | 16 (6.3%) | 32 (6.3%) | |
| Southwest | 36 (14.2%) | 68 (13.4%) | |
| BMI, median (IQR) | 29.9 (8.7) | 29.5 (8.9) | 0.6450 |
| BMI class, n (%) | 0.9256 | ||
| Overweight | 84 (33.1%) | 185 (36.4%) | |
| Class 1 obesity, BMI 30.0–34.9 | 62 (24.4%) | 109 (21.5%) | |
| Class 2 obesity, BMI 35.0–39.9 | 40 (15.7%) | 84 (16.5%) | |
| Class 3 obesity, BMI > 40.0 | 25 (9.8%) | 46 (9.1%) | |
| AUDIT‐C score, n (%) | 0.3885 | ||
| Low | 212 (83.5%) | 411 (80.9%) | |
| High | 42 (16.5%) | 97 (19.1%) | |
| Diabetes, n (%) | 161 (63.4%) | 324 (63.8%) | 0.9152 |
| Tobacco use, n (%) | 0.9276 | ||
| Current smoker | 59 (23.2%) | 111 (21.9%) | |
| Former smoker | 78 (30.7%) | 167 (32.9%) | |
| Never smoker | 112 (44.1%) | 219 (43.1%) | |
| Alcohol‐associated cirrhosis, n (%) | 110 (43.3%) | 222 (43.7%) | 0.177 |
| Cirrhosis comorbidity, n (%) | 0.6346 | ||
| 0 | 15 (5.9%) | 30 (5.9%) | |
| 1+0 | 53 (20.9%) | 93 (18.3%) | |
| 1+1 | 55 (21.7%) | 120 (23.6%) | |
| 3+0 | 10 (3.9%) | 25 (4.9%) | |
| 3+1 | 121 (47.6%) | 234 (46.1%) | |
| 5+0 | – | 2 (0.4%) | |
| 5+1 | – | 4 (0.8%) | |
| eCTP class, n (%) | 0.9370 | ||
| A | 195 (76.8%) | 394 (77.6%) | |
| B | 55 (21.7%) | 105 (20.7%) | |
| C | 4 (1.6%) | 9 (1.8%) | |
| Lab results at diagnosis of COVID‐19, median (IQR) | |||
| Alanine aminotransferase (IU/ml) | 42.2 (48.0) | 41.0 (51.9) | 0.6806 |
| Platelet count (×10E9/L) | 150.7 (89.8) | 152.5 (104.9) | 0.7194 |
| Creatinine (mg/dL) | 1.0 (0.5) | 1.0 (0.4) | 0.2835 |
| Total bilirubin (mg/dL) | 0.8 (0.6) | 0.8 (0.7) | 0.7164 |
| International normalized ratio | 1.1 (0.3) | 1.1 (0.3) | 0.8397 |
| MELD‐Na score | 9.0 (7.0) | 8.0 (6.0) | 0.1378 |
| Treatment of COVID‐19, n (%) | |||
| Dexamethasone, n (%) | 42 (16.5%) | 84 (16.5%) | 1.0000 |
| Hydroxychloroquine, n (%) | 3 (1.2%) | 7 (1.4%) | 0.8219 |
| Remdesivir, n (%) | 33 (13.0%) | 66 (13.0%) | 1.0000 |
| Tocilizumab, n (%) | 3 (1.2%) | 4 (0.8%) | 0.5913 |
| Cause of death, n (%) | <0.0001 | ||
| COVID‐19 | 7 (2.8%) | 57 (11.2%) | |
| Cirrhosis | 0 (0.0%) | 15 (3.0%) | |
| Others | 2 (0.8%) | 6 (1.2%) | |
| Cause of hospitalization, n (%) | 0.0163 | ||
| COVID‐19 | 50 (19.7%) | 117 (23.0%) | |
| Cirrhosis | 16 (6.3%) | 21 (4.1%) | |
| Others | 41 (16.1%) | 48 (9.4%) | |
| Prior COVID‐19 infection, n (%) | 38 (15.0%) | 11 (2.2%) | <0.0001 |
| Chronic kidney disease, n (%) | 84 (33.1%) | 169 (33.3%) | 0.9566 |
| HIV, n (%) | 7 (2.8%) | 11 (2.2%) | 0.6128 |
| Cancer, n (%) | 107 (42.1%) | 205 (40.2%) | 0.6022 |
| Solid Organ (nonliver) Transplantation, n (%) | 11 (4.3%) | 9 (1.8%) | 0.0285 |
Bold indicates significance.
Postvaccination COVID‐19
Of the 254 who developed postvaccination COVID‐19, 49.6% developed after the BNT162b2 mRNA, 47.6% after the 1273‐mRNA, and 2.8% after the Ad.26.COV2.S vaccine. The lower number of postvaccination COVID‐19 after the Ad.26.COV2.S vaccine was consistent with low usage of the Ad.26.COV2.S vaccine among veterans.
The median time to postvaccination COVID‐19 after full vaccination was 27.0 days (IQR, 25.0) after the second dose of an mRNA or the first dose of the Ad.26.COV2.S vaccine (Table 2). Of the 172 participants who developed COVID‐19 after partial vaccination, 38 (22.1%) developed between 7 and 14 days, 42 (24.4%) between 14 and 21 days, 38 (22.1%) between 21 and 28 days, and 54 (31.4%) more than 28 days after the first dose of the vaccine. Figure 2 shows the number of patients who developed postvaccination and the unvaccinated cohort over time, in the overall (panel A) and PS‐matched (panel B) cohorts. The number of patients with unvaccinated COVID‐19 exceeded that with postvaccination COVID‐19 throughout the study period.
TABLE 2.
Outcomes among patients with postvaccination and unvaccinated COVID‐19
| Variable | Matched | ||
|---|---|---|---|
| Postvaccination | Unvaccinated | p | |
| (n = 254) | (n = 508) | ||
| Event, n (%) | |||
| Death within 60 days of COVID‐19 | 9 (3.5%) | 78 (15.4%) | <0.0001 |
| COVID‐19‐related death | 7 (2.8%) | 57 (11.2%) | <0.0001 |
| Hospitalization | 107 (42.1%) | 186 (36.6%) | 0.1404 |
| COVID‐19‐related hospitalization | 50 (19.7%) | 117 (23.0%) | 0.2925 |
| Mechanical ventilation | 4 (1.6%) | 29 (5.7%) | 0.0082 |
| Time to event, median (IQR) | |||
| Death within 60 days of COVID‐19 | 16.0 (26.0) | 16.0 (19.0) | 0.9346 |
| COVID‐19‐related death | 16.0 (14.0) | 16.0 (11.0) | 0.9350 |
| Hospitalization | 7.0 (1.0) | 7.0 (2.0) | 0.8331 |
| COVID‐19‐related hospitalization | 7.0 (1.0) | 7.0 (2.0) | 0.0689 |
| Mechanical ventilation | 13.0 (10.0) | 12.0 (11.0) | 0.9492 |
Bold indicates significance.
FIGURE 2.
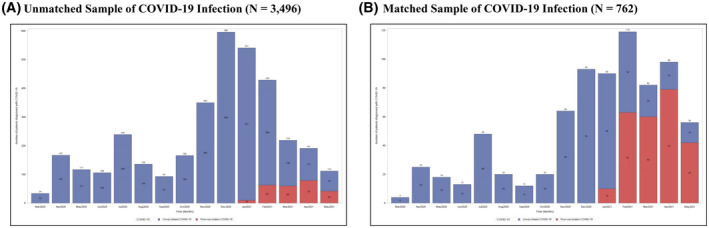
Number of patients diagnosed over time with unvaccinated (blue) and postvaccination COVID‐19 (red) in the (A) overall cohort and (B) PS‐ matched cohort
Among the 508 controls, 504 were unvaccinated at the time of COVID‐19 diagnosis, and 4 were diagnosed within 7 days of the receipt of the first vaccine dose.
The most common COVID‐19‐specific therapies used were dexamethasone (42 [16.5%] postvaccination and 84 [16.5%] unvaccinated COVID‐19, p > 0.99), while the second most common was remdesivir (33 [13.0%] postvaccination and 66 [13.0%] unvaccinated COVID‐19, p > 0.99).
Outcomes
Association of postvaccination COVID‐19 with overall and COVID‐19‐related death
The unadjusted overall mortality within 60 days of diagnosis of COVID‐19 was lower in the postvaccination compared to the unvaccinated PS‐matched cohort (9 [3.5%] vs. 78 [15.4%], p < 0.0001) (Table 2). Similarly, the unadjusted COVID‐19‐related death was also lower in the postvaccination group compared to unvaccinated patients (7 [2.8%] vs. 57 [11.2%], p < 0.0001).
On multivariable Cox regression, older age (aHR, 1.05; 95% CI, 1.02–1.08; p = 0.0002) and use of dexamethasone (aHR, 4.43; 95% CI, 2.27–8.65; p < 0.0001), but not patient location, diabetes mellitus, AUDIT‐C score, alcohol‐associated cirrhosis, CTP class, or MELD‐Na, were associated with mortality within 60 days of COVID‐19 (Table 3). After adjusting for potential confounders, postvaccination COVID‐19 was associated with reduced overall death within 60 days (aHR, 0.21; 95% CI, 0.10–0.42; p < 0.0001; Figure 3).
TABLE 3.
Multivariable HRs for the risk of overall or COVID‐19‐related death in postvaccination versus unvaccinated COVID‐19
| Variable | Overall death | COVID‐19‐related death | ||
|---|---|---|---|---|
| aHR (95% CI) | p | aHR (95% CI) | p | |
| Number of patients | 762 | – | 762 | – |
| Number of events | 87 | – | 64 | – |
| Group | ||||
| Control | REF | REF | ||
| Vaccine | 0.21 (0.10–0.42) | <0.0001 | 0.23 (0.10–0.53) | 0.0005 |
| Location, n (%) | ||||
| Northeast | REF | REF | ||
| Southeast | 1.22 (0.56–2.65) | 0.6128 | 1.55 (0.61–3.93) | 0.3524 |
| Midwest | 1.28 (0.62–2.62) | 0.5038 | 1.47 (0.61–3.53) | 0.3863 |
| South | 0.64 (0.27–1.49) | 0.2974 | 0.83 (0.30–2.30) | 0.7158 |
| Northwest | 1.97 (0.79–4.90) | 0.1435 | 2.00 (0.66–6.04) | 0.2209 |
| Southwest | 1.56 (0.71–3.44) | 0.2671 | 2.23 (0.90–5.53) | 0.0841 |
| Age | 1.05 (1.02–1.08) | 0.0002 | 1.06 (1.03–1.09) | 0.0003 |
| BMI | 1.00 (0.97–1.03) | 0.7580 | 1.01 (0.98–1.04) | 0.5361 |
| Diabetes | ||||
| No | REF | REF | ||
| Yes | 1.07 (0.65–1.74) | 0.7991 | 0.90 (0.52–1.55) | 0.7084 |
| Alcohol | ||||
| No | REF | REF | ||
| Yes | 1.05 (0.63–1.75) | 0.8453 | 1.16 (0.66–2.04) | 0.6173 |
| AUDIT‐C score | ||||
| Low | REF | REF | ||
| High | 1.08 (0.54–2.15) | 0.8289 | 1.05 (0.47–2.33) | 0.9112 |
| eCTP | ||||
| A | REF | REF | ||
| B | 1.18 (0.67–2.09) | 0.5588 | 0.99 (0.51–1.92) | 0.9689 |
| C | 1.08 (0.19–6.10) | 0.9288 | N/A | N/A |
| Dexamethasone | ||||
| No | REF | REF | ||
| Yes | 4.43 (2.27–8.65) | <0.0001 | 3.79 (1.68–8.57) | 0.0014 |
| Remdesivir | ||||
| No | REF | REF | ||
| Yes | 0.62 (0.29–1.33) | 0.2229 | 1.13 (0.47–2.71) | 0.7836 |
| MELD‐Na | 1.03 (0.99–1.08) | 0.1592 | 1.03 (0.98–1.09) | 0.2445 |
Bold indicates significance.
Abbreviations: N/A, not available; REF, reference.
FIGURE 3.
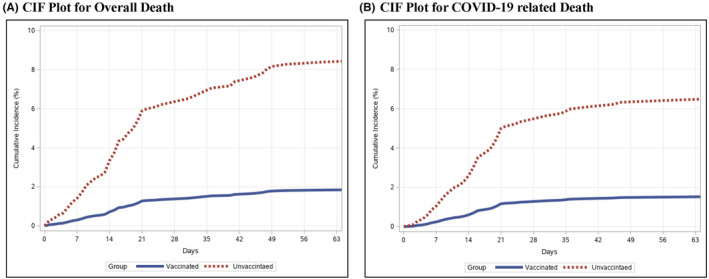
Adjusted time from COVID‐19 diagnosis to death (A) and COVID‐19‐related death (B) among patients with cirrhosis and postvaccination and unvaccinated COVID‐19 infection. Abbreviation: CIF, cumulative incidence function
This association of postvaccination COVID‐19 and reduced mortality was observed after both full (aHR, 0.22; 95% CI, 0.08–0.63; p = 0.005) and partial (aHR, 0.19; 95% CI, 0.07–0.54; p = 0.002) vaccination (Table S1).
Predictors of COVID‐19‐related death were similar to those for overall death and included older age (aHR, 1.06; 95% CI, 1.03–1.09; p = 0.0003) and treatment with dexamethasone (aHR, 3.79; 95% CI, 1.68–8.57; p = 0.001) (Table 3). After adjusting for potential confounders, postvaccination COVID‐19 was associated with reduced COVID‐19‐related death (aHR, 0.23; 95% CI, 0.10–0.53; p = 0.001; Figure 3). This association, too, was observed after both full (aHR, 0.24; 95% CI, 0.08–0.73; p = 0.01) and partial (aHR, 0.26; 95% CI, 0.08–0.80; p = 0.02) vaccination (Table S1).
Association of postvaccination COVID‐19 with hospitalization and mechanical ventilation
The unadjusted rates of hospitalization (107 [42.1%] in postvaccination vs. 186 [36.6%] in unvaccinated COVID‐19, p = 0.14) and COVID‐19‐related hospitalization (50 [19.7%] in postvaccination vs. 117 [23.0%] in unvaccinated COVID‐19, p = 0.29) were similar (Table 2). On multivariable Cox regression, predictors of COVID‐19‐related hospitalization included treatment with dexamethasone (aHR, 2.15; 95% CI, 1.20–3.85; p = 0.01) and remdesivir (aHR, 4.75; 95% CI, 2.67–8.47; p < 0.0001). After adjusting or potential confounders, postvaccination COVID‐19 was not associated with COVID‐19‐related hospitalization (aHR, 0.88; 95% CI, 0.66–1.18; p = 0.41) (Table S2).
The unadjusted rates of mechanical ventilation were lower in postvaccination COVID‐19 compared to unvaccinated participants (4 [1.6%] vs. 29 [5.7%], p < 0.01) (Table 2).
On multivariable Cox regression, the use of dexamethasone was not associated with mechanical ventilation (aHR, 4.76; 95% CI, 0.88–25.66; p = 0.07). Postvaccination COVID‐19 was associated with reduced need for mechanical ventilation within 60 days (aHR, 0.33; 95% CI, 0.11–0.96; p = 0.04) (Table S2).
Stratified analysis comparing postvaccination COVID‐19 following BNT162b2 mRNA and 1273‐mRNA vaccines with overall and COVID‐19‐related death
We performed a stratified analysis among patients who received an mRNA vaccine, to identify differences in mortality between postvaccination COVID‐19 following the BNT162b2 mRNA and 1273‐mRNA vaccines. Postvaccination COVID‐19 following 1273‐mRNA was associated with lower overall (aHR, 0.12; 95% CI, 0.04–0.37; p = 0.0002) and COVID‐19‐related (aHR, 0.15; 95% CI, 0.03–0.61; p = 0.01) mortality. Postvaccination COVID‐19 following the BNT162b2 mRNA vaccine was also associated with a reduction in overall (aHR, 0.27; 95% CI, 0.10–0.71; p = 0.01) and COVID‐19‐related (aHR, 0.27; 95% CI, 0.09–0.80; p = 0.02) mortality (Table S3) However, the differences between the two mRNA vaccines were not statistically significant.
Stratified analysis comparing postvaccination COVID‐19 with overall and COVID‐19‐related death in compensated and decompensated cirrhosis
We analyzed the association of postvaccination COVID‐19 and death, stratified by compensated versus decompensated liver disease. Among patients with compensated cirrhosis, postvaccination COVID‐19 was associated with a reduction in hazard of death (aHR, 0.19; 95% CI, 0.08–0.45; p = 0.0001) and COVID‐19‐related death (aHR, 0.16; 95% CI, 0.06–0.46; p = 0.0001). Among patients with decompensated cirrhosis, postvaccination COVID‐19 was associated with a reduction in hazard of death (aHR, 0.27; 95% CI, 0.08–0.90; p = 0.03) but not COVID‐19‐related death (aHR, 0.51; 95% CI, 0.14–1.88; p = 0.31) (Table S4).
Sensitivity analysis
We performed a sensitivity analysis by restricting inclusion to participants who developed COVID‐19 after the availability of vaccines. Because the vaccines were available on December 18, 2020, the earliest date of postvaccination COVID‐19 after partial vaccination was December 25, 2020, which was selected as the start date for the sensitivity analysis. After adjusting for potential confounders, postvaccination COVID‐19 was associated with reduced overall (aHR, 0.25; 95% CI, 0.11–0.59; p = 0.001) and COVID‐19‐related (aHR, 0.33; 95% CI, 0.13–0.85; p = 0.02) death (Table S5). Similar to the findings in the primary analysis, the association of postvaccination COVID‐19 and reduced mortality was observed after both full (aHR, 0.25; 95% CI, 0.08–0.78; p = 0.001) and partial (aHR, 0.33; 95% CI, 0.13–0.85; p = 0.02) vaccination (Table S6).
To account for patients who developed reinfection after recovery from prior SARS‐CoV‐2 infection, we performed a sensitivity analysis, adjusting for this variable.
Prior SARS‐CoV‐2 infection was not associated with mortality (aHR, 0.23; 95% CI, 0.03–1.75; p = 0.15). A multivariable analysis with inclusion of prior COVID‐19 in the model showed similar associations noted in primary analysis. Postvaccination COVID‐19 remained associated with overall (aHR, 0.24; 95% CI, 0.12–0.50; p = 0.0001) and COVID‐19‐related (aHR, 0.23; 95% CI, 0.10–0.53; p = 0.001) mortality (Table S7).
Clinical course of participants who died after postvaccination COVID‐19
A total of 9 participants (3.5%) died following postvaccination COVID‐19, 4 after full and 5 after partial vaccination. Of these, 7 (2.8%) were COVID‐19‐related, 3 after full and 4 after partial vaccination. The risk factors and clinical characteristics of these patients are shown in Table 4. All 7 participants who died from COVID‐19 followed a similar course, admitted with symptomatic COVID‐19 pneumonia and respiratory failure, with no differences between those fully or partially vaccinated participants. Two other deaths were unrelated to COVID‐19 or liver disease (one following polymicrobial bacteremia unrelated to liver disease and one with pulmonary edema and cardiac arrest following cocaine use).
TABLE 4.
Clinical features of patients who died after full or partial vaccination
| Age/sex | Etiology of cirrhosis | CTP score/MELD‐Na | Comorbidities | Full/partial vaccination | Time from last dose of vaccine to COVID‐19 diagnosis (days) | Time from last vaccine dose to death (days) | Cause of death |
|---|---|---|---|---|---|---|---|
| 68/M | NAFLD | CTP B9/25 | COPD/HTN/obesity/CKD | Fully vaccinated | 77 | 85 | COVID‐19 |
| 63/M | HCV + alcohol | CTP A5/7 | COPD/HTN | Fully vaccinated | 23 | 72 | Non‐COVID‐19 |
| 64/M | NAFLD | CTP B8/21 | COPD/HTN/obesity/CKD | Fully vaccinated | 59 | 76 | COVID‐19 |
| 69/M | Alcohol | CTP A5/7 | COPD/HTN | Fully vaccinated | 35 | 39 | COVID‐19 |
| 69/M | HCV | CTP A5/11 | HTN/obesity | Partially vaccinated | 9 | 16 | COVID‐19 |
| 65/M | NAFLD | CTP A5/6 | HTN/obesity/CKD | Partially vaccinated | 20 | 21 | COVID‐19 |
| 58/M | HCV | CTP A6/10 | HTN/obesity | Partially vaccinated | 26 | 42 | COVID‐19 |
| 48/M | HCV + alcohol | CTP A6/22 | COPD/HTN/CKD | Partially vaccinated | 28 | 62 | Non‐COVID‐19 |
| 67/M | Alcohol | CTP B8/16 | HTN/obesity/CKD | Partially vaccinated | 22 | 57 | COVID‐19 |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HTN, hyptertension.
DISCUSSION
While COVID‐19 vaccines are highly effective in the general population, no vaccine is universally successful in preventing infection.[ 11 ] Patients with cirrhosis can become ill, require hospitalization and/or mechanical ventilation, or even die from COVID‐19 infection after vaccination. We have previously shown the likelihood of COVID‐19 among 20,037 patients with cirrhosis who underwent vaccination within the VA system.[ 1 ] In this analysis, we focused on clinical morbidity and mortality associated with 254 patients who developed postvaccination COVID‐19 and compared their outcomes with those with unvaccinated COVID‐19. The findings indicate that patients with cirrhosis and postvaccination COVID‐19 infection can have serious complications, including hospitalization, mechanical ventilation, or death (n = 9, 3.5% mortality rate), albeit all at lower rates than those with unvaccinated COVID‐19.
The association of postvaccination COVID‐19 and reduced mortality was observed after both full and partial vaccination, suggesting that patients with cirrhosis should be encouraged to take the first dose at the earliest available opportunity.
When the definition of partial vaccination was changed from 7 days to 21 days after the first dose, the association of reduced mortality approached that observed after full vaccination. Although postvaccination COVID‐19 was associated with reduced mortality, COVID‐19‐related mortality, and the need for mechanical ventilation, there was no association of a reduction in overall hospitalization within 60 days or COVID‐19‐associated hospitalization. This discordance is likely because there were no uniform criteria for hospitalization across centers, and patients were admitted for a myriad of reasons including the need for isolation in the absence of caregivers or for close observation in the setting of decompensated cirrhosis. The observation that postvaccination COVID‐19 was associated with reduced need for mechanical ventilation corroborates less severe disease among those participants who were hospitalized.
The Centers for Disease Control and Prevention (CDC) reported that out of 10,262 SARS‐CoV‐2 vaccine breakthrough infections in the United States among the general population, 995 (10%) were hospitalized, and 160 (2%) died.[ 12 ] Hospitalizations and deaths were COVID‐19‐related in 706 (71%) and 132 (82%), respectively. Our data suggest rates that are higher than those in the CDC cohort, with postvaccination COVID‐19 in cirrhosis associated with higher overall (3.5 vs. 2%) and COVID‐19‐related (2.8% vs. 1.3%) mortality, as well as hospitalization (42.1 vs. 10%) and COVID‐19‐related hospitalization (19.7% vs. 6.9%), which is consistent with the higher prevalence of comorbidities among veterans compared to the general population.
Sequencing data were only available in 555 (5%) reported cases in the CDC cohort, with 356 (64%) SARS‐CoV‐2 variants of concern, including B.1.1.7 (199; 56%), B.1.429 (88; 25%), B.1.427 (28; 8%), P.1 (28; 8%), and B.1.351 (13; 4%).[ 12 ]
We noted an association of the use of dexamethasone with increased overall and COVID‐related mortality in our cohort. The magnitude of this association was lower when hospitalization was included in the multivariable model. Only 16.5% of patients in either group received dexamethasone, and we did not control for the timing of administration of the medication. The findings most likely represent confounding by indication because only hospitalized and the sicker patients received dexamethasone. The association of postvaccination COVID‐19 and death did not change if dexamethasone was removed from the multivariable model.
We also performed a stratified analysis of postvaccination COVID‐19, by the type of mRNA vaccine. Postvaccination COVID‐19 following receipt of the mRNA‐1273 vaccine was associated with a numerically greater but statistically insignificant reduction in overall and COVID‐19‐related mortality compared to that following the BNT162b2 mRNA vaccine. Of note, there are emerging data that the 1273‐mRNA vaccine may be slightly more effective at protection against the delta variant, presumably due to the higher dose of vaccine (100 vs. 30 μg)[ 13 ]; however, our cohort may be underpowered to detect small differences between the two mRNA vaccines.[ 13 ]
Postvaccination COVID‐19 can occur due to either primary vaccine failure or breakthrough infections despite vaccine response. Primary vaccine failure occurs when patients do not develop an adequate immune response despite partial or full vaccination.[ 14 ] This has been observed in posttransplant patients, who mount a diminished antibody response to COVID‐19 vaccines.[ 14 ] Chronic liver disease, particularly decompensated cirrhosis, is associated with vaccine hyporesponsiveness to several commonly used vaccines, including hepatitis B,[ 15 ] pneumococcal, and influenza vaccines.[ 16 ] Our previous data suggest that the reduction in COVID‐19 infection associated with vaccination is lower and slower in cirrhosis.[ 1 ] Our stratified analysis based on hepatic compensation indicates that among patients with compensated cirrhosis, postvaccination COVID‐19 was associated with a greater reduction in hazard of death and COVID‐19‐related death compared to decompensated cirrhosis. These data are consistent with the lower association of reduction in COVID‐19 after mRNA vaccines among patients with decompensated cirrhosis.
Breakthrough infections occur after adequate antibody response to the COVID‐19 vaccine and have been attributed to SARS‐CoV2 variants such as the delta variant.[ 17 ] These infections occur despite an adequate antibody response to the vaccine but have been associated with lower viral loads of SARS‐CoV2, which in turn may explain the reduced severity of COVID‐19 infection.[ 18 ] Further studies are needed to identify antibody and T‐cell responses in patients with cirrhosis after COVID‐19 vaccination and the type of variants in postvaccination COVID‐19 infection.
Limitations
We acknowledge the following limitations to our study. While we present a relatively large number of patients with cirrhosis and postvaccination COVID‐19 infections, the numbers reported here are likely to be an undercount as asymptomatic patients were less likely to be screened.[ 19 ] Asymptomatic COVID‐19 may be more common with postvaccination COVID‐19. Due to the retrospective study design, we were unable to account for differences in SARS‐CoV‐2 variants. However, a sensitivity analysis that restricted analysis to a similar point of time during the pandemic for postvaccination and unvaccinated COVID‐19 showed similar results. The veteran cohort is limited in the proportion of women. The majority of postvaccination SARS‐CoV‐2 infections in this study was reported following mRNA vaccines, a reflection of earlier vaccine availability and greater use of mRNA vaccines within the VA system.
Strengths
The data presented have relative strengths. This is a large cohort of postvaccination COVID‐19 infection in a well‐characterized group of patients with cirrhosis. We propensity‐matched the two groups for various factors associated with severe COVID‐19. Granular data were available on each patient, which helped to identify the reasons for testing for COVID‐19, details of treatments, hospitalization, and death by chart review.
In summary, postvaccination COVID‐19 in patients with cirrhosis can be associated with serious outcomes. Therefore, these patients should be counseled to follow self‐protective behavior to minimize risk.
However, our findings that postvaccination COVID‐19 is associated with lower overall and COVID‐19‐related mortality and risk of mechanical ventilation should be encouraging for patients with cirrhosis and the practitioners who care for them. The fact that this association was observed even after partial vaccination may help persuade patients with vaccine hesitancy to receive vaccination.
CONFLICT OF INTEREST
Dr. John received grants from Exelixis, Exact Sciences, Glycotest, GlaxoSmithKline, and Viking. Dr. Martin consults for and received grants from AbbVie. He consults for CLDF, Ultragenix, and Theratech. He received grants from Biovie, Voyage, Durect, and Argon. Dr. Dahman consults for Exact Sciences.
AUTHOR CONTRIBUTIONS
Binu V. John and Bassam Dahman had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Binu V. John and Bassam Dahman. Acquisition, analysis, or interpretation of data: All authors. Statistical analysis: Binu V. John, Yangyang Deng, and Bassam Dahman. Drafting of the manuscript: Binu V. John. Critical revision of the manuscript for important intellectual content: All authors. Obtained funding: Binu V. John and Bassam Dahman. Administrative, technical, or material support: Binu V. John, Yangyang Deng, Kaley B. Schwartz, Tamar H. Taddei, David E. Kaplan, and Bassam Dahman. Supervision: Binu V. John and Bassam Dahman.
DISCLAIMER
The authors prepared this work in their personal capacity. The opinions expressed in this article are the authors’ own and do not reflect the view of the Department of Veterans Affairs or the US government.
Supporting information
Fig S1
Table S1‐7
ACKNOWLEDGMENTS
Services supporting the analysis and interpretation of the data of this research project were generated by the VCU Massey Cancer Center Biostatistics Shared Resource, supported, in part, with funding from National Institutes of Health–National Cancer Institute (NIH‐NCI) Cancer Center support grant P30 CA016059. The VCU Massey Cancer Center Biostatistics Shared Resource and the NIH‐NCI had no role in the design of the current study. We acknowledge data and support from the VA COVID‐19 shared data resource.
John BV, Deng Y, Schwartz KB, Taddei TH, Kaplan DE, Martin P, et al. Postvaccination COVID‐19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. 2022;76:126–138. 10.1002/hep.32337
Funding information
Supported by National Institutes of Health–National Cancer Institute Cancer Center support grant P30 CA016059
REFERENCES
- 1. John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D, et al. Association of BNT162b2 mRNA and 1273‐mRNA vaccines with COVID‐19 infection and hospitalization among patients with cirrhosis. JAMA Internal Med. 2021;181(10):1306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarin SK, Choudhury A, Lau GK, Zheng M‐H, Ji D, Abd‐Elsalam S, et al. Pre‐existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID‐19 Liver Injury Spectrum Study). Hepatol Int. 2020;14(5):690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(2):768–71.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. John BV, Aitcheson G, Schwartz KB, Khakoo NS, Dahman B, Deng Y, et al. Male sex is associated with higher rates of liver‐related mortality in primary biliary cholangitis and cirrhosis. Hepatology. 2021;74(2):879–91. [DOI] [PubMed] [Google Scholar]
- 5. John BV, Khakoo NS, Schwartz KB, Aitchenson G, Levy C, Dahman B, et al. Ursodeoxycholic acid response is associated with reduced mortality in primary biliary cholangitis with compensated cirrhosis. Am J Gastroenterol. 2021;116(9):1913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Center for Disease Control and Prevention‐Stay Up to Date with Your Vaccines . [cited 2021 Dec 24]. https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/stay‐up‐to‐date.html. Accessed January 1, 2022.
- 7. Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jepsen P, Vilstrup H, Lash TL. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology. 2014;146(1):147–56, quiz e15–6. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan DE, Dai F, Aytaman A, Baytarian M, Fox R, Hunt K, et al. Development and performance of an algorithm to estimate the Child‐Turcotte‐Pugh score from a national electronic healthcare database. Clin Gastroenterol Hepatol. 2015;13(13):2333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT‐C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–17. [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention . How CDC monitors COVID‐19 vaccine effectiveness. Updated December 23, 2021. https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/effectiveness/how‐they‐work.html. Accessed December 24, 2021.
- 12. CDC COVID‐19 Vaccine Breakthrough Case Investigations Team . COVID‐19 vaccine breakthrough infections reported to CDC‐United States. MMWR Morb Mortal Wkly Rep. 2021;70:792–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puranik A, Lenehan PJ, Silvert E, Niesen MJM, Corchado‐Garcia J, O'Horo JC, et al. Comparison of two highly‐effective mRNA vaccines for COVID‐19 during periods of alpha and delta variant prevalence. medRxiv. 2021. 10.1101/2021.08.06.21261707 [DOI] [Google Scholar]
- 14. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27(6):e1942. 10.1002/rmv.1942 [DOI] [PubMed] [Google Scholar]
- 16. Leise MD, Talwalkar JA. Immunizations in chronic liver disease: what should be done and what is the evidence. Curr Gastroenterol Rep. 2013;15(1):300. [DOI] [PubMed] [Google Scholar]
- 17. Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, et al. Vaccine breakthrough infections with SARS‐CoV‐2 variants. N Engl J Med. 2021;384(23):2212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levine‐Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, et al. Initial report of decreased SARS‐CoV‐2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27(5):790–2. [DOI] [PubMed] [Google Scholar]
- 19. Aschwanden C. Five reasons why COVID herd immunity is probably impossible. Nature. 2021;591:520–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐7


