Abstract
COVID‐19 significantly impairs survival rates among hematological patients when compared to the general population. Our prospective multicentre project analyzed early administration of anti‐SARS‐CoV‐2 spike protein neutralizing monoclonal antibodies (NmAbs) – bamlanivimab (72%) and casirivimab/imdevimab (28%) – efficacy among hematological patients with early‐stage COVID‐19. Mortality rate was compared to a control cohort of 575 SARS‐CoV‐2 positive hematological patients untreated with any specific anti‐COVID‐19 therapy. 88 hematological patients with lymphomas, acute leukemias, and myeloma as their most frequent underlying diagnoses (72%) were evaluated with a 97 days median follow‐up after NmAb administration. One third of patients (32%) were treated with an anti‐CD20 monoclonal antibody before COVID‐19 diagnosis. Median time between first COVID‐19 symptom and NmAb administration was 2 days. When administering NmAb, 29%, 57%, 11%, 2%, and 1% of our patients had asymptomatic, mild, moderate, severe, and critical degrees of COVID‐19, respectively. 80% of baseline asymptomatic patients remained asymptomatic following NmAb administration. Median duration of COVID‐19 symptoms after NmAb administration was 2.5 days. Progression to severe/critical COVID‐19 occurred among a total of 17% (15/88) of our cases and numerically higher with bamlanivimab versus casirivimab/imdevimab (21% vs. 8%; p = 0.215), and myelomas (29%), lymphomas (17%) and acute leukemias (18%), respectively. During final follow‐up, nine deaths (10%) were recorded ‐ all after bamlanivimab (p = 0.056) with 8% attributed to COVID‐19. Regarding “remdesivir/convalescent plasma naïve” patients, COVID‐19 mortality rates were significantly lower in our NmAbs treated cohort compared to the control cohort of untreated SARS‐CoV‐2 positive hematological patients (6% vs. 16%, p = 0.020), respectively. Our study validated the safety and efficacy of NmAbs early use among hematological patients with newly diagnosed early‐stage COVID‐19 in terms of alleviating infection course and decreasing mortality. Results confirmed a more positive effect of a casirivimab/imdevimab combination versus bamlanivimab monotherapy.
Keywords: anti‐SARS‐CoV‐2 monoclonal neutralizing antibodies, bamlanivimab, casirivimab, COVID‐19, hematological malignancy, imdevimab, SARS‐CoV‐2
1. INTRODUCTION
During randomized trials involving general outpatients, neutralizing spike receptor‐binding protein monoclonal antibodies (NmAbs) against SARS‐CoV‐2 (Severe Acute Respiratory Syndrome‐related Coronavirus‐2) compared to placebos have affirmed effect related to a significant decrease in viral load, outpatient visits, hospitalizations, and COVID‐19‐related deaths (Coronavirus Disease 2019), with an excellent current safety profile. 1 , 2 Similar results were obtained via retrospective case‐control studies regarding frequency reduction of hospitalizations. 3 , 4
Immunocompromized patients are generally more susceptible to COVID‐19 with higher mortality reported than the general population. 5 , 6 To date, only a limited number of retrospective case studies chronicling SARS‐CoV‐2‐positive solid organ transplant patients with no worsening of symptoms and further hospitalization after NmAbs administration have been published. 7 , 8
In literature, robust data on NmAbs effectivity in COVID‐19 positive hematological patients are still lacking. 9 , 10 As a result, we resolved to analyze results of what at the time was dominantly the largest cohort of COVID‐19 immunocompromized hematology patients treated with NmAbs‐bamlanivimab or casirivimab/imdevimab.
2. METHODS
Our prospective study included random successive patients with hematological disease diagnosed with COVID‐19, verified with Reverse Transcription Polymerase Chain Reaction (RT‐PCR) or antigen testing, and subsequently treated with a neutralizing spike receptor‐binding protein monoclonal antibody – bamlanivimab or a casirivimab/imdevimab combination – against SARS‐CoV‐2, administered from 01 March 2021 through 13 May 2021 at eight Czech Republic hematological centers. The project was initiated and implemented on behalf of the Czech Leukemia Study Group for Life. Medicines were administered following temporary regulatory authorization by the Czech Republic Ministry of Health. Disease severity was assessed according to adapted definitions. 11 Data were obtained from source medical documentation covering comorbidities, COVID‐19 diagnostics, therapy, and outcome.
After COVID‐19 antigen or RT‐PCR diagnosis, all patients received a single dose of either 700 mg of bamlanivimab (72%) or 1200 mg of casirivimab/1200 mg of imdevimab (28%).
Research was respective of relevant guidelines and regulations with project approval by the Multicentric Ethics Committee of the Brno University Hospital (Number 01‐290321/EK). All patients involved signed an informed consent form.
Basic statistical methods describing absolute and relative frequency for categorical variables, mean, median, minimum and maximum for continuous variables, respectively, were employed. Categorical parameter relations were evaluated using Fisher's exact tests with p = 0.05 as a statistical significance level. COVID‐19 mortality data were compared with a control cohort of SARS‐CoV‐2 positive hematology patients from participating centers who were not treated with NmAbs or any COVID‐19 specific treatment.
3. RESULTS
A total of 88 hematological patients, with lymphomas, acute leukemias, and myeloma as the most frequent underlying diagnoses (72%; 64/88) were evaluated with a 97 days median follow‐up (mean: 89, range: 8–138) after NmAb administration. One third of patients (32%; 28/88) were treated with an anti‐CD20 monoclonal antibody (rituximab in all cases) during the 2 years prior to COVID‐19 diagnosis. Around a third of patients (36%; 29/81) received at least one dose of SARS‐CoV‐2 vaccine before their COVID‐19 diagnosis. 15% (12/82) of patients had a second COVID‐19 episode when NmAb was administrated. Baseline characteristics are described in Table 1. Among 81 evaluable cases, median time between the first COVID‐19 symptom and NmAb administration was 2 days (mean: 3, range: 0–16). Median time between SARS‐CoV‐2 positivity and NmAb treatment was 1 day. While administering NmAb, 25 (29%), 50 (57%), 10 (11%), 2 (2%), and one (1%) of our patients had an asymptomatic, mild, moderate, severe, or critical degree of COVID‐19, respectively (Figure 1). Following NmAb administration, a total of 11/80 (14%) patients were treated with remdesivir, and 7 (7/80%; 9%) received (CP) together with remdesivir, respectively.
TABLE 1.
Characteristics of COVID‐19 hematological patients enrolled in the study
| Number of patients, n (%) | 88 (100) |
| Age at the time of COVID‐19 diagnosis, years, median; mean (range) | 63; 58 (19–84) |
| Sex, male, n (%) | 48 (55) |
| Comorbidities, n (%) | |
| Hypertension | 32 (36) |
| Diabetes mellitus | 18 (21) |
| Dyslipidemia | 14 (16) |
| Coronary heart disease | 11 (13) |
| Smoking | 8/79 (10) |
| Allergy | 38/82 (46) |
| Pulmonary disease | 7 (8) |
| Obesity | 6 (7) |
| Other | 32 (36) |
| None | 21 (24) |
| Unknown | 4 (5) |
| Underlying disease at baseline, n (%) | |
| Lymphoma | 30 (34) |
| Acute leukemia | 17 (19) |
| Multiple myeloma | 17 (19) |
| Chronic lymphocytic leukemia | 12 (14) |
| Myeloproliferative neoplasias | 4 (5) |
| Chronic myeloid leukemia | 2 (2) |
| Myelodysplastic syndrome | 2 (2) |
| Other hematological diseases | 4 (5) |
| Number of days between diagnosis of hematological disease and COVID‐19, median; mean (range) | 406; 1462 (5–16,367) |
| Last hematological therapy 2 years prior to COVID‐19, n (%) | |
| Anti‐CD20 monoclonal antibody (rituximab in all cases) | 28 (32) |
| Another chemotherapy | 27 (31) |
| Autologous SCT | 8 (9) |
| Allogeneic SCT | 6 (7) |
| Corticosteroids | 4 (5) |
| None | 14 (16) |
| Number of days between last hematological therapy and COVID‐19 diagnosis, median; mean (range) | 19; 74 (0–721) |
| SARS‐CoV‐2 vaccination before COVID‐19 diagnosis, n (%) | |
| Total number of vaccinated patients | 29/81 (36) |
| Number of patients only after 1st dosage of vaccination | 25 (31) |
| Number of days between 1st dosage of vaccination and COVID‐19 diagnosis, median; mean (range) | 15; 19 (6–53) |
| Number of patients after 2nd dosage of vaccination | 4 (5) |
| Number of days between 2nd dosage of vaccination and COVID‐19 diagnosis, median; mean (range) | 22; 33 (5–83) |
| COVID‐19 second episode, n (%) | 12/82 (15) |
| Type of anti‐SARS‐CoV‐2 monoclonal neutralizing antibody, n (%) | |
| Bamlanivimab | 63 (72) |
| Casirivimab/imdevimab | 25 (28) |
| Number of days between COVID‐19 diagnosis and NmAb administration, median; mean (range) | 1; 1.4 (0–6) |
Abbreviations: COVID‐19, coronavirus disease 2019; NmAbs, neutralizing monoclonal antibodies; SARS‐CoV‐2, severe acute respiratory syndrome‐related coronavirus‐2; SCT, stem cell transplantation.
FIGURE 1.
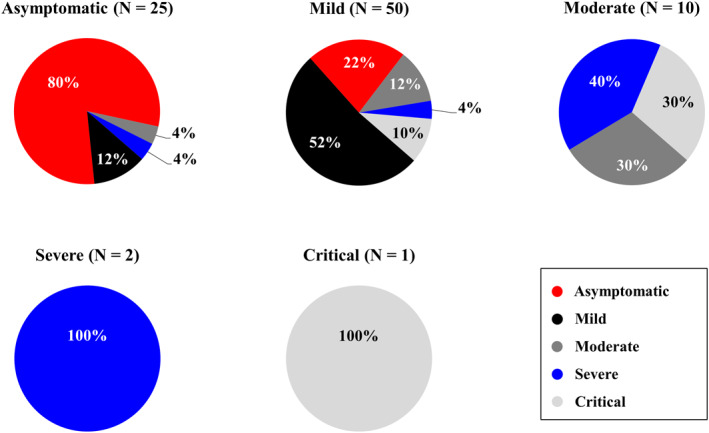
COVID‐19 development overview from initial disease severity to the highest degree during NmAbs administration follow‐up. Figure 1 presents a total of five pie charts divided according to COVID‐19 baseline degree at the time of NmAbs administration. Percentage of COVID‐19's worst degree at follow‐up is indicated in pie chart colors (asymptomatic in red, mild in black, moderate in dark gray, severe in blue, and critical in light gray). COVID‐19, corona virus disease 2019; NmAbs, neutralizing 344 monoclonal antibodies
Regarding extent of infection during follow‐up, 35%, 33%, 12%, 10%, and 10% of the entire group of patients had asymptomatic, mild, moderate, severe, and critical COVID‐19, respectively. Among all baseline asymptomatic patients, 80% (20/25) remained asymptomatic following NmAb administration. With 78 case evaluations, median duration of COVID‐19 symptoms after NmAb administration was 2.5 days (mean: 11, range: 0–101). Progression to severe/critical COVID‐19 occurred among 17% (15/88) of our patients – numerically more frequently yet not significantly in the bamlanivimab versus casirivimab/imdevimab cohort (21% vs. 8%; p = 0.215). Among the entire patient group, COVID‐19's course indicating baseline severity and the specific extent of infection during follow‐up is detailed in Figure 1.
Furthermore, the highest rates of COVID‐19 progression to severe/critical degrees and death were observed among myelomas (29% and 12%), lymphomas (17% and 7%), acute leukemias (18% and 6%), and chronic lymphocytic leukemias (8% and 8%), respectively. Regarding concluding oncological treatment modality, COVID‐19 progression to severe or critical degree and death during follow‐up were most often recorded among patients after induction/reinduction of AL (60% and 20%), autologous stem cell transplantation (SCT; 25% and 13%), and in patients treated with anti‐CD20 monoclonal antibody (14% and 11%), respectively. The data are summarized in Table 2.
TABLE 2.
COVID‐19 progression to severe or critical degree and deaths during follow‐up compared to the control NmAbs untreated cohort
| Cohort of patients treated with NmAbs (n = 88) | Control cohort without NmAbs treatment (n = 575) | ||
|---|---|---|---|
| Progression to severe/critical COVID‐19 | Deaths attributed to COVID‐19 | Deaths attributed to COVID‐19 | |
| Total patients, n (%) | 15/88 (17) | 7/88 (8) | ND |
| Total “remdesivir/CP‐naïve” patients, n (%) | 6/69 (9) | 4/69 (6) | 93/575 (16) |
| Underlying disease at baseline, n (%) | |||
| Lymphoma | 5/30 (17) | 2/30 (7) | 22/114 (19) |
| Acute leukemia | 3/17 (18) | 1/17 (6) | 14/87 (16) |
| Multiple myeloma | 5/17 (29) | 2/17 (12) | 16/88 (18) |
| Chronic lymphocytic leukemia | 1/12 (8) | 1/12 (8) | 21/88 (24) |
| Myeloproliferative neoplasias | 0/4 (0) | 0/4 (0) | 6/57 (11) |
| Chronic myeloid leukemia | 0/2 (0) | 0/2 (0) | 1/53 (2) |
| Myelodysplastic syndrome | 0/2 (0) | 0/2 (0) | 9/34 (27) |
| Other hematological diseases | 1/4 (25) | 1/4 (25) | 4/54 (7) |
| Last hematological therapy prior to COVID‐19, n (%) | |||
| Anti‐CD20 monoclonal antibody | 4/28 (14) | 3/28 (11) | ND |
| Induction/reinduction of AL | 3/5 (60) | 1/5 (20) | ND |
| Autologous SCT | 2/8 (25) | 1/8 (13) | ND |
| Allogeneic SCT | 0/6 (0) | 0/6 (0) | ND |
| RD | 1/1 (100) | 0/1 (0) | ND |
| DRD | 1/4 (25) | 1/4 (25) | ND |
| Corticosteroids | 2/4 (50) | 1/4 (25) | ND |
| BV‐CHP | 1/1 (100) | 0/1 (0) | ND |
| VTD | 1/2 (50) | 0/2 (0) | ND |
| Another chemotherapy | 0/15 (0) | 0/15 (0) | ND |
| None | 0/14 (0) | 0/14 (0) | ND |
| Number of days between last hematological therapy and COVID‐19 diagnosis in patients with progression, median; mean (range) | 11; 17 (0–98) | ND | |
Abbreviations: AL, acute leukemia; BV‐CHP, Brentuximab Vedotin + Cyclophosphamide + Doxorubicin + Prednisone; COVID‐19, coronavirus disease 2019; CP, convalescent plasma; DRD, Daratumumab + Revlimid + Dexamethasone; ND, not done; NmAbs, neutralizing monoclonal antibodies; RD, Revlimid + Dexamethasone; SCT, stem cell transplantation; VTD, Bortezomib + Thalidomide + Dexamethasone.
The proportion of anti‐SARS‐CoV‐2 vaccinated patients was approximately the same in both groups of patients with moderate/severe/critical (Mo/S/C) COVID‐19 when compared to asymptomatic/mild (A/Mi) COVID‐19 at follow‐up (8/28%, 29% vs. 21/60%, 35%; p = 0.631). The median number of days from first vaccination to COVID‐19 diagnosis was yet not significantly but numerically longer in the Mo/S/C group compared to the A/Mi group (21 vs. 14 days; p = 0.542). Prior to COVID‐19 diagnosis, 4% of patients with Mo/S/C worst degree of infection versus 5% of patients with A/Mi worst degree of infection received a second vaccination.
Final evaluation during the last follow‐up affirmed 89% (78/88) asymptomatic patients, one patient (1%; 1/88) with an abiding symptom (dyspnea), and nine deaths (10%; 9/88). All deaths involved patients administered bamlanivimab, with seven fatalities (8%) resulting from COVID‐19. The number of deaths was markedly higher with the cohort given bamlanivimab compared to the group dispensed casirivimab/imdevimab (14% vs. 0%; p = 0.056). Data concerning COVID‐19‐related cohort fatalities are available in Table S1. All three patients with severe or critical COVID‐19 at baseline were still surviving at the time of their last follow‐up.
When comparing COVID‐19‐related mortality in our group of NmAbs‐treated “remdesivir/CP‐naïve” patients (n = 69) with a control cohort of SARS‐CoV‐2 positive hematology patients without any COVID‐19‐specific treatment managed by participating centers (n = 575), significantly fewer patients died in our NmAbs treated group than in the untreated control group (16% vs. 6%, p = 0.020; Table 2).
Adverse side effects were reported in 1 patient (1/63%; 2%) after bamlanivimab (syncope, nausea, fever) and with 1 patient (1/25%; 4%) after casirivimab/imdevimab (burning and eye pain).
4. DISCUSSION
Our study highlights the importance of early administration of anti‐SARS‐CoV‐2 neutralizing NmAbs, bamlanivimab or casirivimab/imdevimab, among hematological patients with freshly diagnosed COVID‐19.
In literature, a number of extensive analyzes have been published detailing substantially higher mortality attributed to COVID‐19 in hematological patients compared to the general population. 5 , 6 As an example, one large meta‐analysis study recorded more than a 30% mortality among individuals with hematological malignancies compared to the 8% mortality in our cohort. 5
Anti‐SARS‐CoV‐2 spike protein NmAbs might generally be effective in substantively decreasing viral load, along with reducing outpatient visits, hospitalizations, and COVID‐19‐related deaths with mild to moderate disease.one to four Of major importance is timely treatment administration from the onset of COVID‐19 symptoms, as our research revealed a shorter median when compared with randomized studies (2.5 days vs. 3–4 days). 1 , 2
Similar results were obtained by a large retrospective case‐control study noting all‐cause mortality reduction at day 21 among a group of high‐risk general patients who received bamlanivimab compared to a control group with mild to moderate COVID‐19 (0.05% vs. 0.4%; Risk Ratio‐RR, 0.13). 3 Furthermore, another real‐life clinical setting confirmed that NmAb (bamlanivimab or casirivimab/imdevimab) treatment significantly decreased hospital in‐patient status among a general population cohort with mild to moderate COVID‐19, especially when treatment was initiated ≤4 days after symptom onset. 4
Regarding immunocompromized patients with a high risk of persistent viral replication and severe infection course, a case study analyzing 25 solid organ transplant patients with mild to moderate COVID‐19 treated with casirivimab/imdevimab and another study from the same center evaluating a total of 10 solid organ transplant patients treated with bamlanivimab were published. 7 , 8 In both studies, no patient experienced progression of symptoms or required hospitalization owing to COVID‐19.
Concerning hematological patients, no extensive cohort studies of COVID‐19 positive hematological patients treated with NmAbs have been published. A single‐center experience of 42 cancer patients in total has been described (76% with hematological malignancy) where mild to moderate COVID‐19 was treated with either bamlanivimab (83%) or casirivimab/imdevimab (17%). 9 The median time to NmAb infusion ‐ 5 days from symptom onset ‐ was longer than in our study. Only 12% of these patients had COVID‐19 deterioration requiring hospitalization ‐‐ all after bamlanivimab ‐‐ from which one died (2%). Similarly by comparison, in our study only 17% of cases progressed to severe/critical COVID‐19 – numerically more frequently in the bamlanivimab versus casirivimab/imdevimab (21% vs. 8%; p = 0.215). Analogous to published data, the highest rates of COVID‐19 progression to a severe/critical degree and death were recorded with myeloma, lymphoma, AL, chronic lymphocytic leukemia, and in patients treated with anti‐CD20 monoclonal antibody, induction/reinduction of AL, and autologous SCT, respectively (Table 2). 5 , 6 Patients with progression of COVID‐19 to severe/critical grade compared with patients without progression received their last oncology treatment at a numerically yet not significantly shorter median of 11 versus 21 days (p = 0.142). Furthermore, 80% of our baseline asymptomatic patients remained asymptomatic throughout follow‐up. Our cohort's COVID‐19 symptoms median duration was only 2.5 days following NmAbs treatment.
Analogous to Puing's study, all deaths in our cohort occurred after bamlanivimab infusion (p = 0.056). All seven patient fatalities attributed to COVID‐19 had either concomitant advanced hematological malignancy, numerous severe comorbidities, or received treatment prior to COVID‐19 with a profound and long‐lasting immunosuppressive effect as indicated in Table S1. The control cohort of SARS‐CoV‐2 positive hematology patients without specific COVID‐19 treatment managed by participating centers exhibited a significantly higher COVID‐19‐related death rate than our analyzed “remdesivir/CP‐naïve” cohort of NmAbs‐treated patients (16% vs. 6%, p = 0.020; Table 2). The highest mortality in the control cohort of patients with lymphoma, AL, high‐risk myelodysplastic syndrome, multiple myeloma, and chronic lymphocytic leukemia correlated with published data. 5 , 6
Significantly, among the 36% of our cohort patients becoming infected with COVID‐19 following vaccination, only 5% were infected after their second dose. Nonetheless, the median time between the first dose of vaccine and COVID‐19 symptom onset was only 15 days among the single‐dose group. Anti‐SARS‐CoV‐2 vaccination before COVID‐19 diagnosis had no effect on the course of infection after NmAbs administration; proportion of vaccinated versus unvaccinated patients was similar in both A/Mi and Mo/S/C groups (35% vs. 29%, p = 0.631) with a longer median time from the first dose in the Mo/S/C group (21 vs. 14 days), respectively. In addition, antibody testing for determining SARS‐CoV‐2 serostatus was not undertaken before the COVID‐19 diagnosis.
Unfortunately, SARS‐CoV‐2 variants are emerging with potential to reduce NmAbs efficacy. Extensive recent analysis has detailed efficacy against many variants of infection with low prophylactic doses of NmAbs in animal models, without the development of resistance, albeit with the exception of bamlanivimab monotherapy and bamlanivimab/etesevimab combination therapy. 12 Therefore, bamlanivimab monotherapy was no longer permitted during the time of Delta variant predominance. Although all patients had not been genotyped at the time of our study, the Alpha variant prevailed in the Czech Republic with potentially preserved susceptibility to bamlanivimab.
Our study's major strength is its focus on a uniform robust multi‐center cohort comprised of hematological patients compared to a control cohort without any COVID‐19 specific treatment. Study constraints concern the regulatory authorization for administration of only the lowest dose of bamlanivimab with a lower efficacy.
5. CONCLUSIONS
Patients with hematological malignancies are at high risk for severe and life‐threatening COVID‐19 exposure. Based on our analysis results, early‐administered anti‐SARS‐CoV‐2 spike protein monoclonal neutralizing antibodies appear to be safe and effective in alleviating infection course and improving prognosis among hematological patients with early‐stage COVID‐19. COVID‐19‐related mortality was substantially 3‐fold lower compared to the control cohort of COVID‐19 untreated hematological patients and was notably associated with advanced hematological disease and comorbidities. Our results should validate the enhanced effect of a casirivimab/imdevimab combination compared to bamlanivimab monotherapy.
CONFLICT OF INTEREST
The authors declare no competing or conflicting interests.
AUTHOR CONTRIBUTIONS
Barbora Weinbergerová, and Jiří Mayer – contributed to study conception and design, implemented material preparation, data collection and analysis; composed and revised manuscript.
Ivo Demel, Benjamin Víšek, Jan Válka, Martin Čerňan, Pavel Jindra, Jan Novák, Lukáš Stejskal, Flóra Kovácsová, Tomáš Kabut, Tomáš Szotkowski, Roman Hájek, Pavel Žák, Petr Cetkovský, Zdeněk Král – contributed to study conception and design; data collection; comments on previous manuscript versions; reviewed and approved the final manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/hon.2974.
Supporting information
Supplementary material 1
ACKNOWLEDGMENTS
Supported by Czech Republic Ministry of Health ‐ conceptual development of research organization (FNBr, 65269705). This study was undertaken as part of our routine work.
Barbora Weinbergerová and Jiří Mayer authors contributed equally to this work.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Chen P, Nirula A, Heller B, et al. SARS‐CoV‐2 neutralizing antibody LY‐CoV555 in outpatients with Covid‐19. N Engl J Med. 2021;384(3):229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with Covid‐19. N Engl J Med. 2021;384(3):238‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganesh R, Pawlowski CF, O’Horo JC, et al. Intravenous bamlanivimab use associates with reduced hospitalization in high‐risk patients with mild to moderate COVID‐19. J Clin Invest. 2021;131(19):e151697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verderese JP, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID‐19: a real‐world experience. Clin Infect Dis. 2021:ciab579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136(25):2881‐2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pagano L, Salmanton‐García J, Marchesi F, et al. COVID‐19 infection in adult patients with hematological malignancies: a European hematology association survey (EPICOVIDEHA). J Hematol Oncol. 2021;14(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhand A, Lobo SA, Wolfe K, Feola N, Nabors C. Bamlanivimab for treatment of COVID‐19 in solid organ transplant recipients: early single‐center experience. Clin Transpl. 2021;35(4):e14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhand A, Lobo SA, Wolfe K, et al. Casirivimab‐imdevimab for treatment of COVID‐19 in solid organ transplant recipients: an early experience. Transplantation. 2021;105(7):e68‐e69. [DOI] [PubMed] [Google Scholar]
- 9. Puing AG, Ho S, Frankel P, et al. SARS‐CoV‐2 specific monoclonal antibody for the treatment of mild‐to‐moderate COVID‐19 in cancer patients: a single‐center experience. J Infect Dis. 2021:jiab406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saultier P, Ninove L, Szepetowski S, et al. Monoclonal antibodies for the treatment of COVID‐19 in a patient with high‐risk acute leukaemia. Br J Haematol. 2021;196. 10.1111/bjh.17756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020;383(18):1757‐1766. [DOI] [PubMed] [Google Scholar]
- 12. Chen RE, Winkler ES, Case JB, et al. In vivo monoclonal antibody efficacy against SARS‐CoV‐2 variant strains. Nature. 2021;596(7870):103‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Data Availability Statement
Research data are not shared.


