Abstract
The past decade has seen significant advances in stroke prevention. These advances include new antithrombotic agents, new options for dyslipidemia treatment, and novel techniques for surgical stroke prevention. In addition, there is greater recognition of the benefits of multi-faceted interventions, including the role of physical activity and dietary modification. Despite these advances, the aging of the population and the high prevalence of key vascular risk factors pose challenges to reducing the burden of stroke. Using an etiology-based framework, current approaches to prevention of cardioembolic, cryptogenic, atherosclerotic, and small vessel disease stroke are outlined in this paper. Special emphasis is given to recent trials of antithrombotic agents, including studies that have tested combination treatments and response according to genetic factors.
Keywords: Ischemic stroke, Transient Ischemic Attack (TIA), Cerebrovascular Disease/Stroke, Thrombosis, Treatment
1. STROKE CLASSIFICATION AND EVALUATION
Classification
Ischemic stroke is defined as an episode of neurological dysfunction caused by focal cerebral, spinal, or retinal cell death attributable to ischemia based on supportive clinical, radiographic or pathologic evidence1. Crucially, stroke is not a disease per se but rather an endpoint of another process that culminates in ischemia with cell death in the central nervous system (CNS). Understanding the risk of subsequent stroke and implementing an appropriate secondary prevention strategy relies on identifying the most likely etiology and, if that is not possible, determining whether any given patient fits in to a therapeutically-relevant subgroup for whom clinical trial data can be evoked.
Numerous classification schemes for ischemic stroke have been proposed to capture this complexity. These include the Atherosclerosis/Small Vessel/Cardiac/Other/Dissection (ASCOD)2 and Causative Classification of Stoke (CCS)3 systems. However, by far the most commonly used scheme in clinical practice is the Trial of Org 10172 in Acute Ischemic Stroke (TOAST) classification system4. Under this system, ischemic stroke etiology can be categorized along 5 major subgroups: 1) Large artery atherosclerosis, 2) Cardioembolism, 3) Small artery occlusion, 4) Stroke of other determined etiology and 5) Cryptogenic. Attribution of stroke etiology to one of these subgroups is based on a history, physical examination and structured diagnostic workup including imaging of the cervical and intracranial vasculature, echocardiography and an assessment of cardiac rhythm.
A. Large artery atherosclerosis:
Stroke arising from large artery atherosclerosis is defined by the presence of ≥50% stenosis or occlusion of an extracranial or intracranial large artery corresponding to the territory of the stroke.
B. Cardioembolism:
Stroke arising from cardioembolism describes an infarct occurring in the presence of a high-risk source of cardioembolism such as atrial fibrillation (AF), left ventricular thrombus, infective endocarditis or recent myocardial infarction. Such infarcts typically bear the hallmarks of remote embolization, i.e., are cortically-based, multifocal and with a predilection for the large cerebral arteries. However, emboli from the heart may also occlude small perforating arteries supplying the pons, thalamus or basal ganglia and so the stroke topography is not a crucial determinant of the etiology. The original system allows for the label of “possible cardioembolic stroke” in the presence of a so-called “medium-risk” cardioembolic source including congestive cardiac failure, mitral valve prolapse or atrial septal aneurysm but this label is not used in clinical practice.
C. Small artery occlusion:
This describes a small stroke (<1.5cm in widest dimension) that occurs in an area of the brain supplied by small perforating vessels e.g the pons, thalamus, or basal ganglia and in a patient with evidence of at least one vascular risk factor. Within this category, strokes arise due to lipohyalinosis of small vessels that branch directly off larger vessels (pontine perforators emanating from the basilar artery or lenticulostriate arteries emanating from the middle cerebral artery).
D. Other determined etiology:
This is a category to describe strokes with a defined, rarer etiology that does not fit in to one of the above three classifications. It includes stroke arising from arterial dissection, exposure to illicit drugs, cerebral vasculitis or an arterial hypercoagulable disorder.
E. Cryptogenic stroke:
In cryptogenic stroke, no clear etiology can be determined. This arises in three scenarios: 1) A patient has had a thorough diagnostic workup and no etiology determined. Patients with lacunar strokes who do not present with classical lacunar syndromes or who exhibit cortical symptoms are classified under this category; 2) A diagnostic workup has yielded two or more plausible etiologies; or 3) A thorough diagnostic workup has not been completed.
Cryptogenic stroke comprises up to 33% of all ischemic strokes5–8. It is a heterogeneous group because it includes patients with multiple high-risk causes and those with no cause identified. The pitfalls in this classification scheme – coupled with the observation that a majority of cryptogenic strokes appear embolic in nature – led to the refined definition of embolic stroke of undetermined source (ESUS)9, 10. ESUS is a subset of cryptogenic stroke and refers to an embolic-appearing stroke for which no high-risk etiology has been determined despite a thorough diagnostic workup (including at least 24 hours of cardiac rhythm monitoring). There is increasing evidence that ESUS patients are heterogeneous, including some with later atrial fibrillation and some with non-stenotic carotid plaques11–13. The ESUS concept and its differentiation from cryptogenic stroke are discussed in more detail in the cryptogenic stroke section.
As further evidence emerges identifying stroke associations as therapeutically relevant entities, current classification systems are likely to further evolve. For instance, the success of recent trials establishing the superiority of percutaneous PFO closure over anti-platelet therapy for secondary prevention of stroke, has led to calls for formally classification of PFO-associated stroke as a subset of cardioembolic stroke14. Similarly, the recognition of atrial dysfunction in the absence of AF as a risk factor for stroke15 and high-risk features of cervical atherosclerosis beyond the degree of luminal stenosis16 will likely to lead to further changes in this classification system over time.
Evaluation
The evaluation of a patient with stroke typically occurs in three partially overlapping phases: 1) Urgent evaluation to determine whether they are a candidate for intravenous thrombolysis (IV tPA) or endovascular thrombectomy (EVT), 2) Confirmation of the diagnosis of ischemic stroke (not strictly necessary for the administration of IV tPA) and 3) Determination of the most likely etiology which, in turn, dictates the optimal secondary prevention strategy. At a minimum, the evaluation must include imaging of the brain via computed tomography (CT) and/or magnetic resonance imaging (MRI), cervical vessel imaging (with cervical vessel ultrasonography, CT angiography or MR angiography), intracranial vessel imaging (with CTA or MRA), transthoracic echocardiography (TTE), electrocardiography (ECG), 24 hours of cardiac telemetry monitoring and an assay of a person’s cholesterol profile and glycated hemoglobin.
If this diagnostic testing has not yielded a high-risk etiology, further evaluation is performed based on an individual patient’s history, examination, stroke topography on imaging and any possible low or medium risk etiologies identified on their initial workup (such as a PFO). Options for further evaluation include:
A. Extended cardiac monitoring:
Two seminal trials examined the use of extended cardiac monitoring in patients with cryptogenic stroke or transient ischemic attack (TIA). EMBRACE17 enrolled patients ≥ 55 years old with cryptogenic stroke or TIA and no history of AF. They were randomized to either usual practice (including an ECG and inpatient cardiac telemetry) or a 30-day cardiac event monitor. The primary endpoint was detection of 30 seconds of AF and this was achieved in 3.2% of patients with usual care and 16.1% of those who wore an extended cardiac monitor. CRYSTAL-AF18 also enrolled patients with cryptogenic stroke/TIA and randomized them to usual care or placement of an insertable cardiac monitor (ICM). By contrast to EMBRACE, CRYSTAL-AF enrolled younger patients (≥ 40 years old) and the primary endpoint was the same. Within the group assigned to usual care, 1.4% of patients were found to have AF within 6 months while 8.9% of those in the ICM group were found to have AF. At 3 years of follow up, detection of AF exceeded 30%.
B. Transesophageal echocardiography (TEE):
TEE provides more detailed imaging than TTE especially of the left atrium, left atrial appendage, valvular structures and the arch of the aorta. In a person with ESUS and no etiology identified, a TEE may be reasonable to examine for the presence of left atrial/ atrial appendage thrombus, atrial myxoma, fibroelastoma or vegetation. It may also be used if PFO is suspected or further detail required on its morphology. One retrospective series found that in patients with ESUS, TEE led to a new finding in 52.5% of cases and effectuated a management change in 16.2% of cases19. Typically, TEE is reserved for patients the below the age of 60 as the yield of this test is low above that threshold.
C. Hypercoagulability testing:
Arterial hypercoagulable disorders can lead to ischemic stroke even in the presence of normal cervical and intracranial vasculature and normal cardiac structure and rhythm. In general, it is of low yield in patients with ischemic stroke20 but should be considered in a person with no other candidate etiology identified especially if they have a history of arterial thrombotic events elsewhere or a family history of arterial thrombotic events. The major arterial hypercoagulable disorders include anti-phospholipid antibody syndrome, disseminated intravascular coagulation, metastatic malignancy and hyperhomocysteinemia. Venous hypercoagulable disorders can be implicated in stroke in the presence of a PFO or large pulmonary arteriovenous fistula (because of paradoxical embolization) and include protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden mutation, prothrombin gene mutation and polycythemia rubra vera. This group also includes hyperhomocysteinemia.
D. Other:
In specific circumstances, other testing may be pursued including MRI with T1 fat suppressed sequences (to examine for arterial dissection not visible on previous testing), diagnostic cerebral angiography and cerebrospinal fluid (CSF) testing (if cerebral vasculitis or reversible cerebral vasoconstriction are suspected) or genetic testing (if an inherited stroke syndrome is suspected). Additional consideration include emerging putative associations with ESUS including occult malignancy21, the carotid web22, and non-stenotic carotid atherosclerosis16 within the early phases of a patient’s diagnostic workup.
2. ANTI-THROMBOTIC THERAPY FOR MINOR STROKE/HIGH-RISK TIA
In general, single anti-platelet therapy is indicated to reduce the risk of subsequent stroke after ischemic stroke or TIA. There has been considerable research examining the efficacy of different individual anti-platelet agents, dual or triple anti-platelet therapy or use of anticoagulation as opposed to anti-platelet therapy. A consistent theme in the literature to-date is that increased intensity of anti-thrombotic therapy reduces the risk of subsequent ischemic stroke but at the cost of an increased risk of hemorrhagic complications. The Warfarin-Aspirin Recurrent Stroke Study (WARSS)23 similarly failed to show a benefit to warfarin compared with aspirin with respect to the primary endpoint of subsequent ischemic stroke or death. However, trials of secondary prevention differed in terms of key aspects of the approach including time from symptom onset to randomization, duration of anti-thrombotic therapy, permitted stroke etiologies and stroke severity. Therefore, considerable interested emerged in identifying a subgroup of patients for whom more intense anti-thrombotic therapy would remain effective without an unacceptably high risk of hemorrhagic complications.
In considering the optimal anti-thrombotic strategy, the first consideration is to assign the most likely underlying etiology for the stroke. In patients with stroke arising from a high-risk cardioembolic source such as AF, anticoagulation is the optimal strategy. In patients with stroke arising from symptomatic ipsilateral extracranial carotid stenosis, the discussion of anti-thrombotic therapy occurs in parallel with consideration of revascularization. In patients with non-cardioembolic stroke or TIA without an indication for revascularization, there is likely a benefit to using a short course of dual anti-platelet therapy provided A) There is a sufficiently high-risk of subsequent stroke and B) The risk of hemorrhagic complications is acceptably low. The risk of intracranial hemorrhage is primarily driven by the volume of infarcted tissue so, in practice, identifying this high recurrence/low hemorrhage risk group means selecting patients with a high-risk TIA (as measured by the ABCD2 score) or a minor ischemic stroke (as measured by a low score on the National Institutes of Health Stroke Scale (NIHSS) at presentation). Two recent clinical trials address this population:
1). Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack (CHANCE):
In this trial, 5,170 patients with minor ischemic stroke or high-risk TIA were randomized to aspirin and clopidogrel (DAPT) for 21 days or aspirin and placebo with treatment with aspirin alone thereafter24. The primary outcome was ischemic or hemorrhagic stroke. There was a lower hazard of ischemic/hemorrhagic stroke in the DAPT group: hazard ratio (HR) 0.68 (95% CI: 0.57–0.81). The difference in groups was accounted for by a lower rate of ischemic stroke in the DAPT group (HR 0.67 (05% CI: 0.56–0.81) whereas there was no difference in the rate of hemorrhagic stroke between the two groups (HR 1.01, 95% CI: 0.38–2.70).
2). Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT):
In this trial, 4,881 patients with minor ischemic stroke or high-risk TIA were randomized to aspirin and clopidogrel for 90 days or aspirin and placebo for 90 days25. The primary outcome was a composite of ischemic stroke, myocardial infarction (MI) or vascular death. There was a lower hazard of this primary outcome in those treated with DAPT than those treated with aspirin alone (HR 0.75, 95% CI: 0.59–0.95). The largest contributor to this composite outcome was ischemic stroke – 267 out of 281 events.
These trials successfully identified a population for whom DAPT was beneficial without an accompanying high risk of hemorrhage. Given the enrollment criteria, these trials enrolled patients with 3 major classifications of ischemic stroke: A) Stroke of small vessel etiology, B) Cryptogenic stroke or C) Stroke associated with large artery atherosclerosis (where the patient was not planned for revascularization). Neither trial included patients treated with intravenous thrombolysis or mechanical thrombectomy. The loading dose of clopidogrel differed between the two trials – 600mg in POINT and 300mg in CHANCE. POINT enrolled patients within 12 hours of symptom onset while CHANCE enrolled patients up to 24 hours after symptom onset. Each trial adopted the same definition of minor ischemic stroke (NIHSS ≤3) and high-risk TIA (ABCD2 score >=4). CHANCE was conducted in an exclusively Chinese population while POINT was conducted in a more geographically and racially diverse population predominantly from the United States but also Europe and Australia.
DAPT with ticagrelor and aspirin was explored in the Acute Stroke or Transient Ischemic Attack Treated with Ticagrelor and ASA for Prevention of Stroke and Death (THALES) trial26. This study randomized 11,016 patients with high-risk TIA/minor stroke to ticagrelor/aspirin or aspirin alone for 30 days. There was a lower hazard of the primary outcome in those treated with ticagrelor/aspirin versus aspirin alone – HR 0.83 (95% CI: 0.71–0.96). By contrast to POINT and CHANCE, there was a high rate of intracranial hemorrhage in the DAPT versus aspirin alone group (HR 3.33, 95% CI: 1.34–8.28).
The CHANCE-2 trial established the superiority of ticagrelor/aspirin versus clopidogrel/aspirin in patients with minor stroke/high-risk TIA and a CRP2C19 (also known as P450 2C19) loss of function allele27. The study enrolled 11,255 patients in China aged 40 years or older with high-risk TIA/minor stroke. All participants had a CRP2C19 loss of function allele4 indicating diminished capacity to convert the prodrug clopidogrel into its active metabolite. High-risk TIA was defined as an ABCD2 score of greater than or equal to 4 while minor stroke was defined as a presenting NIHSS score of 3 or lower. Subjects were randomized within 24 hours of symptom onset to ticagrelor 90mg twice daily plus aspirin 75mg daily versus clopidogrel 75mg daily plus aspirin 75mg daily. Each group received a loading dose of either ticagrelor or clopidogrel. The primary outcome was new ischemic or hemorrhage stroke. 6% of patients randomized to ticagrelor experienced the primary outcome while 7.6% of those randomized to clopidogrel experienced the primary outcome. The rate of moderate or severe bleeding did not differ between the two groups – 0.3% in each while the risk of intracranial hemorrhage was numerically lower in those treated with ticagrelor as opposed to clopidogrel (0.1% vs. 0.2% - HR 0.49, 95% CI: 0.12–1.96).
An alternative combination concept is to combine an anticoagulant with an antiplatelet agent. The Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS)28 trial enrolled patients with either stable coronary artery disease, stable peripheral arterial disease or both. Patients with a stroke within one month prior to screening were excluded. A total of 27,395 participants were randomized to low-dose (2.5mg twice daily) rivaroxaban plus aspirin (100mg once daily), full-dose (5mg twice daily) rivaroxaban alone or aspirin (100mg once daily) alone. The primary efficacy outcome was stroke, myocardial infarction or vascular death. There was a lower hazard of this primary outcome in patients taking low-dose rivaroxaban/aspirin compared with aspirin alone (HR 0.76, 95% CI: 0.66–0.86). Ischemic stroke was a secondary outcome in this trial. There was a lower hazard of stroke in those taking rivaroxaban/aspirin compared with aspirin alone (HR 0.51, 95% CI: 0.38–0.68) and this was the largest contributor to the reduction in the composite outcome in those treated with rivaroxaban and aspirin. Only 3.8% of patients enrolled in this trial had a history of stroke but a secondary analysis found that a benefit to rivaroxaban/aspirin was still evident in this subgroup29. The reduction in subsequent stroke was explained by reduced numbers of cardioembolic strokes and embolic strokes of undetermined source and not strokes associated with large artery atherosclerosis or small vessel disease30. The hypothesis that a low-dose NOAC combined with aspirin is superior to aspirin alone for the prevention of subsequent stroke in patients with recent non-cardioembolic ischemic stroke remains to be tested.
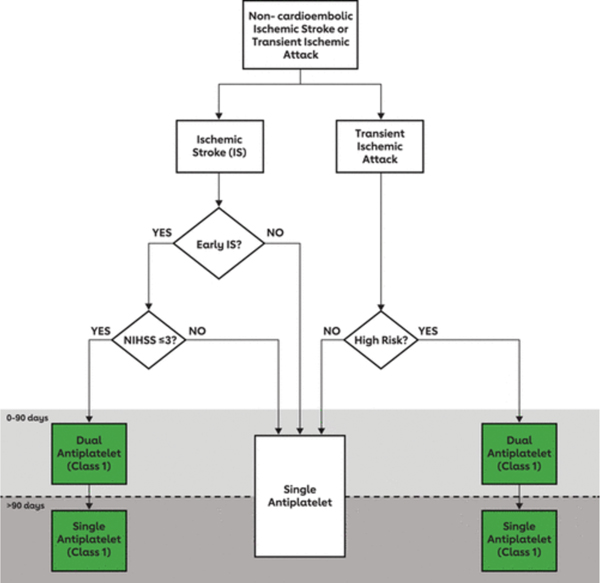
Current American Heart Association/American Stroke Association Ischemic Stroke Secondary Prevention Guidelines endorse the use of DAPT for either 21 or 90 days in patients with non-cardioembolic minor ischemic stroke or high-risk TIA without a strict restriction on either a) use of IV t-PA, b) thrombectomy or c) time from symptom onset to starting DAPT (recommending starting it “ideally within 12–24 hours of symptom onset and at least within 7 days of onset”). Only single anti-platelet therapy is recommended beyond that time point as the risk of subsequent stroke is highest in the immediate wake of minor stroke/TIA and diminishes over time while the risk of hemorrhage is aggregative (Figure 1).
Figure 1.
Schematic diagram for approach to acute antithrombotic therapy
Reproduced with permission from Wolters Kluwer
3. CARDIOEMBOLIC STROKE
Cardioembolic stroke accounts for nearly 30% of ischemic strokes31 is associated with increased mortality and morbidity.32 Furthermore, cardiac sources are responsible for nearly 45% of cryptogenic strokes33, making cardioembolism the most common mechanism for ischemic stroke.
Cardiac sources of embolism include high-risk sources such as atrial fibrillation (AF), valvular heart disease, and endocarditis as well as low-risk sources such as patent foramen ovale. Finding a cardiac source of embolism is of paramount importance as it would often lead to a change in clinical management and secondary prevention strategies. A detailed discussion of these sources is provided below.
Atrial Fibrillation
Background
Atrial fibrillation is the most common cardiac arrythmia and cardioembolic source. The prevalence of AF increases with age, reaching nearly 4% in patients 60 years or older and 8% in patients 80 years or older.34 AF prevalence has increased over the past decade and is expected to continue to increase35 likely due to the aging population and improved detection methods. AF portends an increased risk of ischemic stroke, systemic embolism, and death and studies have shown that anticoagulation is associated with a reduction in these risks.
Secondary prevention in acute ischemic stroke and AF
In patients with acute ischemic stroke in the setting of AF, studies have demonstrated the benefit of anticoagulation therapy for long term secondary prevention and clinical trials comparing direct oral anticoagulants to warfarin showed similar overall efficacy with DOACs with respect to ischemic stroke but lower risk of intracranial hemorrhage with DOACs. The specific anticoagulant and timing of initiation have also been studied. Recent studies including patients treated with oral direct oral anticoagulant showed a similar risk of combined ischemic/hemorrhagic stroke outcome in those whose anticoagulation was started 0–3 days vs. >3 days from the ischemic stroke. These studies are observational and subject to treatment by indication bias and thus ongoing randomized trials will help determine the optimal timing of anticoagulation initiation in patients with acute ischemic stroke and AF.
Other treatments such as left atrial appendage occlusion have not been investigated for secondary prevention but studies of patients with AF comparing LAA occlusion devices such as WATCHMAN to oral anticoagulation showed a similar risk of recurrent stroke or death. Thus, LAA occlusion is another treatment option for secondary prevention in AF, particularly in patients where long term anticoagulation is contraindicated.
Acute Ischemic Stroke in Anticoagulated Patients
While anticoagulation reduces the risk of ischemic stroke in patients with AF, there remains a subgroup of patients who suffer a breakthrough ischemic stroke while on anticoagulation therapy. Studies have shown that this subgroup of patients are at increased recurrence risk and that changing anticoagulation treatment is not associated with a reduction in recurrence risk. In such patients, important strategies include investigating an alternative non-AF related stroke mechanism, determining medication compliance, optimization of secondary prevention strategies are important measures. Furthermore, there is data to suggest a potential benefit of the combination of LAA closure and anticoagulation when compared to anticoagulation alone in patients with AF undergoing cardiac surgery. This approach needs further study in patients with a breakthrough cardioembolic stroke while on oral anticoagulation.
Valvular Heart Disease
Valvular heart disorders, more specifically those affecting the mitral valve are associated with increased ischemic stroke risk. For instance, the Framingham study showed an association between mitral annular calcification and ischemic stroke risk.40 Moreover, the risk of ischemic stroke in patients with rheumatic mitral stenosis has been has been suggested to be due to underlying AF.41
In patients with ischemic stroke in the setting of a mechanical heart valve, diagnostic evaluation to exclude alternative causes as well as valve dysfunction or infection should be considered. Anticoagulation with warfarin is recommended with a target INR of 2–3 for an aortic mechanical valve and 2.5–3.5 for a mitral mechanical valve.42 The addition of low dose aspirin should also be considered in patients with mechanical heart valve and low bleeding risk.42 In such patients, DOACs should be avoided.43 Moreover, in patients with ischemic stroke in the setting of mitral stenosis with or without AF, anticoagulation should be considered.44 Observational studies suggests a potential benefit of DOACs in patients with valvular AF.45 Since patients with valvular AF were excluded from DOAC trials, warfarin remains the preferred treatment in such patients and studies comparing the safety and efficacy of DOACs with warfarin are needed.
Infective Endocarditis
Infective endocarditis of the aortic or mitral valves is an established caused of stroke, where the risk of ischemic stroke starts 4 months before the diagnosis and persists for up to 5 months after the diagnosis.46 Endocarditis is clinically suspected in patients with ischemic stroke and clinical or laboratory concern for a concomitant systemic infection and the diagnosis is confirmed on echocardiography, particularly transesophageal echocardiography.47 The workup in patients with ischemic stroke and endocarditis should also include non-invasive intracranial vascular imaging to screen for mycotic aneurysms and consideration of a diagnostic angiogram in select patients.47
The treatment of endocarditis entails intravenous antimicrobial treatment for at least 6 weeks as well as consideration for surgery in a subgroup of patients. Anticoagulation is avoided in the acute setting but may considered long term if there is an indication after resolution of endocarditis. In addition, measures to reduce the risk of transient bacteremia should be instated.47
Left ventricular dysfunction
Studies have shown an increased ischemic stroke risk in patients with lower ejection fraction in patients with systolic congestive heart failure48, 49 and the stroke risk is particularly higher in those with a prior history of stroke.50 This risk is thought to be mediated by increased occurrence of left ventricular thrombus which is best detected by a cardiac MRI51 or a transthoracic echocardiogram using contrast.52
In patients with low ejection fraction and evidence of LV thrombus, at least 3 months of treatment with warfarin is recommended.53 In patients without LV thrombus, however, there remains equipoise on the best antithrombotic regimen: antiplatelet vs. anticoagulation. The WARCEF trial compared warfarin to aspirin in patients with ejection fraction <35% and showed lower ischemic stroke risk in patients treated with warfarin (HR 0.52 95% CI 0.33–0.82) but higher risk of intracerebral hemorrhage (HR 2.22 95% CI 0.43–11.66), without any significant net benefit (HR 0.93 95% CI 0.79–1.10). This was also the case in patients enrolled in WARCEF who have a history of prior stroke.50 Another post-hoc analysis of the NAVIGATE-ESUS trial showed that in patients with moderate to severe LV dysfunction and ESUS, rivaroxaban was superior to aspirin in reducing the risk of primary outcome (HR 0.36; 95% CI, 0.14–0.93).
Therefore, the best antithrombotic regimen for patients with ischemic stroke and LV dysfunction remains unknown and randomized trials are needed to test direct oral anticoagulants versus aspirin in such patients.
Recent myocardial infarction
Patients with a myocardial infarction (MI) (ST elevation, non-ST elevation, or silent MI) are at increased risk of ischemic stroke, particularly in the first few months after the myocardial infarct.54, 55
In the presence of an LV thrombus, current guidelines recommend anticoagulation with warfarin for at least 3 months.53 In the absence of an LV thrombus, some experts recommend consideration of short-term anticoagulation with warfarin in patients with ischemic stroke and acute anterior ST elevation MI and apical akinesis.44 High quality evidence to support this recommendation, however, is lacking and clinical trials are needed to compare anticoagulation to aspirin in patients with ischemic stroke in the setting of myocardial infarction.
Patent Foramen Ovale
Patent foramen ovale is an intra-septal defect that is present in about 25% of the population.56 Some studies suggested an association between PFO and cryptogenic ischemic stroke57 likely mediated either by paradoxical embolism or via thrombogenesis and clot formation at the PFO site but there remains no high quality data to support this association.
Given the high prevalence of PFO, investigators have proposed the Risk of Paradoxical Embolism (ROPE) score to help determine whether a certain PFO is likely pathogenic or incidental.58 Furthermore, the presence of an atrial septal aneurysm has been shown to portend a higher recurrent stroke risk in patients with PFO.59
Initial PFO trials showed no benefit of PFO closure over medical therapy in patients with ischemic stroke and PFO. These trials however did not select patients based on whether the PFO is pathogenic and some included patients with TIA.59 Analysis of these trials, however, showed a potential benefit of closure in patients with ROPE score ≥ 7, in which the PFO is likely pathogenic.60 More recently, two randomized controlled trials showed a significant benefit in patients with patients with cryptogenic ischemic stroke and a PFO with moderate to severe shunting or atrial septal aneurysm. PFO closure however was associated with increased risk of complications, more commonly atrial fibrillation in 6–7% of patients.59
Controversies on PFO treatment include whether PFO closure is beneficial in a select subset of patients >60 years old with a PFO and no other identifiable stroke mechanism. In addition, it remains unknown whether PFO closure is effective in young patients with clinically covert brain infarcts.
Cardiac tumors
Left sided cardiac tumors are a very rare cause of ischemic stroke.61 The most common ones are atrial myxomas and papillary fibroelastomas.62 Left atrial myxomas are associated with increased stroke risk via embolization of thrombus and/or tumor.63 Papillary fibroelastomas, particularly those that are mobile or that adhere to the aortic valve, are associated with increased thromboembolic risk. 61, 64
Due to the lack of high-quality data, the best treatment strategy for patients with ischemic stroke and left sided cardiac tumors remains unknown. In an observational study, surgical excision has been shown to reduce the risk of recurrent risk.65 Antithrombotic treatment and consideration of tumor resection for left sided tumors are reasonable secondary prevention strategies.53 Tables 1 and 2 provide an overview of cardiac sources of emboli and key AF trials.
Table 1.
Short-term and long-term secondary prevention strategies with cardioembolic stroke causes
| Short term management | Long term management | Areas of Controversy | |
|---|---|---|---|
| Atrial fibrillation | - Oral anticoagulation (prefer DOACs over warfarin) - Optimal initiation timing is unknown, but reasonable to start 2–14 days from stroke - Avoid bridging with heparin or low molecular weight heparin |
- Oral anticoagulation (prefer DOACs over warfarin) - Left atrial Appendage Occlusion as an alternative to oral anticoagulation - Avoid concomitant antiplatelet therapy unless strong indication |
- Optimal timing of anticoagulation initiation - Treatment of ischemic stroke despite anticoagulation therapy |
| Atrial cardiopathy | - Antiplatelet therapy - Cardiac monitoring to look for AF |
- Antiplatelet therapy - Anticoagulation if AF is found on cardiac monitoring |
- Whether apixaban is superior to aspirin in patients with atrial cardiopathy without AF |
| Patent Foramen Ovale | - Antithrombotic therapy - Thorough investigation including cardiac monitoring to look for competing stroke mechanisms |
- PFO closure in patients meeting criteria for positive PFO trials - Antithrombotic therapy in patients not meeting criteria for positive PFO trials |
- The benefit of PFO closure in young patients with clinically covert brain infarcts - The benefit of PFO closure in patients over 60 years without another identifiable stroke mechanism |
| Infective endocarditis | - Intravenous antibiotics - Avoid anticoagulation - Consideration of surgery |
- Warfarin for mechanical heart valve - Anticoagulation for concomitant AF - Preventative measures to reduce the risk of transient bacteremia |
- Timing of surgery in patients with endocarditis and ischemic stroke |
| Recent myocardial infarction | - Antiplatelet therapy - Cardiac monitoring to look for AF - TTE with Definity and/or Cardiac MRI to look for LV thrombus - Warfarin for at least 3 months for LV thrombus - Warfarin for at least 3 months for anterior STEMI with apical hypokinesis |
- Antiplatelet therapy - Warfarin for persistent LV thrombus |
- DOACs vs. warfarin for LV thrombus - DOACs vs. aspirin in patients with ischemic stroke and recent myocardial infarction |
| Left ventricular dysfunction | - Antiplatelet therapy - Cardiac monitoring to look for AF - TTE with Definity and/or Cardiac MRI to look for LV thrombus - Warfarin for at least 3 months for LV thrombus (reasonable to start 4–14 days from stroke) - Consider anticoagulation with warfarin or DOAC patients with EF ≤15% |
- Antiplatelet therapy - Warfarin for persistent LV thrombus - Anticoagulation treatment if AF is found - Consider anticoagulation with warfarin or DOAC patients with EF ≤15% |
- DOAC vs. aspirin in patients with ischemic stroke and EF ≤30% |
| Rheumatic valvular disease | - Anticoagulation, preferably with warfarin, particularly if concomitant AF - Cardiac monitoring to look for AF if not present on initial assessment - Consider initiation at 4–14 days from ischemic stroke - Avoid bridging with heparin or low molecular weight heparin |
- Anticoagulation, preferably with warfarin, particularly if concomitant AF - Consider valve surgery |
- Antiplatelet vs. Anticoagulation in patients without evidence of AF - DOAC vs. warfarin in patients with AF |
| Cardiac tumors | - Antithrombotic therapy - Cardiac monitoring to look for AF if not present on initial assessment - Consider surgical resection when safe from stroke standpoint |
- Antithrombotic therapy - Anticoagulation with DOAC or warfarin if concomitant AF |
- Antiplatelet vs. Anticoagulation therapy for non-surgical patients |
Table 2.
Major atrial fibrillation stroke prevention trials
| Study | Patient population | Comparator groups | Stroke or systemic embolism | Major hemorrhage |
|---|---|---|---|---|
| RE-LY (open label) | Mean age 71 years Mean CHADS2 2.1 Mean TTR 64% History of Stroke or TIA 20% |
Dabigatran 150 mg Dabigatran 110 mg Warfarin |
Dabigatran 150 vs. warfarin: RR 0.66 95% CI 0.53–0.82 Dabigatran 110 vs. warfarin: RR 0.91 95% CI 0.74–1.11 |
Dabigatran 150 vs. warfarin: RR 0.93 95% CI 0.81–1.07 Dabigatran 110 vs. warfarin: RR 0.80 95% CI 0.69–0.93 |
| ROCKET-AF (Randomized controlled) | Mean age 73 years Mean CHADS2 3.5 Mean TTR 55% History of Stroke, systemic embolism or TIA 55% |
Rivaroxaban 20 mg Warfarin |
HR 0.88 95% CI 0.75–1.03 | HR 0.69 95% CI 0.90–1.20 |
| ARISTOTLE (Randomized controlled) | Mean age 70 years Mean CHADS2 2.1 Mean TTR 62% History of Stroke |
Apixaban 5 mg Warfarin |
HR 0.79 95% CI 0.66–0.95 | HR 0.69 95% CI 0.60–0.80 |
| ENGAGE (Randomized controlled) | Median age 72 years Mean CHADS2 2.8 Median TTR 68% History of Stroke or TIA 28% |
Edoxaban 60 mg Edoxaban 30 mg Warfarin |
Edoxaban 60 vs. warfarin: HR 0.79 95% CI 0.63–0.99 Edoxaban 30 vs. warfarin: HR 1.07 95% CI 0.87–1.31 |
Edoxaban 60 vs. warfarin: HR 0.80 95% CI 0.71–0.91 Edoxaban 30 vs. warfarin: HR 0.47 95% CI 0.41–0.55 |
| AVEROES (Randomized controlled) | Mean age 70 years Mean CHADS2 2.0 History of Stroke or TIA 14% |
Apixaban 5 mg Aspirin 81–324 mg |
HR 0.45 95% CI 032–0.62 | HR 1.13 95% CI 0.74–1.75 |
| PREVAIL-AF and PROTECT-AF – 5 year follow up | Mean age 73 years Mean CHADS2 2.3 Mean TTR 62% History of Stroke or TIA 23% |
WATCHMAN Warfarin |
HR 0.96 95% 0CI 0.60–1.54 | HR 0.91 95% CI 0.64–1.29 (Including procedural bleeding) HR 0.48 95% CI 0.32–0.71 (Excluding procedural bleeding) |
4. CRYPTOGENIC STROKE
Based on current worldwide annual ischemic stroke incidence rates66 and considering that a specific cause is not identified in up to 33% of stroke cases5–8, it is estimated that over 2.5 million cryptogenic strokes occur globally per year. As for other types of strokes, the overall management of vascular risk factors and encouragement of a healthier lifestyle are crucial for an effective secondary prevention strategy.
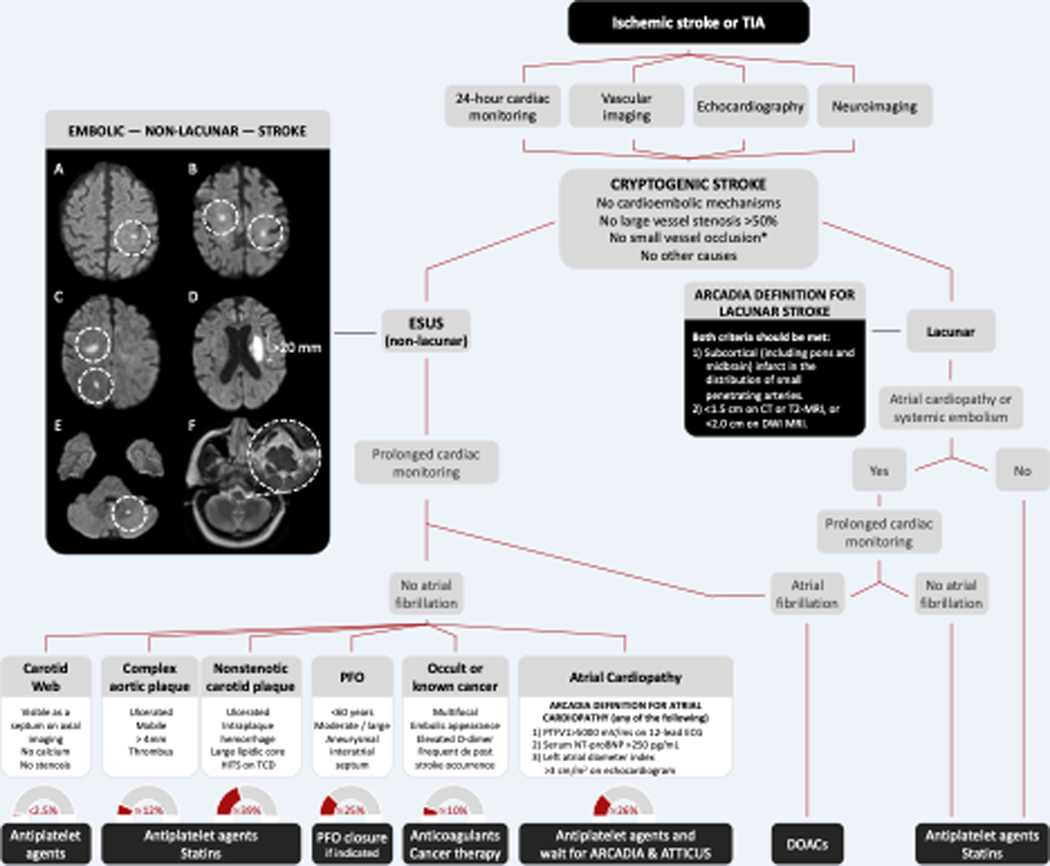
The diagnosis of cryptogenic stroke entails ruling out medium and high-risk cardioembolic sources, >50% stenosis of major extracranial or intracranial arteries, small vessel occlusion, and other less common causes (Figure 2). Based on the distribution and number of brain infarcts on neuroimaging studies, cryptogenic strokes can be further categorized into ESUS (non-lacunar) and lacunar. ESUS accounts for 16% of all ischemic strokes.67 Lacunar cryptogenic strokes are those showing small infarcts involving subcortical brain or brainstem regions in patients who do not present with classical lacunar syndromes or who have cortical symptoms, thus not fulfilling the TOAST criteria for small vessel occlusion.4 The distinction between ESUS and lacunar cryptogenic stroke is sometimes challenging. The criteria used in the AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke (ARCADIA) randomized clinical trial provides a simple and clinically sound approach.39 According to the ARCADIA criteria, lacunar cryptogenic strokes are those involving subcortical cerebral territories of small penetrating arteries, including the pons and midbrain. The established size of lacunar infarcts is <1.5 cm on CT or T2-MRI, or <2.0 cm on diffusion-weighted imaging (DWI) MRI. Embolic infarcts are those involving the cerebral cortex, multiple small subcortical infarcts occurring simultaneously in the same or different vascular territories, lateral medullary infarcts, or cerebellar infarcts.39
Figure 2. Proposed Algorithm for the Diagnosis and Treatment of Cryptogenic Stroke.
Panel A. Classical embolic stroke involving the cerebral cortex. Panel B. Bilateral small subcortical infarcts. Panel C. Small simultaneous subcortical infarcts involving the same vascular territory. Panel D. Single, large (>20 mm) deep infarct. Panel E. Embolic cerebellar infarct. Panel E. Lateral medullary infarct.
ARCADIA, AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke. ESUS, embolic stroke of undetermined source. CT, computed tomography. MRI, magnetic resonance imaging. DWI, diffusion-weighted imaging. PFO, patent foramen ovale. HITS, high-intensity transient signals. TCD, transcranial Doppler ultrasound.
No small vessel occlusion*, clinical TOAST criteria for small occlusion are a traditional lacunar syndrome and the absence of cortical deficits.
Historically, secondary stroke prevention strategies for patients with cryptogenic stroke were mainly focused on the use of antiplatelet therapy and the management of risk factors. More recently, a series of emerging stroke mechanisms have been proposed as the cause of ESUS, expanding secondary prevention options. The identification of these underlying embolic sources requires a tailored and thorough diagnostic approach (Figure 2).
Atrial Fibrillation Detected After Stroke.
The definition of ESUS requires only 24 hours of Holter monitoring. However, it is well established that both AF detection yields and the proportion of patients started on oral anticoagulants increase with longer durations of cardiac monitoring.68 Indeed, AF can be newly diagnosed in 25% of ESUS patients undergoing implantable loop recording.69 However, evidence supporting an association between very prolonged cardiac monitoring (e.g., implantable loop recorders) and lower risk of stroke recurrence is lacking and constitutes an open matter of debate.68 In the meantime and until data from ongoing randomized clinical trials (e.g., FIND-AF2, clinicaltrials.gov, NCT04371055) become available, patients with ESUS and a new diagnosis of AF should be started on DOACs based on their proven efficacy and safety for secondary stroke prevention in the overall AF population.53
Atrial Cardiopathy.
Antiplatelet therapy is the present antithrombotic standard of care for secondary stroke prevention in ESUS patients.70, 71 In the Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source (NAVIGATE-ESUS) trial, rivaroxaban was not superior to aspirin in reducing the risk of recurrent ischemic stroke and resulted in a greater number of major hemorrhages.70 In the Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source (RE-SPECT ESUS) trial, dabigatran was not more effective than aspirin in preventing recurrent ischemic stroke, with no significant differences in major bleeding rates between groups.71 The lack of superiority of direct oral anticoagulants (DOACs) relative to aspirin in ESUS trials highlights the need for a better selection of ESUS patients with higher chances of benefiting from oral anticoagulants. This is the rationale behind ARCADIA, a randomized clinical trial comparing apixaban vs. aspirin for preventing recurrent strokes in ESUS patients with atrial cardiopathy (clinicaltrials.gov, NCT03192215).39 The concept of atrial cardiopathy, also known as atrial substrate, failure, or vulnerability, involves several mechanisms leading to increased risk of embolism, including endothelial dysfunction, local hypercoagulability, and fibrosis.72 Atrial cardiopathy can be diagnosed in at least 26% of ESUS patients when using the ARCADIA criteria.73 This definition entails the presence of at least one of three factors associated with increased risk of stroke independently of AF: P wave terminal force in V1 >5000 mV/ms, serum NT-proBNP >250 pg/mL, or a left atrial diameter index of 3 cm/m2 on echocardiography.39, 72 Apixaban for Treatment of Embolic Stroke of Undetermined Source (ATTICUS) is another ongoing randomized clinical trial comparing apixaban and aspirin (clinicaltrials.gov, NCT02427126).74 The primary outcome is the occurrence of ≥1 ischemic lesions at 12 months after study drug initiation relative to the baseline MRI. If these trials show that apixaban is effective and safe in preventing recurrent strokes in patients with ESUS and atrial cardiopathy, it will result in a paradigm shift in secondary stroke prevention. Meanwhile, patients with criteria for atrial cardiopathy may benefit from more intense cardiac monitoring (beyond the 24 hours required for the ESUS definition), considering that the presence of atrial substrate is associated with higher AF detection and stroke recurrence rates.68
Patent Foramen Ovale.
A PFO can be diagnosed in over 25% of patients with ESUS. Despite the availability of PFO closure as a treatment with proven efficacy and safety for secondary stroke prevention in this population, randomized controlled trials of ESUS have not excluded patients with PFO. As discussed previously, patients in whom a PFO is deemed to have a causal role and who do not have a competing stroke mechanism should undergo closure.
Complex aortic plaque.
Atherosclerotic plaques involving the aortic arch constitute a well-recognized embolic source and are associated with 4-fold increased risk of stroke recurrence at 2.4 years, with annual rates ranging between 7% and 12%.75, 76 The prevalence of complex aortic plaques (ulcerated, with a thickness ≥4 mm, a mobile component or a thrombus) in ESUS patients is approximately 8%.76 To date, there is no evidence that oral anticoagulants are superior to antiplatelet agents in preventing recurrent ischemic strokes in this population. The mainstay of secondary stroke prevention in patients with ESUS and complex aortic plaques is antiplatelet therapy in combination with high-dose statins (goal of LDL cholesterol of <70 mg/dL).53
Nonstenotic Internal Carotid Artery Plaque.
Ipsilateral nonstenotic (<50% stenosis) internal carotid artery plaques have been reported in 39% of ESUS patients77 and are associated with increased risk of progression to severe stenosis and recurrent stroke risk.78 The current standard of care for these patients is the combination of antiplatelet agents and high-dose statins.53 The presence of intraplaque hemorrhage, ulceration, a large necrotic or lipid core are associated with higher embolic risk. The presence of high-intensity transient signals on prolonged transcranial Doppler ultrasound may also help to identify patients with higher odds of stoke recurrence, although more research is needed.79
Carotid Web.
Carotid webs constitute an increasingly recognized stroke mechanism associated with up to 40% recurrence rates.22 The most reliable series reported a prevalence of carotid webs ipsilateral to a large vessel occlusion in up to 2.5% of unselected patients undergoing mechanical thrombectomy.80 Given the lack of data from randomized controlled trials, antiplatelet agents are recommended for secondary stroke prevention.53 In patients with breakthrough strokes on antiplatelet therapy and no competing causes, carotid endarterectomy or stenting may be considered.53
Occult or Active Neoplasms.
Cancer is a well-established cause of stroke and can be diagnosed in up to 10% of patients with ESUS within the year after the stroke.21 Occult cancer should be suspected in ESUS patients with multifocal, embolic appearing strokes and elevated D-Dimer.21 To date, there are no randomized data to guide secondary stroke prevention decisions in patients with ESUS and cancer. Based on the likelihood of hypercoagulability as the main mechanism, anticoagulants are used in clinical practice, but randomized clinical trials are needed.
Other potential stroke mechanisms reported in patients with ESUS include but are not limited to unruptured intracranial aneurysms, left ventricular wall motion abnormalities, decreased left ventricular ejection fraction, cardiac tumors (e.g., papillary fibroelastoma, myxoma), and hypercoagulability in young patients.
Non-ESUS (Lacunar) Cryptogenic Stroke.
A proportion of patients with cryptogenic strokes present with subcortical or brainstem small infarcts, but do not fulfill the TOAST criteria for small vessel occlusion (e.g., a traditional clinical lacunar syndrome and absence of cortical neurological deficits).4 Despite this, the most likely culprit mechanism is small vessel disease. It must be noted that the absence of classical risk factors for small vessel disease such as hypertension, diabetes, and age, does not rule out this diagnosis.81 Alternatively, it has been well documented that a considerable proportion of patients with single, deep, acute brain infarcts have an underlying cardioembolic source, mostly among the elderly population.82 Therefore, highly selected patients with lacunar cryptogenic strokes may still benefit from additional cardiac monitoring. Possible candidates for prolonged cardiac monitoring are those with evidence of concurrent systemic embolism (e.g., renal or splenic infarcts), increased left atrial size, elevated NT-proBNP, and low burden of white matter disease, chronic lacunar infarcts, perivascular spaces, or microhemorrhages. In support of this concept, in the Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE-AF) trial, a new diagnosis of AF was made in 12.6% of patients with a presumed diagnosis of small vessel disease undergoing 12 months of prolonged cardiac monitoring.83 It remains unknown if patients with lacunar cryptogenic strokes benefit from anticoagulation if low-burden AF is detected. As such, the decision to pursue prolonged cardiac monitoring in this population should be weighted on a careful and personalized basis.
5. Secondary Stroke Prevention in Intracranial Atherosclerotic Disease
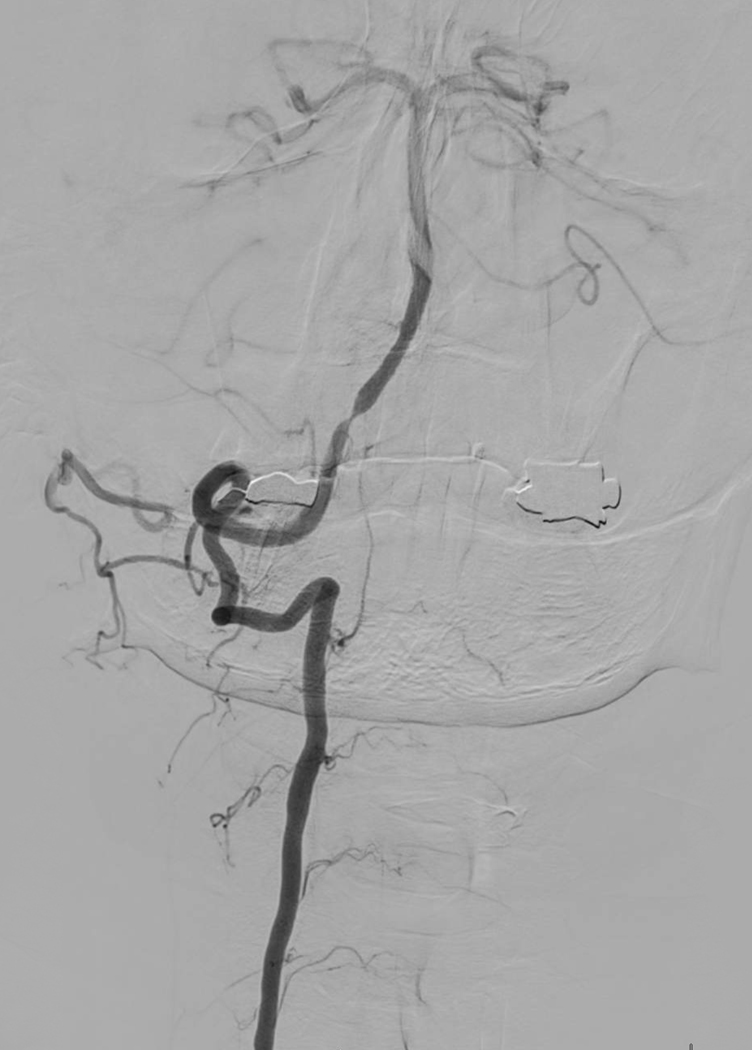
Intracranial atherosclerotic disease (ICAD)(Figure 3) is the most common cause of ischemic stroke worldwide, causing up to 50% of ischemic strokes in Asia and a substantial proportion of strokes in the United States where minorities are affected disproportionately84.
Figure 3.
Intracranial atherosclerosis involving the distal vertebral artery
Stroke Recurrence
Seminal clinical trials have demonstrated that ICAD is the stroke subtype with a high risk of recurrence. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study evaluated patients with a stroke or transient ischemic attack (TIA) in the previous 90 days due to intracranial stenosis of 50–99% and found a 19% risk of recurrent ischemic stroke at 2 years, with 75% occurring in the distribution of the stenotic artery85. The risk of silent infarcts is much higher; in a prospective observational study of patients with recently symptomatic ICAD, 25% had a new infarct at 6–8 weeks, five times higher than clinical events in that early period86.
Markers of higher risk
Certain clinical features are associated with greater risk of stroke recurrence, including women and the early period after the index event87,88. Imaging biomarkers of higher risk have also been identified. After an incident stroke, the risk of recurrent stroke was 25% in those with severe (70–99%) stenosis, compared to only 11% in moderate (50–69%) stenosis89. Multiple infarcts and a border zone pattern are associated with greater risk90,91, suggesting that plaque instability and failure of collaterals may play a role in stroke recurrence. Quantitative flow imaging has described Impaired flow as a risk marker risk in certain patients92.
Antithrombotic treatment
Antithrombotic treatment is a mainstay of management for secondary prevention in ICAD. Anticoagulants were tested in the WASID trial and found not effective; patients treated with warfarin to a goal INR 2–3 had a similar rate of stroke recurrence than those on aspirin 1300 mg/day but a higher rate of major hemorrhagic events and death85. Another trial enrolled ICAD patients within 10 days from onset and randomized them to a low molecular weight heparin or aspirin for 10 days, followed by aspirin alone for 6 months, and found no benefit of anticoagulation in early or delayed stroke recurrence93.
DAPT has become standard of care for the initial three months after an index event. Although the combination of aspirin and clopidogrel vs. aspirin alone have not been tested in an ICAD clinical trial, indirect comparisons of the Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMMPRIS) trial, which enrolled a higher risk population with 70–99% stenosis within 30 days on stroke onset treated with aspirin and clopidogrel, and a similar high-risk cohort from WASID94, suggested that DAPT for 90 days was superior to aspirin in preventing recurrence in this earlier period. Two trials evaluated patients with minor stroke or TIA within 24 hours from onset randomized to DAPT or aspirin and both included subgroups with large vessel disease. The THALES trial evaluated ticagrelor plus aspirin vs. aspirin alone for 30 days in the subgroup with large artery disease. Including those with ICAD, DAPT reduced the risk of recurrent stroke95. However, a subgroup analysis of Chinese patients with ICAD enrolled in the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial did not find a difference between aspirin and clopidogrel vs. aspirin alone in stroke recurrence at 90 days96.
Cilostazol-based regimens of DAPT for ICAD have been tested in Asian populations. The Cilostazol-Aspirin Therapy against Recurrent Stroke with Intracranial Artery Stenosis study (CATHARSIS)97 did not find a benefit of cilostazol plus aspirin vs. aspirin alone. However, the open-label Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS) trial, which compared cilostazol plus aspirin or clopidogrel vs. aspirin or clopidogrel alone in patients 8 to 180 days after symptom onset did find a lower risk of recurrent ischemic stroke (HR 0.49 95%CI 0.31–0.76), but that study had limitations, including the fact that only 29% of the population had ICAD and that the trial was ended prematurely due to slow enrollment98. DAPT with cilostazol plus aspirin vs. clopidogrel plus aspirin was tested in the Trial of Cilostazol in Symptomatic Intracranial Arterial Stenosis II (TOSS-2) trial which enrolled patients with ICAD within 2 weeks; there was no difference in stroke recurrence in the territory of the symptomatic vessel at 7 months with either regimen99. DAPT did not increase hemorrhagic complications in ICAD studies.
Vascular risk factor control
Poorly controlled cholesterol and low-density lipoprotein100, elevated blood pressure101 and inadequate physical activity102 are associated with greater stroke and major cardiovascular event recurrence. Therefore, it is recommended to utilize high-dose statins, engage in at least moderate regular physical activity, and achieve a systolic blood pressure of <130 mmHg53. However, the early post-stroke management of blood pressure after an ICAD-related stroke has not been well studied, and smaller trials have suggested potential higher risk of aggressive blood pressure control in the early window103, particularly in those with hemodynamic impairment104.
Revascularization
Endovascular approaches to ICAD have been tested in two randomized clinical trials with negative results. The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial evaluated high-risk patients with symptoms within 30 days (median 7 days) with high-grade stenosis and randomized patients to intensive medical therapy with or without angioplasty and stenting with a self-expanding stent. It found a greater rate of stroke and death at 30 days of 14.7% in the intervention arm vs. 5.3 in the medical treatment arm105, with no delayed benefit was observed in long-term outcomes106. Similarly, the Vitesse Stent Ischemic Therapy (VISSIT) trial utilizing a balloon-mounted stent found a higher peri-procedural risk with endovascular therapy107. Therefore, angioplasty and stenting should not be recommended as initial treatment of symptomatic ICAD. Although an observational post-market surveillance study of highly selected patients with 70–99% and at least two strokes in the territory of the stenotic vessel treated at least 8 days after symptoms had a lower complication rate than in randomized controlled trials108 the efficacy of this intervention after a second stroke on medical therapy is currently unknown.
Areas of future research
Given the high risk of recurrence in symptomatic ICAD and the failure of recent endovascular trials, there is active research evaluating novel treatment modalities. More intensive antilipidemic treatment with PCSK9 inhibitors (NCT04573777) and testing other antithrombotic regimens, including ticagrelor and low dose direct oral anticoagulants plus aspirin are being evaluated in ongoing studies (NCT05047172). Initial studies of indirect flow bypass with encephaloduroarteriosynangiosis appear to be safe with indications of efficacy109. These interventions need to be tested in larger controlled trials.
6. Stroke prevention in Extracranial atherosclerotic disease
Atherosclerotic carotid artery disease is one of the major causes of ischemic stroke and transient ischemic attack (TIA), accounting for about 10% of cases. Atherosclerotic carotid stenosis occurs primarily at the carotid bifurcation, involving the distal common and the proximal internal carotid artery (ICA). Increasing age, male sex, and diabetes are associated with an increased incidence of carotid stenosis. In a combined analysis of four population-based studies, >50% carotid stenosis was found in 2.3% of men between the ages of 60–69 years, rising to 7.5% above age 80 years110. The corresponding figures for women were 2.0% and 5.0%.
For patients with evidence of extracranial internal carotid artery stenosis who have a recent stroke or TIA in the territory of the stenotic ICA (“symptomatic carotid stenosis”), previous trials showed robust benefit of carotid endarterectomy for severe (70–99%) stenosis111. For patients with moderate stenosis (50–69%), there was modest benefit and there was no demonstrable benefit below 50% stenosis. A major issue with application of these results to contemporary patients is that the trials were initiated more than 30 years ago, during a time when intensity of medical therapy was very different. An example of treatment differences between the first-generation CEA trials and modern practice is found in Table 3.
Table 3.
Developments in medical therapy for carotid stenosis
| Condition | Treatment in first generation carotid stenosis trials | Modern treatment |
|---|---|---|
| Antithrombotic therapy | Aspirin alone | Aspirin + clopidogrel |
| Lipids | Little statin use | High potency statins |
| Blood pressure | No specific target | Systolic blood pressure <130 mm Hg |
| Smoking cessation | No pharmacologic therapy | New pharmacologic treatments |
| Physical activity | No specific target | Benefits understood for regular physical activity (3–4 sessions of aerobic exercise per week) |
| Diabetes | No specific medications for CV risk | Pharmacologic treatments that reduce CV risk, hemoglobin A1C target of <7 |
| High triglyceride levels | No specific treatment | Icosapent ethyl |
For symptomatic patients, a combined analysis of the first-generation trials found several features that were associated with increase benefit from CEA. These included surgery within two weeks of randomization in the trial, male sex, and older age112. Early surgery, with revascularization performed within 2 weeks of the last symptomatic event, is now recommended in guidelines53. For patients who are fit for even earlier surgery, typically reserved for minor strokes, a combined analysis of several trials found a lower complication rate with CEA (1.3% rate of stroke/death) compared to CAS (8.3%)113.
Regarding choice of revascularization procedure, CEA is currently performed much more often than CAS, with CEA accounting for >80% of procedures in a previous national study114. CAS has been associated with a higher complication rate above age 70 years. CEA has been associated with a lower rate of periprocedural stroke but a higher rate of periprocedural myocardial infarction in relation to CAS115.
A newer revascularization option is Transcarotid Artery Revascularization (TCAR). In this procedure, there is direct access achieved in the common carotid artery and flow reversal is instituted. Analysis of a large registry found a lower stroke/death rate with TCAR compared to transfemoral CAS116. In the single arm ROADSTER 2 registry, the 30-day stroke/death rate was 5.0% in symptomatic patients and 1.4% in asymptomatic patients117. However, there has not been a randomized trial comparing TCAR with either CEA or CAS118. Despite growing enthusiasm for TCAR, the AHA/ASA secondary prevention guidelines felt that the efficacy of TCAR for symptomatic patients is not well established53.
Areas for future investigation include assessment of intensive medical therapy for symptomatic patients119. In addition, the role of combination therapy with a direct oral anticoagulant plus antiplatelet therapy for symptomatic carotid stenosis deserves to be tested120. Finally, high quality trials of TCAR are needed to establish whether it is as safe as CEA.
7. Small Vessel Disease (SVD)
SVD causes one fourth of ischemic strokes, most of intracerebral hemorrhages (ICH), and contribute to up to 45% of dementias121. The full spectrum of cerebral SVD includes covert cerebral SVD detected incidentally on brain neuroimaging, and SVD-related clinical presentations with lacunar stroke, cognitive decline or dementia, mood or physical dysfunction. The most prevalent markers of SVD on brain imaging are: white-matter hyperintensities, cerebral microbleeds (CMBs), lacunes and enlarged perivascular spaces.
Management of antithrombotic agents in the context of ischemic stroke related to SVD
Prevention of future ischemic events
In the Secondary Prevention of Small Subcortical Stroke (SPS3) trial of 3020 patients with lacunar ischemic stroke (mean age 63 years), long-term dual versus single antiplatelet therapy increased bleeding and death without reducing recurrent stroke27. Indeed, the pathogenesis of SVD is more complex than just an arteriolar occlusion leading to infarct81. The pathological findings in SVD are arteriolosclerosis, lipohyalinosis, fibrinoid necrosis in which thrombi may not be relevant. So, the usefulness of antiplatelet agent such as aspirin may be challenged. However, since most of the secondary prevention trials lumped together non cardioembolic strokes, it is difficult to build specific recommendation for SVD. Therefore, the general guidance for non-cardioembolic secondary stroke prevention and the use of antiplatelet therapy also applies for stroke caused by SVD53.
As an alternative to aspirin, cilostazol is a phosphodiesterase 3′ inhibitor with mild antiplatelet effects, and several actions targeting processes involved in SVD pathophysiology: endothelial dysfunction, myelin repair, neuroprotection, and inflammation121. In patients with SVD, the balance between the prevention of a recurrent occlusive event versus the risk of ICH is a major concern. In a systematic review, cilostazol decreased recurrent ischemic stroke (17 trials, n=10225, OR=0.68 [95% CI, 0.57–0.81]), hemorrhagic stroke (16 trials, n=9736, OR=0.43 [95% CI, 0.29–0.64]), deaths (OR=0.64 [95% CI, 0.49–0.83]), systemic bleeding (n=8387, OR=0.73 [95% CI, 0.54–0.99])122. To date, the usefulness of cilostazol in non-Asian population in ischemic stroke related to SVD remains uncertain53.
In the context of covert cerebral SVD, one small RCT focused on patients older than 45 years who had at least one silent brain infarct on imaging. They were randomized to either aspirin 100mg (n=36) or placebo (n=47). After four years, the primary endpoint (ischemic stroke, TIA or new silent brain infarction on MRI) occurred in nine controls versus two patients in the aspirin group (p=0.10)123. Data on covert cerebral SVD progression can also be extracted from secondary prevention trials such as SPS3 or primary prevention such as ASPREE124. The 2021 ESO guidelines on covert cerebral SVD reviewed available evidence and concluded that in patients with covert SVD (i.e., who did not have any clinical stroke) antiplatelet agents cannot be recommended to reduce the risk of future stroke, major adverse cardiovascular events (MACE) or cognitive decline125.
SVD is both an ischemic and a hemorrhagic disease
CMBs are markers of the nature and severity of the underlying SVD126 and patients with multiple CMBs may be at increased risk of bleeding, potentially jeopardizing the benefits of antiplatelet treatment after ischemic stroke. Observational data have consistently reported greater rates of future symptomatic ICH in antithrombotic users with CMBs compared to those without CMBs. However, there are inconsistencies in the literature that reflect methodological limitations due to the observational design of these studies, and to the heterogeneity in study populations127. Several MRI subgroup analyses of RCTs (which did not specifically included SVD patients) have reported estimated annualized incident CMB rates of 3–7%, without any apparent differences when comparing between differing antiplatelet monotherapy agents, dual antiplatelet therapy vs. monotherapy, or NOAC treatment vs. aspirin monotherapy127. Four RCTs have assessed interactions between differing antithrombotic regimens and baseline CMBs for clinical outcomes. In patients with recent lacunar stroke participating in the SPS3 trial (n=1278, 30% with CMBs) there were no significant interactions noted between baseline CMB presence and random assignment to combined aspirin/clopidogrel treatment compared with aspirin monotherapy for the outcomes of recurrent stroke and mortality over 3.3 years of follow-up128. In the RESTART trial (ICH patients with concomitant thrombo-embolic/occlusive disease were randomized either to aspirin or to avoid antiplatelet agents), the subgroup analyses of the 235 ICH participants with CMBs showed that, neither the presence, burden, or the location of CMBs influenced the effects of antiplatelet therapy on recurrent sICH129. In the PICASSO trial, the risk of symptomatic ICH was lower with cilostazol than aspirin (0.12%/year vs. 1.49%/year; HR, 0.08; 95%CI 0.01–0.60) in participants with ischemic stroke and baseline CMBs but was not different between the two treatment arms (1.26%/year vs. 0.79%/year; HR 1.60; 95%CI 0.52–4.90) in participants with prior symptomatic ICH (p interaction = 0.011)130. Concerning oral anticoagulation, there were no interaction identified between baseline presence, severity or location of CMBs and rivaroxaban 15 mg daily compared with aspirin for the outcomes of ischemic stroke, ICH or mortality over 11 months of follow-up in NAVIGATE ESUS participants (n=3699, 11% with CMBs)131. Current evidence does not justify withholding these evidence-based treatments from stroke patients solely on the basis of CMBs on MRI.
Other pharmacological interventions
Blood pressure management
BP recommendations for secondary stroke prevention also applies for patients who had a lacunar stroke53. In patients with SVD, intensive management of BP is probably the most effective way to prevent both ischemic and hemorrhagic events as well as cognitive decline. However, in normotensive patients with covert cerebral SVD, systematic BP lowering is currently not recommended125.
Statins
While hyperlipidemia does not play an important role in the development of SVD compared with atheroma, the effect of lipid-lowering therapy on SVD remains unclear. Statins are recommended in secondary prevention guidelines53 with no evidence of different effects in lacunar ischemic stroke.
Lifestyle
As in other types of stroke, long-term prevention of recurrent lacunar stroke includes the control of vascular risk factors. In people with SVD, physical activity may play an important role in secondary stroke prevention125. Few observational data (but no RCTs) focused on the effect of physical activity in SVD patients. An observational analysis of 503 Dutch participants with SVD on neuroimaging without dementia (some participants with history of TIA or minor stroke) suggested that higher baseline physical activity is related to lower incidence of cerebrovascular events (composite endpoint of TIAs, ischemic and hemorrhagic strokes and vascular dementia) (adjusted HR: 0.58, 95%CI: 0.36–0.96) over 9-year follow-up132.
Acknowledgments
DISCLOSURES
Dr. Mac Grory reports no disclosures.
Dr. Yaghi reports a non-funded research collaboration with Medtronic
Dr. Cordonnier reports Boehringer-Ingelheim (Speaker fees), BMS (international RCT steering committee), Amgen (Speaker fees), Astra Zeneca (consulting) and serving as an Associate Editor for Stroke
Dr. Sposato reports Speaker, consulting honoraria, and research grants from Boehringer Ingelheim, Pfizer, Bayer, Daiichi Sankyo and Gore; Chair, WSO Brain & Heart Task Force; Member, Editorial Board of Neurology, Stroke, and JAHA. Neurocardiology section editor, Stroke. Associate Guest Editor, JAHA.
Dr. Romano reports Research salary support to Department of Neurology at the University of Miami from NIH/NINDS for role as PI (MPI) of the MyRIAD study (1R01NS084288) [2014-2020], and from Genentech for role as PI of the Mild and Rapidly Improving Stroke Study (MaRISS) [2013-2020]
Dr. Chaturvedi reports consulting for Astra Zeneca and BrainsGate and serving as an Associate Editor for Stroke, and as an editorial board member of Neurology and Journal of Stroke & Cerebrovascular Disease.
Non-Standard Abbreviations and Acronyms
- AF
Atrial fibrillation
- ARCADIA
AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke
- ARISTOTLE
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
- AVERROES
Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment
- ASCOD
Atherosclerosis/Small Vessel/Cardiac/Other/Dissection
- ATTICUS
Apixaban for Treatment of Embolic Stroke of Undetermined Source
- CAS
Carotid artery stenting
- CATHARSIS
Cilostazol-Aspirin Therapy against Recurrent Stroke with Intracranial Artery Stenosis
- CCS
Causative Classification of Stroke
- CEA
Carotid endarterectomy
- CHANCE
Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack
- COMPASS
Cardiovascular Outcomes for People Using Anticoagulation Strategies
- CRYSTAL AF
Cryptogenic stroke and underlying AF
- DAPT
Dual antiplatelet therapy
- DOAC
Direct oral anticoagulant
- DWI
Diffusion weighted imaging
- EMBRACE
Event monitor belt for recording atrial fibrillation after a cerebral ischemic event
- ENGAGE AF
Global Study to Assess the Safety and Effectiveness of Edoxaban (DU-176b) vs Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation
- ESO
European Stroke Organization
- ESUS
Embolic stroke of undetermined source
- EVT
Endovascular thrombectomy
- ICAD
Intracranial atherosclerotic disease
- ICH
Intracerebral hemorrhage
- LAA
Left atrial appendage
- LV
Left ventricle
- NAVIGATE ESUS
Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source
- NOAC
Novel oral anticoagulant
- POINT
Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke
- PFO
Patent foramen ovale
- PREVAIL
Prospective Randomised Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy
- PROTECT AF
Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation
- RCT
Randomized controlled trial
- RE-LY
Randomized Evaluation of Long-Term Anticoagulant Therapy
- RESPECT ESUS
Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source
- RESTART
Restart or stop antithrombotics Randomized Trial
- ROADSTER
Safety and Efficacy Study for Reverse Flow Used During Carotid Artery Stenting Procedure
- ROCKET AF
Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation
- ROPE
Risk of paradoxical embolism
- SAMMPRIS
Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis
- SPS
Secondary Prevention of Small Subcortical Stroke
- STROKE-AF
Stroke of Known Cause and Underlying Atrial Fibrillation
- SVD
Small vessel disease
- TCAR
Transcarotid artery revascularization
- TEE
Transesophageal echocardiography
- THALES
Acute Stroke or Transient Ischemic Attack Treated with Ticagrelor and ASA for Prevention of Stroke and Death
- TOAST
Trial of Org 10172 in Acute Ischemic Stroke
- TOSS
Trial of Cilostazol in Symptomatic Intracranial Arterial Stenosis
- tPA
Tissue plasminogen activator
- TTE
Transthoracic echocardiogram
- VISSIT
Vitesse Intracranial Stent Study for Ischemic Stroke Therapy
- WARCEF
Warfarin vs Aspirin for reduced cardiac ejection fraction
- WARSS
Warfarin aspirin recurrent stroke study
- WASID
Warfarin-Aspirin Symptomatic Intracranial Disease
REFERENCES
- 1.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME and Hennerici MG. The ASCOD phenotyping of ischemic stroke (Updated ASCO Phenotyping). Cerebrovasc Dis. 2013;36:1–5. [DOI] [PubMed] [Google Scholar]
- 3.Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, Ayata C, Towfighi A, Smith EE, Chong JY et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007;38:2979–84. [DOI] [PubMed] [Google Scholar]
- 4.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL and Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 5.Mac Grory B, Flood SP, Apostolidou E and Yaghi S. Cryptogenic Stroke: Diagnostic Workup and Management. Curr Treat Options Cardiovasc Med. 2019;21:77. [DOI] [PubMed] [Google Scholar]
- 6.Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR and Wolf PA. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382–90. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Yiin GS, Geraghty OC, Schulz UG, Kuker W, Mehta Z, Rothwell PM and Study OV. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saver JL. Cryptogenic Stroke. N Engl J Med. 2016;375:e26. [DOI] [PubMed] [Google Scholar]
- 9.Hart RG, Catanese L, Perera KS, Ntaios G and Connolly SJ. Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update. Stroke. 2017;48:867–872. [DOI] [PubMed] [Google Scholar]
- 10.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, Sacco RL, Connolly SJ and Group CSEIW. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–38. [DOI] [PubMed] [Google Scholar]
- 11.Ntaios G, Perlepe K, Lambrou D, Sirimarco G, Strambo D, Eskandari A, Karagkiozi E, Vemmou A,Korompoki E, Manios E, et al. Identification of patients with embolic stroke of undetermined source and low risk of new incident atrial fibrillation: The AF-ESUS score. Int J Stroke 2021; 16: 29–38 [DOI] [PubMed] [Google Scholar]
- 12.Grosse GM. Sieweke J, Biber S, Ziegler NL, Gabriel MM, Schuppner R, Worthmann H, Bavendiek U, Weissenborn K. Nonstenotic carotid plaque in embolic stroke of unknown source. Stroke 2020; 51: 3737–3741 [DOI] [PubMed] [Google Scholar]
- 13.Kamel H, Merkler AE, Iadecola C, Gupta A and Navi BB. Tailoring the Approach to Embolic Stroke of Undetermined Source: A Review. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elgendy AY, Saver JL, Amin Z, Boudoulas KD, Carroll JD, Elgendy IY, Grunwald IQ, Gertz ZM, Hijazi ZM, Horlick EM et al. Proposal for Updated Nomenclature and Classification of Potential Causative Mechanism in Patent Foramen Ovale-Associated Stroke. JAMA Neurol. 2020;77:878–886. [DOI] [PubMed] [Google Scholar]
- 15.Kamel H, Okin PM, Elkind MS and Iadecola C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke. 2016;47:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta A, Gialdini G, Lerario MP, Baradaran H, Giambrone A, Navi BB, Marshall RS, Iadecola C and Kamel H. Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke. J Am Heart Assoc. 2015;4:e002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O’Donnell M, Laupacis A, Côté R et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–77. [DOI] [PubMed] [Google Scholar]
- 18.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F et al. Cryptogenic stroke and underlying atrial fibrillation. The New England journal of medicine. 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- 19.Katsanos AH, Bhole R, Frogoudaki A, Giannopoulos S, Goyal N, Vrettou AR, Ikonomidis I, Paraskevaidis I, Pappas K, Parissis J et al. The value of transesophageal echocardiography for embolic strokes of undetermined source. Neurology. 2016;87:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bushnell CD and Goldstein LB. Diagnostic testing for coagulopathies in patients with ischemic stroke. Stroke. 2000;31:3067–78. [DOI] [PubMed] [Google Scholar]
- 21.Navi BB, Kasner SE, Elkind MSV, Cushman M, Bang OY and DeAngelis LM. Cancer and Embolic Stroke of Undetermined Source. Stroke. 2021;52:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mac Grory B, Emmer BJ, Roosendaal SD, Zagzag D, Yaghi S and Nossek E. Carotid web: an occult mechanism of embolic stroke. J Neurol Neurosurg Psychiatry. 2020. [DOI] [PubMed] [Google Scholar]
- 23.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–51. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–9. [DOI] [PubMed] [Google Scholar]
- 25.Johnston SC, Easton DJ, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379: 215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, Ladenvall P, Molina CA, Wang Y, for the THALES Investigators. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med 2020; 383: 207–217 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Meng X, Wang A, Xie X, Pan Y, Johnston SC, Li H, Bath PM, Dong Q, Xu A et al. Ticagrelor versus Clopidogrel in CYP2C19 Loss-of-Function Carriers with Stroke or TIA. N Engl J Med. 2021. [DOI] [PubMed] [Google Scholar]
- 28.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 29.Sharma M, Hart RG, Connolly SJ, Bosch J, Shestakovska O, Ng KKH, Catanese L, Keltai K, Aboyans V, Alings M, et al. Stroke Outcomes in the COMPASS Trial. Circulation. 2019;139:1134–1145 [DOI] [PubMed] [Google Scholar]
- 30.Perera KS, Ng KKH, Nayar S, Catanese L, Dyal L, Sharma M, Connolly SJ, Yusuf S, Bosch J, Eikelboom JW, et al. Association Between Low-Dose Rivaroxaban With or Without Aspirin and Ischemic Stroke Subtypes: A Secondary Analysis of the COMPASS Trial. JAMA Neurol. 2020;77:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B and Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–40. [DOI] [PubMed] [Google Scholar]
- 32.Henninger N, Goddeau RP Jr., Karmarkar A, Helenius J and McManus DD. Atrial Fibrillation Is Associated With a Worse 90-Day Outcome Than Other Cardioembolic Stroke Subtypes. Stroke. 2016;47:1486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamel H, Navi BB, Parikh NS, Merkler AE, Okin PM, Devereux RB, Weinsaft JW, Kim J, Cheung JW, Kim LK et al. Machine Learning Prediction of Stroke Mechanism in Embolic Strokes of Undetermined Source. Stroke. 2020;51:e203–e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. [DOI] [PubMed] [Google Scholar]
- 35.Kornej J, Börschel CS, Benjamin EJ and Schnabel RB. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ Res. 2020;127:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer DE, Ziegler PD, Koehler JL, Sarkar S and Passman RS. Temporal Association Between Episodes of Atrial Fibrillation and Risk of Ischemic Stroke. JAMA Cardiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, Van Gelder IC, Hohnloser SH, Carlson M et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–9. [DOI] [PubMed] [Google Scholar]
- 38.Yaghi S, Kamel H and Elkind MSV. Atrial cardiopathy: a mechanism of cryptogenic stroke. Expert review of cardiovascular therapy. 2017;15:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamel H, Longstreth WT Jr., Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, Meinzer C, Dillon C, Ewing I, Spilker JA et al. The AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke randomized trial: Rationale and methods. Int J Stroke. 2019;14:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benjamin EJ, Plehn JF, D’Agostino RB, Belanger AJ, Comai K, Fuller DL, Wolf PA and Levy D. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374–9. [DOI] [PubMed] [Google Scholar]
- 41.Vasconcelos M, Vasconcelos L, Ribeiro V, Campos C, Di-Flora F, Abreu L, Silva L, Diamantino T, Padilha da Silva JL, Esteves WAM et al. Incidence and predictors of stroke in patients with rheumatic heart disease. Heart. 2021;107:748–754. [DOI] [PubMed] [Google Scholar]
- 42.Whitlock RP, Sun JC, Fremes SE, Rubens FD and Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e576S-e600S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–14. [DOI] [PubMed] [Google Scholar]
- 44.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. [DOI] [PubMed] [Google Scholar]
- 45.Dawwas GK, Dietrich E, Cuker A, Barnes GD, Leonard CE and Lewis JD. Effectiveness and Safety of Direct Oral Anticoagulants Versus Warfarin in Patients With Valvular Atrial Fibrillation : A Population-Based Cohort Study. Ann Intern Med. 2021;174:910–919. [DOI] [PubMed] [Google Scholar]
- 46.Merkler AE, Chu SY, Lerario MP, Navi BB and Kamel H. Temporal relationship between infective endocarditis and stroke. Neurology. 2015;85:512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr. Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435–86. [DOI] [PubMed] [Google Scholar]
- 48.Freudenberger RS, Hellkamp AS, Halperin JL, Poole J, Anderson J, Johnson G, Mark DB, Lee KL and Bardy GH. Risk of thromboembolism in heart failure: an analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation. 2007;115:2637–41. [DOI] [PubMed] [Google Scholar]
- 49.Di Tullio MR, Qian M, Thompson JL, Labovitz AJ, Mann DL, Sacco RL, Pullicino PM, Freudenberger RS, Teerlink JR, Graham S et al. Left Ventricular Ejection Fraction and Risk of Stroke and Cardiac Events in Heart Failure: Data From the Warfarin Versus Aspirin in Reduced Ejection Fraction Trial. Stroke. 2016;47:2031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pullicino PM, Qian M, Sacco RL, Freudenberger R, Graham S, Teerlink JR, Mann D, Di Tullio MR, Ponikowski P, Lok DJ et al. Recurrent stroke in the warfarin versus aspirin in reduced cardiac ejection fraction (WARCEF) trial. Cerebrovasc Dis. 2014;38:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takasugi J, Yamagami H, Noguchi T, Morita Y, Tanaka T, Okuno Y, Yasuda S, Toyoda K, Gon Y, Todo K et al. Detection of Left Ventricular Thrombus by Cardiac Magnetic Resonance in Embolic Stroke of Undetermined Source. Stroke. 2017;48:2434–2440. [DOI] [PubMed] [Google Scholar]
- 52.Abdelmoneim SS, Pellikka PA and Mulvagh SL. Contrast echocardiography for assessment of left ventricular thrombi. J Ultrasound Med. 2014;33:1337–44. [DOI] [PubMed] [Google Scholar]
- 53.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. [DOI] [PubMed] [Google Scholar]
- 54.Yaghi S, Pilot M, Song C, Blum CA, Yakhkind A, Silver B, Furie KL, Elkind MS, Sherzai D and Sherzai AZ. Ischemic Stroke Risk After Acute Coronary Syndrome. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merkler AE, Bartz TM, Kamel H, Soliman EZ, Howard V, Psaty BM, Okin PM, Safford MM, Elkind MSV and Longstreth WT Jr., Silent Myocardial Infarction and Subsequent Ischemic Stroke in the Cardiovascular Health Study. Neurology. 2021;97:e436–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hagen PT, Scholz DG and Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clinic proceedings. 1984;59:17–20. [DOI] [PubMed] [Google Scholar]
- 57.Mojadidi MK, Mahmoud AN, Patel NK, Elgendy IY and Meier B. Cryptogenic Stroke and Patent Foramen Ovale: Ready for Prime Time? J Am Coll Cardiol. 2018;72:1183–1185. [DOI] [PubMed] [Google Scholar]
- 58.Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, Homma S, Di Angelantonio E, Di Tullio MR, Lutz JS, Elkind MS, Griffith J et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thaler A, Kvernland A, Kelly S, Song C, Aparicio HJ, Mac Grory B and Yaghi S. Stroke Prevention in Patients with Patent Foramen Ovale. Curr Cardiol Rep. 2021;23:183. [DOI] [PubMed] [Google Scholar]
- 60.Kent DM, Saver JL, Ruthazer R, Furlan AJ, Reisman M, Carroll JD, Smalling RW, Jüni P, Mattle HP, Meier B et al. Risk of Paradoxical Embolism (RoPE)-Estimated Attributable Fraction Correlates With the Benefit of Patent Foramen Ovale Closure: An Analysis of 3 Trials. Stroke. 2020;51:3119–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ and Schaff HV. Embolic potential of cardiac tumors and outcome after resection: a case-control study. Stroke. 2009;40:156–62. [DOI] [PubMed] [Google Scholar]
- 62.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. [DOI] [PubMed] [Google Scholar]
- 63.Valente M, Basso C, Thiene G, Bressan M, Stritoni P, Cocco P and Fasoli G. Fibroelastic papilloma: A not-so-benign cardiac tumor. Cardiovasc Pathol. 1992;1:161–6. [DOI] [PubMed] [Google Scholar]
- 64.Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC and Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146:404–10. [DOI] [PubMed] [Google Scholar]
- 65.Tamin SS, Maleszewski JJ, Scott CG, Khan SK, Edwards WD, Bruce CJ, Oh JK, Pellikka PA and Klarich KW. Prognostic and Bioepidemiologic Implications of Papillary Fibroelastomas. J Am Coll Cardiol. 2015;65:2420–9. [DOI] [PubMed] [Google Scholar]
- 66.Krishnamurthi RV, Ikeda T and Feigin VL. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology. 2020;54:171–179. [DOI] [PubMed] [Google Scholar]
- 67.Perera KS, Vanassche T, Bosch J, Giruparajah M, Swaminathan B, Mattina KR, Berkowitz SD, Arauz A, O’Donnell MJ, Ameriso SF et al. Embolic strokes of undetermined source: Prevalence and patient features in the ESUS Global Registry. Int J Stroke. 2016;11:526–33. [DOI] [PubMed] [Google Scholar]
- 68.Sposato LA, Chaturvedi S, Hsieh CY, Morillo CA and Kamel H. Atrial Fibrillation Detected After Stroke and TIA (AFDAS): A Novel Clinical Concept Challenging Current Views. Under Review. 2021. [DOI] [PubMed] [Google Scholar]
- 69.Tsivgoulis G, Katsanos AH, Köhrmann M, Caso V, Perren F, Palaiodimou L, Deftereos S, Giannopoulos S, Ellul J, Krogias C et al. Duration of Implantable Cardiac Monitoring and Detection of Atrial Fibrillation in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis. Journal of stroke. 2019;21:302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, Swaminathan B, Lavados P, Wang Y, Wang Y et al. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. The New England journal of medicine. 2018;378:2191–2201. [DOI] [PubMed] [Google Scholar]
- 71.Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, Kreuzer J, Cronin L, Cotton D, Grauer C et al. Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source. The New England journal of medicine. 2019;380:1906–1917. [DOI] [PubMed] [Google Scholar]
- 72.Bisbal F, Baranchuk A, Braunwald E, Bayés de Luna A and Bayés-Genís A. Atrial Failure as a Clinical Entity. Journal of the American College of Cardiology. 2020;75:222–232. [DOI] [PubMed] [Google Scholar]
- 73.Jalini S, Rajalingam R, Nisenbaum R, Javier AD, Woo A and Pikula A. Atrial cardiopathy in patients with embolic strokes of unknown source and other stroke etiologies. Neurology. 2019;92:e288–e294. [DOI] [PubMed] [Google Scholar]
- 74.Geisler T, Poli S, Meisner C, Schreieck J, Zuern CS, Nagele T, Brachmann J, Jung W, Gahn G, Schmid E et al. Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): Rationale and study design. Int J Stroke. 2017;12:985–990. [DOI] [PubMed] [Google Scholar]
- 75.Amarenco P, Cohen A, Hommel M, Moulin T, Leys D and Bousser MG. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The New England journal of medicine. 1996;334:1216–21. [DOI] [PubMed] [Google Scholar]
- 76.Ntaios G, Pearce LA, Meseguer E, Endres M, Amarenco P, Ozturk S, Lang W, Bornstein NM, Molina CA, Pagola J et al. Aortic Arch Atherosclerosis in Patients With Embolic Stroke of Undetermined Source: An Exploratory Analysis of the NAVIGATE ESUS Trial. Stroke. 2019;50:3184–3190. [DOI] [PubMed] [Google Scholar]
- 77.Ntaios G, Perlepe K, Sirimarco G, Strambo D, Eskandari A, Karagkiozi E, Vemmou A, Koroboki E, Manios E, Makaritsis K et al. Carotid plaques and detection of atrial fibrillation in embolic stroke of undetermined source. Neurology. 2019;92:e2644–e2652. [DOI] [PubMed] [Google Scholar]
- 78.Singh N, Marko M, Ospel JM, Goyal M and Almekhlafi M. The Risk of Stroke and TIA in Nonstenotic Carotid Plaques: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. 2020;41:1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Higuchi E, Toi S, Shirai Y, Hoshino T, Ishizuka K, Shimizu S, Tsutsumi Y and Kitagawa K. Prevalence of Microembolic Signals in Embolic Stroke of Undetermined Source and Other Subtypes of Ischemic Stroke. Stroke. 2020;51:655–658. [DOI] [PubMed] [Google Scholar]
- 80.Compagne KCJ, van Es A, Berkhemer OA, Borst J, Roos Y, van Oostenbrugge RJ, van Zwam WH, Majoie C, Marquering HA, Dippel DWJ et al. Prevalence of Carotid Web in Patients with Acute Intracranial Stroke Due to Intracranial Large Vessel Occlusion. Radiology. 2018;286:1000–1007. [DOI] [PubMed] [Google Scholar]
- 81.Wardlaw JM, Smith C and Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. [DOI] [PubMed] [Google Scholar]
- 82.Mast H, Thompson JL, Völler H, Mohr JP and Marx P. Cardiac sources of embolism in patients with pial artery infarcts and lacunar lesions. Stroke. 1994;25:776–81. [DOI] [PubMed] [Google Scholar]
- 83.Bernstein RA, Kamel H, Granger CB, Piccini JP, Sethi PP, Katz JM, Vives CA, Ziegler PD, Franco NC and Schwamm LH. Effect of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease: The STROKE-AF Randomized Clinical Trial. JAMA. 2021;325:2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gorelick PB, Wong KS, Bae H-J, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39(8):2396–2399. [DOI] [PubMed] [Google Scholar]
- 85.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG; Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305–1316. [DOI] [PubMed] [Google Scholar]
- 86.Romano JG, Prabhakaran S, Nizam A, Feldmann E, Sangha R, Cotsonis G, Campo-Bustillo I, Koch S, Rundek T, Chimowitz MI, Liebeskind DS; MyRIAD Investigators. Infarct Recurrence in Intracranial Atherosclerosis: Results from the MyRIAD Study. J Stroke Cerebrovasc Dis. 2021;30(2):105504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams JE, Chimowitz MI, Cotsonis GA, Lynn MJ, Waddy SP; WASID Investigators. Gender differences in outcomes among patients with symptomatic intracranial arterial stenosis. Stroke 2007;38:2055–2062. [DOI] [PubMed] [Google Scholar]
- 88.Ovbiagele B, Cruz-Flores S, Lynn MJ, Chimowitz MI; Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Early stroke risk after transient ischemic attack among individuals with symptomatic intracranial artery stenosis. Arch Neurol 2008;65:733–737. [DOI] [PubMed] [Google Scholar]
- 89.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Benesch CG, Sila CA, Jovin TG, Romano JG, Cloft HJ; Warfarin Aspirin Symptomatic Intracranial Disease Trial Investigators. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113(4):555–563. [DOI] [PubMed] [Google Scholar]
- 90.Prabhakaran S, Liebeskind DS, Cotsonis G, Nizam A, Feldmann E, Sangha RS, Campo-Bustillo I, Romano JG; MYRIAD Investigators. Predictors of Early Infarct Recurrence in Patients With Symptomatic Intracranial Atherosclerotic Disease. Stroke. 2021;52(6):1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, Waters MF, Lutsep H, López-Cancio E, Turan TN, Montgomery J, Janis LS, Lane B, Chimowitz MI; SAMMPRIS Investigators. Hemodynamic Markers in the Anterior Circulation as Predictors of Recurrent Stroke in Patients With Intracranial Stenosis. Stroke. 2019;50(1):143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amin-Hanjani S, Pandey DK, Rose-Finnell L, Du X, Richardson D, Thulborn KR, Elkind MS, Zipfel GJ, Liebeskind DS, Silver FL, Kasner SE, Aletich VA, Caplan LR, Derdeyn CP, Gorelick PB, Charbel FT; Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke Study Group. Effect of Hemodynamics on Stroke Risk in Symptomatic Atherosclerotic Vertebrobasilar Occlusive Disease. JAMA Neurol. 2016;73(2):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong KS, Chen C, Ng PW, Tsoi TH, Li HL, Fong WC, Yeung J, Wong CK, Yip KK, Gao H, Wong HB; FISS-tris Study Investigators. Low-molecular-weight heparin compared with aspirin for the treatment of acute ischaemic stroke in Asian patients with large artery occlusive disease: a randomised study. Lancet Neurol. 2007;6(5):407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chimowitz MI, Lynn MJ, Turan TN, Fiorella D, Lane BF, Janis S, Derdeyn CP; SAMMPRIS Investigators. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. J Stroke Cerebrovasc Dis. 2011;20(4):357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, Ladenvall P, Molina CA, Wang Y, Johnston SC; THALES Steering Committee and Investigators*. Ticagrelor Added to Aspirin in Acute Nonsevere Ischemic Stroke or Transient Ischemic Attack of Atherosclerotic Origin. Stroke. 2020;51(12):3504–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu L, Wong KS, Leng X, Pu Y, Wang Y, Jing J, Zou X, Pan Y, Wang A, Meng X, Wang C, Zhao X, Soo Y, Johnston SC, Wang Y; CHANCE Investigators. Dual antiplatelet therapy in stroke and ICAS: Subgroup analysis of CHANCE. Neurology. 2015;85(13):1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uchiyama S, Sakai N, Toi S, Ezura M, Okada Y, Takagi M, Nagai Y, Matsubara Y, Minematsu K, Suzuki N, Tanahashi N, Taki W, Nagata I, Matsumoto M; CATHARSIS Study Group. Final Results of Cilostazol-Aspirin Therapy against Recurrent Stroke with Intracranial Artery Stenosis (CATHARSIS). Cerebrovasc Dis Extra. 2015;5(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Toyoda K, Uchiyama S, Yamaguchi T, Easton JD, Kimura K, Hoshino H, Sakai N, Okada Y, Tanaka K, Origasa H, Naritomi H, Houkin K, Yamaguchi K, Isobe M, Minematsu K; CSPS.com Trial Investigators. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2019;18(6):539–548. [DOI] [PubMed] [Google Scholar]
- 99.Kwon SU, Hong KS, Kang DW, Park JM, Lee JH, Cho YJ, Yu KH, Koo JS, Wong KS, Lee SH, Lee KB, Kim DE, Jeong SW, Bae HJ, Lee BC, Han MK, Rha JH, Kim HY, Mok VC, Lee YS, Kim GM, Suwanwela NC, Yun SC, Nah HW, Kim JS. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke. 2011;42(10):2883–2890. [DOI] [PubMed] [Google Scholar]
- 100.Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, Frankel M, Chimowitz MI; WASID Study Group. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69(22):2063–2068. . [DOI] [PubMed] [Google Scholar]
- 101.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M; Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007. Jun 12;115(23):2969–2975. [DOI] [PubMed] [Google Scholar]
- 102.Turan TN, Nizam A, Lynn MJ, Egan BM, Le NA, Lopes-Virella MF, Hermayer KL, Harrell J, Derdeyn CP, Fiorella D, Janis LS, Lane B, Montgomery J, Chimowitz MI. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology. 2017;88(4):379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park JM, Kim BJ, Kwon SU, Hwang YH, Heo SH, Rha JH, Lee J, Park MS, Kim JT, Song HJ, Park JH, Yu S, Lee SJ, Park TH, Cha JK, Kwon HM, Kim EG, Lee SH, Lee JS, Lee J. Intensive blood pressure control may not be safe in subacute ischemic stroke by intracranial atherosclerosis: a result of randomized trial. J Hypertens. 2018;36(9):1936–1941. [DOI] [PubMed] [Google Scholar]
- 104.Amin-Hanjani S, Turan TN, Du X, Pandey DK, Rose-Finnell L, Richardson D, Elkind MS, Zipfel GJ, Liebeskind DS, Silver FL, Kasner SE, Gorelick PB, Charbel FT, Derdeyn CP; VERiTAS Study Group. Higher Stroke Risk with Lower Blood Pressure in Hemodynamic Vertebrobasilar Disease: Analysis from the VERiTAS Study. J Stroke Cerebrovasc Dis. 2017;26(2):403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GL Jr, Torbey MT, Zaidat OO, Rumboldt Z, Cloft HJ; SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, Montgomery J, Nizam A, Lane BF, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GL Jr, Lynch JR, Zaidat OO, Rumboldt Z, Cloft HJ; Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis Trial Investigators. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383(9914):333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alexander MJ, Zauner A, Chaloupka JC, Baxter B, Callison RC, Gupta R, Song SS, Yu W; WEAVE Trial Sites and Interventionalists. WEAVE Trial: Final Results in 152 On-Label Patients. Stroke. 2019;50(4):889–894. [DOI] [PubMed] [Google Scholar]
- 108.Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, Gupta R, Kirshner H, Megerian JT, Lesko J, Pitzer P, Ramos J, Castonguay AC, Barnwell S, Smith WS, Gress DR; VISSIT Trial Investigators. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015;313(12):1240–1248. [DOI] [PubMed] [Google Scholar]
- 109.Gonzalez NR, Jiang H, Lyden P, Song S, Schlick K, Dumitrascu O, Quintero-Consuegra MD, Toscano JF, Liebeskind DS, Restrepo L, Rao N, Hinman J, Alexander MJ, Schievink W, Piantadosi S, Saver JL. Encephaloduroarteriosynangiosis (EDAS) revascularization for symptomatic intracranial atherosclerotic steno-occlusive (ERSIAS) Phase-II objective performance criterion trial. Int J Stroke. 2021;16(6):701–709. [DOI] [PubMed] [Google Scholar]
- 110.de Weerd M, Greving JP, Hedblad B, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke 2010; 41: 1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chaturvedi S, Bruno A, Feasby T, et al. Carotid endarterectomy-An evidence based review. Neurology 2005; 65: 794–801 [DOI] [PubMed] [Google Scholar]
- 112.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJM, for the Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915–924 [DOI] [PubMed] [Google Scholar]
- 113.Rantner B, Kollerits B, Roubin GS, Ringleb PA, Jansen O, Howard G, Hendrikse J, Halliday A, Gregson J, Eckstein HH, et al. ; Carotid Stenosis Trialists’ Collaboration. Early endarterectomy carries a lower procedural risk than early stenting in patients with symptomatic stenosis of the internal carotid artery: results from 4 randomized controlled trials. Stroke.2017;48:1580–1587. [DOI] [PubMed] [Google Scholar]
- 114.Lichtman JH, Jones MR, Leifheit EC, Sheffet A, Howard G, Lal BK, Howard VJ, Wang Y, Curtis J, Brott TG. Carotid endarterectomy and carotid artery stenting in the US Medicare population, 1999–2014. JAMA 2017; 318: 1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brott TG, Hobson RW 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, et al. ; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schermerhorn ML, Liang P, Eldrup-Jorgensen J, Cronenwett JL, Nolan BW, Kashyap VS, Wang GJ, Motaganahalli RL, Malas MB. Association of transcarotid artery revascularization vs transfemoral carotid artery stenting with stroke or death among patients with carotid artery stenosis. JAMA.2019;322:2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kashyap VS, Schneider PA, Foteh M, Motaganahalli R, Shah R, Eckstein H, Henao S, LaMuraglia G, Stoner MC, Melton J, et al. Early outcomes in the ROADSTER 2 study of transcarotid artery revascularization in patients with significant carotid artery stenosis. Stroke 2020; 51: 2620–2629 [DOI] [PubMed] [Google Scholar]
- 118.de Borst GJ. Transcarotid artery stenting: Hype or hope? Stroke 2022; DOI: 10.1161/STROKEAHA.121.036464 [DOI] [PubMed] [Google Scholar]
- 119.Chaturvedi S. Aggressive medical therapy alone is adequate in certain patients with severe symptomatic carotid stenosis. Stroke 2013; 44: 2957–2958. [DOI] [PubMed] [Google Scholar]
- 120.Anand S, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018; 391: 219–229. [DOI] [PubMed] [Google Scholar]
- 121.Comerota AJ. Effect on platelet function of cilostazol, clopidogrel, and aspirin, each alone or in combination. Atheroscler Suppl. 2005;6:13–19. doi: 10.1016/j.atherosclerosissup.2005.09.005). [DOI] [PubMed] [Google Scholar]
- 122.McHutchinson C, Blair GW, Appleton JP, Chappell FM, Doubal F, Bath PM, Wardlaw JM. Cilostazol for Secondary Prevention of Stroke and Cognitive Decline: Systematic Review and Meta-Analysis. Stroke. 2020;51:2374–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maestrini I, Altieri M, Di Clemente L, Vicenzini E, Pantano P, Raz E, Silvestrini M, Provinciali L, Paolino I, Marini C, et al. Longitudinal Study on Low-Dose Aspirin versus Placebo Administration in Silent Brain Infarcts: The Silence Study. Stroke Res Treat. 2018; 3: 7532403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, Storey E, Shah RC, Lockery JE, Tonkin AM, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med 2018; 379: 1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wardlaw JM, Debette S, Jokinen H, De Leeuw FE, Pantoni L, Chabriat H, Staals J, Doubal F, Rudilosso S, Eppinger S, et al. ESO Guideline on covert cerebral small vessel disease. Eur Stroke J. 2021;6: CXI–CLXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130(Pt 8):1988–2003. [DOI] [PubMed] [Google Scholar]
- 127.Puy L, Pasi M, Rodrigues M, van Veluw SJ, Tsivgoulis G, Shoamanesh A, Cordonnier C. Cerebral microbleeds: from depiction to interpretation. J Neurol Neurosurg Psychiatry. 2021. :jnnp-2020–323951. [DOI] [PubMed] [Google Scholar]
- 128.Shoamanesh A, Pearce LA, Bazan C, Catanese L, McClure LA, Sharma M, Marti-Fabregas J, Anderson DC, Kase CS, Hart RG, et al. Microbleeds in the Secondary Prevention of Small Subcortical Strokes Trial: Stroke, mortality, and treatment interactions. Ann Neurol. 2017. ;82:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Al-Shahi Salman R, Minks DP, Mitra D, Rodrigues MA, Bhatnagar P, du Plessis JC, Joshi Y, Dennis MS, Murray GD, Newby DE, et al. Effects of antiplatelet therapy on stroke risk by brain imaging features of intracerebral haemorrhage and cerebral small vessel diseases: subgroup analyses of the RESTART randomised, open-label trial Lancet Neurol. 2019;18:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park HK, Lee JS, Kim BJ, Park JH, Kim YJ, Yu S, Hwang YH, Rha JH, Heo SH, Ahn SH, et al. Cilostazol versus aspirin in ischemic stroke with cerebral microbleeds versus prior intracerebral hemorrhage. Int J Stroke. 2021;16:1019–1030. [DOI] [PubMed] [Google Scholar]
- 131.Shoamanesh A, Hart RG, Connolly SJ, Kasner SE, Smith EE, Martí-Fàbregas J, Liu YY, Uchiyama S, Mikulik R, Veltkamp R, et al. Microbleeds and the Effect of Anticoagulation in Patients With Embolic Stroke of Undetermined Source: An Exploratory Analysis of the NAVIGATE ESUS Randomized Clinical Trial. JAMA Neurol. 2021; 78:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Landman TR, Thijssen DH, Tuladhar AM, , de Leeuw FE. Relation between physical activity and cerebral small vessel disease: a nine-year prospective cohort study. Int J Stroke. 2021;16:962–971. [DOI] [PubMed] [Google Scholar]