Abstract
To expand our understanding of the role of angiotensin II (ANGII) in coronavirus infectious disease 2019 (COVID‐19), we conducted an international, multicenter registry study to assess the use of ANGII in patients with COVID‐19 compared to patients not receiving ANGII. Critically ill adult patients who were diagnosed with COVID‐19 and received ANGII were matched with COVID‐19 patients not receiving ANGII according to age, respiratory support, history of hypertension, use of angiotensin‐converting enzyme inhibitors and/or ANGII receptor blocker, and date of admission. All outcomes were exploratory in nature and included improvement in oxygenation, duration of organ support, and mortality. In one year, 132 patients were included (65 in the ANGII group and 67 in the control group), and patients were comparable in baseline characteristics. During the first 12 h of infusion, patients in the ANGII had a faster decrease in FiO2 and maintained similar mean arterial pressure levels. Hospital mortality was not statistically significantly different between the groups (53.8% vs. 40.3%; p = 0.226). Within the limitations of such a study design, our findings confirm previous observations of a potentially positive effect of ANGII on blood pressure and FiO2 but no effect on patient‐centered outcomes.
Keywords: biochemical analysis, coronavirus, respiratory tract, SARS coronavirus
Highlights
In patients with coronavirus infectious disease 2019, the physiological effect of angiotensin II (ANGII) on oxygenation was recently assessed in an uncontrolled case series.
During the first 12 h of infusion, patients in the ANGII had a faster decrease in FiO2 and maintained similar mean arterial pressure levels.
Hospital mortality was not statistically significantly different between the groups.
1. INTRODUCTION
Coronavirus infectious disease 2019 (COVID‐19) can cause severe acute respiratory syndrome requiring invasive mechanical ventilation. 1 Once ventilated, some patients develop vasodilatory hypotension and require vasopressor drugs. 2 Modulation of the renin–angiotensin–aldosterone system (RAAS) with vasopressor drugs may affect outcomes because the angiotensin‐converting enzyme (ACE) type 2 is the viral receptor 3 , 4 for the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike protein and because drugs that inhibit the RAAS may affect the expression of ACE2 and, thereby, cell entry by the COVID‐19 virus. 1 , 5
Angiotensin II (ANGII) is a vasopressor approved by the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of catecholamine‐resistant vasodilatory shock 6 and a substrate for ACE2. In Phase III double‐blind randomized trial, when used as rescue vasopressor, ANGII improved blood pressure in catecholamine‐resistant vasodilatory shock. 7 Moreover, it increased survival in patients with a high ANGI/ANGII ratio 8 or a high renin level 9 and, in patients receiving renal replacement therapy (RRT) at randomization, it increased the likelihood of recovery to RRT independence. 10
In patients with COVID‐19, the physiological effect of ANGII on oxygenation was recently assessed in an uncontrolled case series. This case series reported an improvement of PaO2/FiO2 ratio with ANGII. 11 In contrast, more recently, a small case series reported that ANGII was associated with poor outcomes in six COVID‐19 patients. 12 However, these observational assessments lacked controls. Finally, a single‐center study reported increased blood pressure and PaO2/FiO2 ratio, decreased risk of liver dysfunction, and, in patients with abnormal baseline serum creatinine, a suggestive decrease of RRT use. 13
Given such limited data and to expand our understanding of the role of ANGII in COVID‐19‐related hypotension, we conducted an international, multicenter registry study to assess the use of ANGII in patients with COVID‐19 compared to patients not receiving ANGII across different centers in Europe. In particular, we aimed to test the hypothesis that ANGII would be associated with improved PaO2/FiO2 ratios compared with controls as suggested by previous work.
2. METHODS
2.1. Study design
This is a prospective international, multicenter, ANGII registry‐based study, including patient‐centered outcomes. The study was approved by the Alfred Hospital Ethics Committee (Project Number 215/20) and in each local center according to local regulations. A waiver of consent was obtained. The study was registered on ANZCTR (ACTRN12620000620921) and on ClinicalTrials.gov (NCT04408326) and data from some patients from a single center component of this cohort was published previously. 11 , 13
2.2. Patients
Critically ill adult patients who were diagnosed with COVID‐19 and received ANGII infusion were considered for inclusion. In addition, matched control patients were critically ill adults who were diagnosed with COVID‐19 but did not receive ANGII infusion.
The following additional inclusion criteria were considered for the identification of matched controls: 1) receiving vasopressor infusion; and 2) matched to a patient from the ANGII group by the following criteria: date of intensive care unit (ICU) admission (range of ±2 days), age (range of ±2 years), respiratory support at ICU admission (no supplemental oxygen or supplemental oxygen or noninvasive ventilation/high flow nasal cannula or invasive ventilation), history of hypertension, and use of angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs). No exclusion criterion was considered.
2.3. Intervention
ANGII (Giapreza®; La Jolla) infusion was used according to local protocol and clinical criteria. Patients received ANGII either as a second‐line vasopressor in addition to norepinephrine or solely as a first‐line agent.
2.4. Data collection
Medical records were used for data collection. We obtained data on medical history, clinical and laboratory data every 6 h in the first 12 h after the start of the infusion of ANGII (ANGII group) or other vasopressors (in the control group). Daily data collection was restricted to the first 3 days after inclusion, and outcome data. All data were collected by trained investigators independent from the clinical teams. Before analysis, an extensive round of data cleaning was performed to check for data accuracy.
2.5. Clinical outcomes
All outcomes reported in this study are exploratory in nature. Clinical outcomes collected in the registry included the use of organ support during ICU stay (RRT, extracorporeal membrane oxygenation [ECMO], and/or prone positioning), development of complications during hospital stay (stroke, acute myocardial infarction, unexpected cardiac arrest, acute kidney injury, and/or cardiac arrhythmia), and clinical outcomes (ventilator‐free days at Day 28, ICU‐free days at Day 28, hospital‐free days at Day 28, and ICU, hospital, and 60‐day in‐hospital mortality).
2.6. Statistical analysis
A convenience sample was considered for this analysis, with each center including all patients who received ANGII during the study period and one or two controls for every ANGII patient. Continuous variables are presented as medians (Quartile 25%–75%) and categorical variables as numbers and percentages. Baseline and clinical characteristics of the patients were compared among the groups using Fisher's exact tests and Wilcoxon rank‐sum tests.
Continuous variables over different time points were compared between the groups using a mixed‐effect generalized linear model with Gaussian distribution and with group, time, and group × time interaction included as a fixed effect term (time as a continuous variable) and patients included as random effect term to account for the repeated measurements. p‐values from this interaction represent a statistical assessment of whether the trend over time differed among the groups. In addition, a model considering time as a categorical variable was performed, and between‐group comparisons at each time point were estimated with the appropriate contrasts from the model and using a Holm–Bonferroni method to adjust for multiplicity.
Binary outcomes were compared between the groups with a mixed‐effect generalized linear model with binomial distribution and an identity link, and reported as risk difference with 95% confidence interval (CI). Continuous outcomes were compared with a mixed‐effect median regression, considering an interior point algorithm, and reported as the median difference with a 95% CI. Moreover, 60‐day in‐hospital mortality was reported in Kaplan–Meier curves and compared with a (shared‐frailty) Cox proportional hazard model and reported as hazard ratio and 95% CI. The proportional hazard assumption was assessed through Schoenfeld residuals. All models were adjusted for diabetes, ventilatory support (no support, oxygen support, noninvasive ventilation, high flow nasal cannula, and invasive mechanical ventilation), SpO2, and norepinephrine dose at ICU admission, and the hospital was included as a random effect, cluster effect, or frailty. In addition, all analyses assessing FiO2 and PaO2/FiO2 were reassessed considering adjustments for positive end‐expiratory pressure (PEEP) levels and use of prone positioning in addition to the covariates described above.
The rate of missing data is shown in Table S1. No assumption was made for missing data. All analyses were performed in R v.4.0.3 and, for this exploratory study, a p < 0.05 was considered significant.
3. RESULTS
3.1. Patients
From February 2020 to December 2020, 65 patients received ANGII in six centers in Europe, and were matched to 67 controls who did not receive ANGII. Overall, the median age was 61 (53–67), 20.5% were female, median cardiovascular SOFA was 3 (3–4), and the most prevalent comorbidity was hypertension (45.5%) followed by diabetes (21.2%) (Table 1). Overall, 14.4% of patients were treated with ACEIs and 12.7% with ARBs. Median PaO2/FiO2 was 108 (76–141) mmHg, C‐reactive protein was 119 (29–244) mg/dl, median noradrenaline dose was 0.19 (0.10–0.22) µg/kg/m and 95.5% of the patients were receiving mechanical ventilation at inclusion. Both groups were well balanced for baseline characteristics.
Table 1.
Baseline characteristics of the included patients
| Overall (n = 132) | Angiotensin II (n = 65) | Control (n = 67) | p | |
|---|---|---|---|---|
| Age, years | 61 (53–67) | 61 (53–68) | 60 (50–66) | 0.411 |
| Female gender—no. (%) | 27 (20.5) | 10 (15.4) | 17 (25.4) | 0.197 |
| Body mass index, kg/m2 | 27.5 (24.8–32.0) | 27.4 (25.4–30.9) | 27.5 (24.7–34.0) | 0.991 |
| Days between hospital and ICU admission | 2 (0–4) | 2 (0–4) | 2 (0–5) | 0.890 |
| SOFA | ||||
| Respiratory | 3 (3–4) | 3 (3–4) | 3 (3–4) | 0.861 |
| Cardiovascular | 3 (0–4) | 3 (0–4) | 3 (0–4) | 0.177 |
| Renal | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.256 |
| Comorbidities—no. (%) | ||||
| Hypertension | 60 (45.5) | 26 (40.0) | 34 (50.7) | 0.227 |
| Diabetes | 28 (21.2) | 18 (27.7) | 10 (14.9) | 0.090 |
| Chronic respiratory failure | 11/118 (9.3) | 6/60 (10.0) | 5/58 (8.6) | 0.999 |
| Chronic kidney disease | 6/118 (5.1) | 3/59 (5.1) | 3/59 (5.1) | 0.999 |
| Cancer | 6/116 (5.2) | 3/58 (5.2) | 3/58 (5.2) | 0.999 |
| Leukemia | 1/83 (1.2) | 0/41 (0.0) | 1/42 (2.4) | 0.999 |
| Smoking | 6/101 (5.9) | 2/53 (3.8) | 4/48 (8.3) | 0.420 |
| Use of ACE inhibitors | 17/118 (14.4) | 10/62 (16.1) | 7/56 (12.5) | 0.610 |
| Use of ARBs | 15/118 (12.7) | 6/62 (9.7) | 9/56 (16.1) | 0.408 |
| Use of immunosuppression | 1/84 (1.2) | 1/43 (2.3) | 0/41 (0.0) | 0.999 |
| Use of steroids | 3/85 (3.5) | 0/43 (0.0) | 3/42 (7.1) | 0.116 |
| Ventilatory support at ICU admission—no. (%) | 0.088 | |||
| Low‐flow oxygen | 4 (3.0) | 4 (6.2) | 0 (0.0) | |
| Noninvasive ventilation | 2 (1.5) | 1 (1.5) | 1 (1.5) | |
| Invasive ventilation | 126 (95.5) | 60 (92.3) | 66 (98.5) | |
| Vital signs at ICU admission | ||||
| SpO2, % | 93 (86–96) | 91 (85–95) | 94 (88–97) | 0.063 |
| FiO2 | 0.70 (0.60–0.90) | 0.80 (0.60–0.80) | 0.7 (0.6–0.9) | 0.879 |
| Heart rate, bpm | 100 (88–110) | 100 (89–111) | 99 (86–107) | 0.506 |
| Mean arterial pressure, mmHg | 90 (77–99) | 87 (77–99) | 90 (77–98) | 0.960 |
| Respiratory rate, breaths/min | 26 (22–32) | 28 (22–34) | 25 (21–30) | 0.614 |
| Laboratory tests at ICU admission | ||||
| pH | 7.37 (7.30–7.44) | 7.36 (7.29–7.44) | 7.37 (7.31–7.42) | 0.978 |
| PaO2/FiO2 | 108 (76–141) | 107 (76–140) | 110 (76–142) | 0.982 |
| PaCO2, mmHg | 45 (39–53) | 47 (38–53) | 44 (40–53) | 0.878 |
| Lactate, mmol/L | 1.5 (1.1–2.2) | 1.5 (1.2–2.2) | 1.5 (1.1–2.3) | 0.739 |
| Creatinine, mg/dl | 1.04 (0.85–1.33) | 1.10 (0.86–1.40) | 1.01 (0.79–1.30) | 0.390 |
| C‐reactive protein, mg/dl | 119 (29–244) | 123 (25–263) | 119 (43–233) | 0.860 |
| Support at ICU admission | ||||
| Noradrenaline dose, µg/kg/min | 0.19 (0.10–0.22) | 0.20 (0.08–0.30) | 0.15 (0.10–0.20) | 0.207 |
| Use of tocilizumab—no. (%) | 2/123 (1.6) | 2/61 (3.3) | 0/62 (0.0) | 0.244 |
| Use of renal replacement therapy—no. (%) | 1/126 (0.8) | 1/64 (1.6) | 0/62 (0.0) | 0.999 |
| Use of ECMO—no. (%) | 6/127 (4.7) | 3 (4.6) | 3/62 (4.8) | 0.999 |
| Use of prone positioning—no. (%) | 52/125 (41.6) | 29/63 (46.0) | 23/62 (37.1) | 0.366 |
Note: Data are median (Quartile 25th–75th) and N/total (%).
Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin II receptor blockers; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; SOFA, sequential organ failure assessment.
3.2. Clinical characteristics during the infusion
Angiotensin II was used as the first‐line agent in 44.6% of the patients in the ANGII group, and the starting dose was 5 (5–20) ng/kg/min (Table 2). At the start of the infusion, compared with controls at a similar time, the serum creatinine was higher in the ANGII group (Table 2). During the first 12 h of infusion, patients in the ANGII had a faster decrease in FiO2 (p = 0.039) and maintained similar mean arterial pressure levels (Table 2 and Figure S1). PaO2/FiO2 levels at 6 and 12 h were higher in patients in the ANGII group after 6 h of infusion, but all other laboratory tests remained unchanged (Table 2 and Figure S2). The effect on FiO2 and PaO2/FiO2 was maintained after adjustment for PEEP level and use of prone positioning (Table 2).
Table 2.
Characteristics during the first 12 h after the start of the vasopressor infusion a
| At the start of infusion | 6 h after the start of the infusion | 12 h after the start of the infusion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Angiotensin II (n = 65) | Control (n = 67) | p | Angiotensin II (n = 65) | Control (n = 67) | p | Angiotensin II (n = 65) | Control (n = 67) | p | |
| Angiotensin II as a first line agent—no. (%) | 29 (44.6) | – | – | – | – | – | – | – | – |
| Use as a vasopressor | 45 (69.2) | – | – | – | – | – | – | – | |
| Other reason | 20 (30.8) | – | – | – | – | – | – | – | – |
| VTE prophylaxis at start of infusion—no. (%) | 65 (100.0) | 67 (100.0) | – | – | – | – | – | – | – |
| Ventilatory support at start of infusion—no. (%) | 0.999 | – | – | – | – | – | – | ||
| Noninvasive ventilation | 0 (0.0) | 1/66 (1.5) | – | – | – | – | – | – | |
| Invasive ventilation | 65 (100.0) | 65/66 (98.5) | – | – | – | – | – | – | |
| Support at start of infusion | – | – | – | – | – | – | |||
| Norepinephrine dose, µg/kg/min | 0.16 (0.09–0.23) | 0.15 (0.10–0.20) | 0.626 | – | – | – | – | – | – |
| Use of renal replacement therapy—no. (%) | 5/64 (7.8) | 1/62 (1.6) | 0.208 | – | – | – | – | – | – |
| Use of ECMO—no. (%) | 3 (4.6) | 3/62 (4.8) | 0.999 | – | – | – | – | – | – |
| Use of prone positioning—no. (%) | 29/63 (46.0) | 23/62 (37.1) | 0.366 | – | – | – | – | – | – |
| Infusion details | – | – | – | – | – | – | |||
| Dose (ng/kg/min or µg/kg/min)b | 5 (5–20) | 0.08 (0.00–0.15) | – | 20 (0–32) | 0.11 (0.10–0.20) | – | 14 (0–32) | 0.15 (0.04–0.25) | – |
| PEEP, cmH2O | 12 (10–14) | 12 (10–14) | 0.778 | 11 (10–12) | 12 (10–13) | 0.907 | 10 (10–12) | 12 (9–14) | 0.411 |
| FiO2 * | 0.70 (0.52–0.85) | 0.70 (0.60–0.97) | 0.418 | 0.50 (0.48–0.62) | 0.70 (0.50–0.85) | 0.146 | 0.50 (0.43–0.58) | 0.70 (0.50–0.92) | 0.014 |
| SpO2, % | 94 (91–96) | 93 (88–95) | 0.112 | 97 (95–99) | 94 (91–95) | 0.076 | 96 (95–98) | 94 (92–95) | 0.102 |
| Heart rate, bpm | 102 (81–118) | 98 (86–102) | 0.776 | 95 (77–104) | 94 (81–111) | 0.548 | 90 (75–105) | 105 (84–115) | 0.223 |
| Mean arterial pressure, mmHg | 80 (69–94) | 73 (63–89) | 0.079 | 76 (65–87) | 79 (69–86) | 0.934 | 79 (72–81) | 70 (69–87) | 0.857 |
| Respiratory rate, breaths/min | 22 (16–28) | 24 (17–25) | 0.818 | 22 (17–26) | 22 (20–25) | 0.750 | 22 (18–26) | 22 (21–25) | 0.720 |
| pH | 7.36 (7.31–7.44) | 7.36 (7.30–7.41) | 0.461 | 7.34 (7.27–7.38) | 7.37 (7.33–7.38) | 0.203 | 7.34 (7.31–7.36) | 7.37 (7.32–7.42) | 0.230 |
| PaO2/FiO2 ** | 114 (85–153) | 104 (77–131) | 0.350 | 175 (118–204) | 129 (89–165) | 0.023 | 170 (143–203) | 123 (75–149) | 0.030 |
| PaCO2, mmHg | 48 (39–53) | 45 (40–54) | 0.385 | 47 (38–61) | 42 (38–49) | 0.315 | 46 (40–54) | 42 (40–53) | 0.551 |
| Lactate, mmol/L | 1.7 (1.4–2.2) | 1.9 (1.2–2.5) | 0.800 | 1.6 (1.2–2.3) | 1.8 (1.2–2.4) | 0.567 | 1.4 (1.2–2.1) | 1.5 (1.3–2.1) | 0.668 |
| Creatinine, mg/dl | 1.21 (0.92–1.83) | 1.01 (0.77–1.52) | 0.036 | – | – | – | – | – | – |
| C‐reactive protein, mg/dl | 176 (31–247) | 151 (42–74) | 0.710 | ||||||
Note: Data are median (Quartile 25th–75th) and N/total (%).
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; PEEP, positive end‐expiratory pressure; VTE, venous thromboembolism.
Models comparing characteristics over time are mixed‐effect models considering the moment of measurement, group, as well as the group × time interaction as a fixed effect. Moment of measurement was treated as a categorical variable and random intercepts for patients were included to account for the dependency of repeated measures. Between‐group comparisons at each time point were estimated with the appropriate contrasts from the model and using a Holm–Bonferroni method to adjust for multiplicity.
In the angiotensin group, the dose of angiotensin II is reported as ng/kg/min and in the control group, the noradrenaline dose is reported as µg/kg/min.
p values after adjustment for PEEP and prone positioning: 0.321 (start of infusion), 0.135 (6 h), and 0.015 (12 h)
p values after adjustment for PEEP and prone positioning: 0.343 (start of infusion), 0.026 (6 h), and 0.035 (12 h).
3.3. Clinical characteristics during the first 3 days after inclusion
During the first 3 days after inclusion, FiO2 remained lower and mean arterial pressure higher in patients in ANGII group (Figure S3 and Table S2). In addition, in the first 2 days, PaO2/FiO2 were higher in patients in the ANGII group (Figure 1 and Table S2). The effect on FiO2 and PaO2/FiO2 was maintained after adjustment for PEEP level and use of prone positioning (Table S2). Noradrenaline dose on Day 1 was higher in the ANGII group. In addition, the use of prone positioning on Day 1 and PEEP levels on Days 1 and 3 were also higher in the ANGII group (Table S2). All other laboratory tests were similar between the groups.
Figure 1.
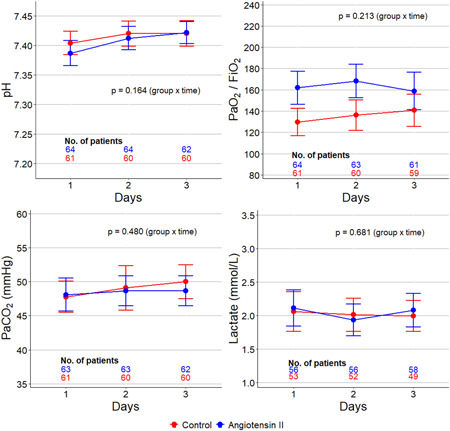
Laboratory tests during the first 3 days after inclusion. The circle is mean and error bars are 95% confidence interval. p values calculated from a mixed‐effect generalized linear model with Gaussian distribution and with the group, time, and group × time interaction included as a fixed effect term and patients included as random effect term to account for the repeated measurements
3.4. Clinical outcomes
Patients in the ANGII group received ANGII for a median of 5 (3–6) days. The need for RRT and ECMO during hospital stay was similar between the groups (Table 3). Overall, prone positioning was used more often in patients in the ANGII group. The incidence of complications during a hospital stay, including stroke and acute myocardial infarction was similar between the groups. After adjustment for confounders, ventilator‐free days at Day 28, ICU and hospital‐free days at Day 28, and ICU and hospital mortality were similar between the groups (Table 3 and Figure 2).
Table 3.
Complications and clinical outcomes
| Overall (n = 132) | Angiotensin II (n = 65) | Control (n = 67) | Effect estimatea (95% CI) | p * | |
|---|---|---|---|---|---|
| Days of use of angiotensin II | 5 (3–6) | 5 (3–6) | – | – | – |
| Organ support during a hospital stay— no. (%) | |||||
| Renal replacement therapy | 32/122 (26.2) | 16/62 (25.8) | 16/60 (26.7) | RD, −4.13 (−19.03 to 10.77) | 0.588 |
| ECMO | 12/124 (9.7) | 5/64 (7.8) | 7/60 (11.7) | RD, −4.15 (−14.84 to 6.55) | 0.449 |
| Prone positioning | 98/124 (79.0) | 54/63 (85.7) | 44/61 (72.1) | RD, 15.40 (0.88–29.92) | 0.040 |
| Complications—no. (%) | |||||
| Stroke | 2/126 (1.6) | 1/65 (1.5) | 1/61 (1.6) | RD, −0.27 (−4.88 to 4.34) | 0.909 |
| Acute myocardial infarction | 1/124 (0.8) | 0/64 (0.0) | 1/60 (1.7) | RD, −1.78 (−5.07 to 1.51) | 0.291 |
| Unexpected cardiac arrest | 14/125 (11.2) | 7/65 (10.8) | 7/60 (11.7) | RD, −4.92 (−15.92 to 6.07) | 0.382 |
| Acute kidney injury | 94/129 (72.9) | 50/65 (76.9) | 44/64 (68.8) | RD, 5.47 (−9.80 to 20.74) | 0.484 |
| Cardiac arrhythmia | 10/127 (7.9) | 5/65 (7.7) | 5/62 (8.1) | RD, −2.10 (−10.15 to 5.94) | 0.609 |
| Clinical outcomes | |||||
| Ventilator‐free days at Day 28 | 0 (0–15) | 0 (0–15) | 1 (0–15) | MD, −0.96 (−4.46 to 2.54) | 0.592 |
| ICU‐free days at Day 28 | 0 (0–12) | 0 (0–10) | 0 (0–13) | MD, 0.00 (−2.33 to 2.34) | 0.999 |
| Hospital‐free days at Day 28 | 0 (0–0) | 0 (0–0) | 0 (0–0) | MD, −0.00 (−3.15 to 3.15) | 0.999 |
| ICU mortality—no. (%) | 57 (43.2) | 33 (50.8) | 24 (35.8) | RD, 10.70 (−6.02 to 27.42) | 0.212 |
| Hospital mortality—no. (%) | 62 (47.0) | 35 (53.8) | 27 (40.3) | RD, 10.65 (−6.49 to 27.80) | 0.226 |
| 60‐Day hospital mortality—no. (%) | 58 (43.9) | 32 (49.2) | 26 (38.8) | HR, 1.18 (0.68–2.03) | 0.560 |
Note: Data are median (Quartile 25th–75th) and N/Total (%).
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; ICU, intensive care unit; MD, median difference; RD, risk difference.
All models adjusted for diabetes, ventilatory support, SpO2, and noradrenaline dose at ICU admission, and considering the hospital of admission as a random effect.
p = 0.950 for Schoenfeld residuals.
Figure 2.
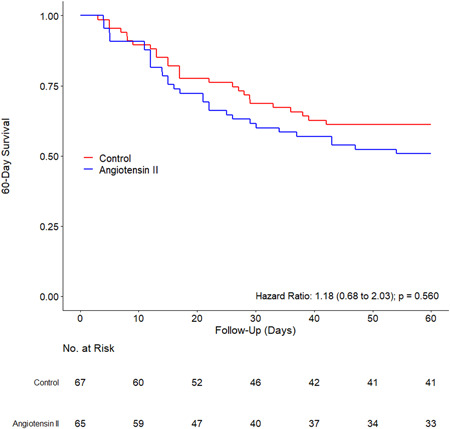
60‐Day survival in each group
4. DISCUSSION
4.1. Key findings
We performed a prospective international, multicenter, registry‐based study of ANGII therapy for vasopressor support in critically ill patients with COVID‐19 and assessed physiological and patient‐centered outcomes compared with matched controls, and, in particular, its effects on PaO2/FiO2 ratio. We found that during the first 12 h of infusion, patients in the ANGII group had a faster decrease in FiO2, and during the first 3 days after inclusion the PaO2/FiO2 ratio was higher, FiO2 remained lower. This effect persisted even after adjustment for the higher PEEP levels and the more common use of prone positioning and was combined with a higher mean arterial pressure. Finally, after adjustment, ventilator, ICU and hospital‐free days, and ICU and hospital mortality were not significantly different.
4.2. Relationship to previous studies
Following the Angiotensin II for the Therapy in High Output Shock (ATHOS) trial, the United States FDA and EMA approved the use of ANGII infusion for the treatment of catecholamine‐resistant (requiring ≥0.2 µg/kg/min of norepinephrine infusion) vasodilatory shock. 6 The median dose reported in our study is consistent with the ATHOS trial criteria for the use of ANGII. In the ATHOS trial mortality at 28 days was 48% for ANGII treated patients. Our observation of a 49% 60‐day mortality is aligned with such findings. RRT, however, was used in approximately 26% of COVID‐19 patients, a lower value than the near 33% seen in the ATHOS trial. 10
The apparent physiological effects on oxygenation observed in this study are in line with a recently reported case series in COVID‐19 patients 11 and a single‐center study of COVID‐19 critically ill subjects. 13 The increase in blood pressure was expected and is also consistent with the ATHOS trial, postmarketing studies of ANGII, 14 , 15 a recent case series on COVID‐19 patients, 16 and the abovecited reports. Only a small uncontrolled single‐center case series reported that five of six ANGII‐treated COVID 19 patients ultimately did not survive hospital discharge. 12 Thus, our study provides the most extensive and only multicentric international controlled assessment of ANGII infusion in the setting of COVID‐19 associated vasodilatory shock.
The rationale for ANGII therapy arises from the potential usefulness of decreasing the expression of the ACE2 receptor and, thereby, reducing the entry of the COVID‐19 virus into cells and its replication. In this regard, ANGII mediates the internalization and degradation of ACE2 into lysosomes through the angiotensin type 1 receptor. 17 Because of concern about the role of the ACE2 receptor in the cell to cell spread of the COVID‐19 virus, several studies have also explored the role of either continuing or discontinuing ACEIs and angiotensin receptor blockers. Such studies have broadly found no detectable effect with either continuation or discontinuation of these medications. 18 , 19 , 20
Finally, it is unknown which vasopressor agent would be safest and/or most appropriate for COVID‐19 patients with vasodilatory shock. In this regard, all advice to clinicians is based on extrapolations from studies in non‐COVID‐19 patients. This is despite the problems with pulmonary hypertension and right ventricular stress seen with COVID‐19 21 and norepinephrine infusion. 22 However, to date, no studies have addressed the comparative safety of norepinephrine therapy in isolation in COVID‐19 patients. Vasopressin use in COVID has only been reported in a case series of 52 patients without clinical outcomes and focusing on viral clearance, which was not affected by its use. 23
4.3. Study implications
Our findings imply that, in a cohort of patients already receiving a median dose of approximately 0.2 µg/kg/min of norepinephrine, ANGII infusion increased blood pressure compared with controls. Moreover, this effect was associated with favorable changes in FiO2. However, there was no signal to suggest a beneficial effect on patient‐centered outcomes.
4.4. Study strengths and limitations
This study has several strengths. It is multicentric and international in design. It has a center and illness severity matched control cases. It presents the only controlled data on vasopressor therapy for COVID‐19 to date. Finally, it reports data on both physiological changes and clinical outcomes.
We acknowledge several limitations. This is not a randomized controlled trial. Thus, no causal inferences can be robustly made on the basis of the evidence provided. It is limited in size and focused on a particular group of severely ill COVID‐19 patients receiving a high median dose of norepinephrine at baseline. The unadjusted mortality rate was numerically higher in patients treated with ANGII. However, the matching of patients was imperfect, the number relatively small, and after adjustment for several key baseline imbalances, this difference was not significant. We did not collect detailed hourly or second hourly physiological data to enable a clearer understanding of the physiological impact of initiating ANGII therapy compared with usual care. Also, we did not collect data regarding treatment with steroids or other drugs. Finally, we did not collect detailed data on renal outcomes and cannot comment on whether ANGII affected renal recovery among survivors.
5. CONCLUSION
In conclusion, we report the findings of a registry‐based case‐matched controlled assessment of ANGII treatment in COVID‐19 patients receiving a high median dose of vasopressor therapy at baseline. Within the limitations of such a study design, our findings confirm previous observations of a potentially positive effect on blood pressure and FiO2 but no effect on patient‐centered outcomes. These observations suggest the need to obtain more controlled information on the use of other vasopressor agents in COVID‐19 patients. Moreover, taken together with studies of ACEI and ARB, they provide further evidence that ACE2 receptor manipulation is unlikely to impact clinical outcomes in COVID‐19 patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Ary S. Neto, Giovanni Landoni, Marlies Ostermann, Lui Forni, Tony Trapani, Kai Zacharowski, Daniel de Backer, and Rinaldo Bellomo designed the study. Ary S. Neto, Nuttha Lumlertgul, Lucas Alvarez‐Belon, Patricia V. Alliegro, and Carolin Wiedenbeck were responsible for the data collection. Ary S. Neto was responsible for data analysis. Rinaldo Bellomo was the study coordinator and supervised the data analysis. All authors reviewed and approved the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.27592.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Barbara Ficial (Department of Critical Care, Guys & St Thomas' Foundation Trust, London, UK) who helped with data collection.
Serpa Neto A, Landoni G, Ostermann M, et al. Angiotensin II infusion in COVID‐19: an international, multicenter, registry‐based study. J Med Virol. 2022;94:2079‐2088. 10.1002/jmv.27592
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 2. Zangrillo A, Beretta L, Scandroglio AM, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID‐19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22:200‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the perfusion conformation. Science. 2020;367:1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Busse LW, Chow JH, McCurdy MT, Khanna AK. COVID‐19 and the RAAS—a potential role for angiotensin II? Crit Care. 2020;24:136‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377:419‐430. [DOI] [PubMed] [Google Scholar]
- 8. Bellomo R, Wunderink RG, Szerlip H, et al. Angiotensin I and angiotensin II concentrations and their ratio in catecholamine‐resistant vasodilatory shock. Crit Care. 2020;24:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellomo R, Forni LG, Busse LW, et al. Renin and survival in patients given angiotensin II for catecholamine‐resistant vasodilatory shock. Am J Respir Crit Care Med. 2020;202:1253‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tumlin JA, Murugan R, Deane AM, et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med. 2018;46:949‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zangrillo A, Landoni G, Beretta L, Morselli F, Serpa Neto A, Bellomo R. Angiotensin II infusion in COVID19‐associated vasodilatory shock: a case series. Crit Care. 2020;24:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinicke U, Adam E, Sonntagbauer M, von Knethen A, Zacharowski K, Neb H. Angiotenin II treamtent in COVID‐19 patients: more risk than benefit? A single‐center experience. Crit Care. 2020;24:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zangrillo A, Colombo S, Scandroglio AM, et al. Angiotensin II infusion and markers of organ function in invasively ventilated COVID‐19 patients. Crit Care Resusc. 2021;23:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alam A, Sovic W, Gill J, et al. Angiotensin II: a review of current literature. J Cardiothor Vasc Anesth. 2021;S1053‐0770(21):00602‐9. [DOI] [PubMed] [Google Scholar]
- 15. Wieruszewski PM, Wittwer ED, Kashani KB, et al. Angiotensin II infusion for shock: a multicenter study of post‐marketing use. Chest. 2021;159:596‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ofosu‐Barko K, Liu Y, Alkhatib Tamimi F, et al. Angiotensin II administration in patients with COVID‐19 shock. Crti Pathw Cardiol. 2021;20:100‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin‐II mediates ACE2 internalization and degradation through an angiotensin‐II type I receptor‐dependent mechanism. Hypertension. 2014;64:1368‐1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. Effect of discontinuing vs continuing angiotensin converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID‐19: a randomized controlled clinical trial. JAMA. 2021;325:254‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loader J, Lampa E, Gustafsson S, Cars T, Sundstrom J. Renin‐angiotensin aldosterone system inhibitors in primary prevention and CPVID‐19. J Am Heart Assoc. 2021;10:e021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bauer A, Schreinlechner M, Sappler N, et al. Discontinuation versus continuation of renin‐angiotensin‐system inhibitors in COVID‐19: a prospective parallel group, randomized, controlled, open label trial. Lancet Respir Med. 2021;9:863‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paternoster G, Bertini P, Innelli P, et al. Right ventricular dysfunction in patients with COVID‐19: a systematic review and meta‐analysis. J Cardiothorac Vasc Anesth. 2021;35:3319‐3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levy B, Klein T, Kimmoun A. Vasopressor use in cardiogenic shock. Curr Opin Crit Care. 2020;26:411‐416. [DOI] [PubMed] [Google Scholar]
- 23. Leisman DE, Mehta A, Li Y. Vasopressin infusion in COVID‐19 critical illness is not associated with impaired viral clearance: a pilot study. Br J Anaesth. 2021;127:e146‐e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


