Abstract
Coronavirus disease 2019 (COVID‐19), caused by a highly pathogenic emerging virus, is called severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Knowledge regarding the pathogenesis of this virus is in infancy; however, investigation on the pathogenic mechanisms of the SARS‐CoV‐2 is underway. In COVID‐19, one of the most remarkable characteristics is the wide range of disease manifestation and severity seen across individuals of different ethnic backgrounds and geographical locations. To effectively manage COVID‐19 in the populations, beyond SARS‐CoV‐2 detection, serological response assessment, and analytic techniques, it is critical to obtain knowledge about at‐risk individuals and comprehend the identified variations in the disease's severity in general and also in the populations' levels. Several factors can contribute to variation in disease presentation, including population density, gender and age differences, and comorbid circumstances including diabetes mellitus, hypertension, and obesity. Genetic factors presumably influence SARS‐CoV‐2 infection susceptibility. Besides this, COVID‐19 has also been linked with a higher risk of mortality in men and certain ethnic groups, revealing that host genetic characteristics may affect the individual risk of death. Also, genetic variants involved in pathologic processes, including virus entrance into cells, antiviral immunity, and inflammatory response, are not entirely understood. Regarding SARS‐CoV‐2 infection characteristics, the present review suggests that various genetic polymorphisms influence virus pathogenicity and host immunity, which might have significant implications for understanding and interpreting the matter of genetics in SARS‐CoV‐2 pathogenicity and customized integrative medical care based on population investigation.
Keywords: ACE2, COVID‐19, genetic diversity, polymorphism, SARS‐CoV‐2, TMPRSS2
Highlights
Genetic factors presumably influence SARS‐CoV‐2 infection susceptibility.
Genetic variants were involved in the pathologic processes of SARS‐CoV‐2 infection.
Various genetic polymorphisms influence virus pathogenicity and host immunity.
Human leukocyte antigens (HLAs) may play a vital role in SARS‐CoV‐2 susceptibility.
Polymorphisms in several genes such as IL‐6, TMPRSS2, IFITM3, CD26, ACE, and DBP were associated with the COVID‐19 severity.
1. INTRODUCTION
COVID‐19, a novel infectious disease discovered in China in December 2019, is triggered by SARS‐CoV‐2, a highly emerging deadly pathogen. 1 , 2 , 3 Fever, sputum production, fatigue, shortness of breath, and cough are the most frequent SARS‐CoV‐2 symptoms. 3 , 4 , 5 , 6 The establishment of SARS‐CoV‐2 disease relies on a particular interplay between Spike glycoprotein and angiotensin‐converting enzyme 2 (ACE2) (from the host). 7 , 8 , 9 The viral spike protein priming is carried out by the cellular serine protease transmembrane protease serine 2 (TMPRSS2), a cleavage that promotes the fusing of membranes (between viral and cellular membranes) viral propagation in the cells infected with SARS‐CoV‐2. 10 , 11 , 12 This procedure might include human exopeptidase CD26, widely known as Dipeptidyl‐peptidase 4 (DPP4), a crucial immunostimulatory component for hijacking and virulence. 13
Age above 60, male gender, and the existence of comorbid metabolic disorders such as being overweight, hypertension, and diabetes have been implicated in the COVID‐19 development and severity. 14 , 15 , 16 Nonetheless, there is a continuous exploration of biological variables related to COVID‐19 evolution. Genetic variables may affect the development and course of infectious diseases, according to evidence. 17 Multiple genetic polymorphisms, mostly single‐nucleotide polymorphisms (SNPs), have been linked to this setting's predisposition to viral respiratory infections. 18 Genetic polymorphism is the hallmark of human biology. 19 Scientists studying disease pathology are completely aware of this, and they often resort to simplifying human disease by establishing animal models that remove this complex facet via years of inbreeding. 19 The immune system is undoubtedly the most significantly influenced by human genetic diversity. 19 , 20 Until recently, the pinpoint significance function of genetic composition in SARS‐CoV‐2 related pathology has been understudied, but pro‐inflammatory‐related processes have been implicated. 8 , 9 , 21 Identifying the host genetic elements implicated in SARS‐CoV‐2 disease might contribute to the emergence of innovative therapeutic approaches for the prophylaxis and clinical monitoring of this disease using a precision medicine strategy. 22 , 23 As there is a paucity of information on genetics and SARS‐CoV‐2 pathogenicity, the objectives of this paper were to explore the role of genetic variants and host polymorphisms in specific viral entry and immune responses related to clinical consequences of COVID‐19 in humans to design future clinical implementation.
2. SARS‐COV‐2 ENTRY MECHANISMS INTO HUMAN CELLS
Initially, investigation for SARS‐CoV‐2 antiviral medications depended solely on the Vero E6 (African green monkey kidney cell line) as the host cell to evaluate inhibitory effects of antiviral candidate drugs on the cytopathic effect (CPE) of the virus. 24 Together with the absence of the expression of TMPRSS2 and ACE2, endogenous unspecified endosomal viral absorption mechanisms are involved for SARS‐CoV‐2 entrance in Vero E6 and several other types of cells. 24 , 25 , 26 As a result, a broad range of compounds that regulate endosomal–lysosomal maturation and autophagy processes have been shown to have potent antiviral activity in Vero E6 cells, which might not apply to original epithelial cells from the lung. This opens the possibility that the antiviral suppression observed in Vero E6 assays by many of these drugs toward this virus is limited and in vitro activity and that they are neither relevant nor translatable as SARS‐CoV‐2 therapies.
SARS‐CoV‐2 and other coronaviruses infect epithelial cells in the mammalian lung by attaching their spike protein to a particular cellular receptor. ACE2, which is present on the lungs and gut epithelial cells and on kidney, heart, and adipose tissues, acts as a cell surface ligand for SARS‐CoV‐2 and the other related SARS‐CoVs. 10 , 12 , 15 , 27 , 28 , 29 , 30 After attaching to the cellular receptor, the spike protein is activated by a host protease at the intersection of its S1 and S2 regions through proteolytic processing. 31 When the newly released S2 domain N‐terminus is inserted further into the cellular membrane, the fusion between viral and cellular membranes leads to the transport of viral RNA further into the host cell cytoplasm, wherein viral proliferation may begin. 31
The spike or S protein (as trimers) is located on the surface of SARS‐CoV‐2 particles, and every one of the S‐protein subunits has two domains, which are designated as S1 and S2 on the S protein's structure. 32 The S1 domain interacts with ACE2 receptors, while the S2 domain promotes membrane fusion, which allows the viral particle to enter the cell. 32 SARS‐CoV‐2 enters the human cell via ACE2 receptors. The S1 domain's receptor‐binding domain (RBD) interacts with ACE2 receptors. 32 An in vitro research revealed that SARS‐CoV‐2 could not infect ACE2 null Vero E6 and Hela cells, implying that this receptor plays a critical role in cellular viral entry. 33 Membrane‐bound TMPRSS2 is required for the cleavage of ACE2 and S‐protein for viral entrance mediating membrane fusion and therefore performs a significant function in SARS‐CoV‐2 pathogenesis. Coronaviruses employ many additional cell surface receptors, plus TMPRSS2 and ACE2, to promote cellular entrance; these include DPP4 and furin for Middle East respiratory syndrome coronavirus (MERS‐CoV), aminopeptidase N (ANPEP) for Human coronavirus 229E (HCoV‐229E), TMPRSS11D for SARS‐CoV, and ST6GAL1 (ST6 Beta‐Galactoside Alpha‐2,6‐Sialyltransferase 1) and ST3GAL4 for Human coronavirus OC43 (HCoV‐OC43) and Human coronavirus HKU1 (HCoV‐HKU1). 34 , 35 , 36 S‐protein of SARS‐CoV‐2 has *80% sequence identity with SARS‐CoV, and most of the remaining positions are conserved. Excluding the insertion of Val483 in SARS‐CoV‐2, the residues across Leu335 and Phe515 of SARS‐CoV‐2 are similar to those spanning Leu322 and Phe501 of SARS‐CoV. This area contains three ACE2 interaction sections, namely CR1, CR2, and CR3, which are located inside a receptor‐binding motif (RBM) of SARS‐CoV‐2 that has *50% sequence similarity with SARS‐CoV. 37
The new SARS‐CoV‐2 virus, like with the SARS, enters cells via the use of the ACE2 protein on the cellular membrane. 38 ACE2 works as an enzyme in the renin‐angiotensin system (RAS), regulating blood pressure, fluid balance, and vascular constriction. Angiotensin I (Ang I) produced by renin degradation is transformed to Ang II by the angiotensin‐converting enzyme ACE, which activates angiotensin II type I receptors (AT1Rs), leading to oxidative stress, pro‐inflammatory signaling, elevated blood pressure, and vasoconstriction. A strong affinity for Ang II exists between the ACE2 enzyme and it, resulting in the formation of Ang II (1–7). As a result, ACE2 mitigates the effects of Ang II and protects against chronic conditions such as cardiovascular disorder, hypertension, and diabetes. 39 Significantly, higher concentrations of Ang II are found in COVID‐19 individuals who have never had an ACE or an ARB, and elevated concentrations are linked with enhanced severity. 40
It is also necessary for SARS‐CoV‐2 virus entrance to be initiated by the serine protease TMPRSS2, which could be partly inhibited in certain cell types by the serine protease inhibitor camostat mesylate, which is also effective in some cell types. 38 It has been observed that when camostat inhibition of TMPRSS2 was coupled with an antagonist of endosomal cysteine proteases, cathepsin B/L, complete inhibition was accomplished. 10
3. IMMUNE RESPONSE TO SARS‐COV‐2
The body's immune system recognizes the entire virus or its surface epitope once inside the target cells, inducing an immune response (innate or adaptive). 2 , 20 , 41 , 42 When a virus is initially detected, immune cells' pathogen recognition receptors (PRRs), mainly Toll‐like receptors (TLRs) 3, 7, and 8, synthesize more interferon (IFN). 1 , 20 The nonstructural proteins of SARS‐CoV and MERS‐CoV disrupt the activity of host innate immunity, affecting total cytokine generation. 43 , 44 The humoral immunity to SARS‐CoV‐2 is similar to other coronavirus diseases, producing IgG and IgM formation. 45 B cells provide an initial response to the N protein when infected with SARS‐CoV.
Nevertheless, antibodies toward S protein may be identified 4–8 days after the onset of first manifestations. 46 , 47 Despite being smaller than S protein, the N protein is highly immunogenic. As it lacks glycosylation sites, it produces N‐specific neutralizing antibodies at the initial stages of severe disease. 48 Individuals infected with SARS‐CoV were shown to have IgA, IgG, and IgM antibodies specific to the virus at different times after the onset of symptoms. 48 Anti‐S‐RBD IgG was found in all 16 SARS‐CoV‐2 cases in an observational research investigation, while anti‐N IgG and anti‐S‐RBD IgM were recognized in 15 cases and anti‐N IgM in 14 cases. 49 According to a research study on pediatric patients, five out of six kids had a protecting humoral immunity, including neutralizing IgG and IgM antibodies against the SARS‐CoV‐2 N and S‐RBD proteins. 50
In responding to viral diseases, our immune system acts in a series of activities that fight the invading virus. 4 , 51 The innate immunity is first activated, which detects and destroys foreign viral material and initiates a signaling cascade that restricts virus transmission to adjacent cells. 51 Although it is difficult to show experimentally, innate immunity may play a significant role in containing SARS‐CoV‐2 in certain instances, such as subclinical or moderate diseases in children and adolescents. 52 T cells that recognize processed Ag expressed by MHC molecules (class I and II) are promptly engaged to eliminate infected cells and coordinate the immune reaction, whereas the IgM reaction proceeds simultaneously. Multivalent antiviral IgM is the first isotype generated toward viral diseases and therefore plays an essential role in the early phases of the disease. 51 During SARS‐CoV‐2 recovery, immunophenotyping of peripheral B cells revealed that both un‐switched IgM+ memory B cells and classical switched B cells are components of the circulatory memory population and may hold steady for months. 53 Convalescent people have a memory of anti‐SARS‐CoV‐2‐reactive T cells. 54 , 55
On the other hand, cross‐reactive T cells were found in a large proportion of pre‐pandemic and seronegative people who had not been subjected to SARS‐CoV‐2, indicating that T cells seem to be more promiscuity than B cells. 54 , 55 , 56 , 57 , 58 According to findings, cross‐reactive IgM memory B cells evoked by prior exposures to pandemic COVID‐19 could be involved in the reaction to SARS‐CoV‐2 in a subset of individuals, while the IgG reaction, particularly to the spike glycoprotein, is particular for SARS‐CoV‐2. 59 , 60 To determine if past reactions to epidemic CoVs offer any degree of protection against symptomatic COVID‐19, additional research must be conducted, and the issue will be explored in more depth later onwards.
3.1. Human genetic diversity and infectious diseases
Human genetic makeup refers to the genetic differences between people and groups and includes alterations spanning from SNPs to massive genome alternations and epigenetic modifications. 61 Thus according to commonly accepted evidence, human genetic variation is believed to be about 0.1%, which means that any two people vary on average by around 1 in 1000 nucleotides. However, some current findings indicate a greater rate of genetic variation in the human community. 62 , 63 This is an interesting topic since it may provide insight into human history and evolution and enhance wellbeing. Individual and population genetic variations may influence diseases predisposition, resistance to disease and pathogens, and responsiveness to pharmacological therapies. Recognizing and tracking such variations is critical for understanding the foundation and processes of different illnesses, finding novel therapeutic strategies, and developing new therapeutics. The distribution of genetic diversity inside and across human societies results from tens of thousands of years of demographic background and evolution that altered the human genome as an adaptive reaction to dietary restrictions, the environment, and pathogens. 64 , 65 Based on the current corpus of findings, newer genomics tools and methods will allow us to comprehend these distinctions better and use the information in biomedicine, from finding novel genes causing the disease to develop novel therapeutics. 66 Infectious diseases have been identified as one of the most significant evolutionary forces that have influenced and shaped the human genome throughout time. Pathogen‐induced infections have been and continue to be the leading cause of death in many parts of the world, acting as a selective pressure that favors genetic variants that confer disease resistance over those that do not. Haldane initially proposed this hypothesis, noting that heterozygosity for some hematological diseases correlates with malaria resistance. 67
Furthermore, naturally occurring resilience to human immunodeficiency virus (HIV) disease is an instance of how the finding of genetic resistance may act as a foundation for future treatment. 61 CCR5‐32 (chemokine receptor 5—CCR5), the cell surface ligand that functions as an HIV coreceptor, has a 32‐base deletion, which results in resistance to HIV. 61 , 68 The existence of this deletion inhibits viral entry and receptor expression on CD4 1T cells, giving complete HIV protection in homozygous carriers and modest resistance toward disease development in heterozygous carriers. 61 Following the finding that suppressing CCR5 expression may render cells resistant to HIV infection, this discovery has opened the path for the development of stem cell‐based therapies. This method was initially shown to be effective over a decade earlier, whenever cases who got stem cells from a CCR5‐32 homozygous donor maintained HIV‐free. 69 A similar method was recently described in another patient, showing the possibility of creating HIV treatments based on decreasing CCR5 expression and the medical importance of finding naturally resistant variations in populations. 70
4. GENETIC CHARACTERISTICS OF THE IMMUNE RESPONSE IN PATIENTS WITH COVID‐19
As among the most studied risk factors for autoimmune disorders, the variation in the human leukocyte antigens (HLAs) gene has been identified as one of the most important. 71 These genes code for MHC molecules, which are responsible for presenting antigens to T‐lymphocytes on the cell surface. 72 The HLA system is among the first to be exposed to exogenous antigens, which indicates its influence on future immunological responses, including autoimmune disorders. 73
According to a study, the HLA‐C*07:29 and B*15:27 alleles have been related to SARS‐CoV‐2 vulnerabilities in the Han ethnicity. 74 MHC‐B*46:01 has the lowest affinity for coronavirus proteins, according to an in silico research, which is why those with the HLA‐B*46:01 genotype may be more vulnerable to COVID‐19, as was previously shown for SARS‐CoV. 75 These molecules of MHC (including MHC‐A*02:02, ‐B*15:03, and ‐C*12:03) demonstrated the most potent ability to express evolutionary conserved SARS‐CoV‐2 peptides carried by human coronaviruses. This suggests that they could offer cross‐protection through T‐cell immunity. 75 In addition, MHC‐A*25:01 and C*01:02, which have a low affinity for coronavirus proteins, were discovered in this investigation. Moreover, Secolin et al. 76 in the Brazilian population determined HLA alleles formerly correlated with the COVID‐19 reaction at loci DQB1 and DRB1. Their findings might cause altering the rate of infection or reaction to infection by SARS‐CoV‐2 and should be further explored in cases with this infection. 76
Six genes in the 3p21.31 region, including SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6, and XCR1, were linked to the severity of the disease in a genome‐wide analysis of individuals from Spain and Italy. 77 The CXCR6 gene, which encodes the chemokine receptor 6 with the CXC motif (CXCR6), which regulates the precise placement of memory CD8+T lymphocytes (lung tissue‐resident) that are specific for respiratory tract infections such as influenza viruses, was found to be expressed at a lower level in the individuals who participated in the research. 78 It has also been shown that upregulation of SLC6A20, which produces sodium/imino‐acid (proline) transporter 1 (SIT1) and physiologically interfaces with ACE2, the cellular ligand of SARS‐CoV‐2, seems to be statistically crucial in patients with COVID‐19. 79 Additional research investigated the HLA‐genotyping of seven distinct HLA locus (including HLA‐A, ‐C, ‐B, ‐DRB1, ‐DQA1, ‐DQB1, and ‐DPB1) and the connection between them and COVID‐19 severity. They identified no link between vulnerability to serious COVID‐19 and the number of HLA‐alleles. CCR9 gene, which generates a chemokine ligand that is both homeostatic and pro‐inflammatory, is one of the genes distinguished among other genes studied. This receptor is found to be implicated in the pathophysiology of many forms of pneumonia, and its production is elevated during the early stages of respiratory inflammation. Several types of pneumonia have been discovered to be mediated by this receptor, and its synthesis is heightened during the initial phases of inflammation in respiratory disease. 80
Lu et al. 81 discovered many genes with mutations that were correlated with an elevated risk of mortality in COVID‐19. Scientists discovered four genes with missense variations when analyzing the genotypes of 193 infected cases: the ERAP2 gene, the BRF2 gene, the TMEM181 gene, and the ALOXE3 gene have three different alleles, which are the rs150892504 allele, the s138763430 allele, and the rs117665206 allele, respectively. 81 Mutations in the ERAP2 gene, which encodes a metalloaminopeptidase implicated in the final processing of antigens for presentation through MHC‐I molecules, are among the mutations described. 81 The analysis of four clinically relevant cases of severe coronavirus infection followed up with whole‐genome sequencing showed the presence of loss‐of‐function mutations in the X‐chromosomal TLR7 gene, which encodes the TLR7, in COVID‐19 cases and their families. 81 Patients' peripheral blood mononuclear cells showed a reduction in type I IFN signaling, which had been demonstrated as a decline in the expression levels of IRF7 proteins (Interferon Regulatory Factor 7), IFNB1 (Interferon Beta 1), and interferon‐stimulated gene 15 (ISG15) after stimulation with imiquimod, a TLR7 agonist, as well as reduced expression of IFN‐, IFN type II 82 (Figure 1). Based on previous studies, researchers have shown that the X chromosome‐specific TLR7 gene supports the innate immunity against coronaviruses. 83 , 84 In a multi‐population analysis, Smatti et al. discovered that multiple genetic variants such as intercellular adhesion molecule 3, IFN‐γ, MBL, IL4, CCL2, CCL5, Furin, TMPRSS2, and CD209 promoter, which could potentially modulate SARS‐CoV‐2 infection, are markedly varying among populations, with the lowest incidence observed among Africans. 85 In another study, Verma et al. 86 discovered that the MUC5B variation rs35705950‐T has a protective function in COVID‐19 disease. Their findings revealed that the existence of rs35705950‐T was related to decreased hospitalizations as well as fewer post‐COVID‐19 pneumonia episodes. 86
Figure 1.
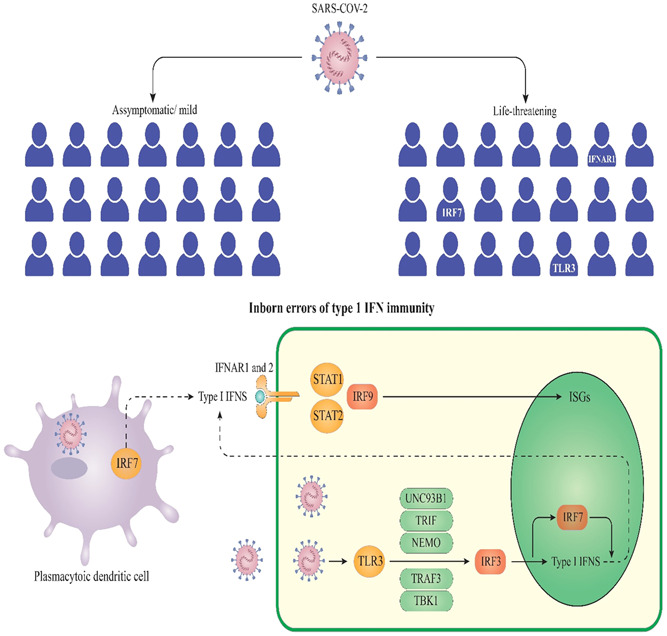
Diversity of type I IFN production and life‐threatening COVID‐19 pneumonia. It has been demonstrated that variants at the TLR3‐ and IRF7‐dependent type I IFN immunity are related to life‐threatening COVID‐19 pneumonia and asymptomatic infection. 87 COVID‐19, coronavirus disease 2019; IRF7, interferon regulatory factor 7; IFN, interferon; TLR3, toll‐like receptor 3 (TLR3)
Moreover, according to the 2020 survey performed by Zhang et al., 88 homozygous heredity of the rs12252 allele of the interferon‐induced transmembrane protein 3 (IFITM3) might be linked with severe illness. IFITM3 produces a protein (an immune effector) that promotes mucosal immune cell longevity by increasing the concentration of CD8+T lymphocytes in the airways, which is vital in viral diseases. 89 , 90 This protein is involved in viral invasion and is linked to the COVID‐19 severity and the cytokine storm. 91
5. OTHER COVID‐19 ASSOCIATED GENES
Interindividual heterogeneity in vulnerability to COVID‐19 disease has been linked to the existence of genetic variants in numerous genes, particularly those that code for proteins involved in the pathogenicity. 92 This section gives a brief overview of the many genes involved in SARS‐CoV‐2 illness and their association with disease severity (Table 1).
Table 1.
Main COVID‐19 associated genes and their polymorphisms
| Gene | Chromosome | Function in COVID‐19 | Polymorphism (SNP) | Study population | Result | References |
|---|---|---|---|---|---|---|
| ACE1 | 17 | ACE‐1 and ACE‐2 are angiotensin peptide cleavers, with ACE‐1 cleaving Ang I and creating Ang II. At the same time, ACE‐2 cleaves Ang II and produces Ang III, which triggers vascular constriction and bronchoconstriction, enhances endothelial dysfunction, fibrosis, and inflammation, and continues the progression of ARDS and lung collapse cases with COVID‐19. | rs1799752 | Korea, Europe | Evidence shows that the ACE1 I/D polymorphism might be implicated in various clinical diseases induced by SARS‐CoV‐2 diseases such as renal damage, pneumonia, ischemic stroke, and immunological reaction, including the cytokine storm. | 93 , 94 |
| ACE 2 | Xp22.2 | It has been discovered that the SARS‐CoV‐2 S protein recognizes the ACE2 ligand. This recognition promotes the virus' binding to the cell. | rs2106806, rs6629110 | Spain | According to the current investigation findings, the minor A allele inside rs2106806 enhances the risk of developing severe COVID‐19. | 95 , 96 |
| ACE 2 | – | – | rs2285666 | India | In this study, researchers discovered for the first time a positive and statistically significant connection between alternative allele (T or A) of rs2285666 with a reduced infection and mortality rate in Indian communities. | 97 |
| ACE 2 | – | – | S19P, I21T/V, E23K, A25T, K26R, T27A, E35D/K, E37K, Y50F, N51D/S, M62V, N64K, K68E, F72V, E75G, M82I, T92I, Q102P, G220S, H239Q, G326E, E329G, G352V, D355N, H378R, Q388L, P389H, E467K, H505R, R514G/*, and Y515C | Europeans, Africans, Asians, and Americans | For the virus to enter and infect the host, these variations played a central role. According to recent research, the uncommon variations of human ACE2 appear to become one of the determining variables linked with adaptation in the fight toward SARS‐CoV‐2. | 98 |
| ACE 2 | – | – | rs61299115, rs11088551, and rs4303794 | Asian and African descents in Israel | The combination of the three SNPs, which are very frequent in the overall population (25%–36%) but are relatively uncommon in the East Asian and Korean populations, suggests that TMPRSS2 is expressed at a lesser rate in these groups. This, together with the differences identified in the promoter of ACE2, might lead to the conclusion that COVID‐19 has varied pathogenesis and outcome in different societies. | 7 |
| TMPRSS2 | 21q22.3 | An additional requirement for viral entrance to the target cell is S priming by cell‐surface proteases, including the TMPRSS2, which also is accomplished by degradation of the virus's S protein at the S1/S2 and S2 domains. | p.Val160Met | Indonesia | However, while the current analysis found no connection between the p.Val160Met variant and illness severity, they did notice a possible connection between the TMPRSS2 (pVal160Met) variation and viral load in subjects with COVID‐19. | 10 |
| TMPRSS2 | – | – | rs383510 | German | This study demonstrated that the intron variation rs383510 in the gene TMPRSS2 is related to an elevated risk of SARS‐CoV‐2 disease in a German population. | 99 |
| IFITM3 | – | – | rs6598045 | Worldwide | A noteworthy finding of this investigation was discovering a significant connection between the death rate of COVID‐19 and the allele frequency of the rs6598045 SNP of the IFITM3 locus. | 100 |
| TMPRSS2 | – | – | rs3787946, rs9983330, rs12329760, rs2298661, and rs9985159 | Italy | Furthermore, while comparing the critically ill patients to the reference persons, it was discovered that the alleles with minor frequency were less frequently identified, indicating that they may have a protective function against the advancement of the disease. Unexpectedly, four of the five SNPs were found to be repeated in two Asian‐derived cohorts, but just two SNPs were found to be replicated in a case series of African descent. Furthermore, we confirmed the relationship between the rs12329760 SNP and Italian nationality in a separate case‐control cohort of Italian origins, which was previously published. These findings clearly imply that the gene 21q22.3 is a new susceptibility locus to an adverse outcome of COVID‐19 and that people of diverse ethnic backgrounds might share the molecular processes behind this genetic propensity. | 101 |
| TMPRSS2 | – | – | p.V197M (p.Val197Met) (rs12329760) | Finland, Italy | It was discovered that there is a frequent variation of the gene p.V197M (p.Val197Met) (rs12329760) which has a detrimental impact on the protease and beneficial effects on the patients. It is thought to play a significant role among two sub‐groups, namely, young men and older women and those with co‐morbidities. The variant amplitude is more prevalent in individuals who were moderately influenced by the disease and did not demand inpatient care or mechanical ventilation than those who were more significantly affected, who mandated mechanical ventilation or intubation. | 102 |
| CD26 | 2 | In recent research, it has been revealed that SARS‐CoV‐2 interacts with the DPP4/CD26 receptor as it enters cells of the respiratory system. It indicates that the link between the S protein (SARS‐CoV‐2) and the DPP4/CD26 is a critical component in the hijacking and pathogenicity of the virus. | rs13015258 | Worldwide | rs13015258, a 5′ UTR polymorphism from CD26, has been shown to have a vital function in controlling the expression of essential regulatory genes, which may be implicated in the internalization of SARS‐CoV‐2. Increased expression of CD26 caused by epigenetic alteration at the rs13015258‐C allele was discovered to be necessary, and this might underlie the increased mortality rate associated with SARS‐CoV‐2 infection among patients with type 2 diabetes. | 103 , 104 |
| IFITM3 | 11 | IFITM has a vital antiviral function in the body since it prevents viruses from crossing through the lipid bilayer of the cell. Variants of the IFITM3 gene are related to clinical responses to influenza and other viruses. | rs12252 | China | In recent years, it has been shown that homozygosity for the C allele of the rs12252 gene in the IFITM3 gene is related to a much more severe infection in an age‐dependent fashion. This supports the hypothesis that IFITM3 plays a function in the disease process and provides the potential for early specialized treatment in at‐risk people, among other things. | 88 , 105 |
| IL‐6 | 7p15.3 | Growing evidence suggests an upsurge of IL‐6 in COVID‐19 critically ill patients, and the presence of this rise is associated with the severity of the illness and the risk of death. | rs1800796/rs1800795 | Worldwide | The polymorphisms in the IL‐6 gene at the rs1800796/rs1800795 loci were studied in communities from India, Mexico, Turkey, Brazil, Russia, Italy, South Africa, the Netherlands, and Greece. The GG genotype was found in most of the communities from these countries, whereas the GC genotype was found in the majority of the societies from China, Spain, Sweden, Poland, Germany, and the United Kingdom. | 106 , 107 |
| Only the Japanese population exhibited the CC genotype for the rs1800796 variant. | ||||||
| HLA | 6 | Considering that HLA plays an essential role in the immunological reactions to pathogens and the pathogenesis of infectious diseases, it has been suggested that HLA diversity in a population might be associated with COVID‐19 occurrence. | HLA‐A*24:02 | China | According to a study conducted in Wuhan, there is a significant association between the HLA‐A*24:02 gene as well as the risk of developing COVID‐19 in the Han population. | 92 , 108 |
| ABO | 9q34.2 | An accumulating body of data shows that the ABO blood type may play a major role in the immunopathology of SARS‐CoV‐2 disease, with group O persons being less likely to test positive and group A persons being more susceptible to infection and more likely to suffer from severe illness. | 3p21.31 and 9q34 | Italy | 9q34 contains the ABO blood group loci, and blood‐group‐specific research discovered a stronger connection between being A‐positive and having a greater chance of contracting covid19 than previously thought. | 109 , 110 |
| DBP | 4q11q13 | It was shown that having higher 25‐hydroxyvitamin D levels was linked with a considerably reduced amount of total lung participation while having low 25‐hydroxyvitamin D levels was related to an increased risk of death. It brought attention to the relevance of the polymorphisms of vitamin D binding protein (DBP), the main transporter of vitamin D, and performs a potentially underappreciated role in the pathophysiology of COVID‐19 infection. | rs7041 and rs4588 | Turkey | Between both the occurrence (per million) and death rates (per million), as well as the GT genotype (P.05), significant positive associations were discovered. In contrast, a significant negative relationship was discovered between both the occurrence (per million) and death rates (per million), as well as the TT genotype atrs7041 locus amongst the communities (p < 0.05). In conclusion, the differences in the occurrence of COVID19 and the death rates associated with it may be linked with the DBP variants rs7041 and rs4588 were shown to have different effects on vitamin D metabolism. | 111 , 112 |
| GSTT1 | 1p13.3 | The GSTT1 and GSTM1 deficient variants are linked with an increased risk of various oxidative stress‐related complex illnesses. It is widely recognized that oxidative stress is a significant contributor to the development of viral respiratory diseases. Pulmonary fibrosis is one of the most serious consequences of COVID‐19 illness, which is characterized by high levels of oxidative stress in the body. | GSTM1 and GSTT1 | India | Patients with the GSTM1+/+ and GSTT1−/− genotypes had a poor survival rate. These findings recommended that patients with the GSTT1−/− genotype in COVID‐19 had a higher death rate. | 113 |
5.1. ACE
ACE is the enzyme that converts angiotensin‐2 to angiotensin (1–7) from 114. It has been shown that nearly all organs, including the lungs, liver, kidney, vascular system, and other organs, express ACE2. 115 , 116 The cellular receptor for SARS‐CoV‐2 infection is considered to be ACE2. 44 The COVID‐19 attaches to target cells via the ACE2 receptor, facilitating COVID‐19 adherence, invading, and penetrating. 117 Several receptors may be utilized, although the virus prefers ACE2 and has lower affinities affinity for Glucose‐Regulate Protein 78 (Grp78) and CD147 as an additional ligand. 116 The amount of ACE2 expression in males is substantially more significant than in females, which may underlie the male preponderance of COVID‐19. 118 , 119 The effects of ACE2 polymorphism on SARS‐CoV‐2 pathogenesis are illustrated in Figure 2.
Figure 2.
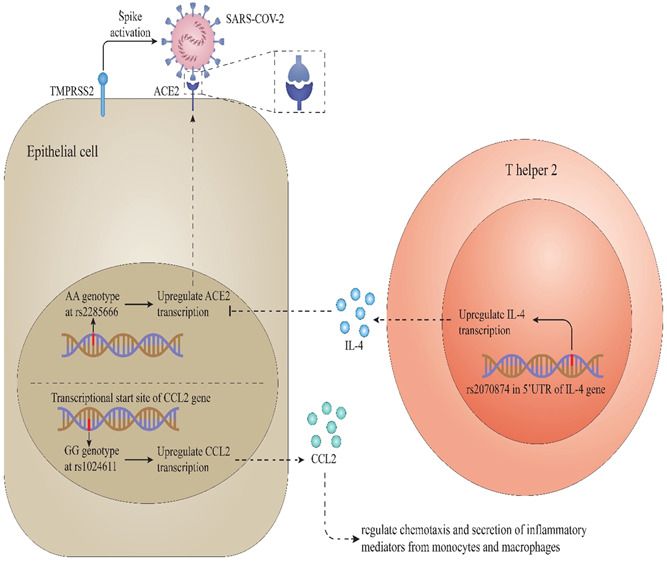
ACE2 polymorphism and effects on SARS‐CoV‐2 entrance and outcomes. It has been found that the AA genotype of the rs2285666 is mediated to higher ACE2 expression that causes more virus attachment and can affect the infection course. 120 Besides this, the GG genotype of this polymorphism is medicated to higher expression of CCL2, a chemokine that regulates the formation of chemotaxis and inflammatory mediators from macrophages and monocytes. ACE2, angiotensin‐converting enzyme 2; CCL2, C‐C Motif Chemokine Ligand 2
Nevertheless, in another investigation, ACE2 activity was not correlated with a male/female disease severity difference amongst Italian subjects with COVID‐19. 121 Another researcher discovered similar findings, revealing no differences in ACE2 gene expression across age or genders (male vs. female) categories. 122 ACE2 gene is highly polymorphic; several polymorphisms in ACE2 have been associated with higher ACE2 protein production and are more prevalent in the East Asian populations. 117 , 123 , 124 It is not just that, but the ACE deleting allele, which is related to changes in ACE synthesis, affects the propagation of the virus and the consequences of COVID‐19 disease, particularly in Asian communities. 125 , 126 According to Stawisk's research, numerous genetic polymorphisms in ACE2 are linked with COVID‐19 susceptibility. 127
Some ACE2 gene variants may also affect neurological sequelae frequencies in COVID‐19 cases. 128 In contrast, Delanghe et al. 129 could not find a connection amongst deletion allele occurrence of the ACE I/D variant in European, North African, and Middle Eastern countries and COVID‐19 disease and mortality rates. There was a low prevalence of polymorphisms in the ACE2 gene in a cohort study of 120 individuals in Madrid, and there was no connection between this gene and COVID‐19. 130 Many variables, including genetic makeup, lifestyle, regional and cultural variations across groups, demographic features of the population (gender, age), and comorbidity, may explain the conflicting findings of these research.
There are 26 exons in the ACE gene, which is located on chromosome 17q35. 131 ACE insertion/deletion (I/D) polymorphism of a 287‐bp Alu repeat sequence of intron 16 may induce alternative splicing in ACE protein, with one active site for the ACE I allele and two active sites for the ACE D allele. 131 The I/D polymorphism accounts for 47% of variations in plasma ACE concentrations, whereas the DD polymorphism is related to the highest concentration in most ethnic groups examined. 132 , 133 The use of ACE blockers enhanced ACE2 levels. 134 Several investigations show a link between the ACE “D” allele and pneumonia in SARS patients and ARDS mortality. Accordingly, in a study by Verma et al., 135 they explored how the ACE1 I/D genotype influenced the disease severity of patients with COVID‐19 in the north Indian community. They discovered that ACE1 DD polymorphism, the abundance of D allele, age above 46, unmarried lifestyle, and incidence of diabetes and hypertension were substantially higher in severe COVID‐19 subjects. The ACE1 ID polymorphism was shown to be positively correlated with socioeconomic COVID‐19 subjects. 135 These data suggest that the ACE1 polymorphism might influence the frequency and outcomes of cases with COVID‐19 and serve as a predictor of COVID‐19 susceptibility and morbidity.
5.2. CD26
The DPP4 is another receptor for coronaviruses, also identified as CD26, which is the primary receptor for MERS‐CoV cell entrance. 136 Furthermore, CD26 has been identified as a cellular ligand for SARS‐CoV‐2 entrance into human cells. 137 Beyond its potential involvement in the entrance of this virus into the target cell, CD26 has been linked to obesity, insulin resistance, and hypertension. 138 DPP4 blockers were used to treat type 2 diabetes mellitus (T2DM) because they enhance the half‐life of glucagon‐like peptide 1 (GLP‐1). 139 Given that diabetes and obesity are essential determinants for COVID‐19 development and disease severity, studying DPP4 protein in these individuals may offer valuable information. According to another research, individuals hospitalized with COVID‐19 had lower levels of DPP4 than a control group. 140 This study even included a group of sepsis subjects who had no changes in DPP4 levels, indicating that the SARS‐CoV‐2 infection causes a reduction in this protein. The DPP4‐encoding genes are heterogeneous; a connection between enzyme levels and specific polymorphism genotypes and pathologies such as T2DM and myocardial infarction has been discovered. 141 , 142 In this line, a study by Posadas‐Sánchez et al. 138 discovered the DPP4 level in a sample of COVID‐19 cases and determined whether these levels are linked to severity and certain clinical observations. Furthermore, a link between the DPP4 rs3788979 polymorphism and COVID‐19 was discovered.
The genetic vulnerability of DPP4 to COVID‐19 was investigated, and a connection was discovered between the rs13015258 missense variation in the CD26 gene and vulnerability to COVID‐19. The in silico analysis also indicates that the S protein of SARS‐CoV‐2 interacts with the targets CD26 and TMPRSS2. 137
5.3. IFITM3
The IFITM proteins are important in antiviral protection in adaptive and innate immunity. 143 The IFITM loci in humans are found on chromosome 11p15.5 and include five genes, one of which is IFITM3. 143 IFITM3 inhibits hemifusion between both the viral envelope and the host cellular membranes in a wide range of enveloped viruses, including Marburg virus, influenza A virus, SARS‐CoV virus, and Ebola virus. 144 IFITM3 suppresses viral‐cell membrane fusion by altering cell membrane flexibility. 145 Previous research has shown that SNPs in the gene IFITM3 might reduce IFITM3's antiviral activity, increasing infection sensitivity and disease severity. 146 In pioneering research, Zhang et al. 88 identified a greater incidence of rs12252 C variants in severe COVID‐19 cases than in moderate cases. It was later demonstrated that C‐allele carriers of the SNP rs12252 in the Spanish population had a twofold higher risk of COVID‐19 relative to a reference population obtained before the pandemic. 105
The reduction of S protein‐mediated entrance by IFITM1, IFITM2, and IFITM3 was shown in functional in vitro experiments for SARS‐CoV‐2. 147 SARS‐CoV‐2 and SARS‐CoV‐1 have an 82% sequence similarity. 148 Kim and Jeong 149 discovered different mortality rates in cases with COVID‐19 from each ethnic group in preliminary research. Significantly, they discovered a significant connection between the COVID‐19 death rate and the genotype frequencies of the IFITM3 gene's rs6598045 SNP. This is the first research to demonstrate a substantial population‐level link between the COVID‐19 mortality rate and the IFITM3 gene's rs6598045 SNP. As a result, mutations in this protein may affect the risk and severity of respiratory diseases, including influenza and COVID‐19 permeability. 146 , 150 Furthermore, IFITMs significantly impact infection in several different ways, including modifying cellular membrane characteristics, changing the lipid/protein structure of acidic subcellular compartments, increasing cholesterol levels on late endosomes and lysosomes, and hindering membrane fusion via a hemagglutinin‐mediated mechanism. 151 In a nutshell, the mechanisms of IFITM‐mediated SARC‐CoV‐2 suppression need more investigation. 152
5.4. TMPRSS2
TMPRSS2 is another human ligand for the SARC‐CoV‐2 that performs a pivotal function in viral entry into the target cell. 10 The study of TMPRSS2 activity in human tissues emphasized the significance of expression in lung tissue and the existence of four polymorphisms (rs383510, rs2070788, rs469390c, and rs464397); these variations are considered to have the potential to affect TMPRSS2 expression and performance. 153 According to Cheng et al.'s research, 154 two genetic polymorphisms in this gene (rs2070788 and rs383510) were strongly linked with the risk of influenza. Furthermore, it was discovered that gender could affect TMPRSS2 gene expression in a large Italian cohort. 92 Several genetic polymorphisms in this gene result in higher TMPRSS2 production and are linked to an increased risk of influenza. 92 Others have been influenced by androgens, which may underlie the gender disparity in severity of COVID‐19. 121 As a result, in German case‐control research, researchers investigated the impact of SNPs in the gene TMPRSS2. Schönfelder et al. 99 genotyped SNPs rs383510, rs12329760, and rs2070788 in the gene TMPRSS2 in 239 positive cases and 253 negative individuals for SARS‐CoV‐2 infection. They investigated the relationship between the SNPs and vulnerability to SARS‐COVID‐19 disease and the disease severity. The characteristics of positive and negative COVID‐19 patients were not different. The CC polymorphism of TMPRSS2 rs383510 was linked to a 1.73‐fold higher risk of SARS‐CoV‐2 disease although not to the disease severity. However, neither TMPRSS2 rs2070788 nor the rs12329760 variants were correlated to the probability of SARS‐CoV‐2 disease or the COVID‐19 severity. The rs383510 CC polymorphism maintained an important predictor of a twofold higher SARS‐CoV‐2 risk of infection in multivariable analysis (MVA). In brief, the investigation by Schönfelder et al. 99 seems to have demonstrated that the intron variant rs383510 in the TMPRSS2 gene is linked with an elevated risk of COVID‐19 in a German population.
Senapati et al. 137 discovered significant associations between three TMPRSS2 gene variations (rs61735794, rs61735792, and rs75603675) and COVID‐19 in Madrid populations, also, they discovered four variants in the TMPRSS2 gene (rs713400, rs77675406, rs11910678, and rs112657409) that control gene expression of TMPRSS2 and impact the chance of COVID‐19 infection.
5.5. GSTT1‐M1
Glutathione S‐transferases (GSTs) are a versatile isoenzyme superfamily that facilitates glutathione coupling with electrophilic chemicals, which detoxifies various endogenous and foreign substances by the cell. 155 GSTs have a key component in detoxifying numerous carcinogens and medicines and the protection of cells toward different kinds of cellular oxidative damage. 156 The different interindividual activities of the GST enzyme are assumed to contribute to the elimination of oxidative stress intermediates by the GST enzyme. 157 It has been discovered that there are eight different types of GST enzymes found inside the cytosol of mammalian tissue (including alpha (α)‐GSTA, mu (μ)‐GSTM, pi (π)‐GSTP, omega (ω)‐GSTO, theta (θ)‐GSTT, sigma (σ)‐GSTS, kappa (κ)‐GSTK), and zeta (ζ)‐GSTZ). 158 The μ (GSTM1: MIM: 600436) and θ (GSTT1: MIM: 138350) are the most frequent variants of GST genes, and they are found on chromosomes 1p13.3 and 22q11.23, correspondingly, on chromosomes 1 and 22. 158 The null genotype (GSTM1−/−) and GSTT1 (GSTT1−/−) genes are linked with decreased enzyme activity and an increased risk of many oxidative stress‐related multifaceted disorders, such as cardio‐respiratory disorders. 158 The GSTT1 and GSTM1 genetic variants result in a full gene deletion, resulting in the complete lack of GSTT1 and GSTM1 enzyme activity in the affected subjects. 159 For example, in research conducted in the North Indian population, Abbas et al. 158 examined the relationship of GSTM1 and/or GSTT1 genotypes with COVID‐19 vulnerability and its consequence. The incidence of GSTM1/, GSTT1/, and GSTM1/GSTT1/ was greater in severe cases than in moderate COVID‐19 cases; however, they did not find a statistically significant relationship between these two factors. In the Cox hazard model, mortality was substantially 2.28‐fold greater in individuals with the GSTT1−/− polymorphism (p = 0.047). Cases with the GSTM1+/+ and GSTT1+/+ polymorphisms died at a lower rate than those with the GSTM1+/+ genotype (p = 0.02). They demonstrated that participants who were GSTT1 null had a greater risk of SARS‐CoV‐2 infection than those with GSTT1 present. In East‐Asian nations, increased COVID‐19 occurrences and deaths were reported, where the GSTT1 null gene was less prevalent than in other genotypes.
5.6. ABO
In humans, the method for classification of the blood system is called the ABO system, which is comprised of A and (B glycoprotein antigens), as well as polyclonal antibodies toward these antigens in those who do not display the antigens. 160 In addition to red blood cells, A and B antigens may be found on various kinds of cells, such as epithelial cells of the gastrointestinal and respiratory system and endothelium lining the blood vessels. 160 As a result, in addition to RBC transfusions, for organ cell, tissue, and cell transplantation, ABO compatibility is required. 160
Previous research has shown that the ABO blood group variation impacts predisposition to COVID‐19, with people in A and O groups getting a more significant and reduced risk, correspondingly. 161 Since then, many investigations have shown a connection between ABO blood groups and SARS‐CoV‐2, among others, such as Zhao et al., 162 Zietz et al., 163 and Zeng et al. 164 A further study by Li et al. 165 analyzed the distribution of ABO blood type in 265 cases with COVID‐19 and 3694 healthy subjects. The percentage of patients belonging to group A was considerably more significant than the proportion of control subjects (39.3% vs. 32.3%, p = 0.017). In contrast, the percentage of cases belonging to group O was substantially lower than the ratio of control subjects (25.7% vs. 33.8%, p = 0.01). 165 In general, the distribution patterns of groups A and O among people of different ages and genders were nearly identical to the general trend. 165 Individuals belonging to groups A and O were shown to have a greater and lower infectious risk, correspondingly, from the other investigations. 166 , 167 In a genome‐wide association study of 1980 cases with COVID‐19 and 1394 Italian controls, researchers discovered two genetic regions on chromosomes 3p21.31 and 9q34 that were associated with predisposition to COVID‐19 disease. Six significant genes are found in the 3p21.31 locus, one of which codes for the transporter Sodium/Imino‐acid (proline) Transporter 1 t (SLC6A20 gene) interacts with the ACE2 protein. 168 The 9q34 locus, which contains the ABO blood type locus, and a blood‐type‐specific study showed a more vital link among A groups and COVID‐19 susceptibility. Moreover, the risk genotypes of the lead variations in 3p21.31 and 9q34.2 were more common in mechanically ventilated cases than to those receiving oxygen augmentation. The connection on 9q34.2 was traced to the ABO gene. 168
In group O, participants had 25% lower blood concentrations of von Willebrand factor (vWF) and factor VIII (FVIII), both of which are required for platelet adhesion, accumulation, and fibrin clot development. 160 The vWF protein is a transporter and stabilizer for FVIII, which is primarily produced in the vascular endothelium and discharged into the circulation. 160 , 169 Individuals in the non‐O group are at a higher risk of pulmonary embolism, venous thromboembolism, and thrombosis. 169 As a result, ABO variation is related to vascular tone, leakage dysregulation, oxidative damage, and cytokine storm production. As a result, the ABO variant may influence the development of COVID‐19 illness via a molecular process that does not require natural antibodies.
5.7. IL‐6
A cytokine that has been identified as pro‐inflammatory is interleukin 6 (IL‐6), which is generated by a wide variety of cells including fibroblasts, keratinocytes, mesangial cells, and macrophages as a response to tissue damage and disease. 170 In instances of SARS‐CoV‐1, elevated concentrations of IL‐6 were found in the acute phase, which was shown to be associated with pulmonary lesions. 171 In response to SARS‐CoV‐1 invading the respiratory system, IL‐6, in particular, can elicit a hyper‐inflammatory (innate) response. 172 Intriguingly, compared to the influenza A virus, SARS‐CoV‐1 can generate higher concentrations of IL‐6 in human epithelial cells. 173 In certain murine viral diseases, IL‐6 is protective and vital in the resolution f infection; but, in others, such as SARS‐CoV‐1, high concentrations of IL‐6 were linked with acute inflammation and were shown to be connected with death in the mice [140]. This also occurs in those diagnosed with SARS‐CoV‐2: special retrospective and meta‐analysis investigations indicate that higher IL‐6 and C‐reactive protein (CRP) are associated with death and severity of disease in comparison to mild form. 174 , 175 , 176 According to growing data, critically severe cases with acute respiratory failure and SARS‐CoV‐2 have either immunological deregulation or a macrophage recruitment disorder, both of which are characterized by pro‐inflammatory cytokines. The immunological deregulation, in particular, is mediated by IL‐6 rather than interleukin‐1 (IL‐1) beta. 176 Increased generation of pro‐inflammatory cytokines through lymphocyte and monocytes dysfunction with CD4 lymphopenia are two critical characteristics of this immune dysregulation. 176 Increased expression of this cytokine was implicated in the development of SARS‐CoV‐2 infection and mortality. 177 , 178 , 179 Research examining the relationship between IL‐6 variants and SARS‐CoV‐2 infection found a link between the IL‐6–174C variant and the severity of pneumonia. 180 Also, it suggested that an anti‐IL‐6R antibody may have been a valuable therapy for SARS‐CoV‐2. In another research, IL‐6 genotypes were shown to predict COVID‐19 severity. 180 , 181 At the same time, Ravi et al. 182 discovered that the IL‐6 rs1800795 G polymorphism was related to SARS‐CoV‐2 diseases incidence and death.
5.8. DBP
Vitamin D binding protein (DBP), which is primarily generated in the liver, regulates the circulatory metabolites of vitamin D (free and total metabolites). 183 , 184 , 185 Indeed, DBP is the most heterogeneous protein, having many variants that significantly impact its biological activities. 183 The two most frequent DBP genotypes, rs4588, and rs7041 have been linked to the development of various clinical disorders, owing to their affinity for vitamin D. 183 According to findings, individuals with the AA genotype at the rs4588 loci had increased plasma concentrations of 25‐hydroxyvitamin D (25(OH)D) in their plasma. 186 , 187 Subjects with the GG genotype had lower 25(OH)D concentrations following the same dosage of vitamin D treatment. 187
Remarkably, two variants, including rs7041 and rs4588, have been liked to obstructive pulmonary disease (COPD). 188 Another finding indicated that the rs7041 loci were more susceptible to hepatitis C infection. 189 In a different population, DBP genetic variants have been significantly correlated with the enhanced vulnerability to illnesses and vitamin D insufficiency; they might even have a function in COVID‐19. 190 , 191 , 192
According to recent research, the rs7041 locus is linked with a greater incidence of SARS‐CoV‐2 diseases and death. 193 As a result, the relationship between DBP and COVID‐19 genetic variation may be due to the pleiotropic modulatory effects of bioavailability vitamin D concentrations. 186 According to a recent study, these SNPs in the DBP gene, particularly the rs7041 locus, are associated with the frequency (GT genotype: r = 0.73, p = 0.02; TT genotype: r = 0.62, p = 0.04) and mortality (GT genotype: r = 0.87, p = 0.01; TT genotype: r = 0.66, p = 0.04) frequencies of SARS‐CoV‐2 in all populations studied. 193 In China, Japan, Nigeria, and Kenya, individuals having a TT polymorphism were more susceptible to COVID‐19. Racial variations in the frequency of these frequent genetic variants may lead to an imbalance in vitamin D metabolism, affecting the severity of acute lower respiratory infections. 193
6. CONCLUSION
The previous findings highlighted the axial role of genetic variants in the susceptivity of many diseases such as cancer, autoimmune diseases, and infectious diseases such as COVID‐19. Concerning the COVID‐19, according to the accumulation of evidence, it seems that the pathogenic fate of COVID‐19 is affected by a wide range of genetic variants and environmental factors. To achieve a decent knowledge of the etiology and pathogenic processes associated with SARS‐CoV‐2 disease, it is crucial to know the factors which are implicated in the disease. Furthermore, this might contribute to predicting the risk of COVID‐19 disease, providing for more effective prevention. The current work demonstrated that a wide range of genes found in cell surface receptors for SARS‐CoV‐2 is linked with an increased risk of getting COVID‐19 infection. A genotype known as “DD” can contribute to explaining the host response's vulnerability to SARS‐CoV‐2 disease, which may result in a broad spectrum of pathophysiological conditions. Therefore, the ACE I/D variant might have been a helpful tool for estimating disease progression and intervention results when applied to the COVID‐19 to create a population‐based treatment development strategy to prevent disease. Also, it seems that the genetic variation in immune cells might influence immunity against SARS‐CoV‐2. All in all, further investigation with larger cohorts and control subjects should be carried out to understand better the relationship between COVID‐19 severity and various polymorphisms and therapeutic response.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Abolfazl Adli had the idea for and planned the study and contributed to the writing of the paper. Mandana Rahimi, Reza Khodaieh, and Niloofar Hashemzaei contributed to the writing of the report. Sayed M. Hosseini contributed to the critical revision of the report. All authors read and approved the final version.
Adli A, Rahimi M, Khodaie R, Hashemzaei N, Hosseini SM. Role of genetic variants and host polymorphisms on COVID‐19: From viral entrance mechanisms to immunological reactions. J Med Virol. 2022;94:1846‐1865. 10.1002/jmv.27615
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mirzaei R, Mahdavi F, Badrzadeh F, et al. The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. Int Immunopharmacol. 2021;90:107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goodarzi P, Mahdavi F, Mirzaei R, et al. Coronavirus disease 2019 (COVID‐19): immunological approaches and emerging pharmacologic treatments. Int Immunopharmacol. 2020:106885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karampoor S, Zahednasab H, Farahmand M, et al. A possible pathogenic role of Syndecan‐1 in the pathogenesis of coronavirus disease 2019 (COVID‐19). Int Immunopharmacol. 2021;97:107684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mirzaei R, Attar A, Papizadeh S, et al. The emerging role of probiotics as a mitigation strategy against coronavirus disease 2019 (COVID‐19). Arch Virol. 2021:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saih A, Baba H, Bouqdayr M, et al. In silico analysis of high‐risk missense variants in human ACE2 gene and susceptibility to SARS‐CoV‐2 infection. BioMed Res Int. 2021;2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laali A, Kermanshah Z, Keyvani H, Kaveh V, Karampoor S. Idiopathic thrombocytopenic purpura as a hematologic manifestation of COVID‐19 infection: a case report. Respir Med Case Rep. 2021;34:101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barash A, Machluf Y, Ariel I, Dekel Y. The pursuit of COVID‐19 biomarkers: putting the spotlight on ACE2 and TMPRSS2 regulatory sequences. Front Med. 2020;7(712). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karampoor S, Hesamizadeh K, Maleki F, et al. A possible pathogenic correlation between neutrophil elastase (NE) enzyme and inflammation in the pathogenesis of coronavirus disease 2019 (COVID‐19). Int Immunopharmacol. 2021;100:108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karampoor S, Hesamizadeh K, Shams Z, et al. The role of lovastatin in the attenuation of COVID‐19. Int Immunopharmacol. 2021;101:108192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwata‐Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93(6):e01815‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mirzaei R, Goodarzi P, Asadi M, et al. Bacterial co‐infections with SARS‐CoV‐2. IUBMB Life. 2020;72(10):2097‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vankadari N, Wilce JA. Emerging COVID‐19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9(1):601‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramos‐Lopez O, Daimiel L, Ramírez de Molina A, Martínez‐Urbistondo D, Vargas JA, Martínez JA. Exploring host genetic polymorphisms involved in SARS‐CoV infection outcomes: implications for personalized medicine in COVID‐19. Int J Genomics. 2020;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keyvani H, Zahednasab H, Sholeh M, Mirzaei R, Esghaei M, Karampoor S. Gender preponderance might be associated with the severity of COVID‐19 infection. J Clin Cell Immunol. 2020;11:598. [Google Scholar]
- 16. Karampoor S, Afrashteh F, Laali A. Persistent hiccups after treatment of COVID‐19 with dexamethasone: a case report. Respir Med Case Rep. 2021;34:101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill AV. Evolution, revolution and heresy in the genetics of infectious disease susceptibility. Philos Trans R Soc B. 2012;367(1590):840‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patarčić I, Gelemanović A, Kirin M, et al. The role of host genetic factors in respiratory tract infectious diseases: systematic review, meta‐analyses and field synopsis. Sci Rep. 2015;5(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin P, Wang E. Polymorphism in clinical immunology—from HLA typing to immunogenetic profiling. J Transl Med. 2003;1(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rasoul M, Rokhsareh M, Mohammad SM, Sajad K, Ahmadreza M. The human immune system against Staphylococcus epidermidis . Crit Rev Immunol. 2019;39(3). [DOI] [PubMed] [Google Scholar]
- 21. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID‐19. J Infect. 2020;80(6):607‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramos‐Lopez O, Milagro FI, Allayee H, et al. Guide for current nutrigenetic, nutrigenomic, and nutriepigenetic approaches for precision nutrition involving the prevention and management of chronic diseases associated with obesity. Lifestyle Genom. 2017;10(1–2):43‐62. [DOI] [PubMed] [Google Scholar]
- 23. Shafaati M, Saidijam M, Soleimani M, et al. A brief review on DNA vaccines in the era of COVID‐19. Future Virol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murgolo N, Therien AG, Howell B, et al. SARS‐CoV‐2 tropism, entry, replication, and propagation: considerations for drug discovery and development. PLoS Pathog. 2021;17(2):e1009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11(5):510‐520. [DOI] [PubMed] [Google Scholar]
- 26. de Vries E, Tscherne DM, Wienholts MJ, et al. Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog. 2011;7(3):e1001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang N, Shen HM. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID‐19. Int J Biol Sci. 2020;16(10):1724‐1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lukassen S, Chua RL, Trefzer T, et al. SARS‐CoV‐2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10):e105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kachuri L, Francis SS, Morrison M, et al. The landscape of host genetic factors involved in infection to common viruses and SARS‐CoV‐2. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Millet JK, Whittaker GR. Physiological and molecular triggers for SARS‐CoV membrane fusion and entry into host cells. Virology. 2018;517:3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Senapati S, Banerjee P, Bhagavatula S, Kushwaha PP, Kumar S. Contributions of human ACE2 and TMPRSS2 in determining host‐pathogen interaction of COVID‐19. J Genet. 2021;100(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bertram S, Glowacka I, Müller MA, et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin‐like protease. J Virol. 2011;85(24):13363‐13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bertram S, Heurich A, Lavender H, et al. Influenza and SARS‐coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7(4):e35876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses – drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS‐CoV‐2 entry by using human ACE2. Cell. 2020;181(4):894‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hussman JP. Cellular and molecular pathways of COVID‐19 and potential points of therapeutic intervention. Front Pharmacol. 2020;11:1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng H, Wang Y, Wang GQ. Organ‐protective effect of angiotensin‐converting enzyme 2 and its effect on the prognosis of COVID‐19. J Med Virol. 2020;92(7):726‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu N, Hong Y, Chen R‐G, Zhu H‐M. High rate of increased level of plasma Angiotensin II and its gender difference in COVID‐19: an analysis of 55 hospitalized patients with COVID‐19 in a single hospital, Wuhan, China. medRxiv. 2020. [Google Scholar]
- 41. Mahdiun F, Mansouri S, Khazaeli P, Mirzaei R. The effect of tobramycin incorporated with bismuth‐ethanedithiol loaded on niosomes on the quorum sensing and biofilm formation of Pseudomonas aeruginosa . Microb Pathog. 2017;107:129‐135. [DOI] [PubMed] [Google Scholar]
- 42. Mirzaei R, Mohammadzadeh R, Mirzaei H, et al. Role of microRNAs in Staphylococcus aureus infection: potential biomarkers and mechanism. IUBMB Life. 2020;72(9):1856‐1869. [DOI] [PubMed] [Google Scholar]
- 43. Mirzaei R, Karampoor S, Sholeh M, Moradi P, Ranjbar R, Ghasemi F. A contemporary review on pathogenesis and immunity of COVID‐19 infection. Mol Biol Rep. 2020;47(7):5365‐5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mirzaei R, Mohammadzadeh R, Mahdavi F, et al. Overview of the current promising approaches for the development of an effective severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccine. Int Immunopharmacol. 2020:106928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shah VK, Firmal P, Alam A, Ganguly D, Chattopadhyay S. Overview of immune response during SARS‐CoV‐2 infection: lessons from the past. Front Immunol. 2020;11:1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tan YJ, Goh PY, Fielding BC, et al. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin Diagn Lab Immunol. 2004;11(2):362‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu HS, Hsieh YC, Su IJ, et al. Early detection of antibodies against various structural proteins of the SARS‐associated coronavirus in SARS patients. J Biomed Sci. 2004;11(1):117‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meyer B, Drosten C, Müller MA. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Xu J, Jia R, et al. Protective humoral immunity in SARS‐CoV‐2 infected pediatric patients. Cell Mol Immunol. 2020;17(7):768‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Castro Dopico X, Ols S, Loré K, Karlsson Hedestam GB. Immunity to SARS‐CoV‐2 induced by infection or vaccination. J Intern Med. 2022;291(1):32‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pierce CA, Sy S, Galen B, et al. Natural mucosal barriers and COVID‐19 in children. JCI Insight. 2021;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Newell KL, Clemmer DC, Cox JB, et al. Switched and unswitched memory B cells detected during SARS‐CoV‐2 convalescence correlate with limited symptom duration. PLoS One. 2021;16(1):e0244855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vernizzi L, Paiardi C, Licata G, et al. Glutamine synthetase 1 increases autophagy lysosomal degradation of mutant huntingtin aggregates in neurons, ameliorating motility in a Drosophila Model for Huntington's Disease. Cells. 2020;9(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183(1):158‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nelde A, Bilich T, Heitmann JS, et al. SARS‐CoV‐2‐derived peptides define heterologous and COVID‐19‐induced T cell recognition. Nat Immunol. 2021;22(1):74‐85. [DOI] [PubMed] [Google Scholar]
- 57. Yaseen FS, Saide K, Kim SH, et al. Promiscuous T‐cell responses to drugs and drug‐haptens. J Allergy Clin Immunol. 2015;136(2):474‐476. [DOI] [PubMed] [Google Scholar]
- 58. Panina‐Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19(12):2237‐2242. [DOI] [PubMed] [Google Scholar]
- 59. Song G, He W‐t, Callaghan S, et al. Cross‐reactive serum and memory B‐cell responses to spike protein in SARS‐CoV‐2 and endemic coronavirus infection. Nat Commun. 2021;12(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ng KW, Faulkner N, Cornish GH, et al. Preexisting and de novo humoral immunity to SARS‐CoV‐2 in humans. Science. 2020;370(6522):1339‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Divac Rankov A, Ljujic M. Chapter 8 – Human genetic diversity in health and disease. In: Ozturk M, Egamberdieva D, Pešić M, eds. Biodiversity and Biomedicine. Academic Press; 2020:123‐136. [Google Scholar]
- 62. Andolfatto P. Adaptive hitchhiking effects on genome variability. Curr Opin Genet Dev. 2001;11(6):635‐641. [DOI] [PubMed] [Google Scholar]
- 63. Redon R, Ishikawa S, Fitch KR, et al. Global variation in copy number in the human genome. Nature. 2006;444(7118):444‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Balaresque PL, Ballereau SJ, Jobling MA. Challenges in human genetic diversity: demographic history and adaptation. Hum Mol Gen. 2007;16(R2):R134‐R139. [DOI] [PubMed] [Google Scholar]
- 65. Jorde LB, Watkins WS, Bamshad M. Population genomics: a bridge from evolutionary history to genetic medicine. Hum Mol Gen. 2001;10(20):2199‐2207. [DOI] [PubMed] [Google Scholar]
- 66. Mirzaei R, Zamani F, Hajibaba M, et al. The pathogenic, therapeutic and diagnostic role of exosomal microRNA in the autoimmune diseases. J Neuroimmunol. 2021;358:577640. [DOI] [PubMed] [Google Scholar]
- 67. Haldane JBS. The rate of mutation of human genes. Hereditas. 1949;35(S1):267‐273. [Google Scholar]
- 68. Karampoor S, Zahednasab H, Amini R, Esghaei M, Sholeh M, Keyvani H. Maraviroc attenuates the pathogenesis of experimental autoimmune encephalitis. Int Immunopharmacol. 2020;80:106138. [DOI] [PubMed] [Google Scholar]
- 69. Allers K, Hütter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011;117(10):2791‐2799. [DOI] [PubMed] [Google Scholar]
- 70. Gupta RK, Abdul‐Jawad S, McCoy LE, et al. HIV‐1 remission following CCR5Δ32/Δ32 haematopoietic stem‐cell transplantation. Nature. 2019;568(7751):244‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Arango M‐T, Perricone C, Kivity S, et al. HLA‐DRB1 the notorious gene in the mosaic of autoimmunity. Immunol Res. 2017;65(1):82‐98. [DOI] [PubMed] [Google Scholar]
- 72. Malkova A, Kudlay D, Kudryavtsev I, Starshinova A, Yablonskiy P, Shoenfeld Y. Immunogenetic predictors of severe COVID‐19. Vaccines. 2021;9(3):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Choo SY. The HLA system: genetics, immunology, clinical testing, and clinical implications. Yonsei Med J. 2007;48(1):11‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin M, Tseng HK, Trejaut JA, et al. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet. 2003;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nguyen A, David JK, Maden SK, et al. Human leukocyte antigen susceptibility map for severe acute respiratory syndrome coronavirus 2. J Virol. 2020;94(13):e00510‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Secolin R, de Araujo TK, Gonsales MC, et al. Genetic variability in COVID‐19‐related genes in the Brazilian population. Hum Genome Var. 2021;8(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid‐19 with respiratory failure. N Engl J Med. 2020;383(16):1522‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wein AN, McMaster SR, Takamura S. CXCR6 regulates localization of tissue‐resident memory CD8 T cells to the airways. J Exp Med. 2019;216(12):2748‐2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kuba K, Imai Y, Ohto‐Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cervantes‐Barragan L, Züst R, Weber F, et al. Control of coronavirus infection through plasmacytoid dendritic‐cell–derived type I interferon. Blood. 2007;109(3):1131‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu C, Gam R, Pandurangan AP, Gough J. Genetic risk factors for death with SARS‐CoV‐2 from the UK Biobank. medRxiv. 2020. [Google Scholar]
- 82. Van Der Made CI, Simons A, Schuurs‐Hoeijmakers J, et al. Presence of genetic variants among young men with severe COVID‐19. JAMA. 2020;324(7):663‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moreno‐Eutimio MA, López‐Macías C, Pastelin‐Palacios R. Bioinformatic analysis and identification of single‐stranded RNA sequences recognized by TLR7/8 in the SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV genomes. Microb Infect. 2020;22(4–5):226‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xiao X, Chakraborti S, Dimitrov AS, Gramatikoff K, Dimitrov DS. The SARS‐CoV S glycoprotein: expression and functional characterization. Biochem Biophys Res Commun. 2003;312(4):1159‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Smatti MK, Al‐Sarraj YA, Albagha O, Yassine HM. Host genetic variants potentially associated with SARS‐CoV‐2: a multi‐population analysis. Front Genet. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Verma A, Minnier J, Huffman JE, et al. A MUC5B gene polymorphism, rs35705950‐T, confers protective effects in COVID‐19 infection. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang Q, Bastard P. Inborn errors of type I IFN immunity in patients with life‐threatening COVID‐19. Science. 2020;370(6515):eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang Y, Qin L, Zhao Y, et al. Interferon‐induced transmembrane protein 3 genetic variant rs12252‐C associated with disease severity in coronavirus disease 2019. J Infect Dis. 2020;222(1):34‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brass AL, Huang IC, Benita Y, et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139(7):1243‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue‐resident memory CD8⁺ T cells during infection with influenza virus due to selective expression of IFITM3. Nat Immunol. 2013;14(3):238‐245. [DOI] [PubMed] [Google Scholar]
- 91. Dai Y‐J, Zhang W‐N, Wang W‐D, He S‐Y, Liang C‐C, Wang D‐W. Comprehensive analysis of two potential novel SARS‐CoV‐2 entries, TMPRSS2 and IFITM3, in healthy individuals and cancer patients. Int J Biol Sci. 2020;16(15):3028‐3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kaltoum ABO. Mutations and polymorphisms in genes involved in the infections by covid 19: a review. Gene Rep. 2021;23:101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yamamoto N, Ariumi Y, Nishida N, et al. SARS‐CoV‐2 infections and COVID‐19 mortalities strongly correlate with ACE1 I/D genotype. Gene. 2020;758:144944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yehualashet AS, Belachew TF. ACEIs and ARBs and their correlation with COVID‐19: a review. Infect Drug Resist. 2020;13:3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ni W, Yang X, Yang D, et al. Role of angiotensin‐converting enzyme 2 (ACE2) in COVID‐19. Crit Care. 2020;24(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Martínez‐Sanz J, Jiménez D, Martínez‐Campelo L, et al. Role of ACE2 genetic polymorphisms in susceptibility to SARS‐CoV‐2 among highly exposed but non infected healthcare workers. Emerg Microbes Infect. 2021;10(1):493‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Srivastava A, Bandopadhyay A, Das D, et al. Genetic association of ACE2 rs2285666 polymorphism with COVID‐19 spatial distribution in India. Front Genet. 2020;11:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Darbani B. The expression and polymorphism of entry machinery for COVID‐19 in human: juxtaposing population groups, gender, and different tissues. Int J Environ Res Public Health. 2020;17(10):3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schönfelder K, Breuckmann K, Elsner C, et al. Transmembrane serine protease 2 polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus type 2 infection: a German case‐control study. Front Genet. 2021;12(585). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim Y‐C, Jeong B‐H. Strong correlation between the case fatality rate of COVID‐19 and the rs6598045 single nucleotide polymorphism (SNP) of the interferon‐induced transmembrane protein 3 (IFITM3) gene at the population‐level. Genes. 2021;12(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Andolfo I, Russo R, Lasorsa VA, et al. Common variants at 21q22. 3 locus influence MX1 gene expression and susceptibility to severe COVID‐19. iScience. 2021;24(4):102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Monticelli M, Hay Mele B, Benetti E, et al. Protective role of a TMPRSS2 variant on severe COVID‐19 outcome in young males and elderly women. Genes. 2021;12(4):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Solerte SB, Di Sabatino A, Galli M, Fiorina P. Dipeptidyl peptidase‐4 (DPP4) inhibition in COVID‐19. Acta Diabetol. 2020;57(7):779‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Senapati S, Kumar S, Singh AK, Banerjee P, Bhagavatula S. Assessment of risk conferred by coding and regulatory variations of TMPRSS2 and CD26 in susceptibility to SARS‐CoV‐2 infection in human. J Genet. 2020;99:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gómez J, Albaiceta GM, Cuesta‐Llavona E, et al. The interferon‐induced transmembrane protein 3 gene (IFITM3) rs12252 C variant is associated with COVID‐19. Cytokine. 2021;137:155354. [DOI] [PubMed] [Google Scholar]
- 106. Rostamian A, Ghazanfari T, Arabkheradmand J, et al. Interleukin‐6 as a potential predictor of COVID‐19 disease severity in hospitalized patients and its association with clinical laboratory routine tests. Immunoregulation. 2020;3(1):29‐36. [Google Scholar]
- 107. Karcioglu Batur L, Hekim N. Correlation between interleukin gene polymorphisms and current prevalence and mortality rates due to novel coronavirus disease 2019 (COVID‐2019) in 23 countries. J Med Virol. 2021;93(10):5853‐5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tavasolian F, Rashidi M, Hatam GR, et al. HLA, immune response, and susceptibility to COVID‐19. Front Immunol. 2021;11:601886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang Y, Garner R, Salehi S, La Rocca M, Duncan D. Association between ABO blood types and coronavirus disease 2019 (COVID‐19), genetic associations, and underlying molecular mechanisms: a literature review of 23 studies. Ann Hematol. 2021;100(5):1123‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ellinghaus D, Degenhardt F, Bujanda L, et al. The ABO blood group locus and a chromosome 3 gene cluster associate with SARS‐CoV‐2 respiratory failure in an Italian‐Spanish genome‐wide association analysis. medRxiv. 2020. [Google Scholar]
- 111. Speeckaert MM, Delanghe JR. A key role for vitamin D binding protein in COVID‐19? Eur J Nutr. 2021;60(4):2259‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Karcioglu Batur L, Hekim N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J Med Virol. 2021;93(3):1409‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Saadat M. An evidence for correlation between the glutathione S‐transferase T1 (GSTT1) polymorphism and outcome of COVID‐19. Clin Chim Acta. 2020;508:213‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9(7):e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin‐converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mariappan V, S RR, Balakrishna Pillai A. Angiotensin‐converting enzyme 2: a protective factor in regulating disease virulence of SARS‐COV‐2. IUBMB Life. 2020;72(12):2533‐2545. [DOI] [PubMed] [Google Scholar]
- 117. Delanghe JR, Speeckaert MM, De Buyzere ML. The host's angiotensin‐converting enzyme polymorphism may explain epidemiological findings in COVID‐19 infections. Clin Chim Acta. 2020;505:192‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single‐cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019‐nCov. BioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID‐19. Cell Death Discov. 2020;6(37). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ghafouri‐Fard S, Noroozi R, Vafaee R, et al. Effects of host genetic variations on response to, susceptibility and severity of respiratory infections. Biomed Pharmacother. 2020;128:110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID‐19 severity in Italy. Aging. 2020;12(11):10087‐10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cai G. Bulk and single‐cell transcriptomics identify tobacco‐use disparity in lung gene expression of ACE2, the receptor of 2019‐nCov. medRxiv. 2020. [Google Scholar]
- 123. Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019‐nCoV/SARS‐CoV‐2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen J, Jiang Q, Xia X, et al. Individual variation of the SARS‐CoV‐2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19(7):e13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sieńko J, Kotowski M, Bogacz A, et al. COVID‐19: the influence of ACE genotype and ACE‐I and ARBs on the course of SARS‐CoV‐2 infection in elderly patients. Clin Interv Aging. 2020;15:1231‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pati A, Mahto H, Padhi S, Panda AK. ACE deletion allele is associated with susceptibility to SARS‐CoV‐2 infection and mortality rate: an epidemiological study in the Asian population. Clin Chim Acta. 2020;510:455‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Stawiski EW, Diwanji D, Suryamohan K, et al. Human ACE2 receptor polymorphisms predict SARS‐CoV‐2 susceptibility. BioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Strafella C, Caputo V, Termine A. Analysis of ACE2 genetic variability among populations highlights a possible link with COVID‐19‐related neurological complications. Genes. 2020;11(7):741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Delanghe JR, Speeckaert MM, De Buyzere ML. COVID‐19 infections are also affected by human ACE1 D/I polymorphism. Clin Chem Lab Med. 2020;58(7):1125‐1126. [DOI] [PubMed] [Google Scholar]
- 130. Torre‐Fuentes L, Matías‐Guiu J, Hernández‐Lorenzo L, et al. ACE2, TMPRSS2, and Furin variants and SARS‐CoV‐2 infection in Madrid, Spain. J Med Virol. 2021;93(2):863‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Verma S, Abbas M, Verma S, et al. Impact of I/D polymorphism of angiotensin‐converting enzyme 1 (ACE1) gene on the severity of COVID‐19 patients. Infect Genet Evol. 2021;91:104801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Clarke NE, Turner AJ. Angiotensin‐converting enzyme 2: the first decade. Int J Hypertens. 2012;2012:307315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. COVID‐19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X‐chromosome in females be protective against SARS‐CoV‐2 compared to the single X‐chromosome in males? Int J Mol Sci. 2020;21(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Li XC, Zhang J, Zhuo JL. The vasoprotective axes of the renin‐angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125(Pt A):21‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Verma S, Abbas M, Verma S, et al. Impact of I/D polymorphism of angiotensin‐converting enzyme 1 (ACE1) gene on the severity of COVID‐19 patients. Infect Genet Evol. 2021;91:104801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Seys LJM, Widagdo W, Verhamme FM, et al. DPP4, the middle east respiratory syndrome coronavirus receptor, is upregulated in lungs of smokers and chronic obstructive pulmonary disease patients. Clin Infect Dis. 2018;66(1):45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Senapati S, Kumar S, Singh AK, Banerjee P, Bhagavatula S. Assessment of risk conferred by coding and regulatory variations of TMPRSS2 and CD26 in susceptibility to SARS‐CoV‐2 infection in human. J Genet. 2020;99(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Posadas‐Sánchez R, Sánchez‐Muñoz F, Guzmán‐Martín CA, et al. Dipeptidylpeptidase‐4 levels and DPP4 gene polymorphisms in patients with COVID‐19. Association with disease and with severity. Life Sci. 2021;276:119410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gurgel Penaforte‐Saboia J, Couri CEB, Vasconcelos Albuquerque N, Lauanna Lima Silva V, Bitar da Cunha Olegario N. Emerging roles of dipeptidyl peptidase‐4 inhibitors in delaying the progression of type 1 diabetes mellitus. Diabetes Metab Syndr Obes. 2021;14:565‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schlicht K, Rohmann N, Geisler C, et al. Circulating levels of soluble dipeptidylpeptidase‐4 are reduced in human subjects hospitalized for severe COVID‐19 infections. Int J Obes. 2020;44(11):2335‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Aghili N, Devaney JM, Alderman LO, Zukowska Z, Epstein SE, Burnett MS. Polymorphisms in dipeptidyl peptidase IV gene are associated with the risk of myocardial infarction in patients with atherosclerosis. Neuropeptides. 2012;46(6):367‐371. [DOI] [PubMed] [Google Scholar]
- 142. Ahmed RH, Huri HZ, Al‐Hamodi Z, Salem SD, Al‐Absi B, Muniandy S. Association of DPP4 gene polymorphisms with type 2 diabetes mellitus in Malaysian subjects. PLoS One. 2016;11(4):e0154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Schönfelder K, Breuckmann K, Elsner C, et al. The influence of IFITM3 polymorphisms on susceptibility to SARS‐CoV‐2 infection and severity of COVID‐19. Cytokine. 2021;142:155492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Diamond MS, Farzan M. The broad‐spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol. 2013;13(1):46‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zani A, Yount JS. Antiviral protection by IFITM3 in vivo. Curr Clin Microbiol Rep. 2018;5(4):229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Everitt AR, Clare S, Pertel T, et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484(7395):519‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Huang IC, Bailey CC, Weyer JL, et al. Distinct patterns of IFITM‐mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7(1):e1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kaur N, Singh R, Dar Z, Bijarnia RK, Dhingra N, Kaur T. Genetic comparison among various coronavirus strains for the identification of potential vaccine targets of SARS‐CoV2. Infect Genet Evol. 2021;89:104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kim YC, Jeong BH. Strong correlation between the case fatality rate of COVID‐19 and the rs6598045 single nucleotide polymorphism (SNP) of the interferon‐induced transmembrane protein 3 (IFITM3) gene at the population‐level. Genes. 2020;12(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Allen EK, Randolph AG, Bhangale T, et al. SNP‐mediated disruption of CTCF binding at the IFITM3 promoter is associated with risk of severe influenza in humans. Nat Med. 2017;23(8):975‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhao T. Interferon‐induced transmembrane protein 3 related to coronavirus disease 2019. J Infect Dis. 2020;222(8):1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID‐19): a literature review. J Infect Public Health. 2020;13(5):667‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Irham LM, Chou WH, Calkins MJ, Adikusuma W, Hsieh SL, Chang WC. Genetic variants that influence SARS‐CoV‐2 receptor TMPRSS2 expression among population cohorts from multiple continents. Biochem Biophys Res Commun. 2020;529(2):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cheng Z, Zhou J, To KK, et al. Identification of TMPRSS2 as a susceptibility gene for severe 2009 pandemic A(H1N1) influenza and A(H7N9) influenza. J Infect Dis. 2015;212(8):1214‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ginsberg G, Smolenski S, Hattis D, Guyton KZ, Johns DO, Sonawane B. Genetic Polymorphism in Glutathione Transferases (GST): population distribution of GSTM1, T1, and P1 conjugating activity. J Toxicol Environ Health Part B. 2009;12(5–6):389‐439. [DOI] [PubMed] [Google Scholar]
- 156. Hayes JD, Strange RC. Glutathione S‐transferase polymorphisms and their biological consequences. Pharmacology. 2000;61(3):154‐166. [DOI] [PubMed] [Google Scholar]
- 157. Dasari S, Ganjayi MS, Meriga B. Glutathione S‐transferase is a good biomarker in acrylamide induced neurotoxicity and genotoxicity. Interdiscip Toxicol. 2018;11(2):115‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Abbas M, Verma S, Verma S, et al. Association of GSTM1 and GSTT1 gene polymorphisms with COVID‐19 susceptibility and its outcome. J Med Virol. 2021;93(9):5446‐5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhang X, Yang J, Liu X, Zhao G, Li X, Xun G. Glutathione S‐transferase gene polymorphisms (GSTT1 and GSTM1) and risk of schizophrenia: a case‐control study in Chinese Han population. Medicine. 2020;99(36):e21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yamamoto F, Yamamoto M, Muñiz‐Diaz E. Blood group ABO polymorphism inhibits SARS‐CoV‐2 infection and affects COVID‐19 progression. Vox Sang. 2021;116(1):15‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cheng Y, Cheng G, Chui CH, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293(12):1450‐1451. [DOI] [PubMed] [Google Scholar]
- 162. Zhao J, Yang Y, Huang H, et al. Relationship between the ABO blood group and the COVID‐19 susceptibility. medRxiv. 2020. 10.1101/2020.03.11.20031096 [DOI] [Google Scholar]
- 163. Zietz M, Zucker J, Tatonetti NP. Associations between blood type and COVID‐19 infection, intubation, and death. Nat Commun. 2020;11(1):5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zeng X, Fan H, Lu D, et al. Association between ABO blood groups and clinical outcome of coronavirus disease 2019: evidence from two cohorts. medRxiv. 2020. [Google Scholar]
- 165. Li J, Wang X, Chen J, Cai Y, Deng A. Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia. Br J Haematol. 2020;190(1):24‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Muñiz‐Diaz E, Llopis J, Parra R, et al. Relationship between the ABO blood group and COVID‐19 susceptibility, severity and mortality in two cohorts of patients. Blood Transfus. 2021;19(1):54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wu B‐B, Gu D‐Z, Yu J‐N, Yang J, Shen W‐Q. Association between ABO blood groups and COVID‐19 infection, severity and demise: a systematic review and meta‐analysis. Infect Genetics Evol. 2020;84:104485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. David E, Frauke D, Luis B, et al. The ABO blood group locus and a chromosome 3 gene cluster associate with SARS‐CoV‐2 respiratory failure in an Italian‐Spanish genome‐wide association analysis. medRxiv. 2020. [Google Scholar]
- 169. Trégouët DA, Heath S, Saut N, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113(21):5298‐5303. [DOI] [PubMed] [Google Scholar]
- 170. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect Biol. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Magro G. SARS‐CoV‐2 and COVID‐19: Is interleukin‐6 (IL‐6) the 'culprit lesion' of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine: X. 2020;2(2):100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wang WK, Chen SY, Liu IJ, et al. Temporal relationship of viral load, ribavirin, interleukin (IL)‐6, IL‐8, and clinical progression in patients with severe acute respiratory syndrome. Clin Infect Dis. 2004;39(7):1071‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Okabayashi T, Kariwa H, Yokota S, et al. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006;78(4):417‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Gong J, Dong H, Xia QS, et al. Correlation analysis between disease severity and inflammation‐related parameters in patients with COVID‐19: a retrospective study. BMC Infect Dis. 2020;20(1):963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ruan Q, Yang K. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan. China. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ulhaq ZS, Soraya GV. Interleukin‐6 as a potential biomarker of COVID‐19 progression. Med Mal Infect. 2020;50(4):382‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ulhaq ZS, Soraya GV. Anti‐IL‐6 receptor antibody treatment for severe COVID‐19 and the potential implication of IL‐6 gene polymorphisms in novel coronavirus pneumonia. Med Clin (Engl Ed). 2020;155(12):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kirtipal N, Bharadwaj S. Interleukin 6 polymorphisms as an indicator of COVID‐19 severity in humans. J Biomol Struct Dyn. 2020:1‐3. [DOI] [PubMed] [Google Scholar]
- 182. Rajkumar RP. Genetic polymorphisms mediating behavioural and immune response to pathogens may moderate the impact of the COVID‐19 pandemic: a pilot study. medRxiv. 2020. [Google Scholar]
- 183. Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol. 2019;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Pt A):132‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Karampoor S, Zahednasab H, Ramagopalan S, Mehrpour M, Tameshkel FS, Keyvani H. 25‐hydroxyvitamin D levels are associated with multiple sclerosis in Iran: a cross‐sectional study. J Neuroimmunol. 2016;290:47‐48. [DOI] [PubMed] [Google Scholar]
- 186. Alshahawey M. A genetic insight into vitamin D binding protein and COVID‐19. Med Hypotheses. 2021;149:110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mehramiz M, Khayyatzadeh SS, Esmaily H, et al. Associations of vitamin D binding protein variants with the vitamin D‐induced increase in serum 25‐hydroxyvitamin D. Clin Nutr ESPEN. 2019;29:59‐64. [DOI] [PubMed] [Google Scholar]
- 188. Khanna R, Nandy D, Senapati S. Systematic review and meta‐analysis to establish the association of common genetic variations in vitamin D binding protein with chronic obstructive pulmonary disease. Front Genet. 2019;10:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Xie CN, Yue M, Huang P, et al. Vitamin D binding protein polymorphisms influence susceptibility to hepatitis C virus infection in a high‐risk Chinese population. Gene. 2018;679:405‐411. [DOI] [PubMed] [Google Scholar]
- 190. Takiar R, Lutsey PL, Zhao D, et al. The associations of 25‐hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms, and race with risk of incident fracture‐related hospitalization: twenty‐year follow‐up in a bi‐ethnic cohort (the ARIC Study). Bone. 2015;78:94‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Michos ED, Misialek JR, Selvin E, et al. 25‐hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: the ARIC study. Atherosclerosis. 2015;241(1):12‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Khan AH, Jafri L, Siddiqui A, Naureen G, Morris H, Moatter T. Polymorphisms in the GC gene for vitamin D binding protein and their association with vitamin D and bone mass in young adults. J Coll Physicians Surg Pak. 2019;29(8):715‐719. [DOI] [PubMed] [Google Scholar]
- 193. Karcioglu Batur L, Hekim N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J Med Virol. 2021;93(3):1409‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study's findings are available from the corresponding author upon reasonable request.


