Abstract
Myocarditis and pericarditis may constitute adverse reactions of mRNA coronavirus disease 2019 (COVID‐19) vaccines. This study aimed to document these reactions and to assess the association with patient sex and age. This is as an observational retrospective study using a case–non‐case design (also called disproportionality study) on inflammatory heart reactions reported with mRNA COVID‐19 vaccines within the World Health Organization (WHO) global safety database (VigiBase), up to June 30, 2021. Results are expressed using reporting odds ratios (RORs) and their 95% confidence interval (95% CI). Of 716,576 reports related to mRNA COVID‐19 vaccines, 2,277 were cases of inflammatory heart reactions, including 1241 (55%) myocarditis and 851 (37%) pericarditis. The main age group was 18–29 years (704, 31%), and mostly male patients (1,555, 68%). Pericarditis onset was delayed compared with myocarditis with a median time to onset of 8 (3–21) vs. 3 (2–6) days, respectively (P = 0.001). Regarding myocarditis, an important disproportionate reporting was observed in adolescents (ROR, 22.3, 95% CI 19.2–25.9) and in 18–29 years old (ROR, 6.6, 95% CI 5.9–7.5) compared with older patients, as well as in male patients (ROR, 9.4, 95% CI 8.3–10.6). Reporting rate of myocarditis was increased in young adults and adolescents. Inflammatory heart reactions may rarely occur shortly following mRNA COVID‐19 vaccination. Although an important disproportionate reporting of myocarditis was observed among adolescents and young adults, particularly in male patients, reporting rates support a very rare risk, that does not seem to compromise the largely positive benefit‐risk balance of these vaccines. Furthermore, this study confirmed the value of disproportionality analyses for estimation of relative risks among subgroups of patients.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Myocarditis and pericarditis cases following mRNA coronavirus disease 2019 (COVID‐19) vaccination have been reported and vaccine causality have been confirmed by the European Medicine Agency and the US Food and Drug Administration.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What are the demographic features of inflammatory heart reactions following mRNA COVID‐19 vaccination?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ In this observational retrospective pharmacovigilance study at a global level, an important disproportionate reporting of myocarditis following mRNA COVID‐19 vaccination was observed among adolescents and young adults, particularly in male patients. Reporting rate supports a very rare incidence, lower than reports of myocarditis related to severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) infection.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Individual risk benefit decision making may be required for the use of COVID‐19 vaccines.
Myocarditis and pericarditis are inflammatory heart conditions that occur in the setting of excessive host immune response to antigenic stimuli. 1 Viral myocarditis has been widely reported after severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) infection and subsequent development of coronavirus disease 2019 (COVID‐19). 2 , 3 In some instances, these inflammatory reactions have been associated with some drug exposure, including vaccines. 4 , 5 , 6 A descriptive analysis of drug‐associated myopericarditis spontaneous reports in the United States (1990–2018) identified that about 0.1% of the cases were related to vaccines. 7 Inflammatory heart reactions following vaccination have been mostly reported with live‐virus vaccines, such as the smallpox vaccine, 5 , 8 , 9 and inactivated influenza vaccines. 10 Myocarditis cases have been reported following mRNA COVID‐19 vaccination and early analyses suggested it may constitute a complication of these vaccines. 11 , 12 , 13
Vaccination is critical to contain the SARS‐CoV‐2 pandemic. The mRNA vaccines represent a new generation of vaccines that can be rapidly responsive to emerging viruses. 14 Pivotal phase III randomized clinical trials were conducted on 35,654 volunteers who were exposed to the active mRNA vaccines. 15 , 16 Up to July 2021, tozinameran (BNT162b2; Pfizer‐BioNTech) and elasomeran (mRNA 1273; Moderna) have been distributed in 104 and 49 countries worldwide, respectively, and more than 341 million doses have been administered in the United States. 17 Rare but severe adverse drug reactions have been highlighted in clinical trials and thereafter reported after mRNA COVID‐19 vaccination, such as anaphylaxis or Bell’s palsy. 18 , 19 , 20 In April and May 2021, a growing signal concerning inflammatory heart reactions has emerged in several regions of the world with early coverage in the press. 21 , 22 , 23 , 24 This risk appears to concern mainly mRNA vaccines and at a less extent other COVID‐19 vaccines, such as viral vector based‐vaccines. 25 A small case series in members of the US Military suggested an incidence below 1 over 100,000 doses administered. 26 Preliminary analysis by national authorities in Europe and in the United States led to confirm this safety signal and a probable causal relationship have been established. 24 , 27 , 28
In this study, we aimed to document the inflammatory heart reactions reported after mRNA COVID‐19 vaccination and to further assess the association with patient sex and age.
METHODS
Study design
This is a retrospective observational study of the inflammatory heart reactions associated with mRNA COVID‐19 vaccines registered in a global pharmacovigilance database.
Data source
VigiBase (https://www.who‐umc.org/vigibase/vigibase/) is the unique World Health Organization (WHO) global database of individual case safety reports. 29 This database is maintained, deduplicated, and deidentified by Uppsala Monitoring Centre (Uppsala, Sweden). VigiBase is the world’s largest pharmacovigilance database, with over 23 million reports of suspected adverse drug reactions gathered from national pharmacovigilance systems and continuously updated. More than 130 countries, over 5 continents submit spontaneous safety reports in this database. Information concerning the reporter, the patient, the suspected and concomitant drugs, and the suspected adverse drug reactions and their seriousness, are reported for each case. Reactions are coded using the hierarchical Medical Dictionary for Regulatory Activities (MedDRA; https://www.meddra.org/).
COVID‐19 Data Tracker (https://covid.cdc.gov/covid‐data‐tracker/) is the official dashboard of the US Center for Disease Control and Prevention, allowing assessment of the number of people vaccinated within the United States.
Ethics
This study has obtained ethics approval from the Cochin University Hospital institutional review board (number AAA‐2021‐08039) in conformity with the French laws and regulations. 30
Data extraction and analysis
Reports including an mRNA COVID‐19 vaccine as the suspected drug in the adverse reaction have been extracted. From that, cases of inflammatory heart reactions (i.e., myocarditis, myopericarditis, pericarditis, and pleuropericarditis), were retrieved using the ad hoc high‐level terms from MedDRA, “noninfectious myocarditis” and “noninfectious pericarditis,” coded as reactions. Cases included in this study were registered up to June 30, 2021, in VigiBase. For each report of interest, the following data were extracted or assessed: demographic features, continent of reporting, type of reporter, month of reporting, patient sex, patient age, time to reaction onset, seriousness, reaction outcome, and type of heart inflammatory reaction. Seriousness was defined, according to the WHO, as the occurrence of death, life‐threatening adverse event, inpatient hospitalization or prolongation of an existing hospitalization, significant disability, or requirement of intervention to prevent any of these. 31
Disproportionality analysis
Disproportionality analyses, also called case–non‐case studies, are similar to case–control studies but for the purpose of pharmacovigilance studies. These studies are nested in a database of spontaneous reports to assess possible disproportionality in reporting. Disproportionality analysis compares the proportion of a specific adverse drug reaction reported in a specific group (e.g., drug exposure, patient age, and patient sex) with the proportion of the same adverse drug reaction for a control group. 32 , 33 , 34 This statistical approach has shown its value to provide an estimation of relative risks of adverse drug reaction, that are correlated in most cases with results from meta‐analyses of clinical trials. 35
To assess an association between inflammatory heart reactions after mRNA COVID‐19 vaccines and patient age or sex, we performed a disproportionality analysis among different patient groups. This analysis was realized in the cohort of mRNA COVID‐19 vaccine reports. Characteristics of interest are patient sex and age groups, specially 12–17 years, 18–29 years, and over 30 years, as reported by safety signals of concern. Cases are reports concerning a specific patient group and that include the terms myocarditis, pericarditis, or pleuropericarditis as reactions after an mRNA COVID‐19 vaccine. Non‐cases are reports concerning a specific patient group that include all other reactions to an mRNA COVID‐19 vaccine. Therefore, our analyses assessed a potential difference of reporting of inflammatory heart reactions secondary to mRNA COVID‐19 vaccines in male compared with female patients and in 12–17 years or 18–29 years old patients compared with patients over 30 years old. Thereafter, sensitivity analyses were performed, restricted to serious reports only, and to reports originating from a healthcare professional.
Statistical analysis
Descriptive analysis of the cases was performed. Quantitative variables were expressed as median ± interquartile range (IQR) and compared using nonparametric analysis. Qualitative variables were expressed in number and percentages. Time to reaction onset was analyzed using a survival Mantel‐Cox model.
Disproportionality in reporting between groups is expressed using reporting odds ratio (ROR) and its 95% confidence interval (95% CI). ROR is a ratio similar in concept to the odds ratio in case–control studies and corresponds to the exposure odds among reported cases of interest over the exposure odds among reported non‐cases. ROR (95% CI) were calculated as , where a is the number of cases reported with mRNA COVID‐19 vaccines in a specific group (e.g., male patients), b is the number of non‐cases (i.e., all other adverse drug reactions) reported with mRNA COVID‐19 vaccines in a specific group, c is the number of cases reported with mRNA COVID‐19 vaccines in a control group (e.g., female patients), and d is the number of non‐cases reported with mRNA COVID‐19 vaccines in a control group.
Reporting rates and their 95% CI were estimated using the total count of vaccinated people in the United States that had a complete vaccination scheme (i.e., received a second dose of a two‐dose vaccine scheme or one dose of a single‐dose vaccine scheme).
RESULTS
Demographics and characteristics of cases
As of the end of June 2021, of a total of 26,258,646 reports registered in the worldwide spontaneous report database VigiBase, 716,576 reports were related to an mRNA COVID‐19 vaccine as a suspected drug, including 495,493 with tozinameran and 221,368 with elasomeran (including 285 reports with both vaccines). Among them, 2,277 cases of inflammatory heart reactions were retrieved, such as 1,241 (54.5%) myocarditis, 851 (37.4%) pericarditis, 167 (7.3%) myopericarditis, and 18 (0.8%) pleuropericarditis (Table 1 ). Cases were largely reported from North America (63.4%) and Europe (35.0%). Of the cases, 1,655 (72.7%) were reported with tozinameran and 622 (27.3%) with elasomeran. Patients were of the younger age bracket (median age 33, (21–54)) mostly from the group between 18 and 29 years old (704, 30.9%), more commonly male patients (1,555, 68.3%). Patient distribution was different according to age and sex (P < 0.0001) for both myocarditis and pericarditis cases (Figure 1 ). Specifically, most of myocarditis cases have been reported in 12–17 and 18–29 years old male patients. Overall, the median time to onset for these inflammatory heart reactions was 3 (2–14) days after vaccine injection. Onset of pericarditis was delayed compared with myocarditis with a median time to onset of 8 (3–21) vs. 3 (2–6) days, respectively (P = 0.001; Figure 2 ). Seventy‐three percent (764) of reported myocarditis cases occurred before day 6 vs. 42% (312) of reported pericarditis cases. Drug other than COVID‐19 mRNA vaccines were suspected in the cardiac reaction onset in 200 (8.7%) patients. Most of the myocarditis (1,015, 81.8%) and pericarditis (492, 57.8%) reported cases required hospitalization; 219 (21.5%) and 101 (20.5%) were life‐threatening, respectively. In 22 patients, the inflammatory heart reaction had a fatal outcome, corresponding to 15 (1.2%) myocarditis cases (median age 60 (56–78) years old), 5 pericarditis cases (median age 71 (67–77) years old), and 2 myopericarditis cases (age 55 and 83). Among these fatal cases, the vaccine was the only suspected drug in all except one case in which atezolizumab, an anti‐PDL1, was another suspected drug.
Table 1.
Characteristics of inflammatory heart reactions reported with mRNA COVID‐19 vaccines within the WHO global safety database
| Reporting characteristics | Tozinameran vaccine (n = 1,655) | Elasomeran vaccine (n = 622) | Overall (n = 2,277) |
|---|---|---|---|
| Continent of reporting | |||
| North America | 942 (56.9%) | 502 (80.7%) | 1444 (63.4%) |
| Europe | 676 (40.8%) | 120 (19.3%) | 796 (35.0%) |
| Oceania | 31 (1.9%) | – | 31 (1.4%) |
| Latin America | 5 (0.3%) | – | 5 (0.2%) |
| Africa | 1 (0.1%) | – | 1 (0.1%) |
| Type of reporter | |||
| Physician | 383 (23.1%) | 75 (12.1%) | 458 (20.1%) |
| Pharmacist | 35 (2.1%) | 9 (1.4%) | 44 (1.9%) |
| Other health professional | 46 (2.8%) | 2 (0.3%) | 48 (2.1%) |
| Consumer | 234 (14.1%) | 34 (5.5%) | 268 (11.8%) |
| Unknown | 957 (57.8%) | 502 (80.7%) | 1459 (64.1%) |
| Sex, male (%) | 1108 (66.9%) | 447 (71.9%) | 1555 (68.3%) |
| Age, years | 33 (19–55) | 31 (22–52) | 33 (21–54) |
| Age, ranges | |||
| 12–17 years | 291 (17.6%) | 3 (0.5%) | 294 (12.9%) |
| 18–29 years | 425 (25.7%) | 279 (44.9%) | 704 (30.9%) |
| 30–39 years | 234 (14.1%) | 103 (16.6%) | 337 (14.8%) |
| 40–49 years | 171 (10.3%) | 64 (10.3%) | 235 (10.3%) |
| 50–59 years | 173 (10.5%) | 50 (8.0%) | 223 (9.8%) |
| 60–64 years | 74 (4.5%) | 30 (4.8%) | 104 (4.6%) |
| 65+ years | 240 (14.5%) | 77 (12.4%) | 317 (13.9%) |
| Unknown | 47 (2.8%) | 16 (2.6%) | 63 (2.8%) |
| Type of reaction | |||
| Myocarditis | 905 (54.7%) | 336 (54.0%) | 1241 (54.5%) |
| Including requiring hospitalization | 573 (63.3%) | 223 (66.4%) | 796 (64.1%) |
| Including life‐threatening condition | 150 (16.6%) | 69 (20.5%) | 219 (17.6%) |
| Including fatal outcome | 11 (1.2%) | 4 (1.2%) | 15 (1.2%) |
| Pericarditis | 621 (37.5%) | 230 (37.0%) | 851 (37.4%) |
| Including requiring hospitalization | 287 (46.2%) | 104 (45.2%) | 391 (45.9%) |
| Including life‐threatening condition | 59 (9.5%) | 42 (18.3%) | 101 (11.9%) |
| Including fatal outcome | 3 (0.5%) | 2 (0.9%) | 5 (0.6%) |
| Myopericarditis | 115 (6.9%) | 52 (8.4%) | 167 (7.3%) |
| Including requiring hospitalization | 71 (61.7%) | 38 (73.1%) | 109 (65.3%) |
| Including life‐threatening condition | 21 (18.3%) | 8 (15.4%) | 29 (17.4%) |
| Including fatal outcome | 1 (0.9%) | 1 (1.9%) | 2 (1.2%) |
| Pleuropericarditis | 14 (0.8%) | 4 (0.6%) | 18 (0.8%) |
| Including requiring hospitalization | 10 (71.4%) | 2 (50.0%) | 12 (66.7%) |
| Including life‐threatening condition | 2 (14.3%) | 1 (25.0%) | 3 (16.7%) |
| Including fatal outcome | – | – | – |
| Time to onset, days | |||
| Myocarditis | 3 [2–7] | 3 [2–4] | 3 [2–6] |
| Pericarditis | 8 [3–20] | 10 [4–33] | 8 [3–21] |
| Myopericarditis | 4 [2–14] | 3 [2–12] | 4 [2–14] |
| Pleuropericarditis | 8 [3–23] | 6 [2–13] | 7 [3–19] |
| Cases with other suspected reported drug a | 155 (9.4%) | 45 (7.2%) | 200 (8.7%) |
Data are presented as N (%) or median (IQR).
COVID‐19, coronavirus disease 2019; IQR, interquartile range; WHO, World Health Organization.
aOther reported suspected drugs are infliximab (1 case with elasomeran), nicotinic acid and hydrocodone/paracetamol (1 case with tozinameran), and antilymphocyte immunoglobulin (1 case with tozinameran).
Figure 1.
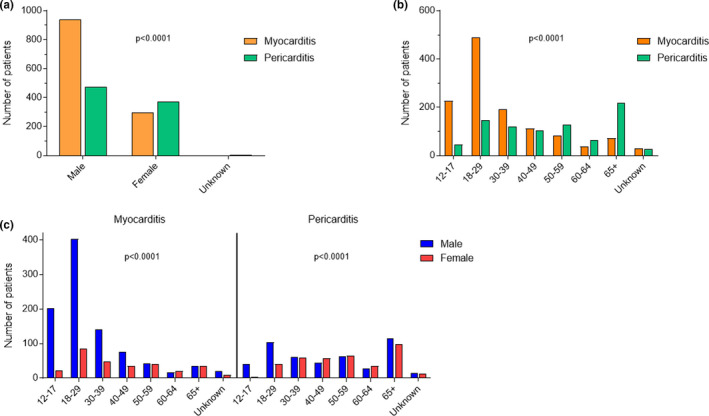
Demographic features of inflammatory heart reactions after mRNA coronavirus disease 2019 (COVID‐19) vaccines reported in the World Health Organization (WHO) global safety database. (a) Sex distribution according to reaction type (myocarditis or pericarditis). (b) Reaction type (myocarditis or pericarditis) distribution according to age. (c) Sex distribution according to reaction type (myocarditis or pericarditis) and age. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
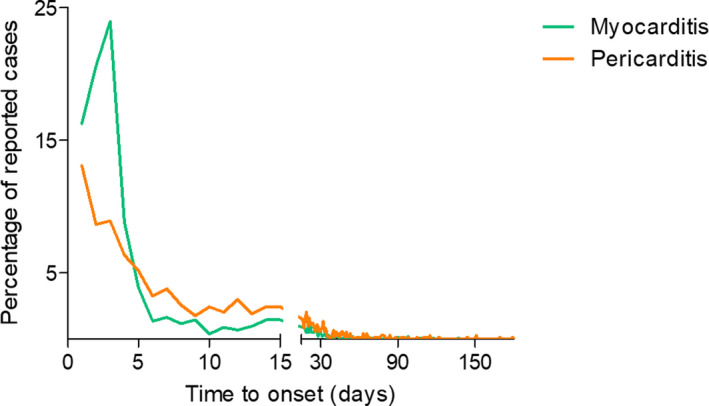
Time to onset according to the type of inflammatory heart reaction. The y‐axis represents the percentage of cases reported when “time to onset” was reported, corresponding to a total of 1,039 myocarditis and 740 pericarditis. Time to onset was earlier for myocarditis. [Colour figure can be viewed at wileyonlinelibrary.com]
Regarding myocarditis cases originating from the United States, they were mainly reported after May 2021, although reactions occur alongside with vaccination achievements (Figure 3b,c ). Reporting rate (95% CI) was overall 0.61 (95% CI 0.57–0.65) per 100,000 fully vaccinated people, with a reporting inflation after April 2021, up to 3.57 (95% CI 3.30–3.86) per 100,000 fully vaccinated people in June 2021 (Figure 3a , Table S2 ). We observed a large increase in reporting rates in 12–17‐year‐old patients (3.69, 95% CI 3.25–4.18 per 100,000 persons) and in 18–29‐year‐old patients (1.97, 95% CI 1.80–2.16 per 100,000 persons) compared with patients over 30 years old (0.21, 95% CI 0.19–0.24 per 100,000 persons) fully vaccinated vaccine recipients.
Figure 3.
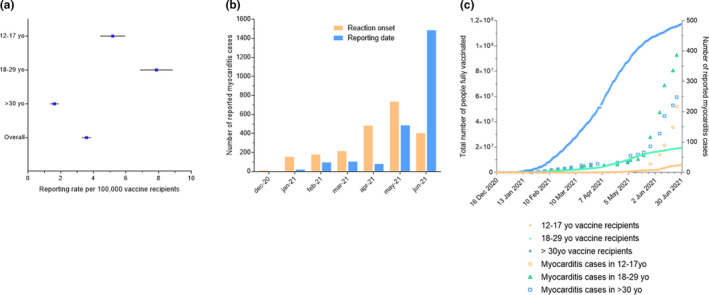
Features of myocarditis reporting in the United States. (a) Myocarditis reporting rate as of June 2021 according to age. Vaccine recipients are defined as the total count of vaccinated people in the United States that had a complete vaccination scheme (i.e., received a second dose of a two‐dose vaccine scheme or one dose of a single‐dose vaccine scheme; see Table S2 for detailed rates according to time). (b) Number of myocarditis cases per month according to reporting date and to reaction onset date. (c) Reported myocarditis cases and total number of people fully vaccinated according to the age group (12–17 years old, 18–29 years old, and over 30 years old). [Colour figure can be viewed at wileyonlinelibrary.com]
Disproportionality analysis
Compared with female patients, male patients were associated with an increased risk of myocarditis reporting (ROR, 9.4, 95% CI 8.3–10.6) and of pericarditis reporting (ROR, 3.7, 95% CI 3.2–4.2; Figure 4 ). Analysis according to age group showed a marked disproportionate reporting of myocarditis after mRNA COVID‐19 vaccines in 12–17‐year‐old (ROR, 22.3, 95% CI 19.2–25.9) and 18–29‐year‐old (ROR, 6.6, 95% CI 5.9–7.5) recipients compared with over 30‐year‐old recipients. Specifically, this increased reporting was more marked in male than in female patients, among all age groups (Figure 4a ). Regarding pericarditis, a disproportionate reporting in male patients among all age groups, compared with female patients was observed (Figure 4b ). Younger male patients belonging to the 12–17 and 18–29‐year‐old groups were also more prone to report pericarditis after mRNA COVID‐19 vaccines compared with the over 30‐year‐old group. This finding not being true in female patients in whom no disproportionality in pericarditis reporting was observed according to age. Further sensitivity analyses restricted to serious reports and to reports originating form healthcare professionals showed consistent results (Tables S3–S6).
Figure 4.
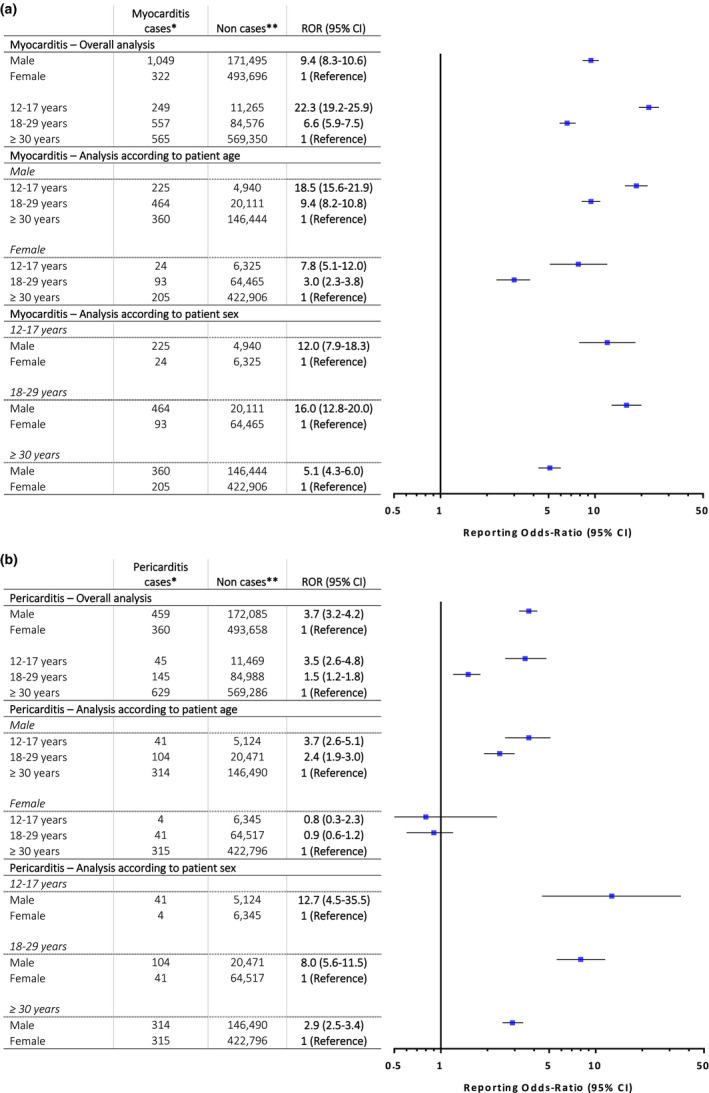
Reporting odds ratios for myocarditis and pericarditis in mRNA coronavirus disease 2019 (COVID‐19) vaccine recipients according to sex and age within the World Health Organization (WHO) global safety database. (a) Myocarditis. (b) Pericarditis. Case–non‐case approach is similar to case–control method but for the purposes of pharmacovigilance studies. Disproportionality in adverse drug reaction reporting between groups is expressed using RORs and their 95% CI. ROR is a ratio similar in concept to the odds ratio in case‐control studies and corresponds to the exposure odds among reported cases of inflammatory heart disorders over the exposure odds among reported non‐cases. A lower bound of the 95% CI of the ROR over 1.0 suggests that inflammatory heart disorders are more frequently reported in COVID‐19 mRNA vaccine recipients specific group compared with control group. We performed the main analysis and a secondary analysis stratified according to age groups and sex. *Inflammatory heart disorder cases were individual case safety reports were retrieved using the high level terms “noninfectious myocarditis” and “noninfectious pericarditis” according to the Medical Dictionary for Regulatory Activities (MedDRA, https://www.meddra.org/), coded as reactions. Cases of myopericarditis have been considered as myocarditis. **Non‐cases were reports containing any other reaction. ROR, reporting odds‐ratio; 95% CI, 95% confidence interval. [Colour figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
In this global pharmacovigilance retrospective observational study, we report the largest case series of inflammatory heart reactions following mRNA COVID‐19 vaccination to date. Our global analysis show that myocarditis is highly likely to be reported in adolescents and young adults below 30 years old. Furthermore, we also observed a dramatically increased disproportionate reporting risk in male patients, among all age groups. These myocarditis and pericarditis cases mostly required hospitalization, some were life‐threatening, and, in older patients, occasionally fatal. As of June 2021, we observed overall 3.57 (from 3.30 to 3.86) myocarditis reports per 100,000 fully vaccinated persons in the United States; this rate being higher in adolescents (5.15, from 4.45 to 5.95) and young adults (7.82, 6.89 to 8.86). This supports that myocarditis may appear very rarely following mRNA vaccination, even in young people, although our analysis cannot assess accurate incidence.
Overall, our findings are consistent with the experience first reported by the Israeli Ministry of Health and thereafter by the US Centers for Disease Control and Prevention (CDC). 21 , 35 In our study, among the US population, 3.57 (from 3.30 to 3.86) myocarditis reports per 100,000 persons fully vaccinated have been observed overall as of June 2021. Although our approach cannot accurately assess reaction incidence owing to under‐reporting, this reporting rate is consistent with the findings from a national study in Israel, where the excess risk of myocarditis in the 42 days following the administration of an mRNA COVID‐19 vaccine has been assessed at 2.7 (from 1.0 to 4.6) events per 100,000 persons. 36 Noteworthy, the authors mentioned that this risk was lower than that expected following infection by SARS‐CoV‐2. One major limitation of this study was the lack of risk estimates according to age and sex group. 37 Our analysis allows to estimate a relative risk according to patient age and sex. We found a major increased reporting for myocarditis in male patients, and in adolescents and young adults below 30 years old, supporting a much higher risk in these groups. In a comparable manner, an analysis from the CDC found that observed myocarditis reports were higher than expected case rates for male patients compared with female patients, and higher at younger ages compared with older ages. 38 Otherwise, one could hypothesize a current background risk for myocarditis probably lower than usual along with the widespread adoption of community‐based mitigation measures to reduce the transmission of SARS‐CoV‐2 and thereafter other respiratory infection. 39 , 40
Myocarditis and pericarditis have been previously reported after vaccination, especially smallpox and influenza vaccines. 7 Potential mechanisms are currently unknown, but several hypotheses may be considered. The first is that incidence appears to be higher in situations related to higher immune reactivity (e.g., younger patient population, after second dose, etc.). This may be related to greater adaptive immune response in younger individuals, which may lead to greater increases of CD4+ Th17+ cell populations, predisposing individuals to developing myocarditis. It would be interesting to see if the recently reported mRNA diagnostic of Th17 activation in myocarditis is also positive in these patients. 41 Second, is the possibility that mRNA in these vaccines may enhance autoimmunity. 42 The mRNA is known to be a self‐adjuvant for innate immune responses, and this may help to explain their immunogenicity, and trigger excessive immune responses in some individuals, especially when there may be presence of a cross‐reacting antigen. Optimization of mRNA dose delivered is needed to elicit appropriate immune response while minimizing undesirable inflammatory reactions, re‐assessing the dosing frequency and time interval between doses, as well as optimization of all ingredients associated with the mRNA, in order to achieve the most beneficial outcome of effective immunization with the least number of adverse effects to all different population demographics. Finally, it has been hypothesized that myocarditis can occur due to direct cell invasion via the spike protein interacting with the angiotensin‐converting enzyme 2 (ACE2) widely expressed and prevalent in cardiomyocytes. 43 , 44 However, in case of COVID‐19 related myocarditis, SARS‐CoV‐2 has not been found in cardiomyocytes, but only in the remaining myocardium, thus the cell injury was thought to be due to the generalized inflammatory response to COVID‐19, part of which is Th1 activation. 45 In two recently reported cases of myocarditis following mRNA vaccination, only inflammatory infiltration was assessed in the myocardium, suggesting that the ACE2 hypothesis is probably not relevant. 46
The reason for male predominance in myocarditis cases following mRNA COVID‐19 vaccination is unknown. Although, female patients usually generate higher overall antibody levels and more adverse events following vaccination, male patients have increased enhanced type‐1 immune responses. 47 These differences may be driven by sex hormone differences and testosterone is thought to play a role in commitment to a Th1 response. 38 Hence, sex differences observed in myocarditis could suggest a mechanism for myocarditis occurrence involving Th1 activation.
This retrospective pharmacovigilance analysis has some limitations and strengths. Spontaneous reports will likely feature under‐reporting of total real‐world cases as well as variable data quality, all of which are inherent to any pharmacovigilance system. 48 Hence, as a limitation of this study, it is not possible to accurately assess incidence of inflammatory heart reactions following mRNA COVID‐19 vaccination. However, in the current context of a highly communicated risk at a global level, such as the myocarditis risk as of June 2021, the reporting rate may be relevant to approximate incidence. Another limitation is the quality of the data reported in pharmacovigilance databases, that could result in some cases in misdiagnosis. However, it appears not likely that this precludes our analysis according to patient age and sex, which was consistent after considering only reports originating from healthcare professionals. Furthermore, VigiBase, covering more than 90% of the world’s population, provides a unique opportunity to analyze rare adverse events at a global scale. Disproportionality analysis on VigiBase has proven its value in detecting increased risk of events. 32 Our study confirmed the value of disproportionality analyses in relative risk estimation among subgroups of patients. Our analysis highlights an increased risk of myocarditis reporting in young male patients. On one hand, myocarditis has suffered from a notoriety bias after media communication. On the other hand, non‐cases reporting may be inflated as well, resulting in an underestimation of this risk. Besides, it is not clear whether, in the current pandemic setting, the expected rate of myocarditis is different from the historical rate of 0.95 to 2.16 per 100,000 persons among the US population. 49 Finally, it is not likely that study groups in our disproportionality analysis, based on patient age and sex, may have been imbalanced in relation with these limitations. Sensitivity analyses showing consistent results strengthen our findings.
We report a global analysis on inflammatory heart reactions occurring after mRNA COVID‐19 vaccination. Reporting risk of myocarditis is highly increased in adolescents or young adults, and in male patients. However, owing hundreds of millions of doses administered worldwide to date, the excess risk of myocarditis following mRNA COVID‐19 vaccination, supported by the rate of reporting in our study and a recent study, appeared very rare. 36 Foremost, it has been shown that SARS‐CoV‐2 infection is itself a very strong risk factor for myocarditis, higher than following vaccination. 36 Absolute risk may greatly increase with the achievements of the mass immunization campaign and the enlargement of vaccination to young adults. Adapting the dosage, timing, and protocol of mass immunization in young adults and children, especially male patients, should be discussed. 50 Nevertheless, benefits of COVID‐19 vaccines still very largely outweigh their risks given the current prevalence of SARS‐CoV‐2 infections around the world. As COVID‐19 vaccines’ administration increases worldwide, physicians and the public should be aware of these very rare inflammatory heart reactions, and follow appropriate monitoring and treatment procedures as with other cases of myocarditis. 51 Further studies are needed to support individual risk‐benefit decision making about the use of COVID‐19 vaccines. In the future, as newer formulations are being developed, such as for vaccine boosters, the dose and timing for the at‐risk cohort for cardiac complications should be considered to maximize the benefit to risk ratio.
FUNDING
This work is supported in part by research grants from the Canadian Institutes of Health Research, Heart & Stroke Foundation of Canada, Genome Canada, and University of Ottawa of Heart Institute.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
L.C., A.B., M.A.‐K., and P.L.L. wrote the manuscript. L.C., A.B., and P.L.L. designed the research. L.C. and A.B. performed the research. L.C., A.B., P.P.L., M.A.‐K., T.S.K., G.N., J.R., M.‐D.D., M.‐B.V.‐R., J.M., F.S., and J.‐M.T. analyzed the data.
Supporting information
Table S1‐S6
ACKNOWLEDGMENTS
The authors would like to thank all the healthcare workers and the French Pharmacovigilance Network involved in the current large immunization campaign and in its safety monitoring, VigiBase is a fully deidentified database maintained by the Uppsala Monitoring Center (UMC). The authors are indebted to the National Pharmacovigilance centers that contributed data. Information from VigiBase comes from a variety of sources, and the probability that the suspected adverse effect is drug‐related is not the same in all cases. The information does not represent the opinion of the Uppsala Monitoring Center (UMC) or the World Health Organization and only reflects the authors’ opinion. According to VigiBase access rules, no specific ethical approval is needed. VigiBase access is granted to national and regional pharmacovigilance centers such our teams.
DATA AVAILABILITY STATEMENT
Aggregated data of spontaneous reports are available at http://www.vigiaccess.org/.
- 1. Epelman, S. , Liu, P.P. & Mann, D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 15, 117–129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joy, G. et al. Prospective case‐control study of cardiovascular abnormalities 6 months following mild COVID‐19 in healthcare workers. JACC Cardiovasc. Imaging 14, 2155–2166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daniels, C.J. et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS‐CoV‐2 infection: results from the big ten COVID‐19 cardiac registry. JAMA Cardiol. 6, 1078 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma, R. , Wang, Q. , Meng, D. , Li, K. & Zhang, Y. Immune checkpoint inhibitors‐related myocarditis in patients with cancer: an analysis of international spontaneous reporting systems. BMC Cancer 21, 38 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eckart, R.E. et al. Incidence and follow‐up of inflammatory cardiac complications after smallpox vaccination. J. Am. Coll. Cardiol. 44, 201–205 (2004). [DOI] [PubMed] [Google Scholar]
- 6. Griffin, J.M. , Woznica, E. , Gilotra, N.A. & Nucifora, F.C. Clozapine‐associated myocarditis: a protocol for monitoring upon clozapine initiation and recommendations for how to conduct a clozapine rechallenge. J. Clin. Psychopharmacol. 41, 180–185 (2021). [DOI] [PubMed] [Google Scholar]
- 7. Su, J.R. et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990–2018. Vaccine 39, 839–845 (2021). [DOI] [PubMed] [Google Scholar]
- 8. Mei, R. et al. Myocarditis and pericarditis after immunization: Gaining insights through the Vaccine Adverse Event Reporting System. Int. J. Cardiol. 273, 183–186 (2018). [DOI] [PubMed] [Google Scholar]
- 9. Casey, C.G. et al. Adverse events associated with smallpox vaccination in the United States, January‐October 2003. JAMA 294, 2734–2743 (2005). [DOI] [PubMed] [Google Scholar]
- 10. Gatti, M. et al. Influenza vaccination and myo‐pericarditis in patients receiving immune checkpoint inhibitors: investigating the likelihood of interaction through the vaccine adverse event reporting system and VigiBase. Vaccines 9, 19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watkins, K. , Griffin, G. , Septaric, K. & Simon, E.L. Myocarditis after BNT162b2 vaccination in a healthy male. Am. J. Emerg. Med. 6757, 815.e1–815.e2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abu Mouch, S. et al. Myocarditis following COVID‐19 mRNA vaccination. Vaccine 39, 3790–3793 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larson, K.F. et al. Myocarditis after BNT162b2 and mRNA‐1273 vaccination. Circulation 144, 506–508 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson, N.A.C. , Kester, K.E. , Casimiro, D. , Gurunathan, S. & DeRosa, F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines 5, 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baden, L.R. et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N. Engl. J. Med. 384, 403–416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polack, F.P. et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CDC Covid data tracker. Centers for Disease Control and Prevention (2020). <https://covid.cdc.gov/covid‐data‐tracker>. [Google Scholar]
- 18. Soeiro, T. et al. Type I interferons as the potential mechanism linking mRNA COVID‐19 vaccines to Bell’s palsy. Therapie 76, 365–367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimabukuro, T.T. , Cole, M. & Su, J.R. Reports of anaphylaxis after receipt of mRNA COVID‐19 vaccines in the US‐December 14, 2020‐January 18, 2021. JAMA 325, 1101–1102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Renoud, L. et al. Association of facial paralysis with mRNA COVID‐19 vaccines: a disproportionality analysis using the World Health Organization pharmacovigilance database. JAMA Intern. Med. 181, 1243 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vogel, G. & Couzin‐Frankel, J. Israel reports link between rare cases of heart inflammation and COVID‐19 vaccination in young men. Science Magazine (2021). <https://www.sciencemag.org/news/2021/06/israel‐reports‐link‐between‐rare‐cases‐heart‐inflammation‐and‐covid‐19‐vaccination>. [Google Scholar]
- 22. Diaz, G.A. et al. Myocarditis and pericarditis after vaccination for COVID‐19. JAMA 326, 1210 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. COVID‐19 vaccine benefits still outweigh risks, despite possible rare heart complications. American Heart Association. <https://newsroom.heart.org/news/covid‐19‐vaccine‐benefits‐still‐outweigh‐risks‐despite‐possible‐rare‐heart‐complications>. [Google Scholar]
- 24. Gargano, J.W. et al. Use of mRNA COVID‐19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices – United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 977–982 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerneis, M. , Bihan, K. & Salem, J.‐E. COVID‐19 vaccines and myocarditis. Arch. Cardiovasc. Dis. 114, 515–517 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montgomery, J. et al. Myocarditis following immunization with mRNA COVID‐19 vaccines in members of the US military. JAMA Cardiol. 6, 1202 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. PINHO, A.C . Comirnaty and Spikevax: possible link to very rare cases of myocarditis pericarditis. European Medicines Agency (2021). <https://www.ema.europa.eu/en/news/comirnaty‐spikevax‐possible‐link‐very‐rare‐cases‐myocarditis‐pericarditis>. [Google Scholar]
- 28. Commissioner of the Coronavirus (COVID‐19) Update: June 25, 2021. FDA. <https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐june‐25‐2021>
- 29. Lindquist, M. VigiBase, the WHO Global ICSR database system: basic facts. Drug Inf. J. 42, 409–419 (2008). [Google Scholar]
- 30. Toulouse, E. , Lafont, B. , Granier, S. , Mcgurk, G. & Bazin, J.‐E. French legal approach to patient consent in clinical research. Anaesth. Crit. Care Pain Med. 39, 883–885 (2020). [DOI] [PubMed] [Google Scholar]
- 31. Edwards, I.R. & Aronson, J.K. Adverse drug reactions: definitions, diagnosis, and management. Lancet Lond. Engl. 356, 1255–1259 (2000). [DOI] [PubMed] [Google Scholar]
- 32. Montastruc, J.‐L. , Sommet, A. , Bagheri, H. & Lapeyre‐Mestre, M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br. J. Clin. Pharmacol. 72, 905–908 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Faillie, J.‐L. Case‐non‐case studies: Principle, methods, bias and interpretation. Therapie 74, 225–232 (2019). [DOI] [PubMed] [Google Scholar]
- 34. Khouri, C. et al. Adverse drug reaction risks obtained from meta‐analyses and pharmacovigilance disproportionality analyses are correlated in most cases. J. Clin. Epidemiol. 134, 14–21 (2021). [DOI] [PubMed] [Google Scholar]
- 35. Hause, A.M. et al. COVID‐19 vaccine safety in adolescents aged 12–17 years – United States, December 14, 2020‐July 16, 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 1053–1058 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barda, N. et al. Safety of the BNT162b2 mRNA Covid‐19 vaccine in a nationwide setting. N. Engl. J. Med. 385, 1078–1090 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee, G.M. The importance of context in Covid‐19 vaccine safety. N. Engl. J. Med. 385, 1138–1140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bozkurt, B. , Kamat, I. & Hotez, P.J. Myocarditis with COVID‐19 mRNA vaccines. Circulation 144, 471–484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olsen, S.J. et al. Decreased influenza activity during the COVID‐19 pandemic – United States, Australia, Chile, and South Africa, 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1305–1309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jones, N. How COVID‐19 is changing the cold and flu season. Nature 588, 388–390 (2020). [DOI] [PubMed] [Google Scholar]
- 41. Blanco‐Domínguez, R. et al. A novel circulating MicroRNA for the detection of acute myocarditis. N. Engl. J. Med. 384, 2014–2027 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu, P.P. , Blet, A. , Smyth, D. & Li, H. The science underlying COVID‐19: implications for the cardiovascular system. Circulation 142, 68–78 (2020). [DOI] [PubMed] [Google Scholar]
- 43. Siripanthong, B. et al. Recognizing COVID‐19‐related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 17, 1463–1471 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hikmet, F. et al. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 16, e9610 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mele, D. , Flamigni, F. , Rapezzi, C. & Ferrari, R. Myocarditis in COVID‐19 patients: current problems. Intern. Emerg. Med. 16, 1123–1129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verma, A.K. , Lavine, K.J. & Lin, C.‐Y. Myocarditis after Covid‐19 mRNA Vaccination. N. Engl. J. Med. 385, 1332–1334 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fischinger, S. , Boudreau, C.M. , Butler, A.L. , Streeck, H. & Alter, G. Sex differences in vaccine‐induced humoral immunity. Semin. Immunopathol. 41, 239–249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Navar, A.M. , McNally, E. , Yancy, C.W. , O’Gara, P.T. & Bonow, R.O. Temporal associations between immunization with the COVID‐19 mRNA vaccines and myocarditis: the vaccine safety surveillance system is working. JAMA Cardiol. 6, 1117–1118 (2021). [DOI] [PubMed] [Google Scholar]
- 49. Gubernot, D. et al. Population‐Based background incidence rates of medical conditions for use in safety assessment of COVID‐19 vaccines. Vaccine 39, 3666–3677 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krumholz, H. M . Myocarditis after mRNA COVID‐19 vaccine. NEJM Watch (2021). https://www.jwatch.org/na53825/2021/07/20/myocarditis‐after‐mrna‐covid‐19‐vaccine. [Google Scholar]
- 51. Ammirati, E. et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ. Heart Fail. 13, e007405 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S6
Data Availability Statement
Aggregated data of spontaneous reports are available at http://www.vigiaccess.org/.


