To the Editor,
Zika virus (ZIKV) disease (ZVD) is considered to be one of the significant public health diseases of concern post‐2016 outbreak in Brazil. 1 A mosquito‐borne flavivirus had been reported to be associated with the increased incidence of microcephaly, congenital Zika syndrome, and Guillain‐Barre syndrome. Since its discovery from the Zika forest in Uganda in 1947, several outbreaks of ZVD have been reported from Africa, Southeast Asia, and the Pacific Islands. Besides this, numerous travels associated cases of Zika have been also reported from various countries. 1 India has reported the first case of ZVD from Gujarat. Subsequently, few sporadic cases (Gujarat, Tamil Nadu) and outbreaks of ZVD have been reported from Rajasthan and Madhya Pradesh states during 2017–2018. 2 Recently, more than 100 cases Zika have been detected and confirmed with real‐time reverse transcriptase‐polymerase chain reaction (rRT‐PCR) from the Uttar Pradesh state of India during October–November 2021. 3 The present study reports the detection of ZVD cases amongst healthcare workers in a private hospital of Thiruvananthapuram district, Kerala state, India.
In the midst of the second wave of the COVID‐19 pandemic, an increase in the number of undiagnosed exanthematous fever was noticed among the patients visiting the outpatient department and health care workers of the private hospital in Thiruvananthapuram during the month of May 2021. The health care workers with similar clinical manifestations were screened for the ease of sampling and further follow‐up. During 20–21st May 2021, a total of 19 health care workers (18 female and 1 male, the median age of 24 years; interquartile range: 20–52 years) reported at a median post‐onset date of 4 days with maculopapular morbilliform rash involving the face, trunk and upper limb (94.74%), mild fever (68.42%), myalgia (47.37%), arthralgia (26.32%), conjunctival congestion (26.32%), sore throat (31.58%), headache (15.79%), rhinitis (5.26%), and posterior cervical lymphadenopathy (5.26%). The cases showed mildly raised C‐reactive protein and one case typically showed lymphocytosis (Table 1). The cases were treated with antipyretic and antiallergic drugs for managing the symptoms. All the cases were mild and had no complications during the course of illness. The illness was self‐limiting and the rash subsided within 2–5 days of onset. All the health care workers were vaccinated for measles, mumps, and rubella.
Table 1.
Clinical details of the cases investigated for Zika virus disease from Thiruvananthapuram Kerala India 2021
| Cases investigated for Zika | Age/sex | Day of onset | Post‐onset date | Zika real time RT‐PCR | Zika IgM ELISAa | Clinical presentation | Haematological investigations | Date of recovery | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever (mild) | Maculo‐papular rash | Sore throat | Pruritis | Myalgia | Arthralgia | Rhinitis | Headache | Conjunctival congestion | Posterior cervical lymphadenopathy | Hemoglobin (12–15 gm/dl) | Total leucocyte count (4000–10 000/μl) | Acute neutrophil count (2000–7000/μl) | Acute lymphocyte count (1000–3000/μl) | Platelet (150 000–450 00/μl) | C‐reactive protein (<2 mg/dl) | |||||||
| Case‐1 | 24/F | 01/05/21 | 20 | Neg | Pos | + | + | + | _ | _ | _ | _ | _ | _ | 18/05/21 | |||||||
| Case‐2b, c | 28/F | 17/05/21 | 5 | Pos | Pos | + | + | _ | _ | _ | _ | _ | _ | 21/05/21 | ||||||||
| Case‐3d | 30/F | 15/05/21 | 7 | Pos | Pos | + | + | + | + | 13.8 | 4400 | 2525 | 1288 | 275 000 | 2 | 20/05/21 | ||||||
| Case‐4 | 26/F | 16/05/21 | 6 | Neg | Neg | + | + | + | + | + | + | + | 13.8 | 7200 | 3557 | 2673 | 242 000 | 2 | 31/05/21 | |||
| Case‐5d | 40/F | 15/05/21 | 7 | Pos | Pos | + | 10.8 | 4400 | 2907 | 1033 | 255 000 | 1.7 | 20/05/21 | |||||||||
| Case‐6e | 33/M | 16/05/21 | 6 | Pos | Pos | + | + | + | + | 15 | 4200 | _ | _ | 156 000 | 4.6 | 26/05/21 | ||||||
| Case‐7d | 37/F | 18/05/21 | 4 | Pos | Pos | + | + | + | _ | _ | _ | _ | _ | _ | 22/05/21 | |||||||
| Case‐8e | 21/F | 18/05/21 | 4 | Pos | Neg | + | + | + | + | _ | _ | _ | _ | _ | _ | 24/05/21 | ||||||
| Case‐9f | 28/F | 16/05/21 | 6 | Pos | Pos | + | + | 11.9 | 6200 | 4577 | 1023 | 283 000 | 3.5 | _ | ||||||||
| Case‐10g | 28/F | 16/05/21 | 6 | Pos | Neg | + | + | + | + | + | _ | 5400 | 2828 | 8173 | 189 000 | 0.7 | 21/05/21 | |||||
| Case‐11 | 52/F | 19/05/21 | 3 | Neg | Not done | + | + | + | + | + | 12.1 | 3900 | 2415 | 1055 | 223 000 | 10.1 | 23/05/21 | |||||
| Case‐12e | 24/F | 20/05/21 | 2 | Pos | Neg | + | + | + | _ | _ | _ | _ | _ | _ | 24/05/21 | |||||||
| Case‐13g | 28/F | 20/05/21 | 2 | Pos | Neg | + | + | _ | _ | _ | _ | _ | _ | 24/05/21 | ||||||||
| Case‐14g | 20/F | 20/05/21 | 2 | Pos | Pos | + | + | + | _ | _ | _ | _ | _ | _ | 24/05/21 | |||||||
| Case‐15 | 24/F | 21/05/21 | 1 | Neg | Neg | + | + | + | + | + | 12.7 | 6900 | _ | _ | 210 000 | _ | 24/05/21 | |||||
| Case‐16d | 24/F | 20/05/21 | 2 | Pos | Pos | + | + | + | 15.5 | 5000 | _ | _ | 208 000 | _ | 26/05/21 | |||||||
| Case‐17 | 23/F | 21/05/21 | 1 | Neg | Pos | + | + | + | + | 12 | 4200 | 1706 | 1851 | 205 000 | _ | 21/05/21 | ||||||
| Case‐18 | 29/F | 21/05/21 | 1 | Neg | Neg | + | + | _ | _ | _ | _ | _ | _ | 26/05/21 | ||||||||
| Case‐19g | 23/F | 21/05/21 | 1 | Pos | Not done | + | + | + | _ | _ | _ | _ | _ | _ | 24/05/21 | |||||||
Note: Cases 6, 12, and 13 had dual infection of EBV and ZIKV.
Abbreviations: –, data not available; +, symptoms present; ELISA, enzyme‐linked immunoassay; F, female; IgM, immunoglobulin M; M, male; Neg, negative; Not done, sample was insufficient to perform the assay; Pos, positive; RT‐PCR, reverse transcriptase‐polymerase chain reaction.
Serum samples positive for Zika IgM antibodies by ELISA needs confirmation by neutralization assay.
Urine samples positive for Zika virus by qRT‐PCR.
Antenatal mother with 6 weeks of gestation when detected positive for Zika virus by qRT‐PCR.
EDTA and urine samples positive for Zika virus by qRT‐PCR.
Urine, EDTA blood and serum positive for Zika virus by qRT‐PCR.
Only EDTA positive for Zika virus by qRT‐PCR.
Only serum sample positive for Zika virus by qRT‐PCR.
The clinical specimens (oropharyngeal/nasopharyngeal swabs, serum, EDTA blood, and urine) of these 19 cases were referred to the Indian Council of Medical Research–National Institute of Virology, Pune, India on May 25, 2021 for further virological investigation. All the cases were screened for SARS‐CoV‐2, Measles, Rubella, Enterovirus, Parechovirus (HpeV), human alphaherpesvirus 1 & 2, human alphaherpesvirus 3, human betaherpesvirus 5, human betaherpesvirus 6A & 6B & 7, human gammaherpesvirus 4, human mastaadenovirus A to G, and primate erythroparvovirus 1 by rRT‐PCR. 4 , 5 Out of 19 cases, three cases were found be positive for EBV. On June 9, 2021, a 24‐year‐old pregnant lady from Thiruvananthapuram, presenting with typical symptoms was confirmed to be affected with ZIKV with CDC Trioplex RT‐qPCR (Dengue, Chikungunya, and Zika). 5 This positive Zika case triggered us to retrospectively screen the clinical specimens of all the 19 cases for ZIKV using CDC Trioplex RT‐qPCR. 6
Of all the cases, 13 cases were tested positive for Zika viral RNA (C t ranged: 26.75–37.47) and 10 cases were positive for anti‐Zika IgM antibodies (Table 1). Case‐2 (28 years) reported a spontaneous abortion at 6 weeks of gestation, 21 days after the onset of symptoms. Considering the spontaneous abortion in the very early weeks of pregnancy, the microcephaly in the aborted fetus was difficult to rule out. The complete genomes (>97%) could be retrieved from two Zika positive samples (MCL‐21‐H‐8900 and MCL‐21‐H‐8901) using next‐generation sequencing. The ZIKV strains MCL‐21‐H‐8900 and MCL‐21‐H‐8901 have 99.33% and 99.4% nucleotide similarities with Zika strain from Rajasthan, India (Accession number: MK238037.1), respectively (Figure 1). The three cases which were positive for Epstein–Barr virus (C t value range: 33–35) also had confection with ZIKV.
Figure 1.
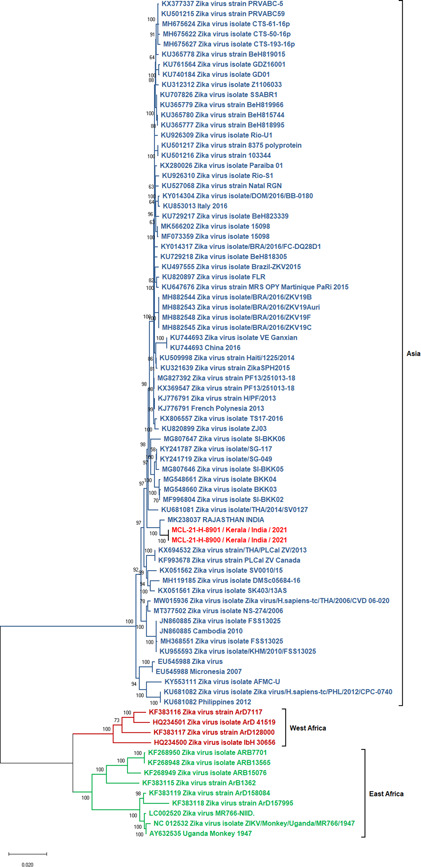
Maximum‐likelihood phylogenetic tree of genome sequences retrieved from two ZIKV positive cases reported in Kerala, India during 2021. ZIKV, Zika virus
This is the first report on confirmation of ZIKV circulation in Kerala state. The cases had no travel history within the last 1 month. Apparently, none of the family members of the cases had a similar clinical presentation. The cases which were sporadically reported during this period probably suggest circulation of the ZIKV in the community as well. The detection of ZIKV amidst the COVID‐19 pandemic has added an extra burden on the public health system of Kerala state. The cocirculation and detection of flavivirus and SARS‐CoV‐2 have been reported in flavivirus endemic areas. 7 , 8 The recent third serosurvey of COVID‐19 by the Indian Council of Medical Research, India demonstrated high seroprevalence in Kerala state (11.6%) compared to the national average of 21%. 9 This data demonstrates the effectiveness of robust surveillance adopted by the state. Similarly, the preparedness and active surveillance of the public health system of Kerala state has helped to timely identify the Zika cases among the healthcare workers. The finding of the study suggests the inclusion of arboviral disease in testing algorithms. In conclusion, the findings of the investigation demonstrate the need for active human and entomological surveillance of Zika across the country to mitigate future outbreaks.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Pragya D. Yadav, Rima R. Sahay, Vettakkara Kandy Muhammed Niyas contributed to study design, data analysis, interpretation, and writing and critical review. Gajanan N. Sapkal, Rajalakshmi Arjun, Anita M. Shete, Deepak Y. Patil, Shailesh D. Pawar contributed to data collection and interpretation. Nivedita Gupta and Priya Abraham contributed to the critical review and finalization of the paper.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the encouragement and support extended by Prof. (Dr.) Balram Bhargava, Secretary to the Government of India Department of Health Research, Ministry of Health & Family Welfare & Director‐General, ICMR New Delhi. The authors are also thankful to the staff of the Indian Council of Medical Research (ICMR)–National Institute of Virology, Pune including Dr. Abhinendra Kumar, Scientist B, Mrs. Savita Patil, Mrs. Triparna Majumdar, Ms. Jyoti Yemul, Mrs. Kaumudi Kalele for extending excellent technical support. Authors are also thankful to the Infection Control Team of KIMSHEALTH, Thiruvananthapuram (Aisha Mubarak, Sreerekha R. Nair, and Bineetha Viswanathan) and Jeffrey Jomes, Nurse practitioner, Infectious Diseases, KIMSHEALTH, Thiruvananthapuram, for their help in data collection. Financial support was provided by the ICMR, New Delhi at ICMR–National Institute of Virology, Pune.
Pragya D. Yadav and Vettakkara K. Muhammed Niyas are equal first authors.
REFERENCES
- 1. Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet. 2017;390(10107):2099‐2109. [DOI] [PubMed] [Google Scholar]
- 2. Gupta N, Yadav PD, Patil DY, Sapkal G. Preparedness of public health‐care system for Zika virus outbreak: an Indian perspective. J Infect Public Health. 2020;13(7):949‐955. [DOI] [PubMed] [Google Scholar]
- 3. Scroll.in . Inside Uttar Pradesh's Zika outbreak: can India's most populous state contain the virus spread? 2021. Accessed November 30, 2021. https://scroll.in/article/1010555/inside-uttar-pradeshs-zika-outbreak-can-indias-most-populous-state-contain-the-virus-spread
- 4. Choudhary M, Vipat V, Jadhav S, et al. Development of in vitro transcribed RNA as positive control for laboratory diagnosis of SARS‐CoV‐2 in India. Indian J Med Res. 2020;151(2‐3):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee CK, Chai CN, Capinpin SM, et al. Evaluation of the Luminex ARIES HSV 1&2 Assay and comparison with the FTD Neuro 9 and in‐house real‐time PCR assays for detecting herpes simplex viruses. Ann Lab Med. 2018;38(5):440‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santiago GA, Vázquez J, Courtney S, et al. Performance of the Trioplex real‐time RT‐PCR assay for detection of Zika, dengue, and chikungunya viruses. Nat Commun. 2018;9(1):1‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masyeni S, Santoso MS, Widyaningsih PD, et al. Serological cross‐reaction and coinfection of dengue and COVID‐19 in Asia: experience from Indonesia. Int J Infect Dis. 2021;102:152‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harapan H, Ryan M, Yohan B, et al. Covid‐19 and dengue: double punches for dengue‐endemic countries in Asia. Rev Med Virol. 2021;31(2):e2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Department of Health & Family Welfare, Government of Kerala. Technical paper COVID 19 ICMR—Serological Surveillance Report. 2021. Accessed November 30, 2021. https://health.kerala.gov.in/pdf/Technical-paper-COVID-19-Sero-Surveillance-Round-3-ICMR.pdf


