Abstract
Spices are natural plant products enriched with the history of being used as herbal medicine for prevention of diseases. India is also known as the ‘Land of Spices’. Out of 109 spices recognized by the International Organization for Standardization (ISO) more than 52–60 spice crops are grown in India. The major spices exported by India are turmeric, cumin, coriander, fenugreek, peppers, etc. The Indian spices are divided into three era viz. early period, middle age and early modern period. Spices are used in beverages, liquors, and pharmaceutical, cosmetic and perfumery products. The major issue with spices is their handling and storage. This review article mainly focuses on two aspects: at the outset the handling and storage of the spices is an essential factor as spices are available in different forms like raw, processed, fresh, whole dried, or pre‐ground dried. Therefore, the need of processing, packaging, storage and handling of the spices is important as the deterioration of spices can lead to the loss of therapeutic activity. Furthermore, many herbal constituents have the capability to enhance the bioavailability of drugs. Therefore, an attempt has been made to throw a light on the bioenhancer activity and therapeutic activity along with their mechanism of action of some Indian spices which are regularly used for cooking purpose on a daily basis to enhance the taste of food. The spices suggested by ministry of AYUSH which is relevant to its medicinal and biological property in treatment and prevention from COVID‐19 are discussed. © 2022 Society of Chemical Industry.
Keywords: Indian spices, handling, storage, bioenhancer activity, therapeutic activity, COVID‐19
INTRODUCTION
Spices have an enriched history for being used as herbal medicine for prevention of diseases. 1 Spices are natural plant products that are a seed, fruit, root, bark, or other plant substance primarily used for flavouring, colouring or preserving food. Spices take an important place in common man’s life right from the kitchen and medicinal uses in homes. As India is blessed with a varied climate each of its states produce some spice or other. India is also known as the ‘Land of Spices’. The major spices exported by India are turmeric (10%), cumin (10%), coriander (9.5%), fenugreek (4.2%), peppers (4%) and others (19%). 2 In the present time, humans are concerned about immunity. Nature gave us food and medicine stores, but we could not use that gift today. In fact, food is the only medicine. According to Hindu scriptures, excellent and scientifically made food makes a healthy body. The food style or habit of people is changing to a greater extent as they are moving towards spicy food because of good taste and aroma. Indian food has traditionally been rich in spices. There is no food in which spices are not used. The use of spices has been practiced in the Indian subcontinent since time immemorial and is still relevant today due to the importance of the spice and its therapeutic properties. States like Kerala, Punjab, Gujarat, Manipur, Mizoram and Uttar Pradesh are considered as the hub for growing spices. 3 Spices are the main ingredients of Ayurvedic formulation used for antipyretic purposes viz. Sudarshan Churna and many more. 4 Spices have flourished in the food industry, and cosmetics and pharmaceutical industries. In the blind race of vitamins and nutrients, we gave preference to frozen fruits, while the truth was that seasonal fruits and vegetables have always been helpful in seasonal changes. Indian food and its traditional recipes have been considered the best for centuries.
History of spices
The history of Indian spice is divided into three periods and they are early period, middle age and early modern period. 5
Early period
The most primitive history about Indian spices is recorded in the ‘Rig ved’ around 6000 bc. Spices are an important group of agricultural commodities. They can be primarily defined as farm products used in various forms; fresh, ripe, dried, broken, powdered, etc. which contributes aroma, taste, flavour and colour to food.
Middle period
Common spices were imported into Western Europe each year during the Late Middle Ages. Spices were among the most demanded and high‐priced products available in Europe in the Middle Ages. Some common spices are pepper, cinnamon, cumin, nutmeg, ginger and cloves. The most exclusive was saffron.
Early modern period
The development kept the spice trade, with America as a late comer with its new seasonings, commercial into the 19th century. Spices are well known as appetizers or preservatives and many of them have prosperous medicinal properties and are used in pharmaceutical, perfumery, cosmetic products and religious rituals, etc. 6
Mechanism of action of spices
Due to variety in cultivation, collection and processing of spices; their mechanism of action varies depending on its structural microscopic, macroscopic and physical and chemical properties of the constituents. The general mechanism of action of bioenhancers is as follows:
Reduction in hydrochloric acid secretion and increase in gastrointestinal blood supply.
Inhibition of gastric intestinal transit (GIT), gastric emptying time and intestinal motility
Modification in GIT epithelial cell membrane permeability
Cholagogue effect, bioenergetics and thermogenic properties.
Suppression of first metabolism and inhibition of drug metabolizing enzyme.
Classification of bioenhancer
Extensive literature is available about the classification and types of bioenhancers. Bioenhancers can belong to any of the classes based on their mechanism of action by which they increase the bioavailability. A general way of classification of bioenhancers is depicted in Fig. 1(A). 7
Figure 1.

(A) Classification of bioenhancers. (B) Flow chart of processing of spices.
Processing and packaging of spices
A spice may be available in several forms: fresh, whole dried, or pre‐ground dried. The processing steps are summarized in Fig. 1(B). 8 The steps involved in processing of spices are:
Sorting and heating: Sorting is the early step where the spices are sorted to remove the unnecessary parts like rotting or mouldy parts. For heating the spices are heated at an average of 80 to 90 °C during an average time of 5 min before being sun dried. 8
Sun drying: Sun drying is one of the important steps of the processing of spices. In this technique sun drying reduces the water activity in the spices and prevents the development of micro‐organism but also reduces the weight of the spices. The duration of sun drying can also control the active compound and colour. 9 In addition the drying of spices on the ground can lead to chemical and microbiological contamination of spices which are unsuitable for consumption. 10
Milling: Sun drying of spices is followed by the milling step. 11 Milling is performed by various methods such as the tradition milling method by the use of cemented millstone, built stone and mortars. Modern mills are also used and they are small mill, plate disc mill.
Packaging: Spices are packed in different types of material such as glass bottles, plastic bottles, plastic bags and jute bags. The use of other packaging materials is also common, these include the use of cardboard boxes, multi‐wall paper bags and plastic sacks that are utilized for enhanced preservation of spices. 12
Storage for spices
Most herbs are marketed in the dried form as a high concentration of water will cause product deterioration over time. The changes in the volatiles depend on factors such as the drying method, the biological characteristics of the plants and their volatile composition. According to the World Health Organization (WHO), there are nearly about 119 plant derived pharmaceutical medicine and about 74% are used in modern medicine which for the same remedial value are used in traditional system of medicine. The major question is about safety, efficacy and quality of herbal drugs. Spices used as or in formulation should be identified and authenticated and be free from insect, pest, fungi and micro‐organisms. In the store house there should be adequate space for testing, approving and rejecting herbs and an orderly placement of store herbs and spices with controlled temperature and humidity. The store house for storage of herbs and spices should be as per good manufacturing practices (GMP). 13
Main factors that cause deterioration of foods during storage 14
Climatic influences that cause physical and chemical changes (ultraviolet light, moisture vapour, oxygen and temperature changes)
Contamination (by micro‐organisms, insects or soils)
Mechanical forces (damage caused by impact, vibration, compression or abrasion)
Tampering or adulteration
Packing of spices
There is no specified or standard procedure for proper storage of raw herbal material to date. It is only the proper handling, packaging and storage of herbs and spices which can conserve the safety, efficacy and quality of herbs and spices. Therefore, the choice of appropriate packaging material is important and depends upon the herbal material as shown in Table 1. 13
Table 1.
Material for specific packaging and storage of raw herbs
| Serial number | Part of plant and category | Packing material |
|---|---|---|
| 1. | Woody in nature like stem, bark, etc. | Gunny bags and woven sacks |
| 2. | Fleshy in nature like fruit, rhizomes, etc. | High gauge high‐density high molecular (HDHM) bags, woven sac with Low density (LD) liner, wooden box |
| 3. | Soft in nature like creepers, leaves, etc. | High gauge HDHM bags, woven sac with LD liner, high gauge polyethylene bags |
| 4. | Flowers, anthers, stigma, petals, seeds, etc. | Corrugated box with polypropylene, woven sack, high‐density poly ethylene (HDPE) container, fibers boards drums |
| 5. | Herbal extracts and compounds | Air tight HDPE containers, corrugated box with polyethylene, woven sack and fiber boards drum with polyethylene bags |
| 6. | Volatile content | Air tight HDPE containers, Air tight carboys, card board box with polyethylene liners |
Description of some of the commonly used Indian spices with their applications as bioenhancer
There are plenty of herbal spices that exist in various parts of India and possess broad spectrum of therapeutic and pharmacological action. A few of them are discussed here.
Black pepper
Cardamom
Cinnamon
Clove
Coriander
Star anise
Fenugreek
Turmeric
(a) Black pepper
Black pepper (Piper nigrum Linn.) is the world’s most common spice and well‐known as the ‘King of Spices.’ The word ‘pepper’ is derived from the Sanskrit pippali, the word for long pepper, via the Latin piper, which was used by the Romans. The English word for pepper is derived from the Old English ‘pipor.’ The Latin word is also the source of German pfeffer, French poivre, Dutch peper, and other similar forms. 15 Pepper is a perennial climber and a inhabitant of south India mainly Kerala, Karnataka and Tamil Nadu and to a certain extent in Andaman and Nicobar Islands, Goa, Pondicherry and north‐eastern states. In its dried form, the fruit is frequently referred to as peppercorns. 16 , 17 , 18 , 19 Peppercorns, and the powdered pepper derived from grinding them, may be described as black pepper and their reported therapeutic activities are listed in Table 2. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25
Table 2.
List of some reported therapeutic activity of spices and their constituents
| Species name (part used) | Scientific name | Activity | Mechanism of action | Reference |
|---|---|---|---|---|
| Black pepper (fruits) | Piper nigrum | Gastrointestinal stimulatory activity | The anti‐spasmodic activity of black pepper and piperine is mediated through activation of opioid receptor along with Ca2+ channel blocking (CCB) effect. | 16 |
| Antibacterial activity | Bactericidal action of pepper appears by alteration of cell membrane permeability result in leakage of intracellular material might cause cell death. | 17 | ||
| Antihistamic activity | The inhibition of histamine release from the mast cells plays an important role in the mechanism of anti‐allergic effect against type I allergy. | 18 | ||
| Analgesic and anti‐inflammatory activity | Piperine possesses inhibition of prostaglandin synthesis and release. | 19 | ||
| Antidepressant | Inhibit monoamine oxidase activity, increase monoamine neurotransmitter levels, and thus produce antidepressant‐like activity in various mouse models of behavioral despair. | 20 | ||
| Anti‐asthamatic activity | H‐1 receptor antagonism | 21 | ||
| Blood pressure lowering | Piperine mediates spasmolytic effect through calcium antagonism. | 22 | ||
| Antidiabetic activity | Enhancement of glucose utilization, so blood glucose levels were significantly decreased in glucose. | 23 | ||
| Hypolipidemic activity | Clearance of lipids from circulation may be due to reactivation of lipolytic enzymes. | 24 | ||
| Anticancer activity | Cytotoxic analysis revealed a dose‐dependent response with maximum cellular inhibition | 25 | ||
| Cardamom (seed pods) | Elettaria cardamomum | Skin disorders (non‐melanoma skin cancer) | Cardamom contains copper and manganese that are required for the activation of superoxide dismutase (SOD) enzyme, which in turn helps in the decrease in the process of lipid peroxidation. Thus, play anticarcinogenic role in preventing the progression of skin carcinogenesis in mice. | 30 |
| Anticonvulsant activity | Cardamom extract increase the level of γ‐aminobutyric acid (GABA) in the blood serum and shows the anticonvulsant activity | 31 | ||
| Blood pressure lowering activity | Cardamom produced a dose‐dependent fall in the arterial blood pressure. Partial blockade of hypotensive responses with atropine indicate that cardamom lowers blood pressure due to the presence of cholinergic and an additional (Ca2+ antagonist) components, as identified in the gut preparations. Blood pressure is the product of peripheral resistance and cardiac output. | 32 | ||
| Diuresis | The diuretic effect of cardamom was confirmed, when it caused significant increase in the urine volume (diuresis) in rats, like furosemide, a standard diuretic. Cardamom also enhanced the urinary excretion of Na+ and K+, similar to that caused by furosemide. | 32 | ||
| Antioxidant activity | The extract showed protection against hydrogen peroxide (H2O2) induced DNA damage and inhibited 2,2'‐azobis(2‐amidinopropane) dihydrochloride (AAPH) induced protein oxidation and lipid peroxidation. Cardamom also exhibited antioxidant effects. | 33 | ||
| Anti‐inflammatory and analgesic | Cardamom extract administration to rats at 50 and 100 mg kg−1 inhibited carrageenan‐induced paw edema, and down‐regulated cytokines such as COX‐2, IL‐6, and TNF‐α and inhibited i‐NOS mediated nitric oxide (NO) generation. | 33 | ||
| Gastro‐ protective activity | A crude methanolic extract (TM), essential oil (EO), petroleum ether soluble (PS) and insoluble (PI) fractions of methanolic extract, were studied in rats at doses of 100–500, 12.5–50, 12.5–150 and 450 mg kg−1, respectively for their ability to inhibit the gastric lesions induced by asprin, ethanol and pylorous ligature. In addition their effects on wall mucus and gastric acid output were recorded. All fractions (TM, EO, PS and PI) significantly inhibited gastric lesions | 34 | ||
| Asthma | The cardamom extract was then studied in isolated tracheal tissues, to elucidate the possible mode of bronchodilator action, where crude extract of cardamom caused relaxation of both carbachol and K+‐induced contractions, like verapamil, a Ca2+ antagonist. | 35 | ||
| Insecticidal activity | The volatile oil from cardamom acts as a potential grain protectant by killing various life stages of the stored product insects attacking wheat, e.g. Tribolium castaneum and Sitophilus zeamais; via contact and fumigant | 36, 37 | ||
| Action: Moreover, it also prevented eggs treated with the oil from developing to the adult stage. Cardamom oil dramatically suppressed egg hatching and larval survival of Tribolium castaneum, thus demonstrating its ovicidal properties. | ||||
| Cinnamon (bark) | Cinnamomum zeylanicum | Anti‐microbial activity | Cinnamomum zeylanicum extract demonstrated significant inhibition effect on Staphylococcus aureus, Entrobacter cloacae, Acinetobacter baumannii [minimum inhibitory concentration (MIC) 0.4 mg mL−1] | 40 |
| Anti‐diabetic activity | The effect of cinnamon extract on glycosylated hemoglobin A1c and fasting blood glucose levels in patients with type 2 diabetes was analyzed. A total of 66 patients with type 2 diabetes were recruited and randomly divided into three groups: placebo and low‐dose and high‐dose supplementation with cinnamon extract at 120 and 360 mg d−1, respectively. | 41 | ||
| Both hemoglobin A1c and fasting blood glucose levels were significantly reduced in patients in the low‐ and high‐dose groups, whereas they were not changed in the placebo group. Thus, significantly improving blood glucose in patients with type 2 diabetes. | ||||
| Cinnamon (bark) | Antioxidant activity | The volatile oil was screened for its potential as an antioxidant by using in vitro models, such as the β‐carotene‐linoleate and phosphomolybdenum complex method. The volatile oil showed 55.94% and 66.9% antioxidant activity at 100 and 200 ppm concentration, respectively. Also, the volatile oil showed good antioxidant capacity, using the formation of the phosphomolybdenum complex. | 42 | |
| Immuno‐modulatory activity | Cinnamon at high dose increases both cell mediated and humoral immunity and at low dose shows effect only on humoral immunity. Cinnamaldehyde is reported to inhibit lymphocyte proliferation and NF‐κ B stimulation. The results of the present study shows that cinnamon is a possible immune system booster. | 43 | ||
| Wound healing activity | The ethanol extract of the bark of C. zeylanicum was evaluated for wound healing activity in Wistar rats. The extract was administered by the oral route at a dose of 250 mg kg−1 and 500 mg kg−1 body weight. The extract significantly enhanced the wound breaking strength in the case of incision wound, the rate of wound contraction and the period of epithelization in the case of excision wound. | 44 | ||
| Clove (flower buds) | Syzygium aromaticum | Antioxidant activity | The antioxidant potential of clove is evaluated against copper induced lipid peroxidant, 2,2‐diphenyl‐1‐picrylhydrazy (DPPH) free radical scavenging capability of methanol extract of clove is examined. The clove exhibited a concentration dependent antioxidant activity. Furthermore, 56% of DPPH free radicals were scavenged with extract equivalent to 100 μg clove. | 46 |
| Antibacterial activity | Antibacterial susceptibility assay shows promising evidence for the antibacterial effect of clove methanolic and ethanolic extract against three foods associated with Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Methanolic extract of clove showed maximum zone of inhibition 24 mm against Staphylococcus aureus while minimum was 19 mm against Pseudomonas aeruginosa. Ethanolic extract of clove showed maximum zone of inhibition 20 mm against Pseudomonas aeruginosa while minimum was 18 mm against Escherichia coli. | 47 | ||
| Anti‐inflammatory activity | The current study clearly demonstrated anti‐inflammatory effect of clove essential oil in vivo, which equals to that of etodolac at 0.025 mL kg−1 and 0.100 mL kg−1 doses and to that of indomethacin at 0.050 mL kg−1 and 0.200 mL kg−1 doses. | 48 | ||
| Hair growth enhancer | Formulation containing clove oil was clinically evaluated on rats back compared with minoxidil standard lotion as a positive control and distilled water as a negative control. The selected formulation was demonstrated to condition hair with grooming and enhanced hair growth with longer lag time compared with minoxidil but after one week the hair growth accelerated. | 49 | ||
| Anti‐cancer activity | Significant reduction in the number of proliferating cells and an increased number of apoptotic cells was noted in these blood pressure‐induced lung lesions following clove treatment. | 50 | ||
|
Coriander (dried seeds) |
Coriandrum sativum |
Antioxidant activity | Three different bioassays were used, namely scavenging of the DPPH radical method, inhibition of 15‐lipoxygenase (15‐LO) and inhibition of Fe2+ induced porcine brain phospholipid peroxidation. Coriander leaves showed stronger antioxidant activity than the seeds, and in both parts of coriander, the ethyl acetate extract contributed to the strongest activity. | 52 |
| Anti‐diabetic activity | Coriander incorporated into the diet (62.5 g kg−1) and drinking water (2.5 g L−1, prepared by 15 min decoction) reduced hyperglycaemia of streptozotocin‐diabetic mice. Insulin secretion by hyperpolarized B‐cells (16.7 mm‐glucose, 25 mm‐KCl) was further enhanced by the presence of extract. | 53 | ||
| Anti‐bacterial activity | Coriander oil exhibited bactericidal activity against almost all bacteria tested, due to membrane permeability with the exception of Bacillus cereus and Enterococcus faecalis. | 54 | ||
|
Star anise (fruit) |
Illicium verum |
Anticancer activity | Diseases induced by free radicals and nicotine can be cured by star anise because it has anticarcinogenic agents. The anticancer action is because of flavonoids, resveratrol and curcumin. They additionally have exhibited remedial potential, including cell‐defensive anti‐inflammatory, and DNA protective properties. This spice also has positive effect on DNA damage, which can be a trigger for cancer, as well as on cancer cell migration. | 58 |
| Insecticidal activity | Insecticidal activity of star anise, the direct contact methods were applied against fruit flies (Drosophila melanogaster). (E)‐Anethole caused 80.3% mortality at 0.159 mg cm−2 at 1 and 3 days treatment in a filter paper diffusion method. Insecticidal properties have also been found in volatile oil of star anise against larva and adults of Tribolium castaneum, Botrytis cinerea and Callosobruchus chinensis. | 59, 60 | ||
| Anti‐inflammatory activity | Star anise anti‐inflammatory action was identified in mice by xylene‐induced auricle edema. The star anise extracts of 10 and 20 mg rough drugs mL−1, clearly decreased the contractility of mice intestinal smooth muscles in 15 min under the effect of acetylcholine and barium chloride. Therefore, it is concluded that aqueous extract of star anise has analgesic and anti‐inflammatory effects on mice intestinal smooth muscles. | 61 | ||
| Anti‐fungal activity | The anti‐fungal activity of star anise was tested against Fusarium solani and F. graminearum and F. oxysporum. Complete inhibition (100%) was examined by using 100 ppm concentration of star anise due to high antifungal action. The growth of F. verticillioides is also completely inhibited at 200 ppm concentration. | 62 | ||
| Fenugreek (leaves and seeds) | Trigonella foenum‐graecum | Anti‐inflammatory and anti‐arthritic activity | Anti‐inflammatory activity was noted with linolenic acid in various acute models involving carrageenan, prostaglanin E2, leukotrienes, and arachidonic acid‐induced inflammation signifying its ability to inhibit both cyclooxygenase and lipoxygenase pathways. Petroleum ether extract of fenugreek seeds show anti‐inflammatory and anti‐arthritic activities in all the models tested. | 63 |
| Fenugreek seed extract attenuated hyperglycaemia was investigated in vitro. FSE stimulated glucose uptake in CHO‐HIRc‐mycGLUT4eGFP cells in a dose‐dependent manner. This effect was shown to be mediated by the translocation of glucose transporter 4 (GLUT4) from the intracellular space to the plasma membrane. | 64 | |||
| The seed extract exhibit scavenging of hydroxyl radical (OH‐) and inhibition of H2O2 induced lipid peroxidation in rat liver. The result shows that the extract of fenugreek seed contains antioxidant property. | 65 | |||
| The effect of fenugreek seeds (Trigonella foenum graecum) compared to omeprazole was studied on ethanol‐induced gastric ulcer. The soluble gel fraction derived from the seeds was more effective than omeprazole in preventing lesion formation. | 66 | |||
| Turmeric (rhizomes) | Curcuma longa | Anti‐ulcer activity | An ethanol extract of turmeric was studied in rats for its ability to inhibit gastric secretion and to protect gastroduodenal mucosa against the injuries caused by pyloric ligation. An oral dose of 500 mg kg−1 of the extract produced significant anti‐ulcerogenic activity in rats subjected to pyloruic ligation. | 68 |
| Anti‐fungal activity | In the experimental animals, turmeric oil (dilution 1:80) was applied by dermal application on the seventh day following dermatophytosis induction with Trichophyton rubrum. An improvement in lesions was observed in 2–5 days and the lesions disappeared 6–7 days after the application of turmeric oil. | 69 | ||
| Antioxidant, anti‐inflammatory activity | Turmeric oil was found to have in vitro antioxidant activity and the half maximal inhibitory concentration (IC50) for scavenging superoxides, hydroxyl radicals, and lipid peroxidation were 135 μg mL−1, 200 μg mL−1, and 400 μg mL−1, respectively. Turmeric oil showed significant reduction in paw thickness in carrageenan, dextran‐induced acute inflammation, and formalin‐induced chronic inflammation. | 70 |
Bioenhancer activity of black pepper
Piperine, the chief plant alkaloid present in Piper nigrum Linn. (Black pepper) and Piper longum Linn. (Long pepper), has bioavailability enhancing activity for some food substances and for some drugs. The bioenhancing dose of piperine is approximately 15 mg person−1 d−1 and no more than 20 mg d−1 in divided doses, which corresponds to several thousands up to 40 000 times less than the median lethal dose (LD50) dose of piperine, as recognized in various experiments on rodents. The effective bioenhancing dose of piperine for drug compounds varies, but a dose of approximately 10% (w/w) of the active drug could be regarded as an appropriate bioenhancing dose for most drugs. 26 The mechanism of action of piperine as bioenhancer is shown in Fig. 2(A), 27 and some reported bioenhancer activity of piperine is shown in Table 3.
Figure 2.
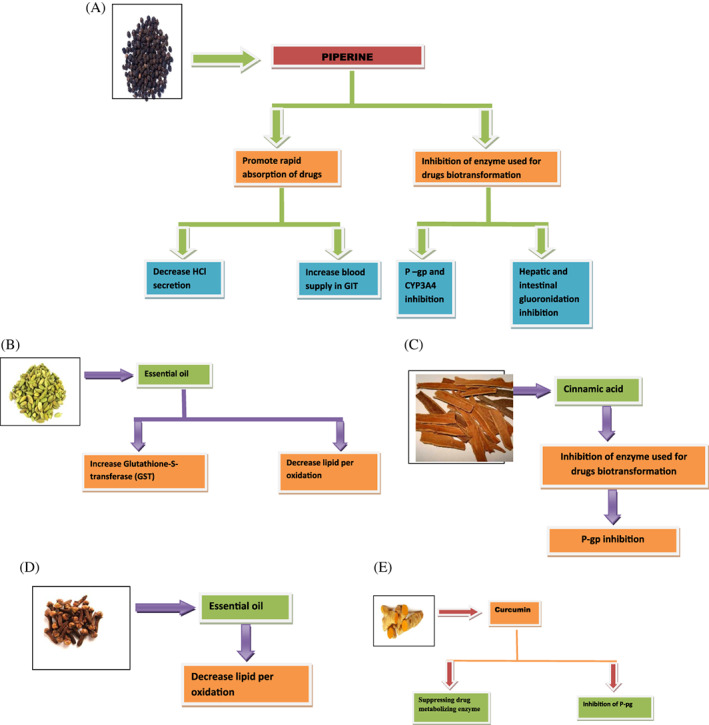
(A) Mechanism of action of black pepper as bioenhancer. (B) Mechanism of action for bioenhancer activity of cardamom. (C) Mechanism of action for bioenhancer activity of cinnamon. (D) Mechanism of action for bioenhancer activity of clove. (E) Mechanism of action of turmeric as bioenhancer.
Table 3.
Reported bioenhancer activity of some constituents of herbal spices
| Herbal spices with drug | Bioenhancement activity | Reference |
|---|---|---|
| Piperine and resveratrol | Bioenhancer activity was studied on mice and the study demonstrated significant improvement in bioavailability of resveratrol. | 27 |
| Piperine and curcumin | The pharmacokinetic of curcumin with piperine was studied on animal and human volunteers. Piperine inhibit the hepatic and intestinal glucuronidation, thus extend the bioavailability of curcumin in both rat and human with no adverse effect. | 28 |
| Piperine and aflatoxin B1 | 10 mg dose of piperine enhance bioavailability of aflatoxin B1 in rat tissue | 29 |
| Cardamom and piroxicam | The penetration index of piroxicam after 1 h pretreatment with 10% cardamom oil was about 340.9‐fold higher than that of non‐pretreatment | 38 |
| Cardamom and indomethacin, diclofanac and piroxicam | The penetration index of piroxicam was extremely increased by 1% cardamom oil about 81.9‐fold compared with that of indomethacin or diclofanac. | 39 |
| Cinnamon and saquinavir | It enhances bioavailability of saquinavir. | 45 |
| Clove oil and carvedilol | It enhances bioavailability of carvedilol. | 51 |
| Curcumin with celiprolol and midazolam | Curcumin suppresses drug metabolizing enzyme CYP3A4 in liver and increase AUC and Cmax of celiprolol and midazolam. | 71 |
(b) Cardamom
Elettaria cardamomum belongs to family Scitaminaceae locally known as ‘elaichi’ is a perennial herb and native to India cultivated in the states of Kerala, Karnataka and Tamil Nadu. It is also cultivated in Pakistan, Burma and Sri Lanka. In South Asia green cardamom is called ‘elaichi’ in Marathi, Hindi and Urdu. It is called elakkaay in Telugu and elam in Tamil. 15 , 28 , 29 , 30 , 31 , 32 Therapeutic activity of cardamom is shown in Table 2.
Bioenhancer activity of cardamom
The essential oil obtained from cardamom acts as a bioenhancer by increasing the glutathione‐S‐transferase (GST) and decreasing lipid per oxidation 33 , 34 , 35 , 36 , 37 as shown in Fig. 2(B). 38 Some of the reported bioenhancer activity of cardamom is listed in Table 3.
(c) Cinnamon
Cinnamon is native to India, Sri Lanka, Bangladesh, and Nepal. The name ‘cinnamon’ comes from Greek kinnámōmon, itself ultimately from Phoenician. The botanical name for the spice, Cinnamomum zeylanicum, is derived from Sri Lanka’s former (colonial) name, Ceylon. In sinhala (Sri Lanka), it is known as kurundu, Sanskrit as tvak or da‐rusita‐, Hindi as dalchini, and in Gujarati as taj. In Malayalam cinnamon is called karuva or elavarngam. 15 It is mostly cultivated in the Western Ghats of Kerala and Tamil Nadu. 39 , 40 , 41 , 42 The therapeutic value of cinnamon is listed into Table 2.
Bioenhancer activity of cinnamon
Cinnamic acid obtained from cinnamon acts as a bioenhancer by inhibiting the enzyme P‐pg used for drug biotransformation as shown in Fig. 2(C)). 43 , 44 , 45 Some of the reported bioenhancer activity of cardamom is listed in the Table 3.
(d) Clove
Cloves are native to Indonesia and are used as a spice in cuisine all over the world. The name derives from the French ‘clou,’ meaning ‘nail’ as the buds vaguely resemble small irregular nails in shape. The spice is used in Ayurveda, Chinese medicine and Western herbalism. 15 , 46 , 47 , 48 , 49 The medicinal property of clove is listed into Table 2.
Bioenhancer activity of clove
Clove oil enhances bioavailability by decreasing lipid per oxidation as shown in Fig. 2(D). 45 Some of the reported bioenhancer activity of clove is listed in the Table 3.
(e) Coriander
Coriandrum sativum belongs to family Umbelliferae. Coriander is native to south‐western Asia and regions west to north Africa. The name ‘coriander’ derives from the French coriander through Latin coriandrum. It is an annual herb commonly used in Middle Eastern, Mediterranean, Indian, Latin American, African and Southeast Asian cuisine. Coriander leaves are referred to as cilantro (United States and Canada, from the Spanish name for the plant), dhania (Indian subcontinent, and increasingly in Britain), kindza (in Georgia), Chinese parsley or Mexican parsley. 50 , 51 , 52 , 53 , 54 All parts of the plant are edible, but the fresh leaves and the dried seeds are the most common parts used in cooking. 14 The medicinal property of coriander is listed in Table 2.
Bioenhancer activity of coriander
According to the literature survey done bioenhancer activity is not reported for coriander.
(f) Star anise
Star anise (Illicium verum) belongs to the plant family Illiciaceae. 55 The generic name llicium comes from a Latin word, ‘alluring’ means – fragrance. The plant is known by different local names in different regions of the world like phoolchakri (Hindi), badiane (French), badian (Urdu), and star anise (English). The cutting of star anise fruit is done after ripening in summer season and then it is dried in sunlight. The flowering is observed from March to May, and its fruit become mature from September to October. 56 Star anise has been utilized frequently in Persian and Mughal Indian biryani rice dishes and curries. It was used as an alternative in commercial drinks for aniseed during the 17th century. 57 Star anise is a well‐known source of carbohydrates, proteins, vitamin A and ascorbic acid. The aromatic odour of Illicium verum is because of the presence of essential oil which is 2.5–3.5% in the fresh fruit and 8–9% in dried material. This scented volatile oil mainly comprises of trans‐anethol and shikimic acid (3,4,5‐trihydroxy‐1‐cyclohexene‐1‐carboxylic acid). Other chemical constituents including sesquiterpenes, phenylpropanoids, lignans, flavonoids, palmitic acid are also present. 58 , 59 , 60 The therapeutic activity of star anise is listed into Table 2.
Bioenhancer activity of star anise
According to the literature survey done bioenhancer activity is not reported for STAR Anis.
(g) Fenugreek
Fenugreek (Trigonella foenum‐graecum) is commonly known as maithray (Bangla, Gujarati), methi or mithi (Hindi, Nepali, Marathi, Urdu and Sanskrit), menthyada soppu (Kannada), ventayam (Tamil), menthulu (Telugu), hilbeh (Arabic), ulluva (Malayalam) and shambalîleh (Persian). The name ‘fenugreek’ or foenum‐graecum is from Latin for ‘Greek hay’. 14 It is one of the most common vegetables grown throughout the country. The seeds can be lightly roasted and ground and used as flavouring, especially in curry dishes. Fresh seed can be sprouted to give tasty sprouts. 61 , 62 , 63 , 64 , 65 Fenugreek contains steroidal saponins, alkaloids (including trigonelline and gentianine), mucilage, protein, vitamins A, B, C and minerals. The therapeutic activity is listed in Table 2.
Bioenhancer activity of fenugreek
According to the literature survey done bioenhancer activity is not reported for fenugreek.
(h) Turmeric
Turmeric is derived from the rhizome of the plant Curcuma longa and has been used as a traditional medicine from ancient times in China and India. 66 , 67 It is also known as kunyit (Indonesian and Malay), besar (Nepali) and haldi or pasupu in some Asian countries. In Assamese it is called halodhi. 14 Turmeric is available in two seasons in India, i.e. February to May and August to October. 6 , 68 , 69 , 70 India is the largest producer, consumer and exporter of turmeric in the world. The therapeutic activity of turmeric is listed into Table 2.
Bioenhancer activity of turmeric
Curcumin is obtained from curcuma longa as it posses the bioenhancer property with various drugs given in Table 3. It acts by suppressing drug metabolizing enzymes and by inhibition of P‐gp as shown in Fig. 2(E). 6 , 71 , 72
Patenting of Indian spices has gained significance worldwide for their therapeutic potential in bioenhancing the activity of various synthetic drugs. Some of the recent findings are listed in Table 4.
Table 4.
Recent patents of some bioenhancers
| Name of bioenhancer | Patent number | Assignee | Date | Title |
|---|---|---|---|---|
| Piperine | US5744161A | Sabinsa Corporation | 4 April 1998 | Use of piperine as a bioavailability enhancer |
| Piperine | USOO5536506A | Sabinsa Corporation | 16 July 1996 | Use of piperine to increase the boavailability of nutritional compounds |
| Zingiber officinale | WO2003049753A1 | Council of Scientific and Industrial Research | 19 June 2003 | Bioavailability enhancing activity of Zingiber officinale Linn. and its extracts/fractions thereof |
| Cuminum Cyminum | WO2003075685A2 | Council of Scientific and Industrial Research | 18 September 2003 | Bioavailability/bioefficacy enhancing activity of cuminum cyminum and extracts and fractions thereof |
| Fenugreek | US7338675B2 | TSI Group Ltd Hong Kong | 4 March 2008 | Fenugreek seed bio‐active compositions and methods for extracting same |
| Fenugreek | WO2005084323A2 | Technical Sourcing International, Inc. | 15 September 2005 | Compositions of bio‐active compounds from fenugreek seed and methods for producing same |
| Star anise | CN105713732B | Shandong Agricultural University | 17 March 2020 | Extraction method of star anise oil |
ROLE OF SPICES IN TREATMENT OF CORONA
The Ministry of AYUSH (Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy), Government of India, recently issued advise suggesting how to fight against the corona virus pandemic in India. While antiviral drugs and vaccines are still being developed, the traditional systems focus more on the initial immunity of the body and to provide a holistic result for the wellbeing of an individual as a synchronized strategy. 72 , 73 , 74 The use of these traditional medicines not only helps cure symptoms but also helps progress immunity and reduces the risk of infections to a huge extent. The potential of these plant‐based remedies in curing and preventing the COVID‐19 infection must be broadly explored to develop globally satisfactory therapeutic remedies along with the modern medicines and vaccines.
Coronaviruses (CoVs) belonging to the family: Coronaviridae are enveloped viruses containing non‐segmented, positive‐stranded genomic RNA. The complete replication cycle takes place in the cytoplasm. Corona viruses can cause a number of diseases, including bronchitis, hepatitis, gastroenteritis, and even death in birds, humans, and other animals. 73 It has been found to assail all types of people, mostly elderly patients having diabetes, hypertension, chronic bronchitis, cerebral infarction, chronic obstructive pulmonary disease, cardiovascular disease, and cancer. CoVs enter into the host cell through interaction between the S protein of the virus species and the receptor of the host cell. It binds with the angiotensin‐converting enzyme 2 receptor from the host cell to create a suitable environment for viral replication. 74 , 75 , 76
Some of recommended species by the AYUSH
AYUSH recommends the use of most herbal spices in the treatment of various diseases and dealing with coronal‐like life threatening viral infections which have no successful available treatment to date. However, to prevent all such infectious diseases herbal spices available in the kitchen is the best remedy. 78 Three such spices namely turmeric, ginger and holybasil are discussed here.
Turmeric
Curcumin, a natural polyphenolic compound, could be a potential treatment choice for patients with CoV disease. Curcumin has shown antiviral activities against a number of different viruses, could be a remedial option for the management of COVID‐19 infection. Previous research has shown that curcumin interacts directly with around 30 proteins, including DNA polymerase, thioredoxin reductase, focal adhesion kinase (FAK), protein kinase (PK), tubulin, and lipoxygenase (LOX). Additionally, curcumin modulates intercellular signaling cascades which are crucial for competent virus replication such as attenuation of NF‐κB and PI3K/Akt signaling. It also affects cellular post‐transcriptional and post‐translational modifications, thereby preventing the viral multiplication by interfering with essential steps in their replication cycle, including genome replication, and viral attachment. A virus does not have all the enzymes needed for its replication as a single unit. The virus uses cellular mechanism for its metabolic processes and reproduction. The antiviral agents should stop the growth of viruses in infected cells without harming the healthy cells. The process of the replication of the viruses, including attachment, penetration, uncoating, genome replication and gene expression are potential therapeutic targets is listed in Table 5.
Table 5.
Mechanism of action of curcumin on virus replication process
| Process | Mechanism of action | Reference |
|---|---|---|
| Viral attachment/penetration | Curcumin could alter the surface protein structure in viruses and block the entry of viruses to the cell. In addition, the positively charged curcumin on the surface is subjected to electrostatic interactions with porcine epidemic diarrhea virus (PEDV) or cell membranes and competing with the virus to bind with the cells. A molecular ducking study indicated that curcumin possesses the better binding capability to the receptors and may inhibit the entry of COVID‐19 virus. | 75 |
| Viral replication |
The effects of curcumin on negative‐strand RNA synthesis by using PEDV as a coronavirus model. They demonstrated that curcumin could inhibit PEDV at the replication step. This evidence supports the potential role of curcumin as a promising antiviral agent. The effect of curcumin on viral replication, by quantification of the number of spike proteins present in cultures of Vero E6 cells infected with SARS‐CoV. Their result demonstrated that the inhibitory effect of curcumin in half maximal concentration (EC50) values was higher than 10 μmol L−1 on SARS‐CoV replication. |
75,76 |
| Potential inhibitory effect of curcumin on viral protease | The drugs that are at present tried for the management of COVID‐19 are protease inhibitors that primarily act on the main protease curcumin that may have the potential to inhibit the COVID‐19 infection by molecular docking. Curcumin showed relatively low binding energies and inhibition constants. They suggested that curcumin could have a potential inhibitory effect on COVID‐19 and could potentially act as a therapeutic agent. | 77 |
| Potential effect of curcumin on interferons |
Interferons play an essential role in the defense against coronavirus infection. These viruses could hinder the induction of interferon in humans. All types of interferons play a role in preventing viral infections. Treatment with cationic carbon dots based on curcumin can suppress PEDV model of coronavirus reproduction by stimulating the production of interferon‐stimulating genes (ISGs) and the cytokines (IL8 and IL6) of Vero cells by triggering the innate immunity of the host. |
78 |
Challenges
Despite the possible beneficial effects and safety profile of curcumin against a range of diseases, the limited bioavailability of this turmeric‐derived compound, especially via oral administration may be a challenging issue.
Ginger (Zingiber officinale)
Gingerol exhibit the highest binding affinity (−15.7591 KJ mol−1) with 5R7Y COVID‐19 main protease essential for replication and reproduction of SARS Cov‐2. Corona viral protease 5R7Y residues such as His164, Glu166, Thr190 and Gln192 from hydrogen‐bonded interaction with phyto compound gingerol, it also forms non‐bonded interaction with the residues of His164, Met 165, Glu166, Leu167, Pro168, Arg188, Gln189 and Thr190.
Molecular interaction between COVID‐19 viral RNA binding protein with gingerol makes hydrogen bonded interaction with Val42, Pro58, Ser60 and Thr68 residues and forms non‐bonded interaction with Arg40, Phe41, Val42, Phe57, Pro58, Lys59, Ser60, Ile66, Thr68 and Ile92 residues of COVID‐19 spike glycol protein. Furthermore, 6‐gingerol possesses antiviral activities. The 6‐gingerol binds with the COVID‐19 main protease active sites. With this binding affinity gingerol possesses excellent drug likeliness parameters with zero violations of different rules and very good absorption, distribution, metabolism, and excretion (ADME) pharmacokinetic properties. 77 Finally, 6‐gingerol proves anti‐viral efficiency against SARS CoV‐2 by showing the highest binding affinity and interaction with multiple targets of COVID‐19 including viral proteases, RNA binding protein and spike protein.
Holybasil
The leaves of this plant are rich in phytonutrients such as antioxidant, flavanol, chlorophyll vitamins and minerals as well as Eugenol, a bioactive compound that has anti‐microbial, antifungal and anti‐bacterial properties and reduces stress and plasma glucose level. It is described as an adaptogenic helping the body to adapt to new demands and stresses and has been used to treat colds, bronchitis, pleurisy, asthma, stress, insect bites and mouth ulcers. Immune response include increased natural killer and T‐helper cell in the well adult and enhanced immune response to viral infection and aids asthmatic symptoms by rising vital capacity. 79
CONCLUSION
It can be concluded that various research studies are conducted on Indian spices across the world. Spices not only add taste and flavour to the food but also play an important role in maintaining health from various diseases. Many herbal compounds have the capability to enhance the bioavailability. The question is whether humans use spices as a medicine. Or are they were used for nutrient absorption and digestion. The mixing of spices in food not only makes it interesting but also ensures the effect on the body of that food. Therefore, an attempt has been made to compile the bioenhancer activity of Indian spices. In the present condition where the world is facing the COVID‐19 pandemic the traditional Ayurvedic systems take longer to have an effect compared to modern medicines, but they also usually have the advantage of lesser side effects and being cost effective. Along with modern medicine, these systems could also be explored as possible treatment/prevention for COVID 19. This review provides scientific evidence which support the use of Indian spices in enhancing the activity of other drugs, prevent and relive from various health problem. Since it is not only necessary to eat the nutrients, it is also necessary for the nutrient to get to the right place. As we know, our immune system is strengthened by nutrients when the body absorbs nutrients properly.
REFERENCES
- 1. Pandey R, Tiwari RK and Shukla SS, Omics: a newer technique in herbal drug standardization and quantification. J Young Pharm 8:76–81 (2016). [Google Scholar]
- 2. Dubey S, Indian spices and their medicinal value. Indian J Pharm Educ Res 51:s330–s332 (2017). [Google Scholar]
- 3. Talukder AS, Kandeepan G and Shukla V, Indian spices: taste of foods. Beverage Food World 43:46–53 (2016). [Google Scholar]
- 4. Bhargava S, Bhargava P, Saraf S, Pandey R, Shukla SS and Garg R, Evaluation of antipyretic activity of sudarshan churna: an ayurvedic formulation. J Res Educ Indian Med 3:2684–2690 (2008). [Google Scholar]
- 5. Mohan S, Rajan SS and Unnikrishnan G, Marketing of Indian Spices as a challenge in India. Int J Bus Manag Invent 2:26–31 (2013). [Google Scholar]
- 6. Kesarwani K and Gupta R, Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed 3:253–266 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jain G and Patil UK, Strategies for enhancement of bioavailability of medicinal agents with natural products. Int J Pharma Sci Res 6:5315–5324 (2015). [Google Scholar]
- 8. Akpo‐Djenontin D, Anihouvi VB, Vissoh VP, Gbaguidi F and Soumanou M, Processing, storage methods and quality attributes of spices and aromatic herbs in the local merchandising chain in Benin. Afr J Agric Res 11:3537–3547 (2016). [Google Scholar]
- 9. Peter KV, in Handbook of Herbs and Spices, Vol. 3, ed. by Peter VK. Wood Head Publishing Ltd., Cambridge, UK, p. 566 (2006). [Google Scholar]
- 10. Salari R, Najafi MBH, Boroushaki MT, Mortazavi SA and Fathi MN, Assessment of the microbiological quality and mycotoxin contamination of Iranian red pepper spice. J. Agric. Sci. Technol. 14:1511–1521 (2012). [Google Scholar]
- 11. Hossain MB, Barry‐Ryan C, Martin‐Diana AB and Brunton NP, Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Sci Environ Health 123:85–91 (2010). [Google Scholar]
- 12. Prashant K, Greening retail: an Indian experience. Int J Retail Distr Manag 42:613–625 (2014). [Google Scholar]
- 13. Masand S, Madan S and Balin SK, Modern concept of storage and packaging of raw herbs used in Ayurvedic. Int J Res Ayurveda Pharm 5:242–245 (2014). [Google Scholar]
- 14. Naik J, Nagalakshmi S, Balasubrahmanyam N, Dhanaraj S and Shankaracharya NB, Packaging and storage studies on commercial varieties of Indian Chillies. J Food Sci Technol 38:227–230 (2001). [Google Scholar]
- 15. Ajaikumar B, Cemile B, Sanjit D, Divya D, Bokyung S and Bharat A, Traditional uses of spices: an overview, in Molecular Targets and Therapeutic Uses of Spices. World Scientific Publishing Co. Pte. Ltd., Singapore, pp. 1–24 (2009). [Google Scholar]
- 16. Mehmood MH and Gilani AH, Pharmacological basis for the pepper and piperine in gastrointestinal disorder. J Med Food 13:1086–1096 (2010). [DOI] [PubMed] [Google Scholar]
- 17. Karsha PV and Lakshmi OB, Antibacterial activity of black pepper with special reference to its mode of action on bacteria. Indian J Nat Prod Resour 1:213–215 (2010). [Google Scholar]
- 18. Noriko H, Shunsuke N, Kazunori I, Kimihisa I, Masashi T, Munekazu I et al., Histamine release inhibitory activity of Piper nigrum leaf. Biol Pharm Bull 31:1973–1976 (2008). [DOI] [PubMed] [Google Scholar]
- 19. Tasleem F, Azhar I, Ali SN, Perveen S and Mahmood ZA, Analgesic and anti‐inflammatory activity of piper nigrum. Asian Pac J Trop Med 7:S461–S468 (2014). [DOI] [PubMed] [Google Scholar]
- 20. Mao Q‐Q, Xian Y‐F, Ip S‐P and Che C‐T, Involvement of serotonergic system in the antidepressant‐like effect of piperine. Prog Neuropsychopharmacol Biol Psychiatry 35:1144–1147 (2011). [DOI] [PubMed] [Google Scholar]
- 21. Kaushik D, Rani R, Kaushik P, Sacher D and Yadav J, In‐ vivo and in‐vitro Antiasthmatic studies of plant piper longum Linn. Int J Pharmacol 8:192–197 (2012). [Google Scholar]
- 22. Taqvi S, Shah A and Gilani A, Blood pressure lowering and vasomodulator effects of Piperine. J Cardiovasc Pharmacol 52:452–458 (2008). [DOI] [PubMed] [Google Scholar]
- 23. Nabi SK, Kasetti RB, Sirasanagandla S, Tilak TK, Kumar MVJ and Rao CA, Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complement Altern Med 13:13–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verma P, Rathore B, Kumar V, Singh RK and Mahdi AA, Hypolipidemic activity of Piper longum in experimental hyperlipidemia. Int J Pharm Sci Res 8:3385–3390 (2017). [Google Scholar]
- 25. Akila P, Chandini R, Yadav AK, Reddy V, Sowmya MN and Madhunapantula S, In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int J Appl Basic Med Res 7:67–72 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Javed S, Ahsan W and Kohli K, The concept of bioenhancers in bioavailability enhancement of drugs – a patent review. JSL 1:143–165 (2016). [Google Scholar]
- 27. Johnson JJ, Nihal M and Siddiqui IA, Enhancing the bioavailability of resveratrol by combining it with piperine. Mol Nutr Food Res 55:1169–1176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shoba G, Joy D, Joseph T, Majeed M, Rajendran R and Srinivas P, Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64:353–356 (1998). [DOI] [PubMed] [Google Scholar]
- 29. Allameh A, Saxena M, Biswas G, Raj HG, Singh J and Srivastava N, Piperine: a plant alkaloid of the piper species enhance the bioavailability of Aflatoxin B1 in rat tissues. Cancer let 61:195–199 (1992). [DOI] [PubMed] [Google Scholar]
- 30. Das I, Acharya A, Berry DL, Sen S, Williams E, Permaul E et al., Anti‐oxidative effects of the spice cardamom against non‐melanoma skin cancer by modulating nuclear factor erythroid‐2‐related factor 2 and NF‐kB signalling pathways. Br J Nutr 108:984–997 (2012). [DOI] [PubMed] [Google Scholar]
- 31. Naik MK and Ali M, Effect of anticonvulsant activity of Elettaria cardamomum seed extract in animal models. IJIPSR 6:130–138 (2018). [Google Scholar]
- 32. Gilani AH, Jabeen Q, Khan A and Shah AJ, Gut modulatory, blood pressure lowering, diuretic and sedative activities of cardamom. J Ethnopharmacol 115:463–472 (2008). [DOI] [PubMed] [Google Scholar]
- 33. Kandikattu HK, Rachitha P, Jayashree GV, Krupashree K, Sukhith M, Majid A et al., Anti‐inflammatory and anti‐oxidant effects of cardamom (Elettaria repens (Sonn.) Baill) and its phytochemical analysis by 4D GCXGC TOF‐MS. Biomed Pharmacother 91:191–201 (2017). [DOI] [PubMed] [Google Scholar]
- 34. Jamal A, Javed K, Aslam M and Jafri MA, Gastro protective effect of cardamom, Elettaria cardamomum Maton. Fruits in rats. J Ethnopharmacol 103:149–153 (2006). [DOI] [PubMed] [Google Scholar]
- 35. Khan A, Khan QJ and Gilani AH, Pharmacological basis for the medicinal use of cardamom in asthma. Bangladesh J Pharmacol 6:34–37 (2011). [Google Scholar]
- 36. Huang Y, Lam SL and Ho SH, Bioactivities of essential oil from Elletaria cardamomum (L.) Maton to Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J Stored Pdts Res 36:107–117 (2000). [Google Scholar]
- 37. Chegini SG and Abbasipour A, Chemical composition and insecticidal effects of the essential oil of cardamom, Elettaria cardamomum on the tomato leaf miner, Tuta absoluta. Toxin Rev 36:12–17 (2017). [Google Scholar]
- 38. Huang YB, Wu PC, Ko HM and Tsai YH, Effect of pre‐treatment by cardamom oil on in vitro percutaneous penetration of piroxicam gel. Int J Pharm 131:137–141 (1996). [Google Scholar]
- 39. Huang YB, Wu PC, Ko HM and Tsai YH, Cardamom oil as a skin permeation enhancer for indomethacin, piroxicam and diclofenac. Int J Pharm 126:111–117 (1995). [Google Scholar]
- 40. Bayoub K, Baibai T, Mountassif D, Retmane A and Soukri A, Antibacterial activities of the crude ethanol extracts of medicinal plants against listeria monocytogenes and some other pathogenic strains. Afr J Biotechnol 9:4251–4258 (2010). [Google Scholar]
- 41. Lu T, Sheng H, Wu J, Cheng Y, Zhu J and Yan C, Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr Res 32:408–412 (2012). [DOI] [PubMed] [Google Scholar]
- 42. Jayaprakasha GK, Rao JM and Sakariah KK, Volatile constituents from Cinnamomum zeylanicum fruit stalks and their antioxidant activities. J Agric Food Chem 51:4344–4348 (2003). [DOI] [PubMed] [Google Scholar]
- 43. Niphade SR, Asad M, Chandrakala GK, Toppo E and Deshmukh P, Immunomodulatory activity of Cinnamomum zeylanicum bark. Pharm Biol 47:1168–1173 (2009). [Google Scholar]
- 44. Kamath JV, Rana AC and Chowdhury AR, Pro‐healing effect of Cinnamomum zeylanicum bark. Phytother Res 17:970–972 (2003). [DOI] [PubMed] [Google Scholar]
- 45. Majumdar SH, Kulkarni AS and Kumbhar SM, Yogvahi (bioenhancer): an Ayurvedic concept used in modern medicines. International Research Journal of Pharmacy and Medical Sciences 1:20–25 (2018). [Google Scholar]
- 46. Dua A, Singh A and Mahajan R, Antioxidants of clove (Syzygium aromaticum) prevent metal induced oxidant damaged of bio molecules. Int Res J Pharm 6:273–278 (2015). [Google Scholar]
- 47. Pandey A and Singh P, Antibacterial activity of Syzygium aromaticum (clove) with metal ion effect against food borne pathogens. Asian J Plant Sci Res 1:69–80 (2011). [Google Scholar]
- 48. Abdurrahman O and Hanefi O, The anti‐inflammatory activity of eugenia caryophyllata essential oil: an animal model of antiinflammatory activity. Eur J Gen Med 2:159–163 (2005). [Google Scholar]
- 49. Shahtalebi MA, Hosseini AS and Safaeian L, Preparation and evaluation of clove oil in emu oil self‐emulsion for hair conditioning and hair loss prevention. J HerbMed Pharmacol 5:72–77 (2016). [Google Scholar]
- 50. Banerjee S, Panda CK and Das S, Clove ( Syzygium aromaticum L.), a potential chemopreventive agent for lung cancer. Carcinogenesis 27:1645–1654 (2006). [DOI] [PubMed] [Google Scholar]
- 51. Ribnicky DM, Poulev A, Schmidt B, Cefalu WT and Raskin I, Evaluation of botanicals for improving human health. Am J Clin Nutr 87:472S–475S (2008). [DOI] [PubMed] [Google Scholar]
- 52. Wangensteen H, Samuelsen AB and Malterud KE, Antioxidant activity in extracts from coriander. Food Chem 88:293–297 (2004). [Google Scholar]
- 53. Gray AM and Flatt PR, Insulin‐releasing and insulin‐like activity of the traditional anti‐diabetic plant Coriandrum sativum (coriander). Br J Nutr 81:203–209 (1999). [DOI] [PubMed] [Google Scholar]
- 54. Silva F, Ferreira S, Queiroz J and Domingues F, Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry. J Med Microbiol 60:1479–1486 (2011). [DOI] [PubMed] [Google Scholar]
- 55. Wang GW, Hu WT, Huang BK and Qin LP, Illicium verum: a review on its botany, traditional use, chemistry and pharmacology. J Ethnopharmacol 136:10–20 (2011). [DOI] [PubMed] [Google Scholar]
- 56. Boota T, Rehman R, Mushtaq A and Kazerooni EG, Star Anise: a review on benefits, biological activities and potential uses. IJCBS 14:110–114 (2018). [Google Scholar]
- 57. Hirschfeld G, Weber L, Renkl A, Kochanek K and Weiss J, Anaphylaxis after oseltamivir (Tamiflu) therapy in a patient with sensitization to star anise and celery, carrot, mugwort, spice syndrome. Allergy 63:243–244 (2008). [DOI] [PubMed] [Google Scholar]
- 58. Huang W‐Y, Cai Y‐Z and Zhang Y, Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer 62:1–20 (2009). [DOI] [PubMed] [Google Scholar]
- 59. Chaubey MK, Fumigant toxicity of essential oils from some common spices against pulse beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). J Oleo Sci 57:171–179 (2008). [DOI] [PubMed] [Google Scholar]
- 60. Shukla J, Tripathi S and Chaubey M, Toxicity of Myristica fragrans and Illicium verum essential oils against flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Electronic Journal of Environmental, Agricultural and Food Chemistry 8:403–407 (2009). [Google Scholar]
- 61. Deng J, Huang L, Xie Y, Du Z, Hao E and Hou X, In the anti‐inflammatory and analgesic effects of star anise, an aromatic herb in South China. Acta Hortic 1125:151–160 (2016). [Google Scholar]
- 62. Aly SE, Sabry BA, Shaheen MS and Hathout AS, Assessment of antimycotoxigenic and antioxidant activity of star anise (Illicium verum) in vitro. J Saudi Soc Agric Sci 15:20–27 (2016). [Google Scholar]
- 63. Pundarikakshudu K, Shah DH, Panchal AH and Bhavsar GC, Anti‐inflammatory activity of fenugreek (Trigonella foenum‐graecum Linn.) seed petroleum ether extract. Indian J Pharmacol 48:441–444 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vijayakumar MV, The hypoglycaemic activity of fenugreek seed extract is mediated through the stimulation of an insulin signalling pathway. Br J Pharmacol 146:41–48 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kaviarasan S, Naik GH, Gangabhagirathi R, Anuradha CV and Priyadarsini KI, In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem 103:31–37 (2007). [Google Scholar]
- 66. Pandian RS, Anuradha CV and Viswanathan P, Gastro protective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J Ethnopharmacol 81:393–397 (2002). [DOI] [PubMed] [Google Scholar]
- 67. Ammon HP and Wahl MA, Pharmacology of Curcuma longa. PlantaMed 57:1–7 (1991). [DOI] [PubMed] [Google Scholar]
- 68. Rafatullah S, Tariq M, Alyaha MA and Mossa JS, Evaluation of turmeric (Curcuma longa) for gastric and duodenal antiulcer activity in rats. J Ethnopharmacol 29:25–34 (1990). [DOI] [PubMed] [Google Scholar]
- 69. Apisaritakul A, Vanittanakom N and Buddhasukh D, Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae). J Ethnopharmacol 49:163–169 (1995). [DOI] [PubMed] [Google Scholar]
- 70. Liju VB, Jeena K and Kuttan R, An evaluation of antioxidant, anti‐inflammatory, and antinociceptive activities of essential oil from Curcuma longa. L Indian J Pharmacol 43:526–531 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anand P, Kunnumakkara AB, Newman RA and Agarwal BB, Bioavailability of curcumin: problems and promise. Mol Pharm 4:807–818 (2007). [DOI] [PubMed] [Google Scholar]
- 72. Singh K and Verma B, The concept of Vyadhikshamatva (immunity) in Ayurveda . Ayurpharm Int J Ayur Alli Sci 1:99–108 (2012). [Google Scholar]
- 73. Singh NA, Kumar P and Jyoti KN, Spices and herbs: potential antiviral preventives and immunity boosters during COVID‐19. Phytother Res 35:1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Walls AC, Park Y‐J, Tortorici MA, Wall A, McGuire AT and Veesler D, Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell 181:281–292 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ting D, Dong N, Fang L, Lu J, Bi J, Xiao S et al., Multisite inhibitors for entric coronavirus: antiviral cationic cardon dots based on curcumin. ACS Appl Nano Mater 1:5451–5459 (2018). [DOI] [PubMed] [Google Scholar]
- 76. Wen CC et al., Specific plant terpenoids and lignoids possess potent antiviral activities aganist severe acute respiratory syndromes coronavirus. J Med Chem 50:4087–4095 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Ting D, Dong N, Fang L, Lu J, Bi J, Xiao S, and Han H. Multisite inhibitors for entric coronavirus: antiviral cationic cardon dots based on curcumin. ACS Appl Nano Mater 1:5451–5459 (2018). [DOI] [PubMed]
- 78. Samuel CE, Antiviral actions of interferons. Clinical Microbiol Rev 14:778–809 (2001). doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed]
- 79. Chiang LC, Ng LT, Cheng PW, Chiang W and Lin CC, Antiviral activity of extract and selected pure constituent of Ocimum Basilicum. Clin Exp Pharmacol Physiol 32:811–816 (2005). [DOI] [PubMed] [Google Scholar]


