Abbreviations
- CK
creatine kinase
- COVID‐19
coronavirus 2019
- IMNM
immune‐mediated necrotizing myopathy
- MRI
Magnetic resonance imaging
- RBD
receptor‐binding domain
- SRP
signal recognition particle
The BNT162b2 mRNA COVID‐19 vaccine (Pfizer, BioNTech) has proven to be safe with rare serious adverse events after vaccination, but there have been reports of postvaccination events, including myocarditis and immune‐mediated diseases, such as Guillain‐Barré syndrome. 1 We report a case of immune‐mediated necrotizing myopathy (IMNM) after BNT162b2 vaccination associated with antibodies against the receptor‐binding domain (RBD) of the severe acute respiratory system coronavirus‐2 (SARS‐CoV‐2) spike protein and autoantibodies to signal recognition particle (SRP).
A previously healthy 55‐year‐old woman received her first dose of BNT162b2 vaccine (Figure 1). The next day she developed myalgia, chills, fatigue, and vague generalized weakness. On day 21, after she received a second dose of BNT162b2 vaccine, the symptoms worsened to progressive weakness, and on day 49 she presented to an emergency department unable to walk. Her serum creatine kinase (CK) level was 7967 IU/L (normal, <190 IU/L). She was treated for rhabdomyolysis with intravenous fluids and discharged home, but she returned on day 56 with persistent symptoms.
FIGURE 1.
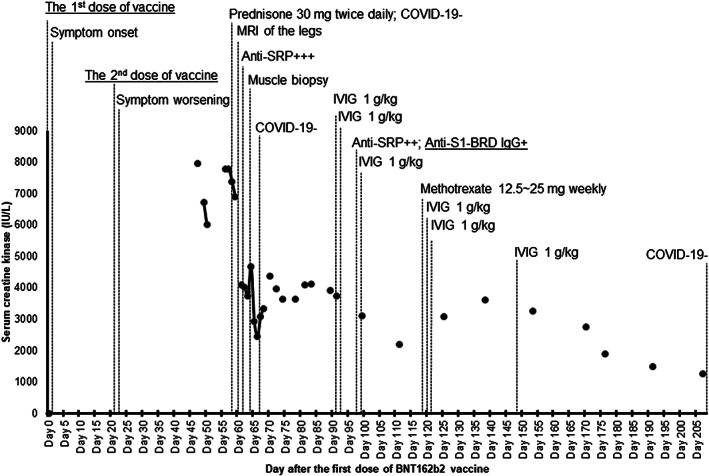
Disease course with associated creatine kinase changes and treatment. The first and second doses of vaccine were injected into the right deltoid muscle. Fatigue and weakness persisted for 3 weeks after the first vaccine dose. The patient had difficulty getting up from a chair and climbing stairs. Anti–signal recognition particle autoantibodies were identified by the immunoblot method on UROIMMUN's EUROBlotMaster. Despite treatment with intravenous immunoglobulin of 1 to 2 g/kg every 4 weeks, high‐dose prednisone, and methotrexate, follow‐up (6‐9 months) assessments demonstrated residual weakness graded 3 to 4/5 on the Medical Research Council scale bilaterally in the proximal muscles of the upper and lower extremities with persistently elevated creatine kinase levels
Neurological examination revealed normal cranial nerve functions. There was symmetrical weakness of the proximal upper and lower extremity muscles, graded 2 to 3/5 on the Medical Research Council (MRC) scale, with preserved strength in the distal muscles and normal sensation. Sensory and motor nerve conduction studies in the upper and lower extremities were normal bilaterally. Needle electromyography showed positive sharp waves, fibrillation potentials, and small‐amplitude motor unit potentials with early recruitment in the right deltoid, triceps, vastus lateralis, tibialis anterior, and gastrocnemius muscles. Magnetic resonance imaging on day 60 demonstrated increased signal intensity on short‐tau inversion recovery images of the thigh muscles, consistent with myositis (Figure 2A‐C).
FIGURE 2.
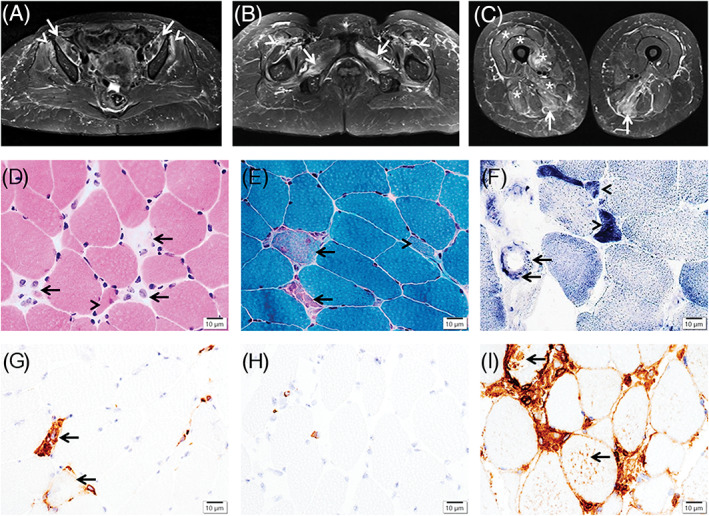
Magnetic resonance imaging of the thigh muscles and immune‐mediated necrotizing myopathy pathology. Axial short‐tau inversion recovery images demonstrate increased signal intensity in the bilateral iliopsoas (A) (arrows), gluteus minimus (A) (arrowheads), adductors and obturator externus (B) (arrows), and semimembranosus (C) (arrows) muscles, and mild intermuscular edema between the rectus femoris and vastus lateralis (B) (arrowheads), with milder involvement of the left vastus medialis, intermedius, and lateralis, as well as right adductor magnus and biceps femoris (C) (asterisk). The vastus lateralis muscle biopsy shows necrotic myofibers in (D) (hematoxylin‐eosin stain) and (E) (Gomori trichrome stain) (arrows), regenerating myofibers in (D‐F) (arrowheads), and capillary endothelial cells containing positive granules in (F) (succinate dehydrogenase stain) (arrows), scattered to aggregated CD68+ macrophages in (G) (arrows), rare CD8+ T cells in (H), and major histocompatibility complex‐1 expression extensively in the necrotic myofibers and focally in some non‐necrotic myofibers in (I) (arrows). Scale bars = 10 μm in (D‐I)
The antinuclear antibody test by indirect immunofluorescence antibody on day 47 was positive (≥1:640; normal, <1:80) with a cytoplasmic (reticular) pattern, suggestive of antibodies to cytoplasmic targets. 2 Anti‐SRP autoantibodies were identified (Figure 1). SARS‐CoV‐2 tests by reverse transcription‐polymerase chain reaction assay of nasopharyngeal swabs were negative on days 58, 67, and 208. On day 98, serum was tested for SARS‐CoV‐2 antibodies using a Food and Drug Administration early‐use‐authorized xMAP assay detecting immunoglobulin G (IgG) antibodies to nucleocapsid, S1, and RBD of S1 (anti–S1‐RBD; Luminex Corp, Austin, Texas), measured as median fluorescence intensity (MFI). The seronegative reference range for all three analytes was 0 to 700 MFI. The patient had high‐titer anti–S1‐RBD IgG antibody (MFI = 2604) but normal‐range antibodies to nucleocapsid (MFI = 109) and S1 (MFI = 294), consistent with a response to the mRNA vaccination.
On day 64, a muscle biopsy of the left vastus lateralis was examined. 3 The muscle showed myopathic changes, including necrotic myofibers; regenerating myofibers, scattered to focally aggregated CD68+ macrophages with rare CD4+ or CD8+ T cells; and focal major histocompatibility complex‐1 expression (Figure 2D‐I), consistent with IMNM. Electron microscopy revealed ultrastructural changes, including previously described mitochondrial abnormalities in IMNM. 4 , 5
Figure 1 summarizes the disease course with the major events, CK changes, and treatment after BNT162b2 vaccination.
Our case of IMNM suggests a probable association/interaction between the vaccine‐induced immune response and anti‐SRP–positive IMNM, given the temporal relationship between the disease course and BNT162b2 vaccination, presence of anti–S1‐RBD, and absence of SARS‐CoV‐2 infection in a patient with anti‐SRP autoantibodies. Anti‐SRP is known to be associated with IMNM, 5 and anti‐SRP autoantibody levels in IMNM can vary with CK levels and disease activities 6 ; in our patient, those autoantibodies are unlikely to have formed before the vaccination, because she was asymptomatic until the vaccination, although prevaccination serum was not available for testing.
The BNT162b2 vaccine, administered in two doses 21 days apart, typically produces high‐titer IgG anti‐RBD antibodies after the first dose, with a further increase after the second dose. 7 In our case, without previous SARS‐CoV‐2 infection, this immune response was evidenced by high‐titer anti–S1‐RBD autoantibodies potentially interacting with the anti‐RSP–associated immune process. The disease course in our case is comparable to that of some other neurological and/or immune‐mediated events seen after the mRNA vaccine. 1 However, billions of doses of COVID‐19 vaccine have been administered throughout the world, many of which are mRNA vaccines such as BNT162b2. Our report has described the experience of one individual among many worldwide who have received not only the BNT162b2 vaccine, but also other COVID‐19 vaccines. Further research is needed to understand the underlying mechanisms. Ongoing surveillance and evaluation of IMNM after COVID vaccination is warranted.
CONFLICT OF INTEREST
M.J.F. is medical director at MitogenDx. M.A.T. is the chief executive officer of the Exerkine Corp, a company currently working on therapies targeting mitochondrial dysfunction. The other authors have no conflicts of interest to declare.
ETHICAL PUBLICATION STATEMENT
We confirm we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The authors thank Katerina Gordon (McMaster University) for performing electron microscopy, and Haiyan Hou and Meifeng Zhang (University of Calgary) for technical assistance. We also thank the study patient, who provided informed consent for all aspects of testing and publication.
Contributor Information
Dubravka Dodig, Email: dubravka.dodig@uhn.ca.
Jian‐Qiang Lu, Email: luj85@mcmaster.ca.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID‐19 mRNA vaccination. JAMA. 2021;326:1390‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Mühlen CA, Garcia‐De La Torre I, Infantino M, et al. How to report the antinuclear antibodies (anti‐cell antibodies) test on HEp‐2 cells: guidelines from the ICAP initiative. Immunol Res. 2021;69(6):594‐608. [DOI] [PubMed] [Google Scholar]
- 3. Lu JQ, Monaco CMF, Hawke TJ, Yan C, Tarnopolsky MA. Increased intra‐mitochondrial lipofuscin aggregates with spherical dense body formation in mitochondrial myopathy. J Neurol Sci. 2020;413:116816. [DOI] [PubMed] [Google Scholar]
- 4. Matsubara S, Bokuda K, Asano Y, et al. Mitophagy in three cases of immune‐mediatednecrotizing myopathy associated with anti‐3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase autoantibodies: ultrastructural and immunohistochemical studies. Neuromuscul Disord. 2018;28:283‐288. [DOI] [PubMed] [Google Scholar]
- 5. Allenbach Y, Benveniste O, Stenzel W, Boyer O. Immune‐mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol. 2020;16:689‐701. [DOI] [PubMed] [Google Scholar]
- 6. Benveniste O, Drouot L, Jouen F, et al. Correlation of anti‐signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum. 2011;63:1961‐1971. [DOI] [PubMed] [Google Scholar]
- 7. Pratesi F, Caruso T, Testa D, et al. BNT162b2 mRNA SARS‐CoV‐2 vaccine elicits high avidity and neutralizing antibodies in healthcare workers. Vaccines (Basel). 2021;9:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


