Abstract
The coagulation markers, pro‐inflammatory cytokines (such as IL‐2R, IL‐6, IL‐10, and TNF‐a) and lymphopenia are associated with the severity of coronavirus disease 2019 (COVID‐19) disease. The use of anti‐inflammatory agents, such as corticosteroids (CS) or tosilizumab (TCZ), has been suggested for the treatment of advanced stage of COVID‐19 and the reduction of mechanical ventilators and mortality. The aim of this meta‐analysis is to determine the role of combination therapy with tocilizumab and steroids in COVID‐19 patients. Relevant studies were found using online international databases, and suitable studies were selected and assessed by two independent researchers. The quality of all papers was determined by a checklist. Heterogeneity assay among the primary studies was evaluated by Cochran's Q‐test and I2 index. The statistical analyses were done using the Stata ver. 14 package (StataCorp) software. Publication bias was estimated through Egger's test, and the impact of each study on the overall estimate was assessed by sensitivity analysis. Five studies were entered into this meta‐analysis. The results of these studies showed that the risk of death for COVID‐19 patients treated with the combination of corticosteroids and tocilizumab compared to tocilizumab and the control group was 0.74 (95% confidence interval [CI]: 0.36–1.50) and 0.48 (95% CI: 0.31–0.74), respectively. This meta‐analysis showed that the risk of death in COVID‐19 patients who were treated with corticosteroids and tocilizumab was lower than the tocilizumab alone and control groups (26% and 52%, respectively).
Keywords: COVID‐19, steroid, therapy, tocilizumab
Highlights
The use of anti‐inflammatory agents, such as corticosteroids (CS) and tosilizumab (TCZ), might be useful for the treatment of advanced stage of COVID‐19. The aim of our study was to determine the role of combination therapy with corticosteroids (CS) and tosilizumab (TCZ). This study showed that the risk of death in COVID‐19 patients who were treated with corticosteroids and tosilizumab was lower than the tosilizumab alone.
1. INTRODUCTION
The novel coronavirus (2019‐nCoV, or COVID‐19) was reported in China's Wuhan city, in December 2019 and spread rapidly as a global health problem. Several syndromes with clinical manifestations are associated with this infection, such as pneumonia, respiratory failure, and death. 1
Generally, most patients have reported mild illness, and approximately 10% of them require the intensive care unit (ICU) due to pneumonia and acute respiratory distress syndrome (ARDS). It is reported that the clinical course of COVID‐19 is characterized by three different stages. 2 In the early stage, viral replication can cause flu‐like symptoms. The second stage is usually accompanied by high fever, () symptoms of pneumonia, and a decreasing () viremia. Most patients show the third and super‐inflammatory phase. 3 , 4 In this stage, it is observed that the levels of markers of inflammation and coagulation (including reactive protein C/CRP, ferritin, and d‐dimer) and pro‐inflammatory cytokines (such as IL‐2R, IL‐6, and TNF‐a) increase. Patients also exhibit lymphopenia. These factors are associated with the severity of COVID‐19 disease. 5 , 6 In addition, Adult Respiratory Distress Syndrome (ARDS) is considered as an acute syndrome of lung inflammation and is the most common cause of death in this group of patients. 7 , 8
Due to the inflammatory status in COVID‐19, steroids have been used as immunomodulatory agents. 9 Recently, several studies have been done on the clinical benefits of steroids and corticosteroids and interferon inhibitors in COVID‐19 patients. 10 In addition regarding viral infection, there are some guidelines that have been suggested for the use of glucocorticoids and other immune‐regulating agents. 11
It is suggested that patients undergoing corticosteroid therapy as well as patients treated with interleukin appear to be better inhibitors than antiviral drugs and antibiotics. 12 In addition, treatment based on combination of anti‐inflammatories (corticoid therapy) and anticoagulants leads to less ventilation, hospitalization, and finally lower morbidity and mortality. 13 It is suggested that the use of anti‐inflammatory agents, such as corticosteroids (CS) or tosilizumab (TCZ), might be useful for the treatment of advanced stage of COVID‐19 to reduce the necessity of mechanical ventilators and lower the risk of mortality. 14 Mechanism of corticosteroid is shown in Figure 1.
Figure 1.
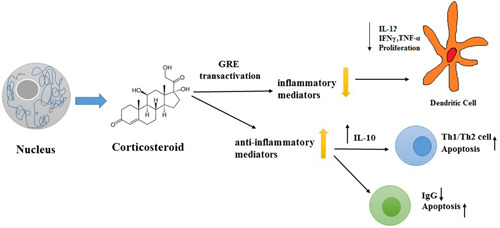
Mechanism of corticosteroid
According to the results of some studies, treatment of COVID‐19 patients with corticosteroids alone or tosilizumab might lead to increased survival. It seems that combination therapy of tosilizumab and corticosteroids can be used to modulating inflammation of lung injury. 15 Therefore, the aim of this meta‐analysis study is to determine the role of combination therapy with tosilizumab and corticosteroids in COVID‐19 patients.
2. MATERIAL AND METHODS
2.1. Search strategy
In this study, the published articles were collected from databases such as Science direct, Pub med, Web of Science, Scopus, Cochrane, and Google scholar between January 2019 and April 2021. The search strategy was performed using English keywords as well as a combination of important, and sensitive words. Search was done using the keywords “Tosilizumab,” “Clinical trial,” “COVID‐19,” “RR,” “SARS‐COV‐2,” “Risk Ratio,” “Steroids,” “Corticosteroids,” “Methylprednisolone” by adding AND, OR in the title and abstract. In addition, the reference of articles was examined for the increase of search sensitivity. Finally, all references are entered into reference management software (EndNote).
2.2. Inclusion criteria
In this study, the search strategy tool (PICO) aimed to determine the impact of patient (P), intervention (I), comparison (C), and outcome (O). “P” signifies COVID‐19 patients in studies; “I” means intervention with tosilizumab and steroids. “C” includes three groups of patients: (1) COVID‐19 patients who were treated with tosilizumab and steroids. (2) COVID‐19 patients who did receive tosilizumab alone 2: COVID‐19 patients who did receive standard of care treatment (control). “O” means the effectiveness of the tosilizumab and steroids in the case group.
All clinical trial and cohort studies were entered into this meta‐analysis for evaluating the efficacy of tosilizumab and steroids in the treatment of COVID‐19.
2.3. Exclusion criteria
1‐ Those studies that scored less than 5 based on the quality assessment checklist. 2‐ Studies that treated patients with other antivirals 5. Case reports or case series studies. Five studies in non‐English languages.
2.4. Selection studies
In this study, the full text or summary of articles, documents, and reports were screened and extracted. First, the irrelevant articles were removed from this study. Then, by reviewing and studying, the case reports and review studies were omitted. Finally, according to inclusion and exclusion criteria, data were extracted from full‐text articles.
2.5. Extracting the data
Screening and data extraction were performed by two independent reviewers based on article title, first author's name, year of study, journal name, place of study, type of study, the total number of patients in TCZ +/ CS group, number of recovered patients in TCZ +/ CS group, number of deaths in TCZ +/ CS group, the total number of patients in the control group, number of recovered patients in the control group, and number of deaths in the control group were entered into the excel.
2.6. Evaluation of quality
According to this point that all the prospective cohort studies entered into a meta‐analysis, the Newcastle–Ottawa scale (NOS) checklist 16 was used for the evaluation of the quality of articles. In this checklist, nine questions were presented that cover the type of study, sample size, study objectives, study population, sampling method, data analysis method, presentation of findings in an appropriate way, and presentation of results based on objectives. Each question was assigned a score. If a study scored at least 5 points, it was included in this study.
2.7. Analysis
Stata ver. 14 package (Stata Corp) was used to analyze the obtained data. Data were weighted and combined based on the inverse variance. Cochran (Q) and I 2 tests were used to determine the heterogeneity index between studies. Based on the heterogeneity results, a random effect model was used to estimate the risk of death in different types of treatment groups. Publication bias was estimated through Egger's test and the impact of each study on the overall estimate was assessed by sensitivity analysis.
3. RESULT
In this study, 1446 articles were found by using different databases. After screening and removing duplicate records, the remaining 331 articles were evaluated for eligibility. Finally, by removing the unrelated records, five studies were included in our meta‐analysis (Figure 2). In addition, according to the NOS checklist, the quality score of the studies was 5 or higher.
Figure 2.
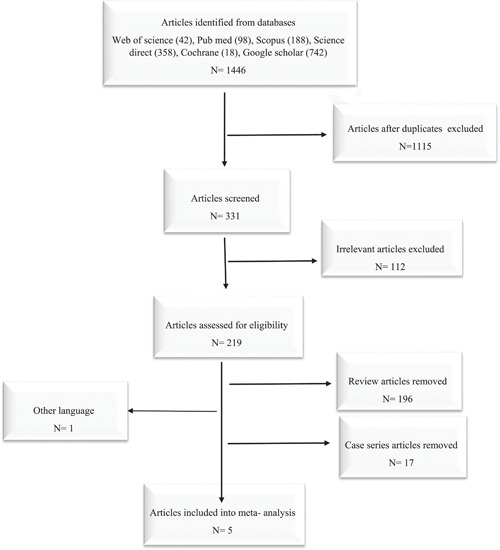
Flowchart of primary studies included in to meta‐analysis
3.1. Corticosteroids and tocilizumab versus tocilizumab
In five studies, COVID‐19 patients treated with corticosteroids and tocilizumab were compared with patients who received tocilizumab alone. 15 , 17 , 18 , 19 , 20 In these five studies, 460 patients received corticosteroids and tocilizumab and 303 patients treated with tocilizumab alone (Table 1). In four studies, 15 , 18 , 19 , 20 the risk of death was lower in the corticosteroids and tocilizumab group than those treated with tocilizumab alone, and the differences in two of these four studies were significant. 15 , 20 . In one study, 17 the risk of death was significantly higher in patients treated with corticosteroids and tocilizumab than the tocilizumab alone group.
Table 1.
Primary studies included in a meta‐analysis (corticosteroids and tocilizumab vs. tocilizumab)
| References | Control number of deaths in group (TCZ) | Control number of patients (TCZ) | Case number of deaths in group (TCZ +/ CS) | Case number of patients (TCZ +/ CS) | Type of study | Area of study | First authors, publication years |
|---|---|---|---|---|---|---|---|
| 15 | 7 | 21 | 23 | 78 | Cohort | Spain | Van den Eynde (2021) |
| 17 | 2 | 88 | 19 | 151 | Cohort | Spain | Rodríguez‐Bano (2021) |
| 18 | 32 | 149 | 13 | 119 | Cohort | Spain | Ruiz‐Antorán (2021) |
| 19 | 4 | 29 | 5 | 56 | Cohort | Italy | Mikulska (2020) |
| 20 | 10 | 16 | 11 | 56 | Observational | Spain | Sanz Herrero (2021) |
| 55 | 303 | 71 | 460 | Total |
Abbreviations: CS, corticosteroids; TCZ, tocilizumab.
Combining the results of these 5 studies using the random‐effects model (I‐square: 72.5%; Q: 14.52; p = 0.006) and inverse variance, the risk of death in the group of corticosteroids and tocilizumab 0.74 (95% CI: 0.36–1.50) was similar to the tocilizumab alone group (Figure 3). Egger test regression also indicated that there was no significant dispersion bias (β = 3.88, p = 0.187).
Figure 3.
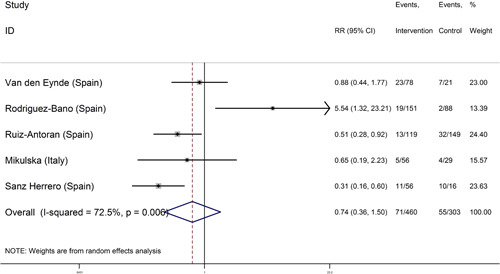
Forest plot of the risk of death in coronavirus disease 2019 (COVID‐19) patients treated with corticosteroids and tocilizumab versus tocilizumab in initial studies and the overall estimate
The results of sensitivity analysis for evaluation of the effect of each initial studies on the overall efficacy of corticosteroids and tocilizumab versus tocilizumab alone showed that only the Rodriguez–Bano study had a significant effect on the overall estimate. After omission of this study, the risk of death in the corticosteroids and tocilizumab group was similar to the tocilizumab alone group 0.53 (95% CI: 0.33–0.83) (Table 2).
Table 2.
Sensitivity analysis for evaluation of the effect of the initial studies on the overall performance estimation of corticosteroids and tocilizumab versus tocilizumab treatment group
| Study omitted | Estimate | [95% Conf. interval] |
|---|---|---|
| Van den Eynde (Spain) | 72801846 | 0.29042196–1.8249683 |
| Rodriguez‐Bano (Spain) | 52766854 | 0.33329785–0.83539116 |
| Ruiz‐Antoran (Spain) | 87784004 | 0.32174429–2.3950796 |
| Mikulska (Italy) | 0.77413857 | 0.33369735–1.7959104 |
| Sanz Herrero (Spain) | 0.95582867 | 0.4291811–2.1287246 |
| Combined | 0.73691619 | 0.36249574–1.4980741 |
3.2. Corticosteroids and tocilizumab versus routine treatment (not treatment with corticosteroids or tocilizumab)
In five studies. 15 , 17 , 18 , 19 , 20 , 21 the corticosteroids and tocilizumab combination‐treated COVID‐19 patients were compared with the control group (received routine treatment) of COVID‐19 patients. The number of samples was 567 in the corticosteroids and tocilizumab group and 890 in the control group (Table 3). In four studies, 15 , 18 , 19 , 20 , 21 the risk of mortality in COVID‐19 patients who received corticosteroids and tocilizumab was significantly lower than the control group. In addition, in Rodríguez–Baño J study, there were no statistically significant differences between the two groups for death (combination of corticosteroids and tocilizumab compared to the control group). 17
Table 3.
Primary studies included in a meta‐analysis (corticosteroids and tocilizumab vs. control)
| Reference | Number of deaths in control group | Number of patients in control group | Case number of deaths in group (TCZ +/ CS) | Case number of patients (TCZ +/ CS) | Type of study | Area of study | First authors, publication years |
|---|---|---|---|---|---|---|---|
| 15 | 69 | 118 | 23 | 78 | Cohort | Spain | Van den Eynde (2021) |
| 17 | 41 | 344 | 19 | 151 | Cohort | Spain | Rodríguez‐Bano (2021) |
| 18 | 40 | 151 | 13 | 119 | Cohort | Spain | Ruiz‐Antorán (2021) |
| 19 | 23 | 66 | 5 | 56 | Cohort | Italy | Mikulska (2020) |
| 12 | 51 | 211 | 13 | 163 | Cohort | Spain | Buzon‐Martin (2021) |
| 224 | 890 | 73 | 567 | Total |
Combining the results of these 5 studies using the random‐effects model (I‐square: 12.83%; Q: 14.52; p = 0.012) and inverse variance, the risk of death in the group of corticosteroids and tocilizumab 0.48 (95% CI: 0.31–0.74) was similar to the control group (Figure 4). Egger test regression also indicated that there was no significant dispersion bias (β = −2.58, p = 0.479). Sensitivity analysis was performed for evaluation of the effect of each study on the overall estimation of the efficacy of corticosteroids and tocilizumab compared to the control group. It is shown that none of the studies had a significant effect on the overall estimation (Table 4).
Figure 4.
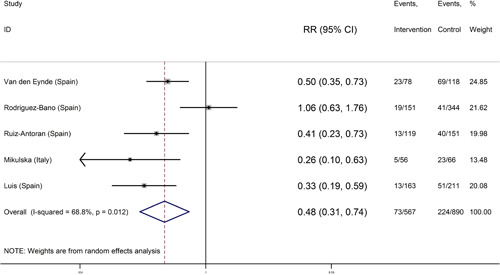
Forest plot of the risk of death in coronavirus disease 2019 (COVID‐19) patients treated with corticosteroids and tocilizumab versus control (routine treatment) in initial studies and the overall estimate
Table 4.
Sensitivity analysis for evaluation of the effect of initial studies on the overall performance estimation of corticosteroids and tocilizumab versus control group
| Study omitted | Estimate | [95% Conf. interval] |
|---|---|---|
| Van den Eynde (Spain) | 0.4577536 | 0.24162339–0.86721057 |
| Rodriguez‐Bano (Spain) | 0.41704786 | 0.32034466–0.54294306 |
| Ruiz‐Antoran (Spain) | 0.48821339 | 0.27956277–0.85258967 |
| Mikulska (Italy) | 0.52491945 | 0.3288298–0.83794242 |
| Buzon‐Martin (Spain) | 0.5208813 | 0.31243503–0.86839598 |
| Combined | 0.47637601 | 0.30570779–0.74232358 |
4. DISCUSSION
The aim of this meta‐analysis study was to evaluate the efficacy of corticosteroids and tocilizumab on the risk of death in COVID‐19 patients. The results showed that the risk of mortality in patients who were treated with corticosteroids and tocilizumab was lower than (26%) in patients treated with tocilizumab alone and control groups. There was no statistically significant difference. By omitting the Rodriguez–Bano, study the risk of death in patients treated with corticosteroids and tocilizumab was significantly lower than (47%) in patients treated with tocilizumab alone. In addition, the risk of mortality in patients who were treated with corticosteroids and tocilizumab was significantly lower than (52%) the control group (the group that received routine treatment).
At the beginning of the epidemic, corticosteroid therapy was one of the most controversial drugs against COVID‐19. 21 In general, steroids, including corticosteroids and glucocorticoids (dexamethasone and methylprednisolone), are used as immunosuppressive therapies in COVID‐19 patients who suffer from severe inflammation by high levels of the pro‐inflammatory cytokines IL‐6 and IL‐1 and play an important role in reducing mortality and the need for, mechanical ventilation. Steroid treatment also increased COVID‐19 patient survival. 22 , 23
Tocilizumab, a monoclonal antibody against IL‐6, is one of the drugs that was recommended at the beginning of the pandemic in China and Italy to treat patients exhibiting, severe inflammation and elevated ferritin and IL‐6 levels (PaO2/FiO2\150). 24 Observational studies in Spain, Italy, and the United States show that tocilizumab reduces ICU admittances, the need for mechanical ventilation, and mortality in patients when compared with those receiving routine treatment. 14 In addition, another study in Italy suggested that the rate of mortality in the tocilizumab group (179 patients) was significantly lower than the control group (365 patients). 25
Another finding reported that the use of corticosteroids or tocilizumab has been widely used in COVID‐19 patients with severe pneumonia. 26 Some studies show that treatment with the combination of corticosteroids and tocilizumab reduces in‐hospital mortality. Also, there was no intubation observed during 48 h. 15 , 27 In addition, in a study conducted in Italy, the use of these two drugs was investigated either separately or in combination to treat COVID‐19 patients. It is found that administration of tocilizumab and steroids led to improve outcomes in COVID‐19 patients. 28 A cohort study by Narain et al. shows that the combination of corticosteroids and tocilizumab for the treatment of patients increase patient survival when compared with the control group or the group receiving corticosteroids alone. 29 Another observational study showed that combination treatment with steroids and tocilizumab had better results than when steroids were added at a later stage. 30 On the other hand Rodríguez–Bano showed that mortality rates were higher in patients receiving a combination of corticosteroids and tocilizumab compared to those that received only tocilizumab. This could be due to different times and different doses of drugs. In current study, high doses of corticosteroids were given to patients with hyperinflammatory. 16
The results of the present studies indicate that combination of corticosteroids and tocilizumab is safe and effective in the treatment of COVID‐19 patients. The effect of this anti‐inflammatory compound treatment requires further RCT studies.
5. CONCLUSION
This meta‐analysis showed that the risk of death in COVID‐19 patients who were treated with corticosteroids and tocilizumab was lower than patients treated with tocilizumab alone and control group (26% and 52%, respectively).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Tahoora Mousavi conducted the literature search and data extraction. Mahmood Moosazadeh performed the statistical analysis and drafted the manuscript. Mahmood Moosazadeh and Tahoora Mousavi revised the final manuscript. All authors reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENT
The authors would like to thank Mazandaran University of Medical Sciences for financial support (IR.MAZUMS.REC.1400.9213).
Moosazadeh M, Mousavi T. Combination therapy of tocilizumab and steroid for COVID‐19 patients: A meta‐analysis. J Med Virol. 2022;94:1350‐1356. 10.1002/jmv.27489
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gou FX, Zhang XS, Yao JX, et al. Epidemiological characteristics of COVID‐19 in Gansu province. Zhonghua liu Xing Bing Xue Za Zhi. 2020;41:E032. [DOI] [PubMed] [Google Scholar]
- 3. Huang X, Wei F, Hu L, Wen L, Chen K. Epidemiology and clinical characteristics of COVID‐19. Arch Iran Med. 2020;23(4):268‐71. [DOI] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. The lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golpour M, Mousavi T, Alimohammadi M, et al. The effectiveness of Colchicine as an anti‐inflammatory drug in the treatment of coronavirus disease 2019: Meta‐analysis. Int J Immunopathol Pharmacol. 2021;3520587384211031763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khalafallah A, Maiwald M, Cox A, et al. Effect of immunoglobulin therapy on the rate of infections in multiple myeloma patients undergoing autologous stem cell transplantation or treated with immunomodulatory agents. Mediterr J Hematol Infect Dis. 2010;2(1):2010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the Cytokine Storm'in COVID‐19. J Infect. 2020;80(6):607‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID‐19). Intensive Care Med. 2020;46(5):854‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buzon‐Martin L, Miguel M‐B, Pedro D‐L, et al. Benefits of early aggressive immunomodulatory therapy (tocilizumab and methylprednisolone) in COVID‐19: single center cohort study of 685 patients. J Transl Autoimmun. 2021:100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;323(18):1824‐1836. [DOI] [PubMed] [Google Scholar]
- 14. Rubio‐Rivas M, Ronda M, Padulles A, et al. Beneficial effect of corticosteroids in preventing mortality in patients receiving tocilizumab to treat severe COVID‐19 illness. Int J Infect Dis. 2020;101:290‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van den Eynde E, Gasch O, Oliva J, et al. Corticosteroids and tocilizumab reduce in‐hospital mortality in severe COVID‐19 pneumonia: a retrospective study in a Spanish hospital. Infect Dis. 2021:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 17. Rodríguez‐Baño J, Pachón J, Carratalà J, et al. Treatment with tocilizumab or corticosteroids for COVID‐19 patients with hyperinflammatory state: a multicentre cohort study (SAM‐COVID‐19). Clin Microbiol Infect. 2021;27(2):244‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruiz‐Antorán B, Sancho‐López A, Torres F, et al. Combination of tocilizumab and steroids to improve mortality in patients with severe COVID‐19 infection: a Spanish, multicenter, cohort study. Infect Dis Ther. 2021;10(1):347‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mikulska M, Nicolini LA, Signori A, et al. Tocilizumab and steroid treatment in patients with COVID‐19 pneumonia. PLoS One. 2020;15(8):e0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanz Herrero F, Puchades Gimeno F, Ortega García P, Ferrer Gómez C, Ocete Mochón MD, García Deltoro M. Methylprednisolone added to tocilizumab reduces mortality in SARS‐CoV‐2 pneumonia: an observational study. J Intern Med. 2021;289(2):259‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buzon‐Martin L, Miguel M‐B, Pedro D‐L, et al. Benefits of early aggressive immunomodulatory therapy (tocilizumab and methylprednisolone) in COVID‐19: single center cohort study of 685 patients. Journal of translational autoimmunity. 2021;4:100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Group TRC. Dexamethasone in hospitalized patients with Covid‐19—preliminary report. N Engl J Med. 2020;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veronese N, Demurtas J, Yang L, et al. Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front Med. 2020;7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970‐10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guaraldi G, Meschiari M, Cozzi‐Lepri A, et al. Tocilizumab in patients with severe COVID‐19: a retrospective cohort study. The Lancet Rheumatology. 2020;2(8):e474‐e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van den Eynde E, Gasch O, Oliva JC, et al. Corticosteroids and tocilizumab reduce in‐hospital mortality in severe COVID‐19 pneumonia: a retrospective study in a Spanish hospital. Infect Dis. 2021;53(4):291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sterne J, Murthy S, Diaz JV, et al, WHO Rapid Evidence Appraisal for COVID‐Therapies (REACT) Working Group . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324(13):1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mikulska M, Nicolini LA, Signori A, et al. Tocilizumab and steroid treatment in patients with COVID‐19 pneumonia. PLoS One. 2020;15(8):e0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Narain S, Stefanov DG, Chau AS, et al. Comparative survival analysis of immunomodulatory therapy for coronavirus disease 2019 cytokine storm. Chest. 2021;159(3):933‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. López‐Medrano F, Pérez‐Jacoiste Asín MA, Fernández‐Ruiz M, et al. Combination therapy with tocilizumab and corticosteroids for aged patients with severe COVID‐19 pneumonia: a single‐center retrospective study. Int J Infect Dis. 2021;105:487‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


