Abstract
It is believed that the subtle equilibrium between tolerance and immunity during the unique biological state of pregnancy, which is characterized by further physiological and hormonal changes, rends pregnant women more vulnerable to coronavirus disease 2019 (COVID‐19). In this retrospective study, confirmed COVID‐19‐positive pregnant women (n = 15) during their third trimester, comprising asymptomatic (n = 7) and mild symptomatic (n = 8), and healthy pregnant controls (n = 20), were enrolled between June 1, 2020 and June 1, 2021 from the Hospital CHR Metz‐Thionville in Metz, France. Vitamin D concentrations, C‐reactive protein (CRP), and oxidative stress markers including superoxide dismutase (SOD), catalase (CAT), reduced (GSH) and oxidized (GSSG) glutathione levels, hydrogen peroxide (H2O2), and the total antioxidant capacity, measured the ferric reducing ability of plasma (FRAP), were evaluated in the serum of patients and controls. Results showed that all pregnant women (patients and controls) enrolled in this study were vitamin D deficient (<20 ng/ml). However, mild COVID‐19 pregnant women were severely vitamin D deficient (<12 ng/ml), which may suggest a link between vitamin D deficiency and the symptomatology of COVID‐19 illness in singleton pregnancy. No differences between the levels of CRP and the majority of the studied oxidative stress markers in COVID‐19‐positive pregnant women (asymptomatic and/or mildly symptomatic patients) versus COVID‐19‐negative pregnant women were found, suggesting the absence or a low magnitude of oxidative stress in pregnant women with COVID‐19. This may also explain the absence of severe courses of COVID‐19 infection. More studies are warranted to investigate the role of vitamin D supplementation and antioxidant‐rich diets in the prevention against severe forms of COVID‐19 in pregnant women.
Keywords: COVID‐19, C‐reactive protein (CRP), oxidative stress, pregnancy, SARS‐CoV‐2, vitamin D
Highlights
-
•
Oxidative status, CRP, and vitamin D levels in pregnant women with and without COVID‐19 during the third trimester.
-
•
Absence or a low level of oxidative stress in pregnant women with asymptomatic to mild symptomatic COVID‐19.
-
•
No differences between the levels of CRP in pregnant women with and without COVID‐19.
-
•
All pregnant women (patients and healthy controls) were vitamin D deficient.
-
•
Mild COVID‐19 pregnant women were severely vitamin D deficient.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a novel betacoronavirus, has been declared as a global pandemic by the World Health Organization (WHO) on March 11, 2020. 1 Until today, the ongoing COVID‐19 pandemic has affected >346 million people and killed >5.5 million people worldwide. It is thought that pregnant women constitute a vulnerable population to COVID‐19 infection, owing to a myriad of adaptive physiological modifications accompanying pregnancy, including a relative immune suppression, edema of respiratory tract mucosa, diaphragm elevation, and increased oxygen consumption. 2 , 3 , 4 Even if it is believed that immunosuppression is fundamental to a successful pregnancy (e.g., to render the mother immunologically tolerant to the fetus), evidence has shown that maternal immune response is rather highly dynamic, as the immunological stages change following the trimesters, for example, switching from an anti‐inflammatory status, a landmark of the second trimester, to a proinflammatory status during the third trimester that is necessary for labor and delivery (reviewed in Reference [5] ). Compared with nonpregnant women of reproductive age, a systematic review has shown that pregnant women present a significantly higher susceptibility to develop severe COVID‐19. 6 Other factors were also considered as playing a significant role in the severity of COVID‐19. This included higher BMI, increased maternal age, less access to health care, comorbidities such as preexisting diabetes and chronic hypertension, vitamin D and nutritional deficiencies, oxidative stress, hyperactive microglia, human genetic variation such as certain mutations in TYK2 and the genomic segment on chromosome 3 that has been inherited from Neanderthals. 6 , 7 , 8 , 9 , 10 For instance, in a large cohort retrospective study, it has been suggested that pregnant women with clinical comorbidities were more susceptible to COVID‐19 infection. 11 It has been further suggested that fetal sex influences maternal immune responses (e.g., maternal titers of IgG antibodies) to SARS‐CoV‐2 during pregnancy. 12 Recently, the capacity of SARS‐CoV‐2 to infect and propagate in the placenta, a fetal‐derived tissue, 12 has been demonstrated. 13 However, no association was found between COVID‐19 and miscarriage, spontaneous preterm delivery, intrauterine fetal SARS‐CoV‐2 infection, and the birth weight of newborns. 3 , 14 , 15 , 16 Nevertheless, in a prospective cohort study, although no SARS‐CoV‐2 vertical transmission to newborns occurred, authors have rather found a high association between COVID‐19 and preeclampsia, preterm delivery, and cesarean section compared with non‐COVID‐19 pregnant women. 4 Such adverse COVID‐19 outcomes including stillbirth were also highlighted in a large cohort study, 17 and it is thought that up to 40% of women with COVID‐19 are concerned by the above‐listed pregnancy complications. 13 Divergences in results between researchers on COVID‐19 outcomes in pregnant women could be explained by several factors. This included the trimester of pregnancy when infection occurred, the numerous forms of COVID‐19, including asymptomatic and mildly to critical illness, the presence of comorbidities such as obesity and diabetes, women's age, SARS‐CoV‐2 viral load, the differences in the control group used in the studies (e.g., non‐COVID‐19 pregnant women or non‐pregnant women), among others.
In this retrospective study, healthy control pregnant women and confirmed COVID‐19 patients without comorbidities including mildly symptomatic and asymptomatic patients were enrolled in their third trimester. We retrospectively evaluated the serum oxidative stress status of pregnant women with and without COVID‐19, their inflammatory status, and also their serum vitamin D levels. To the best of our knowledge, this is the first study that aimed to examine whether oxidative stress disturbances and vitamin D deficiency were associated with confirmed mild COVID‐19 and asymptomatic forms during the third trimester of singleton pregnancy.
2. MATERIALS AND METHODS
2.1. Study design
Twenty healthy women, seven asymptomatic and eight mild COVID‐19‐confirmed positive patients with a single pregnancy from the third trimester were retrospectively enrolled in this study. From June 1, 2020 to June 1, 2021, a total of 453 women have consulted or were being hospitalized in a gynecological service from two maternities (Maternity of Mercy—Metz and Maternity of Bel‐Air—Thionville) in the CHR Metz‐Thionville hospital located in Metz, France. Among this cohort, only 38 women were diagnosed with confirmed COVID‐19 infection, by nasopharyngeal swab and a real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) test. Among them, only COVID‐19‐positive pregnant women in the third trimester of pregnancy (i.e., ≥28 weeks of amenorrhea [WA]) were selected. Twelve COVID‐19‐positive pregnant women were excluded from this study, as serum samples were not collected within 15 days after RT‐PCR positivity. One patient was also excluded because of the insufficient volume of serum. Approximately 1 ml was necessary to carry out the different biochemical tests mentioned below. Thus, only 15 pregnant SARS‐CoV‐2‐infected women from the third trimester with a serum sample (~1 ml) collected within 15 days after the RT‐PCR positivity were included.
The average age of COVID‐19‐positive women (n = 15) included in this retrospective study was 30 years (range: 22−39), with a median gestational age of 37 WA. Among them, seven COVID‐19 pregnant women were diagnosed asymptomatic. Eight COVID‐19 pregnant women were symptomatic, suffering from mild symptomatic form (Table 1).
Table 1.
SARS‐CoV‐2‐infected pregnant women included in the study: age, date of positive RT‐PCR test, comorbidities, and type of COVID‐19
| Patient | Age (years) | RT‐PCR positivity date | Comorbidities | COVID‐19 illness | Symptoms |
|---|---|---|---|---|---|
| 1 | 38 | 34 WA | No | Asymptomatic | / |
| 2 | 33 | 41 WA | No | Asymptomatic | / |
| 3 | 30 | 39 WA | No | Asymptomatic | / |
| 4 | 26 | 36 WA | No | Asymptomatic | / |
| 5 | 31 | 37 WA | No | Asymptomatic | / |
| 6 | 29 | 38 WA | No | Asymptomatic | / |
| 7 | 28 | 39 WA | No | Asymptomatic | / |
| 8 | 27 | 36 WA | No | Mild | Anosmia + ageusia |
| 9 | 31 | 40 WA | No | Mild | Anosmia + ageusia |
| 10 | 25 | 40 WA | No | Mild | Cough + dyspnea |
| 11 | 34 | 35 WA | No | Mild | Dyspnea |
| 12 | 30 | 34 WA | No | Mild | Coughing |
| 13 | 28 | 41 WA | No | Mild | Anosmia + ageusia |
| 14 | 22 | 34 WA | Allergies | Mild | Headache |
| 15 | 39 | 34 WA | No | Mild | Oxygen requirement + dyspnea |
Note: Asymptomatic (n = 7); mild‐COVID‐19 patient (n = 8).
Abbreviations: RT‐PCR, reverse transcriptase‐polymerase chain reaction; WA, weeks of amenorrhea.
As for controls used in this study, 20 COVID‐19‐negative pregnant women were selected from the above cohort, with the following inclusion criteria: singleton pregnancy in the third trimester with a serum sample (~1 ml) collected within 15 days after the RT‐PCR negativity.
The average age of the controls was 31 years (range: 20−35), with a median gestational age of 37.5 WA.
Except for one patient with allergies, all pregnant women controls as well as patients were without any comorbidity.
In this retrospective study, analyzed serum samples were leftovers in CHR Metz‐Thionville hospital laboratories. They were processed in accordance with existing regulations and guidelines of the French Commission for Data Protection (Commission Nationale de l'Informatique et des Libertés). They were completely anonymous, and it was not possible to return to individual patient files. According to the French law, no informed consent is required for processing leftover samples.
2.2. Vitamin D concentrations
Serum 25‐hydroxyvitamin D (25(OH)D) concentrations (ng/ml) were analyzed by a chemiluminescent immunoenzymatic method (two‐step competitive binding), using an Automate UniCel DxI (Beckman Coulter A98856).
2.3. C‐reactive protein levels
C‐reactive protein (CRP) was used as a marker of inflammation. CRP concentrations (mg/L) were evaluated in serums by immunoturbidimetry using an Automate AU (Beckman Coulter A98856).
2.4. Measurement of oxidative stress markers
In this study, all oxidative stress markers were assessed in the serum of pregnant women (controls and patients) by colorimetric assays, using kits purchased from ThermoFisher Scientific according to the manufacturer's instructions. Concentrations were determined using a standard curve provided with each kit. Thus, the activity (U/ml) of superoxide dismutase (SOD) and catalase (CAT), two enzymatic antioxidants, was evaluated at 450 and 560 nm, using a Colorimetric Activity Kit (Reference EIASODC) and a Catalase Colorimetric Activity Kit (Reference EIACATC), respectively. In addition, glutathione levels (μM), including reduced (GSH) and oxidized (GSSG) forms, were evaluated using the Glutathione Colorimetric Detection Kit (Reference EIAGSHC) at 405 nm. The level (μM) of hydrogen peroxide (H2O2), a component of reactive oxygen species (ROS), was assessed at 620 nm, using the Pierce™ Quantitative Peroxide Assay Kit (aqueous) (Reference 23280). Moreover, the total antioxidant capacity (μM) was measured by the mean of the ferric reducing ability of plasma (FRAP) test, at 560 nm, using the Ferric Antioxidant Status Detection kit (Reference EIAFECL2). Colorimetric (OD) measurements were done by a BioRad PR41000 microplate reader.
2.5. Statistical analyses
All data were analyzed based on non‐parametric statistical tests. The Kruskal–Wallis test was used to compare between‐group variables, followed by the Wilcoxon−Mann−Whitney test that was used to compare patients (asymptomatics and/or symptomatics) to the controls. The data were reported as the median (interquartile range [IQR]). Significance was set at p ≤ 0.05. All of the statistical analyses were performed using R statistical software.
3. RESULTS
Serum vitamin D concentrations were significantly lower in mild COVID‐19 pregnant women (10.35 [8.27] ng/ml) compared with healthy pregnant controls (19.02 [8.35] ng/ml) (p < 0.05). However, no significant differences were found between asymptomatic COVID‐19 pregnant women (13.04 [10.74] ng/ml) and healthy pregnant controls (19.02 [8.35] ng/ml) (p > 0.05). Nevertheless, the difference between mild COVID‐19 patients and asymptomatic patients was found significant (p = 0.05). When all COVID‐19‐positive pregnant women (asymptomatic and symptomatic patients) (10.34 [12.30] ng/ml) were considered together, pooled results showed a significantly decreased concentration in COVID‐19‐positive patients compared with controls (p = 0.05) (Figure 1).
Figure 1 .
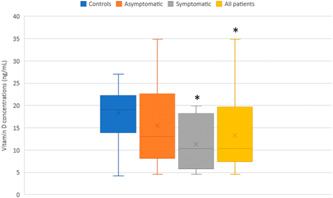
Serum vitamin D (25(OH)D) concentrations (ng/ml) in COVID‐19 pregnant women (n = 15) and non‐COVID pregnant women (n = 19) during the third trimester. *p ≤ 0.05 significantly different from the controls. # p ≤ 0.05 significantly different from asymptomatic COVID‐19 pregnant women. All COVID‐19 patients were constituted by asymptomatic (n = 7) and mild symptomatic (n = 8) patients. Data are shown as box plots, indicating the median and the 25th and 75th percentiles
As for CRP concentrations, no significant differences were found between asymptomatic and/or symptomatic pregnant women and healthy pregnant controls (all p > 0.05) (Figure 2).
Figure 2.
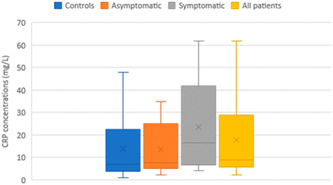
C‐reactive protein (CRP) levels (mg/L) in COVID‐19 pregnant women (n = 14) and non‐COVID pregnant women (n = 12). All COVID‐19 patients were constituted by asymptomatic (n = 6) and mild symptomatic (n = 8) patients. Data are shown as box plots, indicating the median and the 25th and 75th percentiles
Concerning all oxidative stress markers assessed in this study, no significant differences were found between either asymptomatic or symptomatic pregnant women and healthy pregnant controls (all p > 0.05). GSH level was found significantly higher in symptomatic patients compared with asymptomatic (p < 0.05). When all COVID‐19‐positive pregnant women (asymptomatic and symptomatic patients) were considered together, pooled results showed a significantly higher level of GSH and H2O2 in COVID‐19‐positive pregnant women compared with healthy pregnant women (both p < 0.05) (Figure 3).
Figure 3.
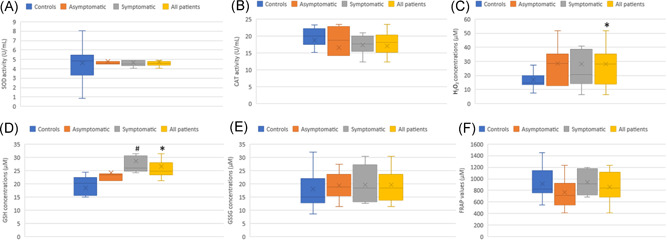
Oxidative stress markers in COVID‐19 pregnant women (n = 15) and non‐COVID pregnant women (n = 19−20) during the third trimester. (A) Superoxide dismutase (SOD) activity expressed in (U/ml). (B) Catalase (CAT) activity expressed in (U/ml). (C) Reduced glutathione levels (GSH) expressed in μM. (D) Oxidized glutathione levels (GSSG) expressed in μM. (E) Hydrogen peroxide (H2O2) concentrations expressed in μM. (F) FRAP values reflecting the total antioxidant capacity (μM) were expressed in μM. *p < 0.05 significantly different from the controls. # p < 0.05 significantly different from asymptomatic COVID‐19 pregnant women. All COVID‐19 patients were constituted by asymptomatic (n = 7) and mild symptomatic (n = 8) patients. Data are shown as box plots, indicating the median and the 25th and 75th percentiles. FRAP, ferric reducing ability of plasma
4. DISCUSSION
Pregnancy is regarded as a unique biological or immunological state that favors increased vulnerability to infections, including more severe COVID‐19. 12 , 18 Vitamin D plays an important role in the immune system and many physiological functions, acting as an anti‐inflammatory, antiviral, and antioxidant agent, among others. 10 , 18 Vitamin D deficiency constitutes a widespread health issue among pregnant women that may aggravate pregnancy outcomes, for example, by increasing the risk of gestational diabetes, pre‐eclampsia, and preterm birth. 19 , 20 , 21 Interestingly, a strong relationship was observed between vitamin D deficiency and both COVID‐19 positivity and COVID‐19 severity. 10 , 22 For example, in a retrospective cohort study enrolling 227 COVID‐19 patients, it was found that 94% had vitamin D deficiency, and it was estimated that severe vitamin D deficiency was 30% higher in COVID‐19 patients than in the general population. 10 In the present study, we have found a significantly lower level of vitamin D in mild COVID‐19 pregnant women compared with pregnant women without COVID‐19. Vitamin D insufficiency, deficiency, and severe deficiency are generally established when the serum 25(OH)D level is below 30 ng/ml, 20 ng/ml, and 12 ng/ml, respectively. 23 This suggests that mild COVID‐19 pregnant women (10.35 ng/ml) had a more severe deficiency compared with asymptomatic COVID‐19 pregnant women (13.04 ng/ml) and non‐COVID‐19 pregnant women (19.02 ng/ml) (Figure 1). When we consider all COVID‐19‐positive pregnant women together, pooled results further showed that SARS‐CoV‐2‐infected pregnant women were severely vitamin D‐deficient compared with uninfected pregnant women (10.34 vs. 19.02 ng/ml) (Figure 1). Our results are in keeping with previous data showing vitamin D deficiency in both pregnant women with and without COVID‐19 (12.46 ng/ml vs. 18.76 ng/ml, respectively). 19 Additionally, pregnant women with moderate/severe COVID‐19 presented severe vitamin D deficiency (9.06 ng/ml) in comparison with pregnant women with mild COVID‐19 (13.69 ng/ml). 19 In contrast, in another study, vitamin D levels were not associated with the severity of COVID‐19 during pregnancy, as similar levels of vitamin D deficiency were found in COVID‐19‐negative‐ and COVID‐19‐positive pregnant women. 24
CRP, an acute‐phase plasma protein synthesized in response to proinflammatory cytokines, constitutes a commonly used clinical biomarker to detect systemic inflammation, 25 , 26 and was found elevated in certain forms of COVID‐19 illness. 27 , 28 It has been argued that in SARS‐CoV‐2‐infected individuals, the hyper‐inflammatory state concerns only patients with moderate/severe to critical COVID‐19 infection but not asymptomatic ones and patients with mild COVID‐19. 27 , 28 In line with this, our results revealed no significant differences between COVID‐19‐positive pregnant women (asymptomatic and/or symptomatic patients) versus healthy pregnant women with regard to CRP values (Figure 2). Despite this observation, our results however showed that 92.8% of SARS‐CoV‐2‐infected pregnant women were diagnosed with inflammation (CRP ≥ 5 mg/L) in contrast to only 75% of control pregnant women. The magnitude of systemic proinflammatory responses to SARS‐CoV‐2 infection is strongly associated with sickness symptoms, and thus with the severity of COVID‐19. 27 , 28
It has been further postulated that oxidative stress, 29 which represents an imbalance of the oxidant/antioxidant equilibrium in favor of the harmful pro‐oxidant state, may play an important role in the cascade of inflammatory reactions during COVID‐19 infection, and in turn, in the severity of the illness. 30 , 31 , 32 , 33 , 34 Oxidative stress and inflammation have a bidirectional relationship, as each of these two harmful conditions can induce the other one, leading to a negative vicious circle. 35 , 36 , 37
To the best of our knowledge, our work constitutes the first study evaluating serum oxidative status of pregnant women with COVID‐19 infection versus healthy control pregnant women during the third trimester of pregnancy. Previously, placental oxidative stress in mothers with COVID‐19 illness has been found. 35 In the present study, we have evaluated oxidative stress markers including GSH, GSSG, CAT, SOD, H2O2, and total antioxidant capacity (FRAP measure) in pregnant women with or without SARS‐CoV‐2 infection (Figure 3). No significant differences were found between either mild COVID‐19 pregnant women or asymptomatic SARS‐CoV‐2‐infected pregnant women compared with healthy pregnant women, suggesting the absence of oxidative stress disturbances in COVID‐19 patients. However, when we consider all COVID‐19‐positive pregnant women together, among all oxidative stress markers, pooled results showed only significant increases of H2O2 production and GSH levels in SARS‐CoV‐2‐infected pregnant women compared with uninfected pregnant women, suggesting the absence or even a low magnitude of oxidative stress in asymptomatic to mild symptomatic COVID‐19 patients. It is important to highlight that our results showed no significant differences among pregnant women with regard to the activity levels of antioxidant‐related enzymes including SOD, which is involved in the detoxification of superoxide radicals (O2 .−), and CAT, which is implicated in the detoxification of H2O2, the major component of intracellular ROS in vivo. 29 The level of GSH, the most prevalent endogenous nonenzymatic antioxidant that is functioning as an important cellular redox buffer antioxidant, 29 even upregulated, it was not depleted, which suggests the absence of oxidative stress disturbances in asymptomatic and symptomatic COVID‐19 pregnant women. This assumption was also highlighted by nonsignificant differences with regard to the oxidized form of GSH (GSSG) among pregnant women groups (Figure 3). In addition, the total antioxidant capacity of serum nonenzymatic antioxidants, measured by FRAP test, showed no difference between pregnant women groups (Figure 3). FRAP measures reflect the contribution of both endogenous nonenzymatic antioxidants such as GSH and uric acid as well as exogenous antioxidants. 38 Among nutrients and non‐nutrients that may contribute to the ferric reducing ability (FRAP) of plasma/serum, we can list, vitamin C, vitamin E, polyphenols, and so forth. Overall, our results were in favor of the absence or even a low level of oxidative stress in COVID‐19‐positive pregnant women compared with healthy pregnant women.
5. STRENGTHS AND LIMITATIONS
The main strengths of the present study were its novelty, its focus on the third trimester of singleton pregnancy, and its design enrolling COVID‐19 patients without comorbidities and controls constituted by healthy pregnant women. However, the main limitation of this retrospective study is the small sample size. In addition, this study did not follow the course of COVID‐19 infection in pregnant patients.
6. CONCLUSION
This study highlighted vitamin D deficiency during the third trimester of pregnancy in both COVID‐19‐negative and COVID‐19‐positive pregnant women. It is important to note that Metz is a city located in the north‐east of France, and it is a less sunny region, which may explain, at least partially, vitamin D deficiency in pregnant women enrolled in this study. However, the deficiency was more severe in mild COVID‐19, which suggests, at least to some extent, a link between vitamin D deficiency and the symptomatology of COVID‐19 illness in pregnancy. Our study has further highlighted the absence or a low magnitude of oxidative stress in COVID‐19‐positive (asymptomatic and symptomatic) pregnant women compared with healthy pregnant controls. This could explain the absence of severe cases of COVID‐19 symptoms in the present study. More studies are necessary to investigate the role of vitamin D supplementation and antioxidant‐rich diet in the prevention against severe forms of COVID‐19 in pregnant women.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
In this retrospective study, analyzed serum samples were leftovers in CHR Metz‐Thionville hospital laboratories. They were processed in accordance with existing regulations and guidelines of the French Commission for Data Protection (Commission Nationale de l'Informatique et des Libertés). They were completely anonymous, and it was not possible to return to individual patient files. According to the French law, no informed consent is required for processing leftover samples.
AUTHOR CONTRIBUTIONS
Conceived the study: Catherine Delamare, Rachid Soulimani, and Jaouad Bouayed. Designed the study: Catherine Delamare and Jaouad Bouayed. Collected samples: Guillaume Schmitt and Catherine Delamare. Performed the experiments: Guillaume Schmitt and Sary Labdouni. Performed statistical analyses and prepared the figures and the table: Guillaume Schmitt. Interpreted data: Guillaume Schmitt, Catherine Delamare, and Jaouad Bouayed. Wrote the manuscript: Jaouad Bouayed. All authors have read and approved the final manuscript.
ACKNOWLEDGMENT
We acknowledge Prof. Torsten Bohn for corrections in the English language.
Schmitt G, Labdouni S, Soulimani R, Delamare C, Bouayed J. Oxidative stress status and vitamin D levels of asymptomatic to mild symptomatic COVID‐19 infections during the third trimester of pregnancy: a retrospective study in Metz, France. J Med Virol. 2022;94:2167‐2173. 10.1002/jmv.27606
DATA AVAILABILITY STATEMENT
The supporting data are available within the article.
REFERENCES
- 1. World Health Organization . WHO timeline—COVID‐19. April 27, 2020. https://www.who.int/news-room/detail/27-04-2020-who-timeline--covid-19
- 2. Cao D, Yin H, Chen J, et al. Clinical analysis of ten pregnant women with COVID‐19 in Wuhan, China: a retrospective study. Int J Infect Dis. 2020;95:294‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antoun L, Taweel NE, Ahmed I, Patni S, Honest H. Maternal COVID‐19 infection, clinical characteristics, pregnancy, and neonatal outcome: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;252:559‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469‐482. [DOI] [PubMed] [Google Scholar]
- 6. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouayed J, Bohn T. The link between microglia and the severity of COVID‐19: the ‘two‐hit’ hypothesis. J Med Virol. 2021;93(7):4111‐4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeberg H, Pääbo S. The major genetic risk factor for severe COVID‐19 is inherited from Neanderthals. Nature. 2020;587(7835):610‐612. [DOI] [PubMed] [Google Scholar]
- 9. Asgari S, Pousaz LA. Human genetic variants identified that affect COVID susceptibility and severity. Nature. 2021;600:390‐391. 10.1038/d41586-021-01773-7 [DOI] [PubMed] [Google Scholar]
- 10. Demir M, Demir F, Aygun H. Vitamin D deficiency is associated with COVID‐19 positivity and severity of the disease. J Med Virol. 2021;93:2992‐2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta P, Kumar S, Sharma SS. SARS‐CoV‐2 prevalence and maternal‐perinatal outcomes among pregnant women admitted for delivery: experience from COVID‐19‐dedicated maternity hospital in Jammu, Jammu and Kashmir (India). J Med Virol. 2021;93(9):5505‐5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ovies C, Semmes EC, Coyne CB. Pregnancy influences immune responses to SARS‐CoV‐2. Sci Transl Med. 2021;27(13617):eabm2070. [DOI] [PubMed] [Google Scholar]
- 13. Fahmi A, Brügger M, Démoulins T, et al. SARS‐CoV‐2 can infect and propagate in human placenta explants. Cell Rep Med. 2021;2:100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223(1):111.e1‐111.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Melo GC, de Araújo KCGM. COVID‐19 infection in pregnant women, preterm delivery, birth weight, and vertical transmission: a systematic review and meta‐analysis. Cad Saude Publica. 2020;36(7):e00087320. [DOI] [PubMed] [Google Scholar]
- 16. Yang P, Wang X, Liu P, et al. Clinical characteristics and risk assessment of newborns born to mothers with COVID‐19. J Clin Virol. 2020;127:104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jering KS, Claggett BL, Cunningham JW, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID‐19. JAMA Intern Med. 2021;181:714‐717. 10.1001/jamainternmed.2020.9241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Getachew B, Tizabi Y. Vitamin D and COVID‐19: role of ACE2, age, gender, and ethnicity. J Med Virol. 2021;93(9):5285‐5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sinaci S, Ocal DF, Yucel, Yetiskin DF. Impact of vitamin D on the course of COVID‐19 during pregnancy: a case control study. J Steroid Biochem Mol Biol. 2021;213:105964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei SQ. Vitamin D and pregnancy outcomes. Curr Opin Obstet Gynecol. 2014;26(6):438‐447. [DOI] [PubMed] [Google Scholar]
- 21. Elsori DH, Hammoud MS. Vitamin D deficiency in mothers, neonates and children. J Steroid Biochem Mol Biol. 2018;175:195‐199. [DOI] [PubMed] [Google Scholar]
- 22. Petrelli F, Luciani A, Perego G, Dognini G, Colombelli PL, Ghidini A. Therapeutic and prognostic role of vitamin D for COVID‐19 infection: a systematic review and meta‐analysis of 43 observational studies. J Steroid Biochem Mol Biol. 2021;211(26):105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lips P, Cashman KD, Lamberg‐Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D defciency: a position statement of the European Calcifed Tissue Society. Eur J Endocrinol. 2019;180:23‐54. [DOI] [PubMed] [Google Scholar]
- 24. Tekin AB, Yassa M, Birol P, et al. Vitamin D status is not associated with clinical severity of COVID‐19 in pregnant women. Eur J Nutr. 2021;28:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bansal T, Pandey A, D D, Asthana AK. C‐reactive protein (CRP) and its association with periodontal disease: a brief review. J Clin Diagn Res. 2014;8(7):ZE21‐ZE24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iddir M, Brito A, Dingeo G, et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID‐19 crisis. Nutrients. 2020;12(6):1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gandhi RT, Lynch JB, del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020;383(18):1757‐1766. [DOI] [PubMed] [Google Scholar]
- 28. Bouayed J, Bohn T. Adapted sickness behavior–why it is not enough to limit the COVID‐19 spread? Brain Behav Immun. 2021;93:4‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bouayed J, Bohn T. Dietary derived antioxidants: implications on health. In: Bouayed J, Bohn T, eds. Nutrition, Well‐Being and Health. Intech; 2012:1‐22. [Google Scholar]
- 30. Derouiche S. Oxidative stress associated with SARS‐Cov‐2 (COVID‐19) increases the severity of the lung disease‐a systematic review. J Infect Dis Epidemiol. 2020;6:121. [Google Scholar]
- 31. Beltrán‐García J, Osca‐Verdegal R, Pallardó FV, et al. Oxidative stress and inflammation in COVID‐19‐associated sepsis: the potential role of anti‐oxidant therapy in avoiding disease progression. Antioxidants. 2020;9(10):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cecchini R, Cecchini AL. SARS‐CoV‐2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karkhanei B, Talebi Ghane E, Mehri F. Evaluation of oxidative stress level: total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID‐19. New Microbes New Infect. 2021;42:100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Menzel A, Samouda H, Dohet F, Loap S, Ellulu MS, Bohn T. Common and novel markers for measuring inflammation and oxidative stress ex vivo in research and clinical practice—which to use regarding disease outcomes? Antioxidants (Basel). 2021;10(3):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mandò C, Savasi VM, Anelli GM. Mitochondrial and oxidative unbalance in placentas from mothers with SARS‐CoV‐2 infection. Antioxidants. 2021;10(10):1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salem S, Leghouchi E, Soulimani R, Bouayed J. Reduction of paw edema and liver oxidative stress in carrageenan‐induced acute inflammation by Lobaria pulmonaria and Parmelia caperata, lichen species, in mice. Int J Vitam Nutr Res. 2021;91(1‐2):143‐151. [DOI] [PubMed] [Google Scholar]
- 37. Jha JC, Ho F, Dan C, Jandeleit‐Dahm K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin Sci. 2018;132(16):1811‐1836. [DOI] [PubMed] [Google Scholar]
- 38. Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. Reversal of oxidative stress‐induced anxiety by inhibition of phosphodiesterase‐2 in mice. J Pharmacol Exp Ther. 2008;326:369‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The supporting data are available within the article.


