Abstract
Limited data are available on the short‐ to midterm levels of antibodies to the CoronaVac vaccine and quantitative change in humoral response after homologous or heterologous booster doses. In this prospective cohort study, we evaluated the anti‐receptor‐binding domain (RBD) immunoglobulin G (IgG) levels after two doses of CoronaVac and heterologous/homologous booster administration among healthcare workers in a university hospital in Turkey. Quantitative anti‐RBD IgG antibody levels were measured at first and fourth months in 560 healthcare workers who had completed two doses of CoronaVac vaccine, and within 2 months after the third dose of CoronaVac or BNT162b2. Participants were asked to complete a questionnaire during the first blood draw. The seropositivity rate was 98.9% and 89.1%, and the median antibody level was 469.2 AU/ml and 166.5 AU/ml at first and fourth month, respectively. In the fourth month, a mean reduction of 61.4% ± 20% in antibody levels was observed in 79.8% of the participants. The presence of chronic disease (odds ratio [OR]: 1.76, 95% confidence interval [CI]: 1.15–2.69) and being in the 36–50 age group (OR: 2.11, 95% CI: 1.39–3.19) were identified as independent predictors for low antibody response. The antibody level increased 104.8‐fold (median: 17 609.4 vs. 168 AU/ml) and 8.7‐fold (median: 1237.9 vs. 141.4 AU/ml) in the participants who received BNT162b2 and CoronaVac, respectively. During the follow‐up, 25 healthcare workers (4.5%) were infected with severe acute respiratory syndrome coronavirus 2. Considering the waning immunity and circulating variants, a single booster dose of messenger RNA vaccine seems reasonable after the inactivated vaccine especially in risk groups.
Keywords: antibody, BNT162b2, CoronaVac, heterologous vaccination, SARS‐CoV‐2
Highlights
The seropositivity rate was quite high with two doses of inactivated CoronaVac vaccine.
Chronic disease and preobesity caused lower antibody response.
There was a significant decrease in the antibody levels at the fourth month after the second dose of CoronaVac.
An increase in antibody levels was detected with both homologous and heterologous booster (messenger RNA vaccine BNT162b2) administration approximately 5 months after the second dose of CoronaVac.
A Heterologous booster with BNT162b2 provided a marked increase in antibody response compared to a homologous booster (104.8‐ vs. 8.7‐fold, respectively).
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, which emerged in December 2019, affected the whole world and caused over 266 million cases and 5 million deaths as of December 9, 2021. 1 Vaccines using different platforms were developed to control the pandemic, and emergency use licenses were granted. CoronaVac 600 SU/0.5 ml (Sinovac Research & Development Co., Ltd.) is an inactivated whole virus vaccine and is administered in two doses with 14 or 28 days apart. Turkey was one of the countries where Phase 3 studies of this vaccine were conducted. 2 While the study was ongoing, emergency use approval was given by the Turkish Medical Drugs and Devices Agency on January 13, 2021, considering the rapidly increasing number of cases and deaths. In accordance with international recommendations, vaccination of healthcare workers and high‐risk groups started first with Turkey becoming one of the few countries that used CoronaVac in mass vaccination after it was produced.
Neutralizing antibody levels are highly predictive of immune protection from symptomatic coronavirus disease 2019 (COVID‐19) after vaccination, 3 but are technically difficult to use in practice. Instead, immunoglobulin G (IgG) antibodies developed against the receptor‐binding domain (RBD) of the spike (S) protein can be used as a predictor in the evaluation of vaccine efficacy. 4 , 5 Studies measuring anti‐S/RBD antibody levels against currently used vaccines are increasing. However, the limited number of studies comparing immunity against different types of vaccines, the differences between the assays used, and the uncertainty of the protective level of antibodies lead to an ambiguous conclusion about the efficacy of different vaccines.
The immune response to a vaccine is associated with several factors. 6 Individuals with immunocompromised and/or chronic disease show a reduced antibody response to vaccines and have a high risk of developing severe COVID‐19 even if vaccinated. 7 , 8 Therefore, evaluation of antibody response may be especially important in determining the need for a booster dose in people with comorbidities.
Recently, booster dosing has been introduced in some countries because of waning protection against infection/disease over time, reduced protection against variants, and inadequate protection with primary series for risk groups. 9 , 10 The U.S. Food and Drug Administration authorized booster doses of the BNT162b2 vaccine to be administered at least 6 months after completion of the primary series for certain populations. 11 In Turkey, the Ministry of Health started to roll out third dose vaccination with CoronaVac or BNT162b2 for healthcare workers on July 1, 2021. Thus, homologous/heterologous booster doses were administered approximately 5 months after the completion of the inactivated vaccine scheme.
The objective of this study is to evaluate the anti‐RBD IgG seropositivity rate and the quantitative change in antibody levels within 3 months who received two doses of inactivated SARS‐CoV‐2 vaccine (CoronaVac), and the effect of heterologous (with BNT162b2 vaccine) or homologous boosting (with CoronaVac) in healthcare workers in a university hospital in Turkey.
2. METHODS
2.1. Study design and population
This prospective cohort study was carried out between March 4, 2021 and September 10, 2021 in healthcare workers of Dokuz Eylul University Hospital, Turkey. Healthcare workers were included in the study if they had received two doses of the Coronavac vaccine, 28 days apart, and excluded if they had been diagnosed with COVID‐19 at any time before the study period.
2.2. Sample size and selection
At the start of the study, data on efficacy were not available as the Coronavac vaccine was newly introduced. The Occupational Health and Safety Unit (OHSU) records of the hospital were examined, and the incidence of COVID‐19 in healthcare workers (n: 3538) in the prevaccine period was determined as 15%, based on the minimum 50% protective limit determined by the World Health Organization for vaccine effectiveness (the disease incidence was calculated as 7.5% in vaccinated people). The sample size was determined as 560 (Openepi, cohort sample size) with a 95% confidence level and 80% power. Systematic sampling was carried out from the vaccinated employee lists for sample selection.
2.3. Data collection and measurement of antibodies
Participants were informed and written consent was obtained. Blood samples were taken in the first and fouth months following the administration of the second dose (between January 14 and January 20, 2021) of CoronaVac. Data on sociodemographic characteristics and factors that may affect vaccine response (including the presence of chronic disease, immunosuppressive‐modulatory therapy, height–weight [body mass index, BMI: underweight: <18.5, normal weight: 18.5–24.9, overweight: 25–29.9, and obesity: >30], smoking, influenza, and pneumococcal vaccination in the last 6 months) were collected with a questionnaire at the first blood collection. The questionnaire was piloted and revised before being used for participants. On July 1, 2021, the third dose booster vaccination scheme (CoronaVac or BNT162b2, depending on the individual preference) started with boosters being given over a 2‐month period (July and August 2021). Between August 23 and September 3, 2021, third blood samples were collected to determine the antibody response from those who did or did not receive the third dose of vaccine. COVID‐19 in the study group is regularly monitored in the OHSU of the hospital. Participants with symptoms of COVID‐19 or those who had come in contact with a known infected person were tested for SARS‐CoV‐2 RNA with a reverse transcriptase‐polymerase chain reaction (PCR) assay using a nasopharyngeal swab sample.
2.4. Laboratory assay
Serum samples were tested for anti‐RBD IgG antibody with a SARS‐CoV‐2 IgG II QUANT (Abbott Diagnostics) assay on the Architect analyzer. Values of ≥50 AU/ml were considered positive according to the recommendation of the manufacturer. The quantitation range of the test was 50–40 000 AU/ml. The values of AU/ml were converted to binding antibody units (BAUs)/ml according to the proposed conversion factor (AU/7) of the manufacturer.
2.5. Outcome and covariates
The outcome variable of the study was quantitative antibody level. Covariates were sociodemographic characteristics of the participants, namely BMI, underlying chronic diseases, immunosuppressive‐modulatory treatment status, smoking, and vaccination with other non‐COVID‐19 vaccines (influenza and pneumococcus). Since the protective antibody level is not yet known, the value of the low anti‐RBD IgG sample (45 BAU/ml) of the “First WHO International Reference Panel for anti‐SARS‐CoV‐2 immunoglobulin (NIBSC code: 20/268),” is accepted as the threshold value for low antibody level for this study. 12 Therefore, 315 AU/ml was chosen as the cutoff level for low antibody status in this study. This value also corresponds to the 33rd percentile in the first‐month antibody results.
2.6. Statistical analysis
Descriptive statistics were generated in numbers and percentages for categorical variables. Conformity of continuous variables to normal distribution was examined visually (by means of histograms and probability graphs) and statistically (by means of Kolmogorov–Smirnov/Shapiro–Wilk tests). If continuous variables fit the normal distribution, the mean and standard deviation were calculated, if not, the median and 25th–75th percentile (Interquartile range) were used. Categorical variables were compared using Pearson χ 2 or Fisher's exact tests. Since the quantitative antibody levels were not normally distributed, the Mann–Whitney U test was used for two groups and the Kruskal–Wallis test for more than two groups. The Bonferroni correction was used when the result was statistically significant in the Kruskal Wallis test by employing a pairwise comparison of groups. The Wilcoxon test was used for quantitative antibody level comparison in dependent groups. The antibody levels (low/high) were converted to dichotomous variables. Whether covariates were risk factors for low antibody response was evaluated by univariate and multivariate logistic regression analysis. Then, independent predictors for the outcome variable were determined using the backward elimination method. The Hosmer–Lemeshow test was used for model fit. Analyses were performed using SPSS 22.0 (IBM Corporation), and p < 0.05 was considered statistically significant.
3. RESULTS
A total of 548 healthcare workers participated in the study (Figure 1). The mean age was 38.7 ± 10.3 (min: 20, max: 65). Age, sex, occupation, presence of chronic disease, immunosuppressive‐modulatory treatment status, BMI, smoking, vaccination status with influenza, and pneumococcal vaccines are shown in Table 1. At least one chronic disease was observed in 146 (26.6%) of the participants, and 17 (3.1%) were using immunosuppressive‐modulatory treatment. Distribution of chronic diseases was hypertension (n: 60, 32%), asthma‐allergy (n: 32, 17.2%), connective tissue disease (n: 25, 13.4%), diabetes mellitus (n: 24, 12.8%), cardiovascular disease (n: 16, 8.6%), cancer (remission/active) (n: 12, 6.5%), chronic lung disease (n: 7, 3.7%), chronic kidney disease (n: 5, 2.8%), chronic neurological disease (n: 3, 1.6%), chronic liver disease (n: 2, 1%), and asplenia (n: 1, 0.5%). Thirty‐four participants had more than one chronic disease.
Figure 1.
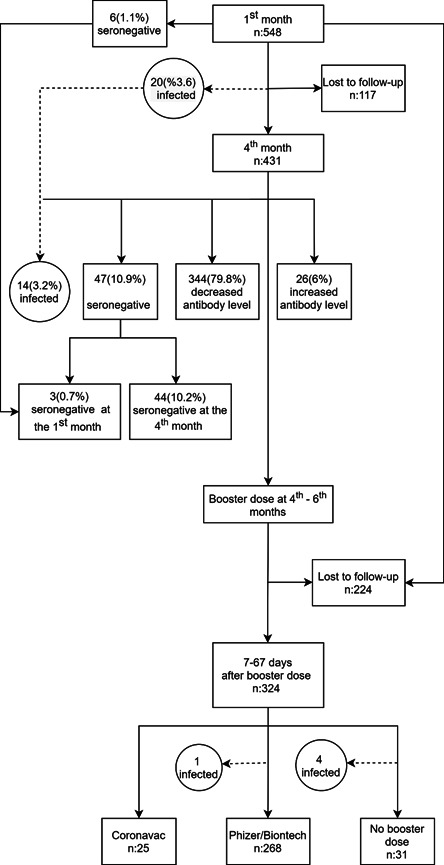
Study population flowchart
Table 1.
Baseline characteristics of study cohort and anti‐RBD IgG antibody level at the first month
| n (%) | Antibody level at first month | p | |
|---|---|---|---|
| AU/ml median (IQR) | |||
| Age groups | |||
| 20–35 | 228 (41.6) | 542.4 (328–941) | |
| 36–50 | 236 (43.1) | 417.5 (203–785) | |
| 51–65 | 84 (15.3) | 441.9 (132–767) | 0.003 * |
| Sex | |||
| Women | 362 (66.1) | 481.9 (250–863) | |
| Men | 186 (33.9) | 452 (251–795) | 0.562 |
| Occupation | |||
| Medical doctor | 191 (34.9) | 547 (322–980) | |
| Nurse | 104 (19) | 479.5 (257–800) | |
| Other healthcare workersa | 253 (46.2) | 422.5 (237–794) | 0.017 ** |
| Presence of chronic disease | |||
| Yes | 146 (26.6) | 355.3 (183–734) | |
| No | 402 (73.4) | 496.1 (275–874) | 0.001 |
| Immunosuppressive‐modulatory therapy | |||
| Yes | 17 (3.1) | 310.4 (88–452) | |
| No | 531 (96.9) | 477.5 (255–831) | 0.005 |
| Body mass index | |||
| Underweight | 13 (2.4) | 371.7 (197–926) | |
| Normal | 262 (48.1) | 563.3 (267–988) | |
| Overweight | 197 (36.1) | 424.9 (235–697) | |
| Obese | 73 (13.4) | 381.2 (214–754) | 0.007 *** |
| Smoking | |||
| Never | 281 (51.3) | 506.4 (264–835) | |
| Current | 172 (31.4) | 430.3 (243–794) | |
| Ever | 95 (17.3) | 463 (242–829) | 0.308 |
| Vaccination status | |||
| Influenza vaccine | |||
| Yes | 65 (11.9) | 502.6 (351–853) | |
| No | 483 (88.1) | 465 (248–822) | 0.106 |
| Pneumococcal vaccine | |||
| Yes | 32 (5.8) | 354.4 (154–634) | |
| No | 516 (94.2) | 478.1 (255–836) | 0.033 |
Note: Bold values indicate significant difference with a p < 0.05.
Abbreviations: IgG, immunoglobulin G; IQR, interquartile range; RBD, receptor‐binding domain.
aMedical secretary, cleaning staff, administrative staff, and so forth.
Between 20–35 age group and 36–50 age group (p = 0.002).
Between medical doctors and other healthcare worker groups (p = 0.013).
Between normal and overweight (p=0.013)
The first‐month evaluation after the second dose of the vaccine showed 98.9% seropositivity, with a median antibody level of 473.6 (251.5–828.6) AU/mL, min: 4, max: 5224.2 AU/ml (Figure 2). There were six seronegative participants (1.1%), four of whom had chronic disease and/or obesity.
Figure 2.
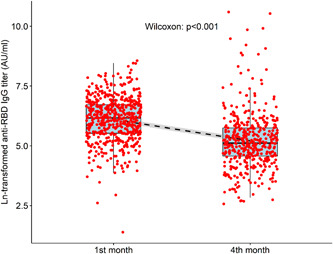
Antibody levels of participants at first and fourth month after second CoronaVac dose. Due to the extreme values of the antibody level in the graphs, the natural logarithm is shown. IgG, immunoglobulin G; RBD, receptor‐binding domain
The first‐month antibody levels were found to be significantly higher in the 20–35 age group (vs. 36–50 age group), in physicians (vs. other healthcare professionals), in participants with normal weight (vs. overweight participants), in people who did not have chronic diseases, and did not use immunosuppressive‐modulatory therapy (Table 1).
In the univariate logistic regression analysis, the presence of chronic disease (odds ratio [OR]: 1.74, 95% confidence interval [CI]: 1.10–2.76) and being in the 36–50 age group (OR: 1.71, 95% CI: 1.07–2.74) were found to be associated with low‐level anti‐RBD IgG (≤314 AU/ml) level (Table 2). In the final model with backward elimination the presence of chronic disease (OR: 1.76, 95% CI: 1.15–2.69, p = 0.008) and being in the 36–50 age group (OR: 2.11, 95% CI: 1.39–3.19, p < 0.001) were determined as independent predictors for low antibody level.
Table 2.
Risk factors of low antibody level a by univariate and multivariate logistic regression models
| Univariate | Multivariateb | |||||
|---|---|---|---|---|---|---|
| Unadjusted odds ratio | 95% CI | p | Adjusted odds ratio | 95% CI | p | |
| Age groups | ||||||
| 20–35 | 1 | 1 | ||||
| 36–50 | 2.42 | 1.62–3.62 | <0.001 | 1.71 | 1.07–2.74 | 0.024 |
| 51–65 | 1.28 | 0.72–2.26 | 0.398 | 0.94 | 0.51–1.76 | 0.866 |
| Sex | ||||||
| Women | 1 | 1 | ||||
| Men | 0.98 | 0.67–1.44 | 0.930 | 1.20 | 0.77–1.86 | 0.409 |
| Occupation | ||||||
| Medical doctor | 1 | 1 | ||||
| Nurse | 1.94 | 1.14–3.30 | 0.013 | 1.54 | 0.84–2.80 | 0.155 |
| Other healthcare workers | 2.15 | 1.40–3.30 | <0.001 | 1.57 | 0.96–2.55 | 0.070 |
| Presence of chronic disease | ||||||
| No | 1 | 1 | ||||
| Yes | 2.01 | 1.35–2.99 | 0.001 | 1.74 | 1.10–2.76 | 0.018 |
| Immunosuppressive‐modulatory therapy | ||||||
| No | 1 | 1 | ||||
| Yes | 2.43 | 0.92–6.41 | 0.072 | 1.75 | 0.60–5.11 | 0.305 |
| Body mass index | ||||||
| Normal–underweight | 1 | 1 | ||||
| Overweight | 1.50 | 1.01–2.22 | 0.041 | 1.24 | 0.81–1.91 | 0.313 |
| Obese | 1.32 | 0.75–2.31 | 0.329 | 0.89 | 0.48–1.63 | 0.710 |
| Influenza vaccine | ||||||
| No | 1 | 1 | ||||
| Yes | 0.59 | 0.32–1.09 | 0.093 | 0.71 | 0.37–1.37 | 0.321 |
| Pneumococcal vaccine | ||||||
| No | 1 | 1 | ||||
| Yes | 1.42 | 0.67–3.03 | 0.355 | 1.20 | 0.54–2.68 | 0.650 |
Note: Participant number in low antibody level group: 175 and high antibody level group: 367. Bold values indicate significant difference with a p < 0.05.
Abbreviations: BAU, binding antibody unit; CI, confidence interval.
Low antibody level: <315 AU/ml (45 BAU/ml).
Adjusted for all variables.
Four months after the second vaccine dose, blood samples were obtained from 431 participants. There was a 21.4% (n: 117) loss in the number of participants compared to the previous blood draw. There was no significant difference between the group of lost participants and the initial group regarding age (p = 0.302), occupation (p = 0.822), presence of chronic disease (p = 0.455), immunosuppressive‐modulator therapy (p = 0.223), and BMI (p = 0.198). However, the rate of dropping out of follow‐up was higher in males (29.6%) than in females (17.1%) (p = 0.001).
When the fourth‐month antibody results were evaluated; the seropositivity rate was 89.1%, with a median antibody level of 166.5 (95.9–313.7) AU/ml, min: 13.1, max: 40 000 AU/ml (Figure 2). Excluding the data of 20 participants (3.6%) infected with SARS‐CoV‐2 during the 4‐month follow‐up, the median and highest antibody levels were 161.8 (92.8–290.6) AU/ml, and 3528.8 AU/ml, respectively.
After excluding patients who were found to be seronegative and who had COVID‐19 infection during follow‐up, 79.8% of the participants (n: 344) had a decrease in antibody levels at the fourth month (Figure 1). The mean percentage decrease was −61.4% ± 20%, min: −95%, max: −0.6%. None of the variables were associated with antibody reduction (data not shown). Antibody levels were increased in 26 cases (6%). The median percentage increase was +40.6 (19.4–66.4), min: 1.5%, max: 149.8 (%).
After the third (booster) dose of vaccine, blood samples were collected from 324 participants. The mean time between vaccination date and blood draw was 50.2 ± 10.8 days, (median 51, min: 7, max: 67). While 82.7% (n: 268) of the participants preferred BNT162b2, 7.7% (n: 25) opted for CoronaVac, and 9.6% (n: 31) did not receive a booster dose. Data from participants who did not receive a third dose of vaccine were not included in the analysis. The seropositivity rate was 100% in people who received the booster vaccine. The antibody results are shown in Table 3 and Figure 3 together with the fourth‐month results. Compared to the fourth month, antibody level increased 104.8‐fold (17609.4 vs. 168 AU/ml) in those vaccinated with BNT162b2, and 8.7‐fold (1237.9 vs. 141.4 AU/ml) in those who received CoronaVac as a booster dose. A 14.2‐fold increase was detected in the BNT162b2 recipients compared to CoronaVac recipients.
Table 3.
Antibody levels of the participants at fourth month after CoronaVac and at 7–67 days after third dose with BNT162b2 or CoronaVac
| Antibody level at fourth month | Antibody level after third dose | p | |
|---|---|---|---|
| AU/ml median (IQR) | AU/ml median (IQR) | ||
| BNT162b2 (n: 268) | 168 (89.5–295.8) | 17609.4 (10518–26981) | <0.001 |
| CoronaVac (n: 25) | 141.4(77.3–286.7) | 1237.9 (349.4–2405.8) | <0.001 |
Note: Bold values indicate significant difference with a p < 0.05.
Abbreviation: IQR, interquartile range.
Figure 3.
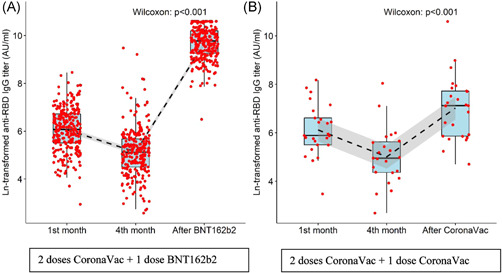
(A) Antibody levels of the participants at the first–fouth months after the second dose CoronaVac and after the third dose with BNT162b2. (B)Antibody levels of the participants at the first–fourth months after the second dose of CoronaVac and after the third dose of CoronaVac. Due to the extreme values of the antibody level in the graphs, the natural logarithm is shown. The dashed lines show the decreasing and increasing trend, the gray area around the line shows the confidence interval. IgG, immunoglobulin G; RBD, receptor‐binding domain
The time between booster dose and blood collection was 7–36 days in 24 (8.2%) and 37–67 days in 269 (91.8%) participants. While the antibody level was significantly higher in people who received the BNT162b2 vaccine in the first group (n: 20) (p < 0.001), there was no significant difference between the two groups of CoronaVac recipients (p = 0.159) (Figure 4 and Table 4).
Figure 4.
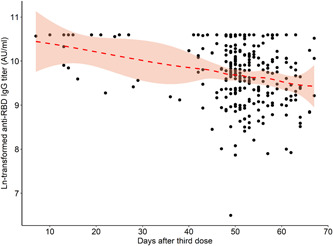
Distribution of antibody levels of the participants according to the time between blood draw and vaccination day with BNT162b2. IgG, immunoglobulin G; RBD, receptor‐binding domain
Table 4.
Antibody levels of two different vaccines according to the time between booster dose and blood draw
| BNT162b2 antibody level | CoronaVac antibody level | |
|---|---|---|
| AU/ml median (IQR) | AU/ml median (IQR) | |
| 7–36 daysa | (n: 20) | (n: 4) |
| 39445 (15728–40000) | 3591 (768–31486) | |
| 37–67 daysa | (n: 248) | (n: 21) |
| 16839 (10048–16839) | 1227 (338–2238) | |
| p | <0.001 | 0.159 |
Note: Bold value indicates significant difference with a p < 0.05.
Abbreviation: IQR, interquartile range.
Blood draw date minus third dose vaccine date.
During the 6‐month follow‐up, 25 healthcare workers (4.5%) were found to be infected with COVID‐19. Twenty of these cases became infected in a 4‐month period after two doses of CoronaVac. Five cases were infected after the fourth month, four of whom did not have a booster vaccine and one of whom had BNT162b2. All were seropositive in the first month of control and had mild disease. No statistically significant difference was found between the first‐month antibody levels of infected and noninfected cases (553.1 vs. 465 AU/ml, p = 0.209). The mean time between the second dose CoronaVac vaccine and COVID‐19 PCR positivity was 85.4 days (median 62, min: 36, max: 175 days).
4. DISCUSSION
High rates of seropositivity (98.9%) were detected at the first month after the initial two‐dose scheme of CoronaVac in this study. Other CoronaVac studies available in healthcare workers showed a seroconversion rate of 98.2%–99.6% on Days 21–30. 13 , 14 This rate is >99.5% with messenger RNA (mRNA) and viral vector vaccines. 15 , 16 Although anti‐RBD antibodies and neutralizing antibodies are closely related and shown to correlate with the efficacy of the vaccine, 4 , 5 the antibody concentration required to prevent the infection or illness is not yet determined, therefore a positive antibody test may not definitely confirm that someone is protected from infection. 17
Antigen and host‐related factors (nutrition, age, sex, etc.) may affect the response to the vaccine. Male sex, advanced age (> 65 years), high BMI ( ≥ 25 kg/m2), immunosuppressive therapy, cancer, and end‐stage renal disease have been reported as factors associated with a low immune response to the COVID‐19 vaccine. 6 In two CoronaVac studies conducted in Turkey, the postvaccine seropositivity was 95.7% and 96.2% in the advanced age group. 13 , 14 Although the highest antibody levels were expected in the young individuals in our study, the high antibody levels detected over the age of 50 showed that the inactivated vaccine can produce a good antibody response in the older age groups as well. Different results have been reported regarding the relationship between sex and vaccine response. Although a lower antibody response is frequently reported in males receiving the mRNA BNT162b2 15 , 18 or CoronaVac vaccine, 19 no difference was found between the first month antibody levels in males and females, and there was no association between male sex and low antibody levels in this study. In the univariate logistic regression analysis, nurses and other healthcare professionals were in the risk group for low antibody response compared to the physician group, but no difference was found between the occupational groups in the multivariate analysis.
Low antibody levels were detected in healthcare workers receiving immunosuppressive therapy. Similar results were reported in other CoronaVac studies. 20 , 21 Therefore, we suggest a booster dose in immunocompromised individuals. Chronic diseases may also cause a low response to COVID‐19 vaccines, but the results of inactivated vaccine studies on this subject are quite different from each other. 14 , 19 Differences could be due to the type of chronic diseases in the study groups. Obesity is another issue that affects the response to the vaccine, as shown in this study. Pellini et al. 22 reported that higher levels of antibodies were detected with mRNA BNT162b2 vaccine in low‐weight and normal‐weight individuals compared to pre‐obese individuals. In our study, being overweight was found to be a risk factor for low antibody levels in the univariate logistic regression analysis, but it was not found as a predictor when the variables were adjusted simultaneously.
In this study, when the responses at the fourth month were compared with the first month ones, antibody levels decreased by 61% and even became seronegative by 10%. In a CoronaVac study, a 56.7% decrease was observed between antibody levels measured at the first and third months after the second dose. 13 A decrease in antibody levels over time after vaccination is also observed with other COVID‐19 vaccines. Individuals who were seronegative at baseline and vaccinated with two doses of mRNA BNT162b2 had a mean antibody level reduction of 37.9% at 3 months, although seropositivity persisted in all participants. 23 In another study with the same vaccine, there was a sharp decrease (56.8%) in the antibody results at the fourth month. 24 In an mRNA‐1273 vaccine study, when the antibody levels at the third and sixth months after the complete vaccination scheme were compared, there was a significant decrease, especially in the individuals who were seronegative at the beginning. 25 Compared to studies done with other vaccine types, antibody reduction and the percentage of seronegativity were greater with CoronaVac. However, the clinical significance of this reduction should be carefully evaluated. Circulating antibody titers are not necessarily predictive for T cell memory. 26 Six months after two doses of an inactivated vaccine, enzyme‐linked immunosorbent assay (ELISA)‐identified anti‐S and anti‐N antibodies exhibited a gradual decline although immune memory based on interferon‐γ‐ secreting T cells persisted. 27 There is also evidence showing that higher levels of antibody response were correlated with a reduced risk of symptomatic infection but did not protect from asymptomatic infection. 28 In our study, although a decrease in antibody level was detected in most of the participants, 6% of them had an increase in the fourth month. This may be due to test variability or asymptomatic infection.
An increase in antibody level was detected with homologous and heterologous booster administration approximately 5 months after the second dose of CoronaVac vaccine in this study. There are only a few studies available regarding the results of booster dose administration after the primary vaccine scheme. In a homologous booster dose study, serum neutralizing antibody levels decreased at eight months after a two‐dose inactivated vaccine, and a higher level of neutralizing antibody titer was obtained when the third dose was administered with the same vaccine. 29 Similarly, in another study, an increase in neutralizing antibodies, ELISA antibodies, and proliferation of T cells after the inactivated vaccine booster suggested that the recall response could be roused by antigen stimulation. 27 As demonstrated in the mentioned studies, an increase in humoral and cellular responses is an expected result of booster doses of the same vaccine. The question this study tried to answer was how heterologous boosting affects antibody levels after the completion of an inactivated vaccine scheme. Antibody levels were found to be significantly higher in those who received the third dose of vaccine with BNT162b2. Studies on this subject have generally focused on heterologous prime‐boost vaccination, and mixed application results of adenoviral vector vaccine ChAdOx1 and mRNA vaccine BNT162b2. A systematic review of these studies showed that heterologous administration of BNT162b2 in ChAdOx1‐primed participants has robust immunogenicity, higher T cell response, and neutralizing activity against variants of concern. 30 An animal study investigating the immunogenic characteristics of homologous or heterologous boost strategies with different vaccine platforms after two doses of inactivated vaccines showed that boosting with recombinant subunit, adenovirus vectored or mRNA vaccine improved both neutralizing antibodies and spike‐specific Th1 cell responses compared to boosting with the third dose of inactivated vaccine. 31 Keskin et al. 32 found that IgG‐S titers were higher in heterologous administration of BNT162b2 group than the homologous booster group in a small number of healthcare workers who are vaccinated with two‐dose CoronaVac. The findings of this study supported the results of these few research on heterologous booster dose administration. The antibody level was also related to the timing of booster doses, being higher in people who received the vaccine approximately 20 days before the blood draw compared to those who received it 53 days before. Although the same individuals were not measured 1 month apart, since both groups were similar in all variables, it can be concluded that there was a decrease in antibody level between 30 and 60 days after BNT162b2 booster.
The incidence rate of COVID‐19 in vaccinated healthcare workers in this study was 48 cases per 1000 people in 6 months. This is the result of vaccination plus other precautions such as using masks and social distancing since healthcare workers continued to use personal protective equipment and other prevention strategies after the vaccination.
There are some limitations of our study. First, the subjects included in the study were not initially screened serologically, and hence those with asymptomatic infections may have inadvertently been included in the study group. Second, participants with asymptomatic infections during the study may not have been detected because they were not regularly screened by PCR. However, considering the antibody levels, the rate of asymptomatic infection was expected to be low. The third is the possibility of selection bias due to participants being lost to follow‐up. A decrease in the number of participants at the second and third blood draw was observed. The main reason could be assumed as the heavy workload due to the pandemic, as well as that some health workers were working in shifts in our hospital and some were on annual leave during the summer period. In the first and fourth months after the inactivated vaccine scheme, there was a 21% loss in participants. All variables, except sex, were similar between the two groups at 4 months. This may cause a biased estimate of antibody levels but we believe the effect may be small, owing to there being no significant difference in antibody levels between men and women at baseline.
CoronaVac is only approved in a small number of countries and is relatively less studied than mRNA or viral vector vaccines. An important strength of this study is that it adds to the limited data in the literature evaluating the short‐to‐medium term antibody response of the inactivated vaccine. The efficient system that tracks PCR positivity, infection dates, and vaccination status of healthcare workers in our OHSU ensured accurate data collection. The major importance of this study is that it provides novel data about the anti‐RBD antibody results of mRNA or inactivated vaccine booster administered after the primary vaccine scheme with CoronaVac is completed.
5. CONCLUSION
Although the seropositivity rate was quite high with two doses of inactivated CoronaVac vaccine, chronic disease and pre‐obesity caused lower antibody response, and there was a significant decrease in the antibody levels within 3 months. An increase was detected in antibody levels with both homologous and heterologous booster doses administered at the earliest 4.5 months after the completion of the inactivated vaccine scheme. However, heterologous booster with mRNA vaccine BNT162b2 provided a marked increase in antibody response compared to homologous booster administration with CoronaVac. A single booster dose of mRNA vaccine seems reasonable after the inactivated vaccine especially in people with risk factors. The effectiveness and duration of high antibody levels should be demonstrated by monitoring the incidence of confirmed COVID‐19 and severe illness with postvaccination surveillance in real life.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Gül Ergör, Derya Çağlayan, Arzu Sayıner, Bülent Kılıç, Alp Ergör, Yücel Demiral, Özgür Appak, and Sema Alp Çavuş conceived and designed the study. Neslişah Şiyve, Ahmet Furkan Süner, Elif Işık, Bülent Kılıç, Derya Çağlayan, Alp Ergör, Yücel Demiral, Çağlar Irmak, Muammer Çelik, Arzu Sayıner, Irmak Güzel, Özgür Appak, and Gamze Öztürk collected data. Derya Çağlayan and Gül Ergör analyzed the original data. Derya Çağlayan, Gül Ergör, and Arzu Sayıner wrote the manuscript.All authors critically reviewed the manuscript, and accept responsibility to submit for publication.
ETHICS STATEMENT
Dokuz Eylül University Clinical ResearchEthics Committee (no: 2021/07‐01), Turkish Medicines and Medical Devices Agency Clinical Research Department (code: 21‐AKD‐33), and TR Ministry of Health General Directorate of Health Services (2021‐02‐12T15_05_18).
ACKNOWLEDGMENTS
This study was supported by the Dokuz Eylul University Department of Scientific Research Projects (DEÜ‐BAP). The authors would like to thank Gözde C. Yıldırım and Salih Keskin for acquisition of the data, Semih Küçükgüçlü and Mehmet A. Özcan for administrative support, and Ahmet N. Emecen for preparing the figures.
Çağlayan D, Süner AF, Şiyve N, et al. An analysis of antibody response following the second dose of CoronaVac and humoral response after booster dose with BNT162b2 or CoronaVac among healthcare workers in Turkey. J Med Virol. 2022;94:2212‐2221. 10.1002/jmv.27620
Preliminary findings of this study were presented as an oral presentation at the 6th National Congress of Clinical Microbiology between October 20–24, 2021.
DATA AVAILABILITY STATEMENT
The raw anonymous participant data will be available immediately following publication and ending 1 year following article publication upon request to the corresponding author. Proposals will be reviewed and approved by the researchers on the basis of scientific merit. Once the proposal has been approved, data can be transferred through a secure online platform after signing a data access agreement and a confidentiality agreement.
REFERENCES
- 1. World Health Organization . Coronavirus (COVID‐19) dashboard. Accessed December 9, 2021. https://covid19.who.int/
- 2. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398:213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27:1205‐1211. [DOI] [PubMed] [Google Scholar]
- 4. Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA‐1273 COVID‐19 vaccine efficacy clinical trial. Science. 2022;375(6567):43–50. 10.1126/science.abm3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Earle KA, Ambrosino DM, Fiore‐Gartland A, et al. Evidence for antibody as a protective correlate for COVID‐19 vaccines. Vaccine. 2021;39:4423‐4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lippi G, Henry BM, Plebani M. Anti‐SARS‐CoV‐2 antibodies testing in recipients of COVID‐19 vaccination: why, when, and how? Diagnostics. 2021;11:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID‐19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8:e583‐e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hippisley‐Cox J, Coupland CA, Mehta N, et al. Risk prediction of covid‐19 related death and hospital admission in adults after covid‐19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bar‐On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 Vaccine Booster against Covid‐19 in Israel. N Engl J Med. 2021;385:1393‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Interim statement on COVID‐19 vaccine booster doses. Accessed September 25, 2021. https://www.who.int/news/item/10-08-2021-interim-statement-on-covid-19-vaccine-booster-doses
- 11. U.S. Food and Drug Administration . FDA authorizes booster dose of Pfizer‐BioNTech COVID‐19 vaccine for certain populations. September 22, 2021. Accessed September 25, 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations
- 12. World Health Organization Reference Panel . First WHO international reference panel for anti‐SARS‐CoV‐2 immunoglubulin NIBSC code: 20/268 instructions for use (Version 3.0); 2020.
- 13. Kara E, Tanir F, Demirhindi H, et al. Humoral immune response in inactivated SARS‐CoV‐2 vaccine: when should a booster dose be administered? medRxiv. 2021. 10.1101/2021.07.08.21260194 [DOI] [PubMed] [Google Scholar]
- 14. Bayram A, Demirbakan H, Günel Karadeniz P, Erdoğan M, Koçer I. Quantitation of antibodies against SARS‐CoV‐2 spike protein after two doses of CoronaVac in healthcare workers. J Med Virol. 2021;93:5560‐5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salvagno GL, Henry BM, di Piazza G, et al. Anti‐SARS‐CoV‐2 receptor‐binding domain total antibodies response in seropositive and seronegative healthcare workers undergoing COVID‐19 mRNA BNT162b2 vaccination. Diagnostics. 2021;11:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eyre DW, Lumley SF, Wei J, et al. Quantitative SARS‐CoV‐2 anti‐spike responses to Pfizer‐BioNTech and Oxford‐AstraZeneca vaccines by previous infection status. Clin Microbiol Infect Dis. 2021;27:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bausch D, Hampton L, Perkins M, Saville M, Gavi . COVID‐19: why we can't use antibody tests to show that vaccines are working. August 17, 2021. Accessed September 26, 2021. https://www.gavi.org/vaccineswork/covid-19-why-we-cant-use-antibody-tests-show-vaccines-are-working
- 18. Vassilaki N, Gargalionis AN, Bletsa A, et al. Impact of age and sex on antibody response following the second dose of COVID‐19 BNT162b2 mRNA vaccine in Greek healthcare workers. Microorganisms. 2021;9:1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yalçın TY, Topçu Dİ, Doğan Ö, et al. Immunogenicity after two doses of inactivated virus vaccine in healthcare workers with and without previous COVID‐19 infection: prospective observational study. J Med Virol. 2022;94:279‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seyahi E, Bakhdiyarli G, Oztas M, et al. Antibody response to inactivated COVID‐19 vaccine (CoronaVac) in immune‐mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int. 2021;41:1429‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medeiros‐Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744‐1751. [DOI] [PubMed] [Google Scholar]
- 22. Pellini R, Venuti A, Pimpinelli F, et al. Early onset of SARS‐COV‐2 antibodies after first dose of BNT162b2: correlation with age, gender and BMI. Vaccines. 2021;9:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Favresse J, Bayart J‐L, Mullier F, et al. Antibody titres decline 3‐month post‐vaccination with BNT162b2. Emerg Microbes Infect. 2021;10:1495‐1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ragone C, Meola S, Fiorillo PC, et al. HLA does not impact on short‐medium‐term antibody response to preventive anti‐SARS‐Cov‐2 vaccine. Front Immunol. 2021;12:734689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tré‐Hardy M, Cupaiolo R, Wilmet A, et al. Immunogenicity of mRNA‐1273 COVID vaccine after 6 months surveillance in health care workers; a third dose is necessary. Journal of Infection. 2021;83(5):556–564. 10.1016/j.jinf.2021.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liao Y, Zhang Y, Zhao H, et al. Intensified antibody response elicited by boost suggests immune memory in individuals administered two doses of SARS‐CoV‐2 inactivated vaccine. Emerg Microbes Infect. 2021;10:1112‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27:2032‐2040. 10.1038/s41591-021-01540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yue L, Xie T, Yang T, et al. A third booster dose may be necessary to mitigate neutralizing antibody fading after inoculation with two doses of an inactivated SARS‐CoV‐2 vaccine. J Med Virol. 2022;94:35‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiu N‐C, Chi H, Tu Y‐K, et al. To mix or not to mix? A rapid systematic review of heterologous prime‐boost covid‐19 vaccination. Expert Rev Vaccines. 2021;20:1211‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J, He Q, An C, et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS‐CoV‐2 vaccine. Emerg Microbes Infect. 2021;10:1598‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keskin AU, Bolukcu S, Ciragil P, Topkaya AE. SARS‐CoV‐2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two‐dose CoronaVac vaccine regimen. J Med Virol. 2022;94(1):39‐41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw anonymous participant data will be available immediately following publication and ending 1 year following article publication upon request to the corresponding author. Proposals will be reviewed and approved by the researchers on the basis of scientific merit. Once the proposal has been approved, data can be transferred through a secure online platform after signing a data access agreement and a confidentiality agreement.


