Abstract
Immune cell dysregulation and lymphopenia characterize COVID‐19 pathology in moderate to severe disease. While underlying inflammatory factors have been extensively studied, homeostatic and mucosal migratory signatures remain largely unexplored as causative factors. In this study, we evaluated the association of circulating IL‐6, soluble mucosal addressin cell adhesion molecule (sMAdCAM), and IL‐15 with cellular dysfunction characterizing mild and hypoxemic stages of COVID‐19. A cohort of SARS‐CoV‐2 infected individuals (n = 130) at various stages of disease progression together with healthy controls (n = 16) were recruited from COVID Care Centres (CCCs) across Mumbai, India. Multiparametric flow cytometry was used to perform in‐depth immune subset characterization and to measure plasma IL‐6 levels. sMAdCAM, IL‐15 levels were quantified using ELISA. Distinct depletion profiles, with relative sparing of CD8 effector memory and CD4+ regulatory T cells, were observed in hypoxemic disease within the lymphocyte compartment. An apparent increase in the frequency of intermediate monocytes characterized both mild as well as hypoxemic disease. IL‐6 levels inversely correlated with those of sMAdCAM and both markers showed converse associations with observed lympho‐depletion suggesting opposing roles in pathogenesis. Interestingly, IL‐15, a key cytokine involved in lymphocyte activation and homeostasis, was detected in symptomatic individuals but not in healthy controls or asymptomatic cases. Further, plasma IL‐15 levels negatively correlated with T, B, and NK count suggesting a compensatory production of this cytokine in response to the profound lymphopenia. Finally, higher levels of plasma IL‐15 and IL‐6, but not sMAdCAM, were associated with a longer duration of hospitalization.
Keywords: SARS‐CoV‐2, COVID‐19, IL‐15, sMAdCAM, Lymphopenia, Symptomatic infection
Graphical Abstract
A mucosal and lymphocyte migration marker (sMAdCAM) and IL‐15, a cytokine regulating leukocyte activation and homeostasis, are associated with different states of immune pathology following COVID‐19.
Abreviations
- AM
asymptomatic mild
- CBA
cytometric bead array
- CCCs
COVID care centres
- CLIA
chemiluminescence immunoassay
- ELISA
enzyme linked immunosorbent assay
- IL‐15
interleukin‐15
- IL‐6
interleukin‐6
- LPS
lipopolysaccharide
- MCGM
municipal corporation of Greater Mumbai
- MD
moderate
- NK cells
natural killer cells
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SM
symptomatic mild
- sMAdCAM
soluble mucosal addressin cell adhesion molecule
- SN
seronegative
- SpO2
oxygen saturation
- SV
severe
- TCM
central memory
- TEM
effector memory
- TN
naïve
- Treg
CD4+ regulatory T cells
- TTD
terminally differentiated.
1. INTRODUCTION
The Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic continues to pose a global health crisis in spite of ongoing interventions such as vaccination. 1 , 2 Pathology of COVID‐19 displays varied clinical manifestations ranging from no symptoms to critical systemic disease. 3 , 4 , 5 Hypoxemia is a key signature that discriminates between mild and moderate to severe disease. 6 , 7 The role of lymphopenia as a defining cellular immune correlate of moderate to severe disease has been well established. 8 However, underlying immune homeostatic mechanisms that might contribute to this phenotype remain largely unexplored. 9 , 10 Understanding and identifying such relationships, in the context of vaccination history, would help to guide therapeutic efforts and to ensure optimal disease management of COVID‐19.The Gastrointestinal (GI) tract is a potential extrapulmonary site for SARS‐CoV‐2 infection and replication. 11 , 12 Endothelial venules of gut express Mucosal Addressin cell adhesion molecule (MAdCAM) that facilitate homing of immune cells to the gut. We have previously reported altered levels of its soluble form (sMAdCAM) in the blood of SARS‐CoV‐2 infected individuals that inversely correlated with plasma IL‐6 and positively with antiviral humoral responses. 13 Further, levels of ICAM‐1 and VCAM‐1 have also been reported to be altered in COVID‐19, linked to lung pathology. 14 , 15 , 16 IL‐6 is a key inflammatory cytokine controlling immune cell proliferation and differentiation. 17 It has been reported to be a key mediator of systemic inflammation in respiratory diseases, including asthma and COVID‐19. 18 , 19 IL‐15, an important homeostatic and activating cytokine that is critical for proliferation, survival, and function of lymphoid cells, especially NK cells and cytotoxic CD8 T cells. 20 , 21 Also, its role in MERS‐CoV infection has been previously documented as being essential fordevelopment of antiviral NK cells and CD8 T cells. 22 Thus, in this study we evaluated the contribution of key inflammatory, cellular homeostatic, and mucosal migratory markers in distinct stages of COVID‐19 pathogenesis. Our results highlight associationsof IL‐6, IL‐15, and sMAdCAM with lymphopenia together with a heretofore undescribed role fordetectable plasma IL‐15 as a marker associated with symptomatic progression.
2. MATERIALS and METHODS
2.1. Study population, setting, and data collection
Sixteen seronegative controls and a total of 130 in‐patientindividuals were recruited, following informed consent, for the study from the COVID Care Centres (CCCs) associated with BYL Nair hospitaland T N medical college, Municipal Corporation of Greater Mumbai (MCGM), Mumbai following approval of institutional ethics committees. The study protocols were approved by ICMR‐NIRRHInstitutional Ethics Committee. We obtained demographic data, clinical history at presentation, and laboratory results during admission.
Blood samples for the study were handled in accordance with ICMR guidelines for biosafety.1–3 ml of whole blood was collected in EDTA vacutainers. Aliquots of whole blood were processed for absolute cell count and immunophenotyping as described below. Plasma was separated by centrifugation at 400 g for 10 min. IgG and IgM antibodies against SARS‐CoV‐2 were detected in fresh plasma samples using Rapid test fromVoxpress (Voxtur Bio LTD, India) and Chemiluminescence immunoassay (CLIA) directed against SARS ‐CoV‐2 anti‐NC IgG. The remaining plasma samples were aliquoted and stored at −800C until batch analysis of cytokines, LPS (Lipopolysaccharide), and Soluble MAdCAM (sMAdCAM).
2.2. Assay for an absolute count of T cells, B cells, and NK cells
BD multitest six colour TBNK reagent and BD Trucount™ tubes were used to enumerate absolute count of T cells, CD4 T cells, CD8 T cells, B cells, and Natural killer (NK) cells using 50 μl of fresh EDTA stabilised bloodfollowing stain/lyse/no‐wash protocol. Data acquisition was performed on BD FACS Aria Fusion (SORP)Flow cytometer (BD Biosciences) where 5000 events gated on beads count were acquired. Data analysis was carried out using FlowJosoftware (BD biosciences). Absolute count for different CD4+ and CD8+memory T cell subsets (naïve, central memory, effector memory, and terminally differentiated) were calculated based on concurrently obtained absolute CD4+ and CD8+ T cell counts, respectively.
2.3. Flow cytometry
Immunophenotyping of fresh peripheral whole blood with following fluorescently labelled monoclonal antibodies, anti‐CD3 (Clone:SK7), anti‐CD8 (Clone: SK1), anti‐CD4 (Clone: RPA‐T4), anti‐CD127 (Clone: HIL‐7R‐M21), anti‐CD25 (Clone: M‐A251), anti‐CCR7 (Clone: 150503), anti‐CD45RA (Clone: HI100), anti‐CD14 (Clone: M5E2 ) and anti‐16 (Clone: 3G8) was performed using stain/lyse/wash protocol as described earlier. 23 Data acquisition was performed on BD FACS Aria Fusion (SORP) flow cytometer (BD Biosciences) where at least 100,000 events in lymphocyte scattergate were acquired. Data analysis was carried out using FlowJosoftware. IL‐6 determination was carried out using a cytometric bead array (CBA, BD Biosciences) to quantify cytokine levels in the plasma of study participants as per manufacturers’ protocol.
2.4. ELISA
Plasma IL‐15, sMAdCAM‐1, and LPS levels were quantified using Human IL‐15 DuoSet ELISA kit (R&D Systems), Human MAdCAM‐1 DuoSet ELISA kit (R&D Systems), and Human Lipopolysaccharides ELISA kit (MyBioSource) respectively, following manufacturers’ recommendations. The values below the level of detection were assigned as zero.
2.5. Statistics
Statistical analysis was performed in GraphPad Prism 8 using non‐parametric tests. Statistical significance of differences among groups was assessed usingnon‐parametric one‐way ANOVA (Kruskal–Wallis test)followed by a post hoc Dunn's test. Spearman's rank‐order correlation was used to analyse the association between participant attributes that had detectable values in our assays. Kaplan–Meier curves were compared using Log‐rank (Mantel‐Cox) test. Statistical significance was accepted at p < 0.05. Receiver operating characteristic (ROC) curvewas generated for IL‐15 levelsand used to discriminate between comparator groups.
3. RESULTS & DISCUSSION
In this study we describe cellular immune signatures characterizing mild (asymptomatic as well as symptomatic) and hypoxemic (moderate and severe) COVID‐19 disease. A total of 130admitted hospital patients, laboratory‐confirmed positive for SARS‐CoV‐2 infection by quantitative RT‐PCR of the throat and nasal swab sampleswere recruited for the study. According to the clinical management protocol for COVID‐19 (Version 3) issued byMinistry of Health and Family Welfare, Government of India, patients were recruited into different groups based on clinical severity. 7 The patients without evidence of breathlessness or hypoxia were classified into the Mild group. Mild patients were further segregated into Asymptomatic Mild (AM; n = 47) and Symptomatic Mild (SM; n = 62) where SMs presented mild symptoms like fever, cough, sore throat, headache, etc. Patients with oxygen saturation (SpO2) of < 93% on room air who required oxygen support to correct hypoxemia, were classified as having Moderate (MD; n = 10) COVID‐19, whereas patients with oxygen saturation (SpO2) of < 90% on room air were considered as having Severe (SV; n = 11) COVID‐19. In addition, a total of16healthy participants with no apparent history of COVID‐19 and seronegative (SN) by Rapid antibody test, were also recruited as controls. The demographic and clinical characteristics of all the study participants are summarised in Table 1.
TABLE 1.
Abridged clinical data of study participants
| Seronegative Healthy | Asymptomatic mild | Symptomatic mild | Moderate | Severe | |
|---|---|---|---|---|---|
| Number ofParticipants | 16 | 47 | −62 | 10 | 11 |
| Age a (years) | 26 (24–46) | 39 (18–75) | 37 (18–71) | 64 (47–75) | 66 (31–81) |
| Gender (M/F) | 8/8 | 33/14 | 41/16 | 6/4 | 9/2 |
| Duration of Hospitalisation a (days) | – | 8 (1–41) | 7 (1–41) | 12 (2–27) | 18 (8–38) |
Data are expressed as the median (range). SpO2 levels at the time of recruitment are reflected in the table.
3.1. Lymphopenia with relative sparing of Treg and CD8+ effector memory subsets define hypoxemic COVID‐19 progression
The absolute counts of total lymphocytes and T cells (both CD4+ and CD8+) in MD and SV groups were significantly decreased compared to AM, SM, and SN (Figure 1A and Supplementary figure S1). Additionally, the SV group also showed a trend towards depletion of total lymphocytes compared to the MD group. Similar depletion profiles (in MD and SV groups) were observed for both T (CD4+ and CD8+) and non T (B and NK) cell compartments. However, no significant difference was observed among mild patients (AM&SM) and compared toSN (Figure 1B‐G). No apparent difference in the frequency of these lymphocyte subsets was observed except fora significant decrease for total T lymphocytesand a significant increase for total non‐T lymphocytes in both MD and SVcompared to other groups (Figure 1H‐M). This suggested a pan lymphopenic phenotype as has been well described, 24 , 25 , 26 , 27 in hypoxemic COVID‐19 affected individuals. Additionally, to evaluate the impact of lymphopenia on the T cell compartment, the distribution of CD4+ and CD8+ T cell subsets was also evaluated. 23 Based on the surface expression of CD45RA and CCR7, CD4+ and CD8+ T cells were differentiated into Naïve (TN; CD45RA+CCR7+), Central memory (TCM; CD45RA‐CCR7+), Effector memory (TEM; CD45RA‐CCR7‐) and Terminally differentiated (TTD; CD45RA+CCR7‐) T cells (Supplementary figure S2). While both MD and SV groups had significant depletion of absolute count for all CD4+ T cell subsets (Figure 2A), our findingsdo not support preferential depletion of any particular subsets in the CD4+ T cell compartment (Figure 2B). In the CD8+ T cell compartment, a comparison of absolute counts revealed profound depletion of all subsets in hypoxemic (MD and SV) individuals (Figure 2C). In contrast to the CD4+ T cell compartment, however, weobserved a relatively lower frequency of naïve (CD8+ TN) and concomitant elevation of effector memory (CD8+ TEM) frequency (Figure 2D). This reflected a distinct depletion profile for CD8+ T cells with relative sparing of effector memory cells. These results extend the limited data available for T cell subsets in a stratified manner to include distinct stages of COVID‐19 pathogenesis. 10 , 28
FIGURE 1.
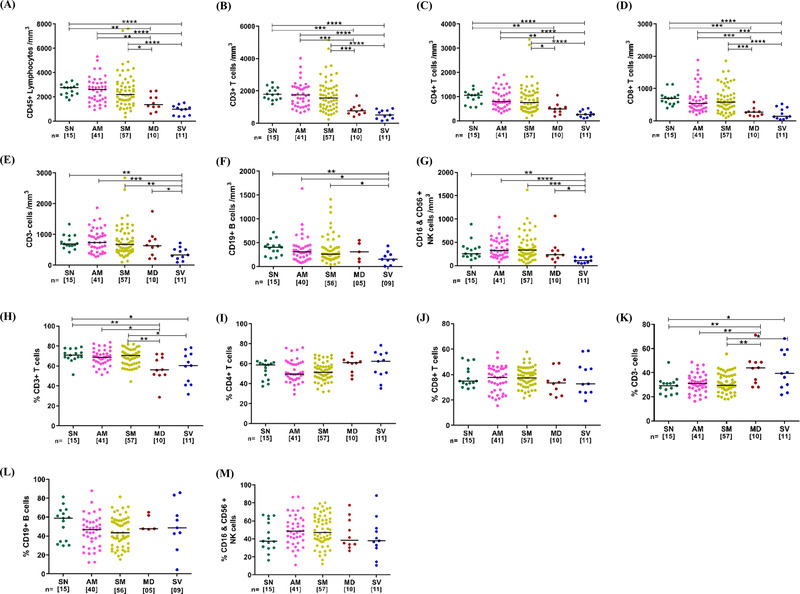
Absolute numbers and frequency distribution of T lymphocytes, CD4+ and CD8+ T cells, Non‐T lymphocytes, B cells, and NK cells among study participants. (A‐G) Variation across seronegative (SN), asymptomatic mild (AM), symptomatic mild (SM), moderate (MD) and severe (SV) groups in counts of (A) CD45+ Total Lymphocytes (B) CD3+ T cells, (C) CD4+ T cells, (D) CD8+ T cells, (E) Non‐T lymphocytes (CD3‐ T cells), (F) CD19+ B cells and (G) CD16+& CD56+ NK cells. (H‐M) Variation across different study groups in frequency of (H) CD3+ T cells (I) CD4+ T cells (J) CD8+ T cells (K) CD3‐ T cells (L) CD19+ B cells and (M) CD16+& CD56+ NK cells. Statistical significance was calculated by non‐parametric one way ANOVA (Kruskal–Wallis test) followed by post hoc Dunn's‐test; *, p < 0.05; **, p < 0.01, ***,p < 0.001; and ****,p < 0.001
FIGURE 2.
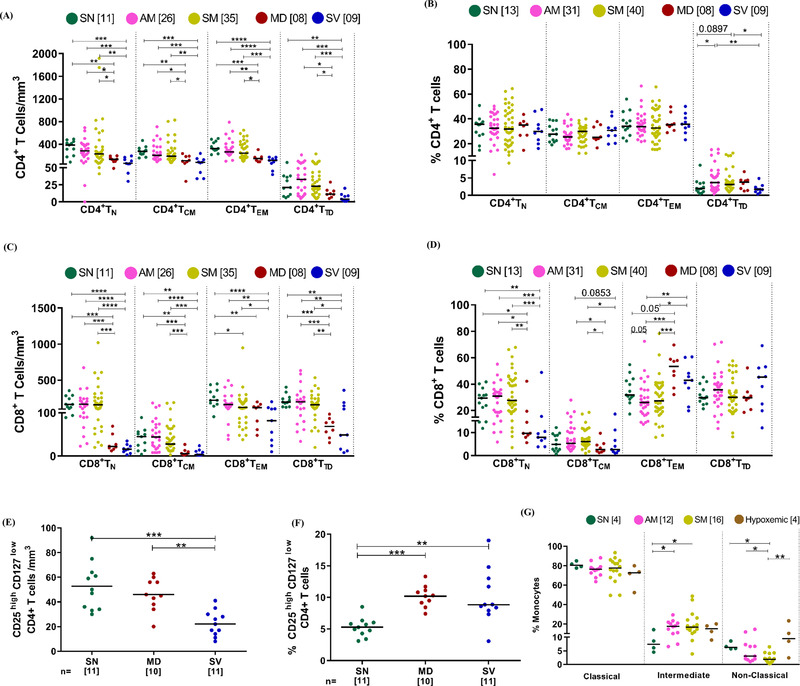
Distribution of different immune subsets among study participants (A‐B) Variation across seronegative (SN), asymptomatic mild (AM), symptomatic mild (SM), moderate (MD), and severe (SV) groups in absolute count and frequency of CD4+ T cells subsets (N = Naïve, CM = central memory, EM = Effector Memory and TD = Terminally differentiated), respectively. (C‐D) Variation across different study groups in absolute count and frequency of CD8+ T cells subsets, respectively. (E‐F) Variation across different groups in Tregs count (E) and frequency(F). (G) Variation in frequency of monocyte subsets across different study groups. Statistical significance was calculated by non‐parametric one way ANOVA (Kruskal–Wallis test) followed by post hoc Dunn'stest; *, p < 0.05; **, p < 0.01, ***,p < 0.001; and ****,p< 0.001
The CD4+ T regulatory cell (Treg) subset represents a critical part of the response to viral infections. 29 , 30 Interestingly, while memory subsets of CD4+ T cell compartment seemed to be equally depleted in hypoxemic individuals, we report here depletion in Treg count that was exacerbated in the SV group (Figure 2E). Also, elevated frequency of CD4+ T regulatory cells in hypoxemic individuals indicated selective preservation of this subset (Figure 2F). As has been reported very recently this elevated frequency may represent suppressive (protective) as well as inflammatory roles played by these cells, markedly higher, in severe disease. 31 Monocytes have been known to modulate inflammatory responses to a variety of viral infections. 32 , 33 When this subset was evaluated, we observed an elevated frequency of the intermediate (CD14+CD16++) subset that defined both mild (AM, SM) and hypoxemic patients (Figure 2G). Also, the concomitant decrease in the frequency of non‐classical monocytes was observed in mild infection irrespective of symptoms. Thus, these results highlight the importance of monitoring monocyte frequency dysregulation as a possible early marker of COVID‐19 that is apparent even in asymptomatic and non‐hypoxemic individuals.
3.2. Contribution of sMAdCAM and IL‐6 to COVID‐19 associated lymphopenia
In an effort to understand factors influencing the observed cellular depletion and dysfunction in our cohort we evaluated plasma markers that could influence inflammation, mucosal leukocyte migration, and homeostasis. We thus undertook the evaluation of circulating IL‐6 and sMAdCAMlevels in our cohort. Indeed, with respect to the latter, we have recently highlighted a role for the modulation of sMAdCAM and expression of its receptor integrin α4β7 present on monocytes, lymphocytes in the context of HIV pathogenesis. 34 As expected, we observed elevated IL‐6 levels, with the highest being observed in hypoxemic individuals, in plasma of all groups compared to seronegative controls (Figure 3A). Additionally, extending our previously reported results 23 obtained with sMAdCAM in mild infection, we observed a progressive decline in these levels across mild as well as hypoxemic patients that seemed converse to the pattern observed for IL‐6 (Figure 3B). Indeed, the levels of these 2 markers were significantly negatively correlated (Figure 3C). Next, correlation analysis was undertaken to delineate putative relationships between the aforementioned soluble markers and cellular subsets described above (Supplementary Figure S3). Intriguingly and reflective of their apparently divergent relationship, both IL‐6 and sMAdCAM showed significant opposing correlations with absolute counts of lymphocytes, T cells (CD4+ and CD8+), B cells, and NK cells supporting their clear, albeit, opposing roles in COVID‐19 associated lymphopenia (Figure 3 D‐I). We also noted a heretofore unreported positive correlation of sMAdCAM levels with CD8+ effector memory T cell counts (Supplementary Figure S4A). This observation concurs with recent data 36 demonstrating lower counts of CD8+ memory T cellsexpressing receptors for sMAdCAM (integrin α4β7) in COVID‐19. Taken together, these results highlight a clear role for altered mucosal homing in the pathogenesis of this disease. Notably, elevated circulating LPS (a marker for gut inflammation), in the context of SARS‐CoV‐2 infection, has only been reported for severe and ICU patients. 37 In our study we also observed increased plasma LPS levels in hypoxemic individuals compared to seronegative controls (Supplementary Figure S4B) as opposed to our previous work with a cohort of mild COVID‐19. 35 Furthermore, and possibly related to altered monocyte frequencies observed ex vivo (Figure 2G), a negative correlation of sMAdCAM levels occurred with frequencies of intermediate monocytes (Supplementary Figure S4C). Importantly, there was no significant correlation between circulating LPS levels with sMAdCAM (Supplementary Figure S4D). These results suggest that the altered trajectory of sMAdCAM levels is less related to gut persistence mediated inflammation and more to altered mucosal leukocyte migration. Taken together, our results show that IL‐6 and sMAdCAM may contribute towards disparate aspects of disease progression where circulating levels of the former are more closely related to inflammatory sequelae and development of anti‐viral B cell responses 38 , 39 and those of the latter with the restoration of mucosal homeostasis of leukocytes.
FIGURE 3.
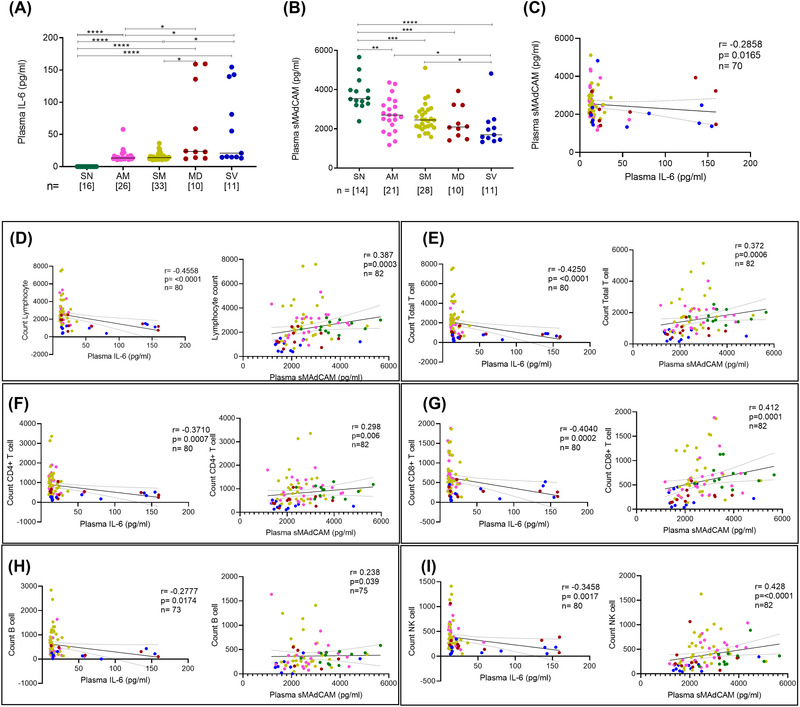
Evaluation of IL‐6 and sMAdCAM along with correlation of cellular immune subsets in study participantsVariation in levels of (A) IL‐6 and (B) sMAdCAM in seronegative(SN), asymptomatic mild (AM), symptomatic mild (SM), moderate (MD) and severe (SV) groups. Relationship between (C) IL‐6 and sMAdCAM and their association with different cellular subsets (D) Lymphocyte count (E) Total T cell count (F) CD4+ T cell count (G) CD8+ T cell count (H) B cell count (I) NK cell count. Statistical significance was calculated by non‐parametric one‐way ANOVA (Kruskal–Wallis test) followed by post hoc Dunn'stest; *, p < 0.05; **, p < 0.01, ***,p< 0.001; and ****,p < 0.001. Correlation analysis was performed using non‐parametric Spearman Rank Correlation test
3.3. Plasma IL‐15 levels discriminate symptomatic and AM COVID‐19
While most immune markers of pathogenesis in COVID‐19 have been reported to be associated with mild, moderate, and severe disease, a marker that clearly discriminates between symptomatic and asymptomatic pathogenesis remains elusive. Having observed distinct depletion profiles of CD4+ and CD8+ T cells, the relative sparing of CD8+ effector memory T cells, and non‐T cell lymphopenia (B and NK cells) we surmised that IL‐15, a major homeostatic and activation cytokine for these subsets needed to be evaluated in COVID‐19 pathogenesis. Thus a set of 10 samples, randomly selected from each were assayed for plasma IL‐15 levels. Also, a single additional sample from the (AM) group was included due to a particularly high level of IL‐6 observed in this individual (Figure 3A). We observed elevated levels of circulating IL‐15 in lymphopenic, hypoxemic individuals and remarkably, as shown in Figure 4A, in symptomatic mildbut, not asymptomatic patients. All hypoxemic individuals had detectable levels of IL‐15 followed by 70% of individuals with symptomatic infection and only 18% (2 out of 11) with asymptomatic infection (Figure 4B). Indeed, of the 2 asymptomatic individuals that had detectable levels of IL‐15 at sampling, one later progressed to hypoxemia. No individuals within the SN control group had detectable levels of plasma IL‐15. Our study is the first to demonstrate the presence of plasma IL‐15 as such a marker where detection of this cytokine is clearly segregated based on the symptomatic status of patients. We observed significant negative correlations between depleted subsets (T, B, NK) and circulating IL‐15 levels across all groups of COVID‐19 affected individuals (Figure 4C‐H and Supplementary Figure S3) indicative of its role in homeostatic restorative production 40 , 41 , 42 in the face of systemic depletion of these subsets. Also, as observed for IL‐6, sMAdCAM levels were negatively correlated with those of IL‐15 (Figure 4I).
FIGURE 4.
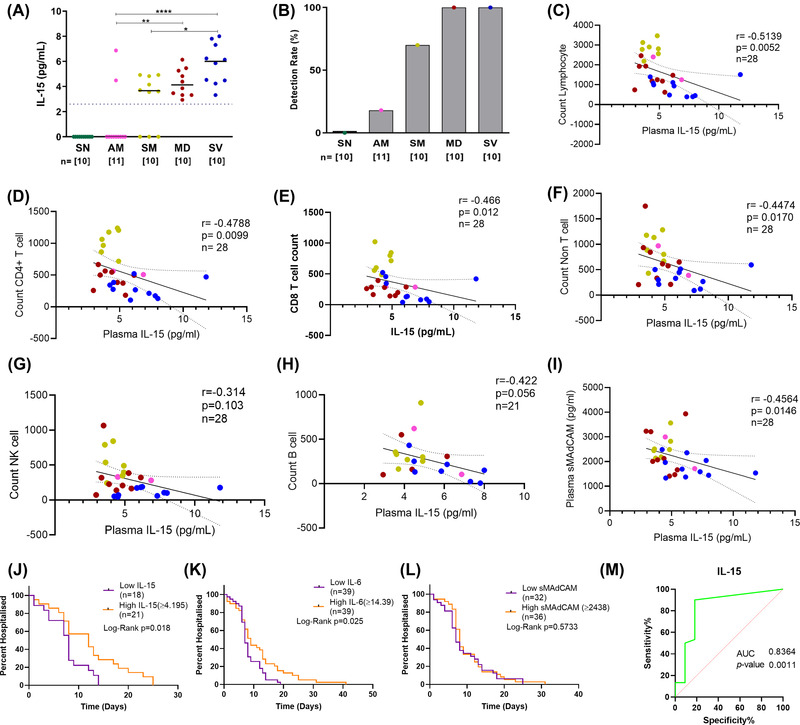
Distribution of IL‐15 and its correlation with immune cells among study participants. (A) Variation in levels of IL‐15 in Seronegative (SN), asymptomatic mild (AM), symptomatic mild (SM), moderate (MD), and severe (SV) groups. The dotted line indicates the limit of detection. (B) Responder rate. Association of IL‐15 and with different cellular subsets (C) Lymphocyte count (D) Total T cell count (E) CD4+ T cell count (F) CD8+ T cell count (G) B cell count (H) NK cell count (I) Soluble MAdCAM. (J‐L) Kaplan Meier plots depicting hospitalization duration post recruitment associated with plasma levels of (J) IL‐15 (K) sMAdCAM and (L) IL‐6. Individuals were categorized into a low and a high group on the basis of median levels. (M) Receiver operating characteristic (ROC) curve analysis of IL‐15 level to discriminate between asymptomatic(AM) and symptomatic (SM, MD, and SV)COVID‐19 infected patients.Area under curve(AUC)0.8364; 95%CI 0.6716 to 1.000; P = 0.0011. Statistical significance was calculated by non‐parametric one‐way ANOVA (Kruskal–Wallis test) followed by post hoc Dunn'stest; *, p < 0.05; **, p < 0.01, ***,p < 0.001; and ****,p < 0.001. Correlation analysis was performed using the non‐parametric Spearman Rank Correlation test. For Kaplan Meier comparisons, Log‐rank (Mantel‐Cox) test was used
Finally, in a rudimentary analysis, we evaluated the possible prognostic value of our single determinations of IL‐15 together with IL‐6 and sMAdCAM in predicting the duration of hospitalization independently. For this analysis, days of hospitalization post sampling, including those from individuals with undetectable values for these markers (considered as 0) were plotted as shown in Figure 4J‐L. Participants were grouped into Low and High categories based on median values of these markers. Proportional hazards analysis revealed significantly longer hospitalization for individuals in the high IL‐15 and IL‐6 groups. This value was above >4.20 pg/ml of IL‐15 and >14.39 pg/ml for IL‐6.Next, an ROC analysis was undertaken with the assumption(as discussed above) that detectable IL‐15 levels can differentiate between symptomatic COVID‐19 infection and asymptomatic infection. We considered asymptomatic participants as a‘control group'compared to symptomatic (including moderate and severe) study participants. We are happy to report that ROC analysis showed significant discrimination based onplasma IL‐15 levels (AUC = 0.8364;p= 0.0011) between these groups(Figure 4 M). Circulating IL‐15 levels have been implicated as a contributory factor to hospitalization time, disease severity, and mortality in some settings. 43 , 44 Indeed, preliminary analysis in our cohort, with respect to days of hospitalization supported this finding. Our study further demonstrates the utility of using it as an early biomarker predicting progression to symptomatic and severe disease.
In summation, our results posit a role for inflammatory, homeostatic, and migratory immune mediators in COVID‐19 associated lymphopenia, of which, plasma IL‐15 detection may be an early prognostic marker.
AUTHOR CONTRIBUTION
Sample processing, performing experiments, data analysis, and figure generation (Amit Kumar Singh, NandiniKasarpalkar, ShilpaBhowmick). Participant recruitment, sample processing, and data analysis (Gaurav Paradkar, MayurTalreja). Sample processing (Abhishek Tiwari, HarshaPalav, SnehalKaginkar).Participantrecruitment (Rajiv Kulkarni, Karan Shah). Sample collection (AshwiniPatil, VarshaKalsurkar). Performed experiments and Data analysis (Sachee Agrawal, Jayanthi Shastri).Scholarly advice (Rajesh Dere, Ramesh Bharmal, SmitaMahale). Conceived study, analysed data, compiled results, and wrote manuscript (Vikrant Bhorand Vainav Patel). All authors revised the manuscript and gave final approval for publication.
Supporting information
Supplementary informaion
ACKNOWLEDGEMENT
We are extremely grateful to Director General, Indian Council of Medical Research (ICMR), Ministry for Health & Family Welfare, Government of India for encouraging and supporting with resources, the pursuit of important research questions relevant to COVID‐19 pathogenesis. We would also like to thank Dr.NooraPathan, Dr. Mahesh Chaurasia, Dr.RasikaSatpute, and Dr.DharmeshBalsarkar for facilitating the recruitment of study participants and Mr. OmkarArekar for the assistance provided in data entry. Also, none of this work would have been possible without the active participation and support of participants to whom we are grateful.
Singh Amit Kumar, Kasarpalkar Nandini, Bhowmick Shilpa, et al. Opposing roles for sMAdCAM and IL‐15 in COVID‐19 associated cellular immune pathology. J Leukoc Biol. 2022;111:1287–1295. 10.1002/JLB.3COVBCR0621-300R
Amit Kumar Singh, Nandini Kasarpalkar, and Shilpa Bhowmick: Joint first authors.
Vikrant M. Bhor and Vainav Patel: Joint Corresponding authors.
Funding information
We acknowledge Institutional intramural support from ICMR‐NIRRH and support from DBT Wellcome Trust India Alliance Team Science Grant IA/TSG/19/1/600019
Contributor Information
Vikrant M. Bhor, Email: bhorv@nirrh.res.in.
Vainav Patel, Email: patelv@nirrh.res.in.
REFERENCES
- 1. COVID‐19 Map ‐ Johns Hopkins Coronavirus Resource Center.
- 2. MoHFW. MoHFW Home . Minist. Heal. Fam. Welfare, Gov. India 2020;
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. www.thelancet.com 2020;395:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA ‐ J Am Med Assoc. 2020;324:782‐793. [DOI] [PubMed] [Google Scholar]
- 5. WHO . Clinical management clinical management : living guidance COVID‐19. World Heal. Organ. 2021; [Google Scholar]
- 6. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020;383:1757‐176. 10.1056/nejmcp2009249. [DOI] [PubMed] [Google Scholar]
- 7. Ministry of Health and Family Welfare . Clinical management protocol : COVID‐19. Version 32020;13.
- 8. Huang I, Pranata R. Lymphopenia in severe coronavirus disease‐2019 (COVID‐19): systematic review and meta‐analysis. J Intensive Care. 2020;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arunachalam PS, Wimmers F, Mok CKP, et al. Systems biological assessment of immunity to mild versus severe COVID‐19 infection in humans. Science. 2020;369:1210‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID‐19 patients reveals distinct immunotypes with therapeutic implications. Science (80‐ ). 2020;369:eabc8511. 10.1126/SCIENCE.ABC8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamers MM, Beumer J, Van Der Vaart J, et al. SARS‐CoV‐2 productively infects human gut enterocytes. Science (80‐ ). 2020;369:50‐54. 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Livanos AE, Jha D, Cossarini F, et al. Intestinal host response to SARS‐CoV‐2 infection and COVID‐19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021;160:2435‐2450.e34. 10.1053/j.gastro.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jagtap D, Bhor VM, Bhowmick S, et al. sMAdCAM: iL‐6 ratio influences disease progression and anti‐viral responses in SARS‐CoV‐2 infection. Front Immunol. 2021;12:619906. 10.3389/fimmu.2021.619906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tong M, Jiang Yu, Xia Da, et al. Elevated expression of serum endothelial cell adhesion molecules in COVID‐19 patients. J Infect Dis. 2020;222:894‐898. 10.1093/infdis/jiaa349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spadaro S, Fogagnolo A, Campo G, et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID‐19 ICU patients. Crit Care. 2021;25:74. 10.1186/s13054-021-03499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calabretta E, Moraleda JM, Iacobelli M, et al. COVID‐19‐induced endotheliitis: emerging evidence and possible therapeutic strategies. Br J Haematol. 2021;193:43‐51. 10.1111/bjh.17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirano T. IL‐6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33:127‐148. 10.1093/intimm/dxaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peters MC, Mcgrath KW, Hawkins GA, et al. Plasma interleukin‐6 concentrations, metabolic dysfunction, and asthma severity: a cross‐sectional analysis of two cohorts. Lancet Respir Med. 2016;4:574‐584. 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID‐19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verbist KC, Klonowski KD. Functions of IL‐15 in anti‐viral immunity: multiplicity and variety. Cytokine. 2012;59:467‐478. 10.1016/j.cyto.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang M, Wen B, Anton OM, et al. IL‐15 enhanced antibody‐dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc Natl Acad Sci U S A. 2018;115:E10915‐E10924. 10.1073/pnas.1811615115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS‐CoV infection in humans is associated with a pro‐inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8‐13. 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh AK, Salwe S, Padwal V, et al. Delineation of homeostatic immune signatures defining viremic non‐progression in HIV‐1 infection. Front Immunol. 2020;11:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID‐19). Front Immunol. 2020;11:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen G, Wu Di, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang W, Berube J, Mcnamara M, et al. Lymphocyte subset counts in COVID‐19 patients: a meta‐analysis. Cytom Part A. 2020;97:772‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Padgett LE, et al. Interplay of monocytes and T lymphocytes in COVID‐19 severity. bioRxiv. 2020. 10.1101/2020.07.17.209304. bioRxiv. [DOI] [Google Scholar]
- 29. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541‐566. [DOI] [PubMed] [Google Scholar]
- 30. Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013;121:29‐37. 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- 31. Galván‐Peña S, et al. Profound Treg perturbations correlate with COVID‐19 severity. bioRxiv. 2020. 10.1101/2020.12.11.416180. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kennedy JJ, Steain M, Slobedman B, Abendroth A. Infection and functional modulation of human monocytes and macrophages by varicella‐zoster virus. J Virol. 2018;93. 10.1128/jvi.01887-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prabhu VM, Singh AK, Padwal V, Nagar V, Patil P, Patel V. Monocyte based correlates of immune activation and viremia in HIV‐infected long‐term non‐progressors. Front Immunol. 2019;10:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kasarpalkar NJ, Bhowmick S, Patel V, et al. Frequency of effector memory cells expressing integrin α 4 β 7 is associated with TGF‐ β 1 levels in therapy naïve HIV infected women with low CD4 + T cell count. Front Immunol. 2021;12:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jagtap D, et al. sMAdCAM:iL‐6 ratio influences disease progression and anti‐viral responses in SARS‐CoV‐2 infection Dhanashree. medRxiv. 10.1101/2020.10.13.2018294. published online ahead of print: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Müller TM, Becker E, Wiendl M, et al. Circulat0ing adaptive immune cells expressing the gut homing marker α4β7 integrin are decreased in COVID‐19. Front Immunol. 2021;12:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arunachalam PS, Wimmers F, Mok CKP, et al. Systems biological assessment of immunity to mild versus severe COVID‐19 infection in humans. Science. 2020;6261:1‐18. Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dienz O, Eaton SM, Bond JP, et al. The induction of antibody production by IL‐6 is indirectly mediated by IL‐21 produced by CD4 + T cells. J Exp Med. 2009;206:69‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eto D, Lao C, Ditoro D, et al. IL‐21 and IL‐6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bergamaschi C, Rosati M, Jalah R, et al. Intracellular interaction of interleukin‐15 with its receptor α during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283:4189‐4199. [DOI] [PubMed] [Google Scholar]
- 41. Inoue S, Unsinger J, Davis CG, et al. IL‐15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401‐9. 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kandikattu HK, Venkateshaiah SU, Kumar S, Mishra A. IL‐15 immunotherapy is a viable strategy for COVID‐19. Cytokine Growth Factor Rev. 2020;54:24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abers MS, Delmonte OM, Ricotta EE, et al. An immune‐based biomarker signature is associated with mortality in COVID‐19 patients. JCI Insight. 2021;6:e144455. 10.1172/jci.insight.144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Angioni R, Sánchez‐Rodríguez R, Munari F, et al. Age‐severity matched cytokine profiling reveals specific signatures in Covid‐19 patients. Cell Death Dis. 2020;11:957. 10.1038/s41419-020-03151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary informaion


