Abstract
Background
Quaternary ammonium compounds (QACs), commonly used in cleaning, disinfecting, and personal care products, have recently gained worldwide attention due to the massive use of disinfectants during the COVID-19 pandemic. However, despite extensive use of these chemicals, no studies have focused on the analysis of QACs in human milk, a major route of exposure for infants.
Objective
Our objectives were to identify and measure QACs in breast milk and evaluate early-life exposure to this group of compounds for nursing infants.
Methods
Eighteen QACs, including 6 benzylalkyldimethyl ammonium compounds (BACs, with alkyl chain lengths of C8-C18), 6 dialkyldimethyl ammonium compounds (DDACs, C8-C18), and 6 alkyltrimethyl ammonium compounds (ATMACs, C8-C18), were measured in breast milk samples collected from U.S. mothers. Daily lactational intake was estimated based on the determined concentrations for 0–12 month old nursing infants.
Results
Thirteen of the 18 QACs were detected in breast milk and 7 of them were found in more than half of the samples. The total QAC concentrations (ΣQAC) ranged from 0.33 to 7.4 ng/mL (median 1.5 ng/mL). The most abundant QAC was C14-BAC with a median concentration of 0.45 ng/mL. The highest median ΣQAC estimated daily intake (EDI) was determined for <1-month old infants based on the average (using the median concentration) and high (using the 95th percentile concentration) exposure scenarios (230 and 750 ng/kg body weight/day, respectively).
Significance
Our findings provide the first evidence of the detection of several QACs in breast milk and identify breastfeeding as an exposure pathway to QACs for nursing infants.
Impact statement
Our findings provide the first evidence of QAC occurrence in breast milk and identify breastfeeding as one of the exposure pathways to QACs for nursing infants.
Keywords: Early-life Exposure, Emerging Contaminants, Biomonitoring, Child Exposure/Health
Introduction
Quaternary ammonium compounds (QACs) are a large group of organic substances used as disinfectants and surfactants in a range of applications, including but not limited to cleaning, disinfecting, and personal care products, pharmaceuticals, pesticides, and biomedical materials [1]. The three most well-known QAC groups include benzylalkyldimethyl ammonium compounds (BACs), dialkyldimethyl ammonium compounds (DDACs), and alkyltrimethyl ammonium compounds (ATMACs), some of which were produced in volumes ranging from 10 to 50 million pounds in the United States in 2015 [1]. After the ban of triclosan use in antibacterial soap by the United States Food and Drug Administration in 2016, some QACs have been used as its replacements [1, 2]. Moreover, the use of QACs as disinfectants has increased since the outbreak of the coronavirus disease 2019 (COVID-19) due to their effectiveness against SARS-2-CoV [1]. This pattern of increased use may continue even beyond the pandemic as the global consumption of disinfecting products is predicted to increase at a rate of 10% per year from 2020 to 2027 [3].
QACs have been detected in the environment, including wastewater sludge, surface waters, sediments, and soil [4–10]. They have also been found in foodstuff (e.g., fruits, milk, and vegetables), dust, and air, suggesting that humans can be exposed to QACs via diet, dust ingestion, dermal absorption, and inhalation [11–18]. However, despite extensive use, there is very limited data on human exposure to and biomonitoring of QACs. The only two existing biomonitoring studies detected several QACs (e.g., C12- and C14-BACs) in blood and have demonstrated that these compounds can bioaccumulate in the human body [17, 19]. Moreover, the latest research indicates that human exposure to QACs has increased during the COVID-19 pandemic [16, 17]. However, there are no studies on the occurrence of QACs in human breast milk, a major nutritional source for infants.
There is a growing concern regarding the toxicity of some QACs. Most studies on sub-chronic toxicity of BACs and DDACs have shown that exposure to these compounds can result in skin irritation [20–22] and adverse respiratory effects [23–26]. Recent in vitro, in vivo, and epidemiological studies on the chronic QAC exposure show that exposure to BACs and DDACs is associated with immunotoxicity [19], metabolic disorders [27, 28], and reproductive toxicity [29, 30]. Furthermore, BACs can cross the placenta and alter cholesterol and lipid homeostasis in neonatal mice brains after gestational exposure [31], suggesting that BACs are potential neurotoxicants.
Infants are more vulnerable to adverse effects of environmental exposures due to their rapid development and growth [32, 33]; therefore, it is critical to evaluate the early-life environmental exposures. Breastfeeding is recognized as a significant exposure pathway to many environmental contaminants for nursing infants [34–36]. For the first time, this study reports the levels of eighteen QACs, including 6 BACs (with alkyl chain lengths of C8-C18), 6 DDACs (C8-C18), and 6 ATMACs (C8-C18), in breast milk samples collected from U.S. mothers, as well as estimates the daily lactational intake of these compounds for nursing infants.
Materials and methods
Recruitment and sample collection
Forty-eight primiparous women pregnant and planning to breastfeed or currently breastfeeding and residing in Seattle, Washington, United States were recruited over social media channels and via parenting groups and paper flyers during March-October 2019 (before the COVID-19 pandemic) [36]. Breast milk was manually extracted into a provided empty glass jar pre-cleaned with water, isopropyl alcohol, and methanol (breast pumps were not used). After collection, the samples were taken from participants within 24 h and stored at −4 oC until shipment to Indiana University, where they were stored at −20 oC until analysis. Information on demographics and socioeconomic status, as well as the use of household cleaners, disinfectants, personal care products, and the household disinfection frequency was also collected (Table 1). Ingredient information for each product listed in surveys was searched online. The products were grouped based on the following categories: cleaning products without QACs, cleaning products with QACs, personal care products with ATMACs, and personal care products without ATMACs. The QACs-containing products included sprays (squirt bottles) and wipes.
Table 1.
Summary of demographic characteristics and the use of disinfecting and personal care products for participants in this study (n = 48).
| Characteristics | N | Percentage, % | Characteristics | N | Percentage, % |
|---|---|---|---|---|---|
| Demographic factors | Disinfecting habits | ||||
| Age (years) | Disinfectant use | ||||
| <33 | 17 | 36 | Yes | 21 | 44 |
| >33 | 29 | 60 | No | 26 | 54 |
| Missing | 2 | 4 | Missing | 1 | 2 |
| Education completed | Disinfecting frequency (n = 21) | ||||
| College | 17 | 36 | More than once a week | 8 | 38 |
| Advanced degree | 29 | 60 | Less than once a week | 11 | 52 |
| Missing | 2 | 4 | Missing | 2 | 10 |
| Census tract median income | Disinfectant type (n = 21) | ||||
| Low-income | 1 | 2 | Wipes | 13 | 62 |
| Lower middle | 8 | 16 | Sprays | 8 | 38 |
| Middle | 20 | 42 | Missing | 0 | 0 |
| Upper middle | 18 | 38 | Personal care product use | ||
| Missing | 1 | 2 | Using ATMAC- containing products (n = 48) | ||
| Residence status in Seattle (years) | Yes | 10 | 21 | ||
| <20 | 33 | 69 | No | 38 | 79 |
| >20 | 13 | 27 | Frequency (n = 10) | ||
| Missing | 2 | 4 | 2–5 times a week | 7 | 70 |
| Child’s age at time of collection | 5–10 times a week | 3 | 30 | ||
| <6 months | 19 | 40 | |||
| >6 months | 24 | 50 | |||
| Missing | 5 | 10 | |||
| Maternal BMI (kg/m2) | |||||
| Underweight, <18.5 | 0 | 0 | |||
| Normal, 18.5–24.9 | 28 | 58 | |||
| Overweight, 25–29.9 | 10 | 21 | |||
| Obese, >30 | 6 | 13 | |||
| Missing | 4 | 8 | |||
Sample analysis
Two mL of breast milk (thawed at room temperature) were spiked with surrogate standards (d7-C12-BAC and d9-C10-ATMAC) and ultrasonicated in 4 mL of acetonitrile for 1 h. The supernatant was transferred to a new tube after the sample was centrifuged (3000 rpm, 5 min). The residue was re-extracted twice, and supernatants were combined, concentrated to 2 mL under N2 in a water bath at 40 °C and diluted with 4 mL of 5% ammonium hydroxide in water (v/v). The sample was loaded on an Oasis WCX cartridge (6 cc, 150 mg, 30 µm) preconditioned with 6 mL of methanol and 6 mL of water. After washing with 3 mL of 5% ammonium hydroxide in water (v/v) and 3 mL of 10% methanol in water (v/v), the cartridges were dried under vacuum. The target analytes were eluted with 6 mL of 2% formic acid in methanol (v/v). The extract was evaporated to dryness using N2, redissolved in 500 μL of acetonitrile, filtered, and spiked with the internal standard (d7-C14-BAC).
Instrumental analysis
The target compounds were identified and quantified on an ultra-performance liquid chromatograph coupled to a triple-quadrupole mass spectrometer (Agilent 1290 Infinity II UPLC – 6470 QQQ-MS) in the positive electrospray ionization (ESI+) mode using a previously developed method [17]. An Acquity UPLC BEH C18 column (50 mm, 2.1 mm i.d., 1.7 μm thickness, Waters, Milford, MA) was used for the UPLC separation of the target analytes. A delay column (ZORBAX RR Eclipse Plus C18, 50 mm, 4.6 mm i.d., 3.5 µm thickness, Agilent, Palo Alto, CA) was set up to reduce the background contamination from the instrument. The mobile phase consisted of water (A) and acetonitrile (B), both containing 0.1% formic acid. The flow rate was 0.4 mL/min. The following gradient was employed: 10% B for 0.5 min initially, ramped to 100% B at 6 min and held for 4 min, returned to 10% B at 10.5 min and equilibrated for 3.5 min after every run. The injection volume was 5 μL. The nebulizer, gas flow, gas temperature, capillary voltage, sheath gas temperature, and sheath gas flow, were set at 25 psi, 10 L/min, 300 °C, 3500 V, 350 °C, and 12 L/min, respectively. The data acquisition was conducted under a multiple reaction monitoring (MRM) mode and the optimized MRM transitions, fragmentors, and collision energies are presented in Table S1.
Quality assurance and quality control
All glassware was heated at 500 °C for 8 h in a muffle furnace before use. Procedural blanks were used to monitor background contamination in the laboratory (n = 5). In addition, field blanks (n = 2) were collected to check background contamination during sampling. Method quantification limits (MQLs) were set as ten times the standard deviation of the target analyte levels detected in the blanks. For compounds not detected in the blanks, MQLs were based on a signal-to-noise ratio of ten. All data were blank corrected by subtracting average blank levels from the sample levels. The blank levels for some analytes (e.g., C14- and C18-DDACs and C14-ATMAC) were elevated; however, the levels in samples were still higher than in blanks, hence the detection frequencies did not significantly decrease after blank-correction. Still, the data for these analytes should be considered with caution. Procedural and field blank levels for all analytes are included in Table S2.
Quantification of the detected target analytes was performed by isotope dilution using calibration curves with concentration ranges of 0.1 − 10 ng/mL and correlation coefficients in linearity tests were all >0.99. The absolute average recoveries for the spiked samples ranged from 41 ± 0.5% (mean ± standard error) to 149 ± 5% and are given in Table S3. The average recoveries of the surrogate standards were 80 ± 0.7% and 73 ± 0.5% for d7-C12-BAC and d9-C10-ATMAC, respectively (Table S4). The concentrations were not corrected based on surrogate recoveries.
Data analysis
Lactational estimated daily intakes (EDIs, ng/kg body weight [bw] /day) of QACs were calculated using Eq. 1:
| 1 |
where C is a median concentration of a QAC in breast milk in ng/mL and FIR is a food ingestion rate (mL/kg bw/day) representing the average daily intake of breast milk (150, 140, 110, and 83 mL/kg bw/day for <1, 1–3, 3–6, and 6–12 month old infants, respectively) [37].
Statistical analyses were conducted using IBM SPSS Statistics 24 and Minitab 13 and plots were generated using Sigma Plot 13. Correlation heatmaps and hierarchical clustering were done using Pearson correlation analysis in R studio. No significant correlation was found between the lipid content and QAC concentrations in breast milk (Table S5); hence concentrations are given in ng/mL. Concentrations below MQLs were replaced with MQL/2 values for the descriptive statistics and correlation analyses. The significance level was set at p < 0.05.
Results
Population characteristics
A description of the participants’ demographic characteristics is presented in Table 1. All mothers were breastfeeding their first child. Most of the participants were Caucasian and lived in or around Seattle, Washington (the average [with standard error] residence time was 13 ± 11 years). Participants’ age ranged from 24 to 42 years old (average 34 ± 4.0 years). Ninety-six percent of the mothers had attained higher education and 80% lived in middle or upper-middle income neighborhoods. Fifty-eight percent had a normal BMI (18.5–24.9), while 34% were overweight or obese. No significant correlation was found between the QAC concentrations and demographic characteristics, possibly due to the limited sample size.
Characterization of QAC levels in breast milk
The detection frequencies and concentrations of the 18 QACs measured in breast milk are given in Table 2. Thirteen of the 18 QACs were detected in breast milk and 7 were found in more than half of the samples. All analyzed samples had at least one QAC detected above the limit of detection. The total QAC concentrations (ΣQAC, the sum of the 13 detected QACs) ranged from 0.33 to 7.4 ng/mL with a median concentration of 1.5 ng/mL. BACs were the most abundant QACs found in these samples with a median ΣBAC concentration (the sum of 5 detected BACs) of 0.92 ng/mL accounting for 71% of the ΣQAC concentration, followed by ATMACs detected at a median ∑ATMAC [the sum of 6 detected ATMACs] concentration of 0.44 ng/mL (29% of the ΣQAC concentration). DDACs were detected in 15% of the samples and at lower concentrations (<2.2–2.8 ng/mL). C10-C16 BACs and C14- and C16-ATMACs were detected in the majority of the samples (67–88%). The most abundant QAC was C14-BAC with a median concentration of 0.45 ng/mL, followed by C12-BAC (median 0.38 ng/mL), C16-ATMAC (0.21 ng/mL), and C14-ATMAC (0.16 ng/mL). These four compounds accounted for 92% of the ΣQAC concentrations. C10- and C16-BACs and C18-ATMAC were detected at significantly lower concentrations (p < 0.05 based on a one-way analysis of variance [ANOVA], Table 2 and Fig. 1). Although C10-DDAC was only detected in 8% of the samples, high concentrations of C10-DDAC were exclusively found in the samples collected from the mothers who reported using QAC-containing disinfectants. C18-BAC, C8- and C10-DDACs, and C8-C12 ATMACs were found in less than half of the samples, and C8-BAC and C12-C18 DDACs were not detected.
Table 2.
Detection frequencies (DF, %), the mean (with their standard errors), minimum, 25th percentile, median, 75th percentile, 95th percentile, and maximum concentrations of each QAC measured in breast milk (ng/mL; n = 48) and their contributions (contr, %) to the ∑QAC concentrations.
| DF | Mean ± SE | Min | 25th | Median | 75th | 95th | Max | Contr. | |
|---|---|---|---|---|---|---|---|---|---|
| % | ng/mL | ng/mL | ng/mL | ng/mL | ng/mL | ng/mL | ng/mL | % | |
| BACs | |||||||||
| C8-BAC | 0 | <MQL | --* | ||||||
| C10-BAC | 79 | 0.0047 ± 0.00035 | <MQL | 0.0031 | 0.0049 | 0.0054 | 0.0098 | 0.011 | 0.37 |
| C12-BAC | 81 | 0.51 ± 0.067 | <MQL | 0.14 | 0.38 | 0.69 | 1.5 | 2.2 | 29 |
| C14-BAC | 67 | 0.56 ± 0.064 | <MQL | <MQL | 0.45 | 0.80 | 1.3 | 2.1 | 35 |
| C16-BAC | 69 | 0.12 ± 0.018 | <MQL | <MQL | 0.088 | 0.17 | 0.32 | 0.52 | 6.7 |
| C18-BAC | 10 | 0.34 ± 0.18 | <MQL | <MQL | <MQL | <MQL | 0.87 | 0.95 | --* |
| ∑BAC | 90 | 1.2 ± 0.14 | <MQL | 0.40 | 0.92 | 1.7 | 3.3 | 4.1 | 71 |
| DDACs | |||||||||
| C8-DDAC | 8 | 0.25 ± 0.23 | <MQL | <MQL | <MQL | <MQL | 0.81 | 0.94 | --* |
| C10-DDAC | 8 | 1.5 ± 0.44 | <MQL | <MQL | <MQL | <MQL | 2.6 | 2.8 | --* |
| C12-DDAC | 0 | <MQL | --* | ||||||
| C14-DDAC | 0 | <MQL | --* | ||||||
| C16-DDAC | 0 | <MQL | --* | ||||||
| C18-DDAC | 0 | <MQL | --* | ||||||
| ∑DDAC | 15 | 1.0 ± 0.35 | <MQL | <MQL | <MQL | <MQL | 2.3 | 2.8 | --* |
| ATMACs | |||||||||
| C8-ATMAC | 6 | 0.054 ± 0.016 | <MQL | <MQL | <MQL | <MQL | 0.082 | 0.087 | --* |
| C10-ATMAC | 6 | 0.093 ± 0.042 | <MQL | <MQL | <MQL | <MQL | 0.16 | 0.17 | --* |
| C12-ATMAC | 44 | 0.062 ± 0.020 | <MQL | <MQL | <MQL | 0.079 | 0.17 | 0.43 | --* |
| C14-ATMAC | 85 | 0.21 ± 0.032 | <MQL | 0.073 | 0.16 | 0.24 | 0.54 | 1.4 | 12 |
| C16-ATMAC | 88 | 0.39 ± 0.099 | <MQL | 0.13 | 0.21 | 0.31 | 1.4 | 4.4 | 16 |
| C18-ATMAC | 65 | 0.031 ± 0.0059 | <MQL | <MQL | 0.013 | 0.027 | 0.11 | 0.19 | 1.0 |
| ∑ATMAC | 98 | 0.67 ± 0.11 | <MQL | 0.28 | 0.44 | 0.63 | 2.0 | 4.6 | 29 |
| ∑QACs | 100 | 2.0 ± 0.24 | 0.33 | 0.88 | 1.5 | 2.9 | 5.0 | 7.4 | 100 |
*Percent contribution to the ∑QAC concentration was not calculated because of DF < 50%. <MQL: concentrations below the method quantification limit; SE standard error. Some concentrations are below the blank levels because the data was blank-corrected by subtracting the average blank levels from sample levels.
Fig. 1. Concentrations of QACs detected in more than 50% of the breast milk samples (ng/mL).
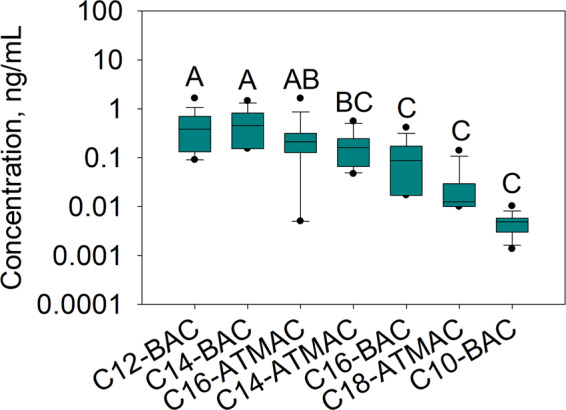
Concentrations are shown as box plots, representing the 25th and 75th percentiles; black lines represent the median and the whiskers represent the 10th and 90th percentiles; the dots represent the 5th and 95th percentiles. The letters show the results of the one-way analysis of variance (ANOVA) and the concentrations are ranked from the highest to the lowest in alphabetical order (A stands for the highest and C for the lowest). The concentrations sharing the same letter are not statistically different at p < 0.05 (e.g., C12-BAC, C14-BAC, and C16-ATMAC).
The individual QACs with detection frequencies over 50% were clustered into two major groups based on the correlation heatmaps and hierarchical clustering (Fig. S1). The first group consisted of C10-C16 BACs and C14-ATMAC (r: 0.44–0.96, p < 0.05) and the other cluster included C16- and C18-ATMACs (r: 0.33–0.44, p < 0.05).
Effect of product use
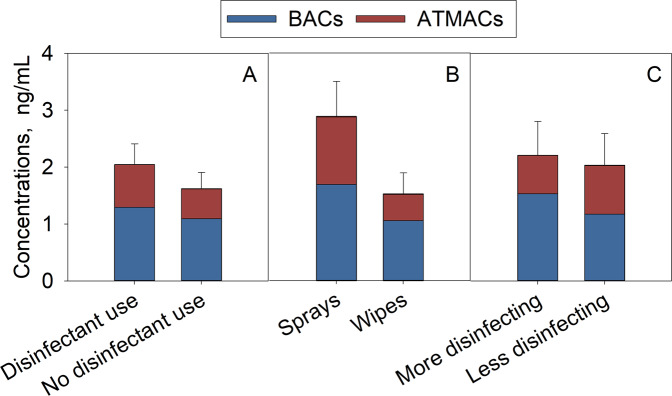
Forty-two percent of mothers in this study indicated that they regularly used QAC-containing disinfecting products in their homes. Among those using such products, sixty-two percent used disinfecting wipes, while the rest used sprays (squirt bottles). More than half of mothers reported disinfecting less than once a week. Overall, the mean ∑QAC concentration in breast milk from the mothers who used QAC-containing disinfecting products was 1.3 times higher than that in mothers who did not disinfect or used disinfectants without QACs (mean ± standard error [95% CI], 2.04 ± 0.36 [1.42–2.67] vs. 1.62 ± 0.29 [1.24–2.33] ng/mL, Fig. 2). The mean ∑QAC concentration in breast milk from mothers who used disinfecting sprays was almost 2 times higher compared to that who used wipes (2.88 ± 0.63 [1.89–3.88] vs. 1.52 ± 0.38 [0.74–2.30] ng/mL; p < 0.05). Mothers who disinfected more frequently (more than once per week) had a slightly higher ∑QAC concentration in their breast milk than those who disinfected less often (2.21 ± 0.60 [1.02–3.39] vs. 2.03 ± 0.56 [1.02–3.04] ng/mL). A similar trend was found for the ΣBAC concentrations. Higher ∑ATMAC concentrations were found in the group of mothers who used QAC-containing disinfecting products, specifically in those who used disinfecting sprays (Fig. 2). No relationship was found between the ∑ATMAC concentrations and the use of personal care products that list ATMACs as the main ingredients.
Fig. 2. The ∑BAC and ∑ATMAC concentrations (mean ± standard error, ng/mL) in breast milk samples grouped based on mothers’ disinfection habits.
A Levels in breast milk collected from mothers who do (n = 21) vs. those who do not disinfect or use (n = 26) QAC-containing disinfecting products. B Levels in breast milk collected from mothers who use sprays (n = 8) vs. wipes (n = 13). C Levels in mothers who disinfect more frequently (more than once a week, n = 8) vs. those who disinfect less frequently (less than once a week, n = 11).
Exposure assessment
Lactational estimated daily intakes (EDIs) of QACs for infants of <1, 1–3, 3–6, and 6–12 month old are presented in Table 3. The highest median ΣQAC EDI was found for the <1 month old infants (230 ng/kg bw/day), followed by that for the 1–3 month old (210 ng/kg bw/day), 3–6 month old (170 ng/kg bw/day), and 6–12 month old (120 ng/kg bw/day) infants. This decline can be explained by the increase in the body weight and decrease in breast milk consumption with age. The median EDI for C14-BAC was the highest for all age groups (37–68 ng/kg bw/day), followed by the EDI for C12-BAC (32–57 ng/kg bw/day). In the high-exposure scenario (based on the 95th percentile concentrations), the ΣQAC EDI increased to 420–750 ng/kg bw/day.
Table 3.
Lactational estimated daily intakes (EDIs, ng/kg bw/day) of QACs detected in more than 50% of the smples for infants at ages of <1, 1–3, 3–6, 6–12 month old.
| Average* exposure scenario, ng/kg bw/day | High* exposure scenario, ng/kg bw/day | |||||||
|---|---|---|---|---|---|---|---|---|
| <1 month | 1–3 month | 3–6 month | 6–12 month | <1 month | 1–3 month | 3–6 month | 6–12 month | |
| C10-BAC | 0.47 | 0.43 | 0.34 | 0.26 | 1.5 | 1.4 | 1.1 | 0.81 |
| C12-BAC | 57 | 53 | 42 | 32 | 230 | 210 | 170 | 120 |
| C14-BAC | 68 | 63 | 50 | 37 | 200 | 180 | 140 | 110 |
| C16-BAC | 13 | 12 | 9.7 | 7.3 | 48 | 45 | 35 | 27 |
| C14-ATMAC | 24 | 22 | 18 | 13 | 81 | 76 | 59 | 45 |
| C16-ATMAC | 32 | 29 | 23 | 17 | 210 | 200 | 150 | 120 |
| C18-ATMAC | 2.0 | 1.8 | 1.4 | 1.1 | 17 | 15 | 12 | 9.1 |
| ∑QACs | 230 | 210 | 170 | 120 | 750 | 700 | 550 | 420 |
*The average and high exposure scenarios were calculated based on the median and 95th percentile concentrations, respectively.
Discussion
Our findings indicate widepsread detection of QACs in breast milk with at least one QAC found in each sample and 7 QACs detected in more than half of the samples. As this is the first study reporting the occurrence of QACs in breast milk, a direct comparison of our results with other studies is not possible. When comparing the QAC levels in this study to the previously reported levels of emerging contaminants, the median ΣQAC concentration (1.5 ng/mL) was lower than that of organophosphate esters (medians 3.5–3.9 ng/mL) [34], comparable to that of melamine derivatives (1.4 ng/mL) [38], but 10 times higher than the median concentration of per- and polyfluoroalkyl substances (PFAS) in breast milk from U.S. mothers (0.12 ng/mL) [17]. Although information about the route and magnitude of exposure to QACs is limited, these findings suggest that certain QACs may accumulate in breast milk at comparable or higher levels than some of the other ubiquitous environmental contaminants.
Two recent studies have reported a frequent detection of QACs in human blood, demonstrating widespread human exposure to QACs in the general U.S. population [17, 19]. The median ΣQAC concentration measured in breast milk was two times lower than that found in human blood collected before the pandemic (sampling year 2019; median 3.4 ng/mL) [17]. This may be explained by the strong binding affinity of QACs to blood proteins, thus allowing only a fraction of QACs to be transported to breast milk [17]. The QAC profiles in breast milk and blood were somewhat different: breast milk was more enriched with BACs, while blood had almost equal contributions from both BACs and ATMACs (73 and 27% vs. 48 and 50%, respectively) [17]. In both cases, DDACs constituted only a minor fraction of the ∑QAC concentrations. The different QAC profiles in blood and breast milk could be explained by a compound-specific lactational transfer. However, this comparison should be considered with caution because these blood and breast milk samples were not paired, thus these differences could be confounded by other factors, such as sampling time, demographic characteristics, and exposure levels.
The most abundant QACs found in these breast milk samples were BACs and ATMACs. Previous studies have shown that BACs are the major ingredients in disinfecting products [16, 39], while ATMACs are mainly used in cosmetics and hair conditioners [1, 40, 41]. Interestingly, strong positive correlations were found between C10–C16 BACs and C14-ATMAC concentrations in breast milk. Similarly, C14-ATMAC was the most abundant ATMAC in some cleaning products analyzed in our previous study, and a strong correlation was observed among the dust levels of C14-ATMAC and C10–C16 BACs [16]. These results suggest that cleaning products could be a potential source of ATMACs in breast milk.
Our results suggest that disinfecting practices may have an effect on the QAC levels in breast milk. The mean ∑QAC concentration in breast milk from mothers who used disinfecting products were higher than those who did not use such products or used disinfectants without QACs. Especially, higher levels of QACs were found in mothers who used disinfecting sprays compared to mothers who used wipes. This could be related to the previously reported higher content of QACs in sprays (1.66% by weight) compared to wipes (0.135%) [16]. In addition, mothers who disinfected more often had a somewhat higher mean ∑QAC concentration in their breast milk than those who disinfected less frequently. Taken together, these results suggest that the use of disinfecting products that contain QACs may affect the levels in breast milk. The lack of a significant relationship between the ∑ATMAC concentrations and the use of personal care products that list ATMACs as the main ingredients, could be because C20–C22 ATMACs commonly found in hair conditioners were not analyzed in this study.
The QAC intake in the present study was more than 3 orders of magnitude lower than the intake through surface-to-hand-to-mouth transfer reported for 3-year-old toddlers, but higher than the estimated dermal absorption through handwashing estimated for the same age group [39]. The ΣQAC EDI was up to 5 times lower than that estimated based on the accidental dust ingestion for toddlers (210–620 ng/kg bw/day) [16]. Generally, the C12-BAC EDIs in both the average and high exposure scenarios were below the reference dose (105 ng/kg bw/day) established by the European Food Safety Authority (EFSA) [42]. However, this reference dose does not account for the potential adverse effects of the chronic exposure to QACs [42].
Conclusions
This is the first study reporting the detection of QACs in breast milk and identifying breastfeeding as an exposure pathway to QACs in nursing infants. The limitations of this study include a small sample size covering a limited geographic area and a quite homogenous population. Information on the specifics of milk collection (e.g., timing, cleaning of the breast and hands, etc.) was not collected. Nonetheless, our findings demonstrate widespread exposure to QACs: at least one QAC was found in each breast milk sample and 7 QACs were found in more than half of the samples. We also found higher QAC levels in breast milk from mothers who used QAC-containing disinfecting products and disinfected more often. Considering a significant increase in the use of QACs as common disinfectants during the COVID-19 pandemic, our findings warrant future research on QAC toxicity and human health effects.
Supplementary information
Acknowledgements
The authors thank the participants for their generosity in sharing their time and breast milk for this study. Carina Wells and Mikyla Sakurai provided significant contributions in recruiting participants and collecting samples, Siobhan Kelly contributed greatly in result communication, and Christina Gu assisted the compilation of the questionnaire.
Author contributions
GZ: Conceptualization, Lab and Data Analysis, Writing - Original draft preparation; ES: Data collection, Writing - Reviewing and Editing; SS: Data evaluation, Writing- Reviewing and Editing; AS: Supervision, Conceptualization; Writing- Reviewing and Editing.
Funding
GZ and AS were supported by the National Institute of Environmental Health Science 2R01ES019620-06A1. Sample collection was supported by Toxic Free Future (Seattle, WA).
Competing interests
All authors declare no competing interests.
Ethics approval and consent to participate
All recruitment and sample collection protocols were approved by the Indiana University Institutional Review Board.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41370-022-00439-4.
References
- 1.Hora PI, Pati SG, McNamara PJ, Arnold WA. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ Sci Technol Lett. 2020;7:622–631. doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- 2.McNamara PJ, Levy SB. Triclosan: an instructive tale. Antimicrob Agents Chemother. 2016;60:7015–6. doi: 10.1128/AAC.02105-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surface disinfectant market size to reach USD 1,547.7 million by 2027; Rising prevalence of hospital-acquired infections to boost growth, Fortune Business Insights, June, 2020.
- 4.Zhang C, Cui F, Zeng GM, Jiang M, Yang ZZ, Yu ZG, et al. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci Total Environ. 2015;518:352–62. doi: 10.1016/j.scitotenv.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Mulder I, Siemens J, Sentek V, Amelung W, Smalla K, Jechalke S. Quaternary ammonium compounds in soil: Implications for antibiotic resistance development. Rev Environ Sci Biotechnol. 2017;17:159–85. doi: 10.1007/s11157-017-9457-7. [DOI] [Google Scholar]
- 6.Jardak K, Drogui P, Daghrir R. Surfactants in aquatic and terrestrial environment: Occurrence, behavior, and treatment processes. Environ Sci Pollut Res. 2016;23:3195–216. doi: 10.1007/s11356-015-5803-x. [DOI] [PubMed] [Google Scholar]
- 7.Harrison KR, Kappell AD, McNamara PJ. Benzalkonium chloride alters phenotypic and genotypic antibiotic resistance profiles in a source water used for drinking water treatment. Environ Pollut. 2020;257:113472. doi: 10.1016/j.envpol.2019.113472. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Doherty AC, Brownawell B, Lara-Martin PA. Distribution and diagenetic fate of synthetic surfactants and their metabolites in sewage-impacted estuarine sediments. Environ Pollut. 2018;242:209–18. doi: 10.1016/j.envpol.2018.06.064. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Brownawell BJ. Quaternary ammonium compounds in urban estuarine sediment environments - A class of contaminants in need of increased attention? Environ Sci Technol. 2010;44:7561–8. doi: 10.1021/es1011669. [DOI] [PubMed] [Google Scholar]
- 10.Pati SG, Arnold WA. Comprehensive screening of quaternary ammonium surfactants and ionic liquids in wastewater effluents and lake sediments. Environ Sci Process Impacts. 2020;22:430–41. doi: 10.1039/C9EM00554D. [DOI] [PubMed] [Google Scholar]
- 11.Xian Y, Dong H, Wu Y, Guo X, Hou X, Wang B. QuEChERS-based purification method coupled to ultrahigh performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) to determine six quaternary ammonium compounds (QACs) in dairy products. Food Chem. 2016;212:96–103. doi: 10.1016/j.foodchem.2016.05.151. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Malik S, Duncan TV, Jablonski JE. High throughput quantification of quaternary ammonium cations in food simulants by flow-injection mass spectrometry. J AOAC Int. 2018;101:1873–80. doi: 10.5740/jaoacint.18-0091. [DOI] [PubMed] [Google Scholar]
- 13.Slimani K, Feret A, Pirotais Y, Maris P, Abjean JP, Hurtaud-Pessel D. Liquid chromatography-tandem mass spectrometry multiresidue method for the analysis of quaternary ammonium compounds in cheese and milk products: Development and validation using the total error approach. J Chromatogr A. 2017;1517:86–96. doi: 10.1016/j.chroma.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 14.BfR opinion No 032/2012, Health assessment of benzalkonium chloride residues in food. 2012.
- 15.BfR opinion No 027/2012, Health assessment of didecyldimethylammonium chloride (DDAC) residues in food. 2012.
- 16.Zheng G, Filippelli GM, Salamova A. Increased indoor exposure to commonly used disinfectants during the COVID-19 pandemic. Environ Sci Technol Lett. 2020;7:760–5. doi: 10.1021/acs.estlett.0c00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng G, Salamova A. Bioaccumulation of quaternary ammonium compounds: Evidence of increased exposure during the COVID-19 pandemic. Environ Sci Technol. 2021;55:14689–98. doi: 10.1021/acs.est.1c01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent G, Kopferschmitt-Kubler MC, Mirabel P, Pauli G, Millet M. Sampling and analysis of quaternary ammonium compounds (QACs) traces in indoor atmosphere. Environ Monit Assess. 2007;133:25–30. doi: 10.1007/s10661-006-9556-3. [DOI] [PubMed] [Google Scholar]
- 19.Hrubec TC, Seguin RP, Xu L, Cortopassi GA, Datta S, Hanlon AL, et al. Altered toxicological endpoints in humans from common quaternary ammonium compound disinfectant exposure. Toxicol Rep. 2021;8:646–56. doi: 10.1016/j.toxrep.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson SE, Shane H, Long C, Lukomska E, Meade BJ, Marshall NB. Evaluation of the irritancy and hypersensitivity potential following topical application of didecyldimethylammonium chloride. J Immunotoxicol. 2016;13:557–66. doi: 10.3109/1547691X.2016.1140854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US EPA, Alkyl Dimethyl benzyl ammonium chloride (ADBAC) final work plan, 2017, Available at: https://www.regulations.gov/contentStreamer?documentId=EPA-HQ-OPP-2015-0737-0004&contentType=pdf. (accessed August 2020).
- 22.US EPA, Didecyl dimethyl ammonium chloride (DDAC) fnal work plan, 2017, Available at: https://www.regulations.gov/contentStreamer?documentId=EPA-HQ-OPP-2015-0740-0004&contentType=pdf. (accessed August 2020).
- 23.LaKind JS, Goodman M. Methodological evaluation of human research on asthmagenicity and occupational cleaning: A case study of quaternary ammonium compounds (“quats”) Allergy Asthma Clin Immunol. 2019;15:69. doi: 10.1186/s13223-019-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumas O, Varraso R, Boggs KM, Quinot C, Zock JP, Henneberger PK, et al. Association of occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Netw open. 2019;2:e1913563. doi: 10.1001/jamanetworkopen.2019.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walters GI, Burge PS, Moore VC, Robertson AS. Cleaning agent occupational asthma in the West Midlands, UK: 2000–16. Occup Med. 2018;68:530–6. doi: 10.1093/occmed/kqy113. [DOI] [PubMed] [Google Scholar]
- 26.Association of Occupational and Environmental Clinics, Revised protocol: Criteria for designating substances as occupational asthmagens on the AOEC List of exposure codes, 2008, Available at: http://www.aoec.org/content/Asthmagen_Protocol_10-25-08.pdf. (accessed November 2021).
- 27.Herron J, Hines KM, Xu L. Assessment of altered cholesterol homeostasis by xenobiotics using ultra-high performance liquid chromatography-tandem mass spectrometry. Curr Protoc Toxicol. 2018;78:65–5. doi: 10.1002/cptx.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herron J, Reese RC, Tallman KA, Narayanaswamy R, Porter NA, Xu L. Identification of environmental quaternary ammonium compounds as direct inhibitors of cholesterol biosynthesis. Toxicol Sci. 2016;151:261–70. doi: 10.1093/toxsci/kfw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melin VE, Melin TE, Dessify BJ, Nguyen CT, Shea CS, Hrubec TC. Quaternary ammonium disinfectants cause subfertility in mice by targeting both male and female reproductive processes. Reprod Toxicol. 2016;59:159–66. doi: 10.1016/j.reprotox.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Melin VE, Potineni H, Hunt P, Griswold J, Siems B, Werre SR, et al. Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Reprod Toxicol. 2014;50:163–70. doi: 10.1016/j.reprotox.2014.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hrubec TC, Melin VE, Shea CS, Ferguson EE, Garofola C, Repine CM, et al. Ambient and dosed exposure to quaternary ammonium disinfectants causes neural tube defects in rodents. Birth Defects Res. 2017;109:1166–78. doi: 10.1002/bdr2.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sly JL, Carpenter DO. Special vulnerability of children to environmental exposures. Rev Environ Health. 2012;27:151–7. doi: 10.1515/reveh-2012-0024. [DOI] [PubMed] [Google Scholar]
- 33.Makri A, Goveia M, Balbus J, Parkin R. Children’s susceptibility to chemicals: A review by developmental stage. J Toxicol Environ Health B Crit Rev. 2004;7:417–35. doi: 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- 34.Zheng G, Schreder E, Dempsey JC, Uding N, Chu V, Andres G, et al. Organophosphate esters and their metabolites in breast milk from the United States: Breastfeeding is an important exposure pathway for infants. Environ Sci Technol Lett. 2021;8:224–30. doi: 10.1021/acs.estlett.0c00916. [DOI] [Google Scholar]
- 35.Lehmann GM, LaKind JS, Davis MH, Hines EP, Marchitti SA, Alcala C, et al. Environmental chemicals in breast milk and formula: Exposure and risk assessment implications. Environ Health Perspect. 2018;126:096001. doi: 10.1289/EHP1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng G, Schreder E, Dempsey JC, Uding N, Chu V, Andres G, et al. Per- and polyfluoroalkyl substances (PFAS) in breast milk: Concerning trends for current-use PFAS. Environ Sci Technol. 2021;55:7510–20. doi: 10.1021/acs.est.0c06978. [DOI] [PubMed] [Google Scholar]
- 37.U.S. EPA, Exposure factors handbook, 2011 ed.; Washington, DC. 2011.
- 38.Zhu H, Kannan K. Occurrence of melamine and its derivatives in breast milk from the United States and its implications for exposure in infants. Environ Sci Technol. 2019;53:7859–65. doi: 10.1021/acs.est.9b02040. [DOI] [PubMed] [Google Scholar]
- 39.Li D, Sangion A, Li L. Evaluating consumer exposure to disinfecting chemicals against coronavirus disease 2019 (COVID-19) and associated health risks. Environ Int. 2020;145:106108. [DOI] [PMC free article] [PubMed]
- 40.Becker LC, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31:296S–341S. doi: 10.1177/1091581812467378. [DOI] [PubMed] [Google Scholar]
- 41.Rahn OVE, Van Eseltine WP. Quaternary ammonium compounds. Annu Rev Microbiol. 1947;1:173–92. doi: 10.1146/annurev.mi.01.100147.001133. [DOI] [Google Scholar]
- 42.European Food Safety Authority, Reasoned opinion on the dietary risk assessment for proposed temporary maximum residue levels (MRLs) of didecyldimethylammonium chloride (DDAC) and benzalkonium chloride (BAC). EFSA J. 2014;12:3675.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.