Abstract
The entire world has been suffering from the coronavirus disease 2019 (COVID‐19) pandemic since March 11, 2020. More than a year later, the COVID‐19 vaccination brought hope to control this viral pandemic. Here, we review the unknowns of the COVID‐19 vaccination, such as its longevity, asymptomatic spread, long‐term side effects, and its efficacy on immunocompromised patients. In addition, we discuss challenges associated with the COVID‐19 vaccination, such as the global access and distribution of vaccine doses, adherence to hygiene guidelines after vaccination, the emergence of novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) variants, and vaccine resistance. Despite all these challenges and the fact that the end of the COVID‐19 pandemic is still unclear, vaccines have brought great hope for the world, with several reports indicating a significant decline in the risk of COVID19‐related infection and hospitalizations.
Keywords: COVID‐19, COVID‐19 vaccination, global assessment, global challenges, herd immunity, SARS‐CoV‐2
Highlights
-
1.
Vaccination plays a pivotal role in reducing the death toll of the COVID‐19 infection.
-
2.
The immunity provided by vaccines is still disputable leading to some challenges like the asymptomatic spread; therefore, staying strict to hygiene guidelines is necessary.
-
3.
Hence, COVID‐19 vaccines' related long‐term side effects are still unknown; cautions should be taken when injected to various individuals of the community.
-
4.
COVID‐19 vaccines' effectiveness are strongly related to their effect on emergent COVID‐19 variants, but until now approved vaccines can still show some protective effects against new variants.
1. INTRODUCTION
The outbreak of coronavirus disease (COVID)‐19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was reported for the first time to the World Health Organization by the Chinese authorities in late December 2019. On March 11, the COVID‐19 pandemic was declared. 1 By mid‐November 2021, over 254 million SARS‐CoV‐2 infections were identified, with over 5 million confirmed fatal cases. 2
COVID‐19 has impacted the entire world, which is feeling the full brunt of the current pandemic. It is negatively affecting the international economy from the primary sectors such as agriculture, and petroleum, and oil to the secondary sectors, including the manufacturing industry and the tertiary sectors like education and finance industry. 3
For instance, the social consequences of the pandemic have increased domestic violence, including emotional, physical, and sexual abuse, prejudice toward individuals of Asian descent, adverse changes to dietary habits, as well as substance use and abuse since the beginning of the pandemic. 3 , 4 , 5 , 6
Furthermore, the COVID19 pandemic has also caused psychological and physical burnout of healthcare personnel. Significant changes have also been made in the healthcare facilities to attend to the substantial number of infected patients. 7 , 8
It is worth mentioning, most countries have taken hygiene guidelines and travel restrictions as their primary strategy in curbing this pandemic; however, the effectiveness of this strategy depends on the governments' strictness of these rules and measures and the general public's adherence to them. 9 , 10
Consequently, the remaining potential player against this dilemma is herd immunity, defined as having 50%–85% of the population immunized either by being naturally infected with COVID‐19 or by vaccination.
By November 2021, over twenty vaccines were approved in different parts of the world, including adenoviral vector vaccines, mRNA vaccines, subunit vaccines, vaccines based on inactivated SARS‐CoV‐2, and a DNA vaccine.
Vaccination serves as a two‐edged sword as it is the best tool for curbing this pandemic and bringing ambiguity, uncertainty, and challenges that the world might face during its providence or later unknown long effects. For instance, the data delivered from vaccine clinical trials on safety profile, immunogenicity, and efficacy in preventing symptomatic infection are limited to the context of these trials and not for their massive use. 11 , 12 This review aims to revisit the unknowns, challenges, and hopes of the COVID‐19 vaccination program and potential measures to overcome them.
2. THE ROAD TO COVID‐19 VACCINES
Cases of COVID‐19 were first reported in late December 2019 in the city of Wuhan in China's Hubei province. 13 Till October 2020, there were 201 Covid‐19 vaccine candidates. However, only 45 of the known vaccine candidates were in the preclinical phase. 14 From the latest WHO report published in February 2021.
A total of 74 and 184 vaccines are in clinical and preclinical development, respectively. Among them, 24 (32%) are protein subunit vaccines, and only 1% is live attenuated vaccine. 15
Different components of SARS‐Cov2 are potential vaccine targets. To better understand the road to the COVID‐19 vaccine development, we must first define all stages involved in developing a vaccine. The entire world is experiencing tremendous difficulties through the challenge of developing vaccines in merely 1–2 years, whereas a complete cycle of vaccine development requires 10–15 years. 16
There are six primary stages in vaccine development: (a) Preclinical; (b) Clinical‐phase1; (c) Clinical‐phase2; (d) Clinical‐phase3; (e) approval; and (f) manufacturing postmarketing surveillance. 16 For COVID‐19 vaccines, some of these phases were merged, and the final step was shortened for the timely provision of the vaccines. Thus, the WHO Emergency Use Listing (EUL) and the United States Food and Drug (FDA) Emergency Use Approval (EUA) were established to bridge the gap between the rapid manufactured unlicensed vaccines and the urgent need for safe and efficient vaccines against the COVID‐19 pandemic. For getting a EUL or a EUA, vaccine manufacturers should submit all data related to their product's trials to the WHO or the FDA for approval. In December 2020, the first FDA's EUA was issued for the Pfizer‐BioNTech COVID‐19 vaccine, aka BNT162b2. 17
Currently, eight vaccines have the WHO's EUA which their technologies are as follows; mRNA‐based vaccines using the selected modified sequences of spike protein gene, for example, mRNA‐1273 (Moderna) and the BNT162b2 (BioNTech/Pfizer) 18 ; then the non‐replicating adenovirus vector‐based DNA vaccines, for example, AZD1222/ChAdOx1 (Oxford/AstraZeneca), JNJ‐78436735/AD26.COV2.S (Janssen/Johnson & Johnson), Ad5‐nCoV (Cansino Biologics), and ChAdOx1_nCoV19 (Covishield) 19 ; finally, the inactivated virus vaccines, for example, BIBP‐CorV (Sinopharm) and CoronaVac (Sinovac Biotech). 20
In addition, other vaccines are widely used, but they merely have national approval, like Sputnik V (Gamaleya Research Institute), NVX‐CoV2373 (Novavax), and Covaxin (Bharat Biotech). 15 For more information on vaccines with either national or EUL approval (Table 1).
Table 1.
Vaccines with national and EUL approval
| Manufacturer/WHO EUL holder | Name of vaccine | NRA of record | Technology | EOI acception |
|---|---|---|---|---|
| BioNTech/Pfizer | BNT162b2 | EMA | mRNA‐based | Accepted |
| Moderna | mRNA‐1273 | EMA | mRNA‐based | Accepted |
| Oxford/AstraZeneca | AZD1222/ChAdOx1 | EMA | non‐replicating viral vector | Accepted |
| Covishield | ChAdOx1_nCoV19 | DCGI | non‐replicating viral vector | Accepted |
| Janssen/Johnson & Johnson | JNJ‐78436735/AD26.COV2.S | EMA | non‐replicating viral vector | Accepted |
| Cansino Biologics | Ad5‐nCoV | NMPA | non‐replicating viral vector | Accepted |
| Sinopharm | BIBP‐CorV | NMPA | Inactivated virus | Accepted |
| Sinovac Biotech | CoronaVac | NMPA | Inactivated virus | Accepted |
| Gamaleya Research Institute of Epidemiology and Microbiology | Sputnik V | Russian NRA | non‐replicating viral vector | Pending |
| Novovax | NVX‐CoV2373/Covovax | EMA | Spike (S)protein subunit | Pending |
| CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI) | CVnCoV/CV07050101(CureVac) | EMA | mRNA‐based | Pending |
| Vector State Research Centre of Virology and Biotechnology | EpiVacCorona | Russian NRA | Peptide antigen | Pending |
| Zhifei Longcom, China | Recombinant Novel Coronavirus Vaccine(CHO Cell) | NMPA | Recombinant protein subunit | Pending |
| IMBCAMS, China | SARS‐CoV‐2 Vaccine, Inactivated (Vero Cell) | NMPA | Inactivated virus | Pending |
| Bharat Biotech, India | COVAXIN | DCGI | Inactivated virus | Pending |
| Clover Biopharmaceuticals | SCB‐2019 | EMA | Spike (S)‐Trimer fusion proteinprotein subunit | Pending |
| BioCubaFarma | Cuba Soberana 01, Soberana 02, and Soberana Plus | CECMED | Spike (S) protein conjugated chemically to meningococcal B or tetanus toxoid or Aluminum | Pending |
| Sinopharm/WIBP | Inactivated SARS‐CoV‐2 Vaccine (Vero Cell) | NMPA | Inactivated virus | Pending |
All the above‐mentioned COVID‐19 vaccines use the viral spike protein as their targeted antigen except for the inactivated vaccines group, which targets the virus as a whole. Likewise, all vaccines induce both cellular and humoral responses with the exception of inactivated vaccines. 16
3. THE UNKNOWNS
3.1. Vaccination longevity
Vaccines are developed to train the immune system for providing immunity against infections. Many vaccines are primarily targeted to prevent disease and may not necessarily protect against specific infections. 21 Even those that provide “sterilizing immunity” may wane in the long run. However, protection against disease or disease progression (severity) may persist due to immune memory. 22 The protective immunity through humoral and cellular response against SARS‐CoV‐2 in humans in natural and experimental settings has not been fully understood since most studies were performed in animal models. 23 However, selected leading vaccines have demonstrated encouraging efficacy rates.
Data suggests that about 95% of subjects retain substantial immune memory fairly stable up to 8 months of natural infection provided by antigen‐specific antibodies, memory B cells, and T cells then decline modestly. 24 Regardless of type, it takes time to develop effective immunity after vaccination. Experts say, even a single dose of any vaccine can provide protection eventually. 25 For example, a study found that the AZD1222 vaccine can provide 76% protection up to 12 weeks after a single dose and subsequently decline the transmission rate by 67%. 26
A recent study revealed that vaccines should have at least 70% efficacy to halt an epidemic and for an ongoing epidemic, 80% efficacy is needed without any other measures when vaccination covers at least 75% of the population. 27 Therefore, preventive measures (social distancing, personal hygiene, handwashing, etc.) and adopting a policy to ensure vaccination coverage would undoubtedly challenge the pandemic in time. More across the border, vaccine trials should be performed to assure the longevity of various vaccines.
3.2. Asymptomatic spread
The median incubation period for COVID‐19 is approximately five days, and those who develop symptoms will do so within 11.5 days. One of the most worrying aspects of this pandemic is the substantial number of undocumented infections (asymptomatic individuals) that facilitate the rapid spread of SARS‐CoV‐2. This imposes a significant public health issue even though it is expected for any pandemic to show a higher frequency of asymptomatic infections. 28 Currently, hundreds of molecular tests and immunoassays have been rapidly developed to diagnose the COVID‐19. If the laboratory testing capacity does not monitor asymptomatic subjects in a given population, these individuals can still contribute to the recurrence and disease spread. 29 They might have the same viral load as symptomatic subjects. 29
Vaccination is well‐known to play an essential role in controlling the spread of infectious diseases. However, the COVID19 is yet unlikely to end, although vaccine programs have advanced in numerous countries. We are currently facing the challenge of scaling up production to attend to the global demand for vaccination doses. A global strategy is still missing to ensure faster affordability and sustainable financing of COVID‐19 vaccines in developing countries that might lack the resources to buy the required quantities of vaccines. Thus, still with a high level of asymptomatic and unvaccinated subjects circulating worldwide, this can pose a challenge to the efficacy of the vaccination in controlling the COVID19 pandemic. In addition, several questions remain to be answered on several levels, such as accurate reinfection risk before or after the second dosage, reinfection possibility regardless of onset yet asymptomatically, and reinfection possibility with the chance to transmit disease to others. 29
COVID‐19 infection is a respiratory disease with viral replication and shedding starting in the upper respiratory airways. Therefore, all of its replication and infectivity stages should be targeted via vaccination to control its infectivity and spread. Despite these challenges, the current COVID‐19 vaccines have been shown to limit viremia and COVID‐19 related syndromes via IgG response induction. However, they lack local mucosal IgA response, which is mainly responsible for the disease transmissibility. 30 Hence, until more knowledge is gathered on the mucosal immunity following systemic vaccination, the virus transmission among asymptomatic vaccinated individuals through droplets cannot be excluded. Thus, still demanding the precaution measures started since the beginning of the pandemic.
In December 2020, the clinical trial with the BNT162b2 vaccine showed 95% protection against infection, and safety was surveilled over two months with similar results to other viral vaccines. 31 This first trial was followed by several studies evaluating the efficacy and safety of other candidate vaccines against COVID19, which adopted SARS‐CoV‐2 confirmed symptomatic cases as their primary efficacy endpoint. 32 However, these studies did not evaluate asymptomatic individuals, 32 posing a great hurdle to scientifically answer whether vaccinated asymptomatic individuals transmit the disease or not. In this context, although significantly lower viral load following the inoculation of mRNA vaccines indicates that vaccination also leads to lower viral transmissibility. However, studies are needed to address this issue. Another critical question that remains unanswered is whether vaccination will limit reinfection by SARS‐CoV‐2 with the same or new variants that have recently emerged. 33 , 34 , 35 In addition, future studies need to be performed to determine if the administration of a seasonal vaccine against COVID19 will be required to avoid the outbreak of a new pandemic.
Overall, it is safe to say that vaccines can limit the intensity of the disease for those who would be severely infected to mild infection or perhaps asymptomatic and possibly reduce viral load in asymptomatic individuals to decrease infectiousness. This will contribute to lowering the pandemic spread but not totally eradicating it as it was learned from previous pandemics.
3.3. Long‐term side effects of COVID‐19 vaccination
A study in the United States found that issues related to safety, efficacy, misleading information, the politicization of scientific themes, the accelerated timeline for vaccine development, the distrust in scientific and medical communities, and longstanding institutional racist practices were identified as the reasons for hesitance and resistance to COVID‐19 vaccines. 36 The most definitive source of this hesitancy is vaccine side effects which can be defined as phenotypic responses of the human organism to drug treatment or simply adverse drug reactions. 37 Side effects are an indication of vaccine antigenicity and immunogenicity; therefore, illustrating the vaccine's success in provoking immune responses. Vaccine side effects can be grouped as short and long‐term based on their time of eruption after vaccination.
On the one hand, currently approved COVID‐19 vaccines reported two types of short‐term side effects: injection site side effects like pain redness and swelling at the injection site and/or associated lymph nodes; and systemic side effects like headache, malaise or joint pain, fever or chills, nausea, and vomiting. 38 , 39
On the other hand, as COVID‐19 vaccines are newly approved and used, their long‐term side effects are still unknown. However, some immunological hypotheses and case reports can provide a possible perspective on these long‐term side effects. SARS‐CoV‐2 spike glycoprotein may mimic some human peptide‐protein sequences; thus, some authors suggested a probability of cross‐reaction. 40 As indicated by Talotta et al. individuals with strong immune responses, especially young women or those who already have a genetic background of autoimmunity, are more vulnerable to autoimmune diseases when being vaccinated with nucleic acid vaccines than other individuals. Nonetheless, this risk is identical to other vaccines. 31 , 41
Subsequently, as explained by Goldman et al., taking adenoviral vector‐based vaccines might induce the production of platelet factor 4 (PF4) autoantibodies resulting in thrombotic thrombocytopenia. This PF4 immunogenicity is facilitated by heparin sulfate proteoglycans released from damaged endothelial cells. 42 However, an exact mechanism via which some COVID‐19 vaccines can induce rare thrombotic thrombocytopenia events remains to be elucidated ‐ some hypotheses to be tested have been recently put forward. Thus, it will be essential to perform future studies to monitor if long‐term side effects of COVID19 vaccination will be developed or not.
3.4. COVID‐19 vaccines for immunocompromised patients
According to recent data, immunocompromised individuals, including subjects with solid organ and hematologic malignancies or undergoing stem cell or solid organ transplantation, as well as patients with inborn errors of immunity or dermatologic and systemic autoimmune diseases, have shown no increased susceptibility to the COVID‐19 infection compared to the general population. However, their disease severity, the dynamics, and the persistence of seroconversion after infection might differ. In addition, this group is often characterized by significantly higher mortality rates. 43 , 44
According to the meta‐analysis of Gresham et al., 45 patients with dermatologic immune disorders treated with interleukin (IL)‐17 inhibitor (brodalumab, ixekizumab, and secukinumab) or IL‐4/13 inhibitor (dupilumab), or anti‐CD20 or methotrexate show relatively normal seroconversion rate after COVID19 vaccination. However, those treated with TNF alpha inhibitors (adalimumab, certolizumab, and etanercept) could not efficiently produce antibodies. Azathioprine and JAK inhibitor‐treated patients also showed a decreased immune response. Moreover, based on Boyarsky and his colleagues, 46 most of the renal transplant patients receiving antimetabolites and alkylating agents (tacrolimus gt; mycophenolate mofetil gt; systemic steroids gt; sirolimus gt; everolimus) were prone to decline and delay immune response due to severely disturbed primary and secondary immune activity. 46
In contrast, mTORi everolimus preserves humoral immune responses. Therefore, patients receiving this therapy would possibly not experience immune response disturbance after COVID‐19 infection or vaccination. It is worth mentioning that immunocompromised patients were excluded from the Phase 3 trials of vaccinations launched in 2020; thus, no remarkable evidence on the efficacy of standard vaccination protocols of this group of patients is currently known. 43 , 44
In this context, it will be a challenge to investigate the impact of vaccination on this group of patients, and unique vaccination protocols should be designed. Here, regular T and B cell activity and the antibody response need to be evaluated to assess the longevity of seroconversion and immunity after vaccination.
4. THE CHALLENGES
4.1. Global access and distribution of vaccine doses
According to Bloomberg et al., seven are now available for public use out of the most promising vaccines. So far, more than 25% of the world population is now fully vaccinated, and 3.84 billion doses have been administered at least across 103 countries at the rate of 33 652 947 doses a day on 25 July, 2021. 47 , 48 , 49
Before the market supply of COVID‐19 vaccines, the WHO with other international organizations such as the Global Alliance for Vaccines and Immunizations (Gavi) and Coalition for Epidemic Preparedness Innovations (CEPI) have made efforts to establish a system to regulate the right and equitable distribution of vaccine named the COVID‐19 Vaccines Global Access (COVAX) Facility. The reports elaborated that 156 countries came forward to join this plan for purchasing and distributing vaccines. The process through which this system decided to distribute the vaccine aimed “to end the acute phase of the pandemic by the end of 2021” and was disclosed by the end of 2020. 50 COVAX collaborators voluntarily participated in this campaign according to their resources and risk factors collectively for investing in vaccine development and preparing the infrastructure required for vaccine distribution. The most crucial aspect of COVAX was to ensure the transparency of vaccines' finance allocation.
In September 2020, the WHO, under COVAX, outlined the vaccine distribution policy. 51 A fair proportional distribution of vaccines through COVAX was established, trying to guarantee a fair distribution by avoiding competition among countries, getting the maximum doses of vaccines for their own citizens. Ethically, this proportional distribution was considered wrong, especially for countries that did not contribute to vaccine development. Finally, global equity ensures that fair vaccine allocation assumes the unique epidemic risks and the needs of all countries, particularly low and middle‐income countries. 52
In addition, the WHO's Strategic Advisory Group of Experts (SAGE) on immunization has released an essential document for vaccine allocation and prioritization based on age (starting with people over the age of 65) and frontline workers. 53
Although these efforts significantly contributed to building a fair vaccination distribution plan, 54 the world has witnessed that income has been an important factor shaping rates of administration of COVID‐19 vaccine doses, i.e., high‐income countries have achieved the fastest vaccine roll‐outs to date based on COVID‐19 vaccine doses administered per 100 people. 49 By October 2021, COVAX has accomplished only 15.5% of its annual plan of vaccine distribution. At the same time, developed countries with high vaccine uptake were already pursuing a strategy of booster doses (Figure 1). As highlighted recently, optimizing the immunity level of wealthy populations cannot come at the expense of low‐income regions that suffer from vaccine unavailability. 56 This is because unvaccinated individuals remain the main drivers of the pandemic, SARS‐CoV‐2 transmission, and evolution. 56
Figure 1.
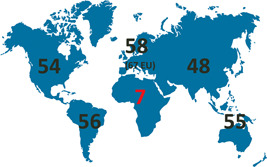
The COVID‐19 vaccine inequity as of November 2021. Only 7% of the African population have received full vaccination, while developed countries (e.g., the European Union with 67% of the population fully vaccinated), with high vaccine uptake, were already recommending booster doses. Based on data by ref. 55 This inequity strongly advocates better support of vaccine aid in low‐income countries and emphasizes that initial vaccinations over booster strategies must be prioritized 56
4.2. Adherence to hygiene guidelines after the vaccination
Until different types of vaccines induce herd immunity, governmental authorities have recommended that hygiene guidelines should be followed to inhibit the spread of SARS‐CoV‐2 infection.
The minimum time needed to induce an optimal level of immunity was observed in vaccines produced by Pfizer‐BioNTech and Novavax companies seven days after the seconnd dose, aka 28 days from the first dose. 31 , 57 Ad26.COV2.S and Ad5‐nCoV vaccines mediated immunity after 28 days but with a single dose. 55 , 56
Nevertheless, the maximum time needed for the induction of immunity was 42 days 58 , 59 (Table 2).
Table 2.
The time needed for immunity induction of approved vaccines
| Vaccine | Developer/country | Doses | Efficacy | Time to induce an optimal level of immunity | Trial Phase |
|---|---|---|---|---|---|
| BNT162b2 mRNA 31 | Pfizer‐BioNTech (Germany‐USA) | Dose1 Day 0 | 95% credible interval, 90.3–97.6 | 7 days after the second dose; Day 28 | Phase III multinational, placebo‐controlled, observer‐blinded |
| Dose2 Day 21 | |||||
| mRNA‐1273 58 | Moderna (USA) | Dose1 Day0, | 94.1% credible interval 89.3%–96.8%; | 14 days after the second dose; Day 42 | Phase III randomized, observer‐blinded, placebo‐controlled trial |
| Dose2 day28 | |||||
| Gam‐COVID‐Vac (Sputnik V) 60 | The Gamaleya Research Institute (Russia) | Dose1 Day 0 | 91.6% credible interval 85.6–95.2 | 1–7 days after the second dose; Day 28 (Cellular immune response). | Phase III randomized control trial |
| Dose2 day 21 | 2‐ day42 (humoral immune response) | ||||
| ChAdOx1 nCoV‐19 (AZD1222) Vaccine 61 | Oxford‐AstraZeneca (UK, Sweden) | Dose1 Day 0 | Efficacy was 81.3% credible interval 60.3–91.2] at ≥12 weeks and 55·1% credible interval [33.0–69.9] at <6 weeks) | Within 90 days from a single standard dose of vaccine | A pooled analysis of 4 Phase III randomized control trials in the UK, Brazil, and South Africa. |
| Dose2 either 6 weeks or 12 weeks | |||||
| Ad26.COV2.S 62 | Johnson & Johnson (USA) | Single‐dose | 72% in the United States, 64% in South Africa, 61% in Latin America | Day 29 after the first vaccine dose. | Phase 1–2a Trial |
| Convidecia (aka, Ad5‐nCoV) 63 | CanSino Biologics, in collaboration with the Institute of Biology of China's Academy of Military Medical Sciences (China) | Single‐dose | 62.1% 64 | 28 days after a single dose | Phase II randomized control trial |
| NVX‐CoV2373 57 | Novavax (USA) | Dose1 Day 0 | 89% in the UK but 49% in South Africa | 7 days after the second dose; Day 28 | 2a/b, multicentre randomized, observer‐blinded, placebo‐controlled |
| Dose 2 Day 21 | |||||
| BBIBP‐CorV 20 | Sinopharm (Chiana) | Dose 1 Day0 | 79.34% 65 | 1–7 days after the second dose; Day 28 (neutralizing antibody) | randomized, double‐blind, placebo‐controlled, phase 1/2 trial |
| Dose 2 day 21 | 2 ‐ Day 42 (humoral immune response) | ||||
| CoronaVac (formerly PiCoVacc) 66 | Sinovac Biotech(China) | Dose 1 Day 0 | 50.65% in Brazil trial, 91.25% in Turkey trial 65 | 14 days after the second dose; Day 28 | Phase II randomized control trial |
| Dose 2 Day 14 | |||||
| Covaxin (aka BBV152 A, B, C) 59 | Bharat Biotech (India) | Dose 1 Day0 | 80.6% | 14 days after the second dose; Day 42 | Phase III randomized control trial |
| Dose 2 Day 28 |
It is worth mentioning, due to the emergence of new COVID‐19 variants or the asymptomatic spread, 67 staying strict to hygiene guidelines is a vital strategy. A study published by Hacisuleyman et al. showed two individuals infected with COVID‐19 new variants after vaccination, despite having a considerable level of antibodies. This effect was not observed due to the dysfunction of the antibodies, but for their insufficiency. 68
Thus, a sandwich strategy of hygiene guidelines and vaccination may contribute to better limiting SARS‐CoV‐2 spread.
4.3. Emergence of novel SARS‐CoV‐2 variants
The coronaviruses belong to RNA viruses that present a high mutation rate, typically exceeding DNA viruses. 69 Compared to influenza viruses, coronaviruses, including SARS‐CoV‐2, as the latter's genomes are replicated by an RNA polymerase that contains a proofreading domain, maintaining relatively high accuracy in virus transcription. 70 , 71 Although the global spread and rapid transmission of SARS‐CoV‐2 provide the virus with numerous opportunities for the emergence of favorable mutations and natural selection, most of the mutations have no impact on pathogenicity. COVID‐19 vaccines have been focused on the spike protein as the main vaccine antigen since it mediates the entrance into host cells. 72 As reported at the beginning of 2021, a total of 9654 mutations in spike protein, corresponding to 400 distinct mutation sites, have been detected. Of these, 44 mutations affect the receptor‐binding domain (RBD). 73 The COVID‐19 vaccines are based on or encode the full‐length spike protein, often (e.g., BNT162b2 by BioNTech/Pfizer, mRNA‐1273 by Moderna, Ad26.COV2.S by Johnson & Johnson, NVX‐CoV2373 by Novavax) is stabilized in prefusion conformation in RBD locked in the “up” position representing a receptor‐accessible state, which is highly immunogenic. 74 , 75 , 76 Therefore, it is likely that single amino acid substitutions or deletions, also in RBD, will not evade the induced immunity to a large extent. Among numerous SARS‐CoV‐2 variants, only four, B.1.617.2, B.1.1.7, B.1.351, and P.1 lineage, have been classified so far as variants of concern (Table 3). The third phase clinical trials of the first COVID‐19 vaccines were unable to evaluate the efficacy against these three variants as their identification occurred after the authorization. 31 , 58 , 80 In such a situation, the neutralization assays using sera of vaccinated individuals and pseudotype virus were employed. 81
Table 3.
| Lineage | Earliest documented samples | The most important sense mutations in spike protein gene |
|---|---|---|
| B.1.1.7 | September 2020 | N501Y, D614G, P681H |
| (Alpha) | UK | |
| B.1.351 | May 2020 | K417N, E484K, N501Y, D614G, A701V |
| (Beta) | South Africa | |
| P.1 | November 2020 | K417T, E484K, N501Y, D614G, H655Y |
| (Gamma) | Brazil | |
| B.1.617.2 | October 2020 | L452R, T478K, D614G, P681R |
| (Delta) | India |
Abbreviation: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
It has been shown that the highly transmissible B.1.1.7 and P.1 variant pose no risk for vaccine effectiveness. 82 , 83 , 84 , 85 , 86 In agreement, the third phase clinical trials of novel vaccine candidates seem to confirm this possibility. B.1.617.2, which has a significantly increased transmissibility (with a basic reproduction number of 6–8) and became a dominant variant in numerous regions by mid‐2021, reveals only the modest effect on the neutralization function of vaccine‐induced antibodies. 87 Therefore, instead of significant immune evasion, B.1.617.2 is increasing the risk of breakthrough infections due to mutations in spike protein that increase its affinity to ACE‐2 receptor and enhance membrane fusion. 88 Importantly, a large post‐authorization study encompassing 3.5 million individuals has shown that although the efficacy of mRNA vaccine against infection is lower for B.1.617.2 variant compared to other SARS‐CoV‐2 variants, and is also more impacted by the time passing from the second vaccine dose, the efficacy against hospitalization remains very high during the 6 months at the level of 93%. 89 As long as the serum levels of neutralization antibodies decrease within a few months following the completion of the initial vaccination regime (Figure 2), the protection against severe COVID‐19 remains high due to adaptive cellular immune response that is retained and is recognizing B.1.617.2 variant well. 90 , 91 Moreover, the data shows that administration of the booster dose of mRNA vaccine at least 5–6 months after the completion of the initial vaccination regime is inducing a robust antibody response that neutralizes the B.1.617.2 variant and decreases the risk of infection. 92 , 93 All in all, these findings show that there is no need to optimize the mRNA vaccines due to the emergence of more transmissible B.1.617.2 variant, but rather invest in booster dosing strategies.
Figure 2.
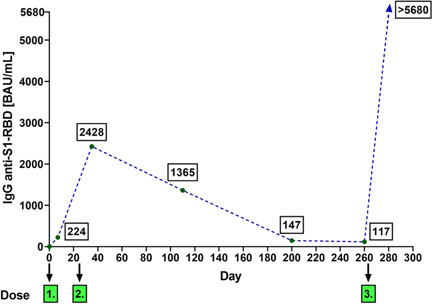
An example of dynamics of serum neutralizing IgG anti‐S1‐RBD antibodies levels induced by administration of BNT162b2 vaccine in 36‐years old male with no immune deficiency. Note the decreasing levels over the course of 8 months after a second dose and a significant rise (above the upper limit of detection in the assay) after a third (booster) dose
In turn, B.1.351 variant, first identified in South Africa, has been evidenced partially escaped monoclonal antibody neutralization. This phenomenon is mainly linked to the E484K mutation in RBD, which is strengthened in the presence of K417N and N501Y. 94 However, clinical trials conducted in South Africa when B.1.351 became the dominant variant in this country indicated that a two‐dose regimen of AZD1222 (AstraZeneca) vaccination does not protect from the mild‐to‐moderate COVID‐19 caused by B.1.351. 95 In turn, Ad26.COV2.S vaccination provided lower but still acceptable efficacy. 96 The mRNA vaccines appear to retain high efficacy against this variant, as shown in the clinical trial of the BNT162b2 vaccine up to 6 months from the second dose. 97 Taken together, this indicates that E484K promotes at least a partial capacity for viral immune escape. Apart from B.1.351 and P.1., this mutation has emerged in other SARS‐CoV‐2 variants, for example, B.1.526 lineage (identified in New York state). 98 Importantly, however, none of these variants became dominant by mid‐November 2021. In South Africa, B.1.351 has been dominated by B.1.617.2. In Europe, B.1.617.2 variant has dominated over B.1.1.7, including a version of B.1.1.7 possessing E484K mutation, ultimately leading to de‐escalation of B.1.1.7 lineage. 99
It remains unknown whether the variants of concern emerged due to a longstanding infection in immunocompromised individuals due to prolonged treatment with convalescent plasma or monoclonal antibodies, 83 or/and as a consequence of random mutations due to a large number of infections in the population. In this context, it seems reasonable to prioritize immunosuppressed individuals' vaccination to lower the risk of virus evolution due to its prolonged host persistence. 100 , 101 Furthermore, because prolonged viral shedding was also observed in children, including asymptomatic cases, it cannot be excluded that they also may be a good host for novel mutations to emerge. Currently, no vaccine has been authorized for administration to children and adolescents, although the first clinical trials targeting these groups were initiated (e.g., NCT04796896 and NCT04800133).
The prediction of the further trajectory of SARS‐CoV‐2 mutations is challenging. However, considering that current vaccines retain the efficacy against the B.1.1.7 strain, which rapidly became dominant in various geographical regions, 102 it can be speculated that as the percentage of vaccinated individuals will continuously increase, the B.1.351 and others possessing E484K mutation may be on the rise. One should note that E484K has also emerged in B.1.1.7 as discovered in February in the UK. 103 In the long term, this does not necessarily indicate an epidemiological threat. Even though antibody response may be partially evaded, there is no evidence that cellular responses mediated by cytotoxic T cells) are affected by these new mutations in the spike protein.
Moreover, the vaccinated individuals also generate non‐neutralizing antibodies involved in immunological mechanisms such as antibody‐dependent cell‐mediated cytotoxicity and opsonization, which are essential for clearance of virus and virus‐infected cells and stimulation of downstream adaptive immune responses via antigen presentation and secretion of inflammatory mediators. 104 , 105 As a result, infections in vaccinated individuals may likely be mild and not lead to a surge of hospitalizations. Nevertheless, considering the continuous emergence of new SARS‐CoV‐2 variants, the currently authorized vaccines may need to be periodically updated to avoid potential clinical efficacy loss. For vaccines developed using the mRNA and vector platforms, this can be achieved in a reasonable time and steps to prepare and test novel versions. Multivalent vaccine candidates have also been initiated. 96 , 106 Meanwhile, it is pivotal to continue tracking the SARS‐CoV‐2 mutations and variants, take local actions to slow variants transmission, and pursue research on their potential effects for the COVID‐19 vaccines in use.
4.4. Vaccine acceptance and resistance: Challenges to be overcome
Despite solid evidence that vaccination prevents severe disease and decreases new infections, some gray areas have raised apprehensions. The ambivalence towards COVID‐vaccination seems as prevalent as the pandemic itself, 107 with their development at unprecedented speeds 108 as a key concern. COVID‐19 vaccines have only been recently introduced worldwide, in a much faster period of time compared to a typical vaccine development, which can take 15 years or more. 109 Therefore, acceptance of vaccines against SARS‐CoV‐2 represented a public health challenge due to scientific‐sounding misinformation such as false information that vaccine trial participants have died after taking a candidate COVID‐19 vaccine through social media platforms. Reduced available data of safety and efficiency also contributed to this scenario. 110
Vaccines are used as prophylactic, given before the infection in an individual, recruiting a more robust immune system to produce potent multi‐targeted attacks against viruses. Increasing the time between the vaccine production and the evolving resistance gives a vaccine a longer time to remain effective and produce higher immunity levels against the virus. 111
Studies have shown that some viruses and bacteria were able to evolve resistance over the vaccines such HBV, S. pneumoniae, Bordetella pertussis, Yersinia ruckeri, and Marek's Disease Virus, time after reducing the number of infections. 113
Vaccine chances of resistance are low, and they are attributed to the absence of one of three essential features that vaccines possess, that is, the vaccine needs to target multiple virus epitopes simultaneously, inducing a redundant and evolutionarily robust immune response; it must suppress pathogen growth and eliminate transmission, and it should provide protection against all circulating serotypes of a given pathogen. 112 Thus, considering the current COVID19 pandemic context that we reviewed here, it will be necessary to develop long‐term studies that monitor the evolution of the immune protection induced by COVID19 vaccines. 113 The combination of vaccine and drug development against COVID19 must be continued. 111 One should, however, note that, as shown in countries dominated by the B.1.617.2 variant, there is a significant negative correlation between fully vaccinated rate and mutation frequency (Mf), with the highest values of Mf observed for populations with vaccination rates below 10%. 114 This finding strongly advocates vaccinating the unvaccinated individuals as a priority in suppressing the mutation of SARS‐CoV‐2. 56
4.5. Ethical challenges
The COVID‐19 pandemic that has caught the world unawares has raised various issues regarding pandemic and vaccination ethics. These concerns cover a wide range of topics, such as testing and trial, distribution, prioritization, beneficence and maleficence, cultural and religious differences, politics and diplomacy, surveillance, and life and death, among others. In addition, the prevalence of misinformation and the promotion of “alternative” cures or remedies do nothing to win the fight against anti‐vaxxers.
As a modified iteration of the classic Trolley Problem – one that fits the existing COVID‐19 vaccination predicament – one can look at the ethical dilemma as such:
COVID‐19 is rampaging (the trolley on the track). There is one available vaccine shot (the lever). There are two people who can be vaccinated. One is an elderly person who, if healthy, has a few more years to live that can be spent to enjoy what life has left to offer (the one). The other is a 30‐year‐old medical professional who, if healthy, can potentially help a great number of people in the next few decades (the many). The side effects of the vaccine are a risk to both. To whom will you give the vaccine?
Putting this thought experiment in the context of the real world makes the problem more complicated because then, one would be presented with more choices. In many instances, some of these choices would seem to be better choices than others. Some would say that the benefits of the vaccine outweigh the risks and that the disadvantaged must be prioritized above everyone else. 115 Others would raise questions on the power of the State to legally compel its citizens to be vaccinated or to decide to donate a portion of State‐acquired vaccines to poorer countries despite the fact that some of its own citizens have yet to be vaccinated. 116 There are also those who would consider the political and diplomatic implications of exploiting the pandemic and the vaccine race to secure a better position on the world stage. 117 These are just some of the ethical challenges that confront the ongoing vaccination efforts.
The ethical challenges posed by this pandemic are one that is often put at the back seat so as to prioritize economic, political, and health concerns. However, these issues are as important as any if the aim is to have an effective, efficient, and humane COVID‐19 vaccination response on a global scale. Ultimately, for the sake of putting an end to the rampage of COVID‐19, it is paramount to keep in mind that the scientific and medical issues and responses relating to vaccine roll‐out must be appreciated side by side with the equally relevant ethical ones.
5. THE HOPES AND CONCLUSIONS
Although the road to ending the COVID‐19 pandemic is still uncertain, vaccines have brought the world hope, with studies pointing towards a significant decline in the risk of COVID19‐related infection and hospitalizations. However, there is evidence that the efficacy of vaccines against infection decrease in time. Eradication of SARS‐CoV‐2 is unlikely; therefore, one should note that emphasis must be placed on decreasing COVID‐19 severity and deaths, while prevention of infection should be seen as a secondary goal and may also require development and implementation of vaccines based on novel approaches or optimization of vaccines for novel SARS‐CoV‐2 variants. Nevertheless, the evolution of SARS‐CoV‐2 must remain monitored globally.
Of note, the pandemic has invoked international goal‐oriented collaborations. Exceptional efforts by scientists, with years of advanced research, along with the enormous funding and the faltering global economy 118 are some factors that have led to the emergence of hundreds of projects within a year. 119 These projects bear scope for improvement in terms of potency, safety, and effectiveness of novel vaccines, and possibly, development of vaccines better‐suited for distinct population groups and variations in the viral genome as well. 120 However, understanding vaccine‐hesitancy is imperative for achieving desired levels of immunization. Addressing the scientific, medical, and ethical challenges that follow vaccine development (inequity, recipient prioritization, etc.) may enable better implementation. Thus, vaccination can control the spread of the COVID‐19 infection, in parallel to the multiple epidemiologic factors, and the several social measurements applied during this pandemic.
CONFLICT OF INTERESTS
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
AUTHOR CONTRIBUTIONS
Piotr Rzymski conceived the manuscript. Kawthar Mohamed, Piotr Rzymski, Md Shahidul Islam, Amjad Khan, Ayesha Mushtaq, Rangarirai Makuku, Mariya Ivanovska, Sara A. Makka, Orsolya Cseprekal, Fareeda Hashem, and Essouma Mickael prepared the first draft of this manuscript. Kawthar Mohamed, Leander Marquez, Orsolya Cseprekal, Irene Ling, Amanuel Godana Arero, Igor Salerno Filgueiras, Dennyson Leandro M. Fonseca, Kseniia Minakova, Eduardo Rodríguez‐Román, Sunny O. Abarikwu, Attig‐Bahar Faten, Giulia Grancini, and Otavio Cabral‐Marques prepared the final draft of the manuscript. Eventually, Nima Rezaei supervised the project and critically appraised it.
ACKNOWLEDGMENTS
We acknowledge the São Paulo Research Foundation (FAPESP grants 2018/18886‐9, 2020/01688‐0, and 2020/07069‐0 to OCM) for financial support.
Mohamed K, Rzymski P, Islam MS, et al. COVID‐19 vaccinations: the unknowns, challenges, and hopes. J Med Virol. 2022;94:1336‐1349. 10.1002/jmv.27487
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon reasonable request
REFERENCES
- 1. Mohamed K, Rodríguez‐Román E, Rahmani F, et al. Borderless collaboration is needed for COVID‐19: a disease that knows no borders. Infect Control Hosp Epidemiol. 2020;41:1245‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weekly epidemiological update on COVID‐19 ‐ 20 July 2021. Accessed 26, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---20-july-2021
- 3. Nicola M, Alsafi Z, Sohrabi C, et al. The socio‐economic implications of the coronavirus pandemic (COVID‐19): a review. Int J Surg. 2020;78:185‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor S, Paluszek MM, Rachor GS, McKay D, Asmundson GJG. Substance use and abuse, COVID‐19‐related distress, and disregard for social distancing: a network analysis. Addict Behav. 2021;114:106754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sidor A, Rzymski P. Dietary choices and habits during COVID‐19 lockdown: experience from Poland. Nutrients. 2020;12:1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rzymski P, Nowicki M. COVID‐19‐related prejudice toward Asian medical students: a consequence of SARS‐CoV‐2 fears in Poland. J Infect Public Health. 2020;13:873‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohamed K, Rezaei N, Rodríguez‐Román E, et al. International efforts to save healthcare personnel during COVID‐19. Acta Biomed. 2020;91:e2020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rezaei N. COVID‐19 affects healthy pediatricians more than pediatric patients. Infect Control Hosp Epidemiol. 2020;41:1106‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nejadghaderi SA, Saghazadeh A, Rezaei N. Health care policies and COVID‐19 prevalence: is there any association? Int J Health Serv. 2021;52:20731421993940‐22. [DOI] [PubMed] [Google Scholar]
- 10. Jabbari P, Taraghikhah N, Jabbari F, Ebrahimi S, Rezaei N. Adherence of the general public to self‐protection guidelines during the COVID‐19 pandemic. Disaster Med Public Health Prep. 2020:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20:615‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID‐19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS‐CoV‐2. Lancet Infect Dis. 2021;21:e26‐e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan JF, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rawat K, Kumari P, Saha L. COVID‐19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021;892:173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO news updates. Accessed 21 May, 2021. https://www.who.int/news-room/news-updates
- 16. Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID‐19. Front Immunol. 2020;11:585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Office of the Commissioner . FDA takes key action in fight against COVID‐19 by issuing emergency use authorization for first COVID‐19 vaccine. 2020. Accessed May 21, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
- 18. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS‐CoV‐2 ‐ preliminary report. N Engl J Med. 2020;383:1920‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koch T, Dahlke C, Fathi A, et al. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open‐label, phase 1 trial. Lancet Infect Dis. 2020;20:827‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO vaccination greatly reduces disease, disability, death and inequity worldwide. 2011. [DOI] [PMC free article] [PubMed]
- 22. Banatvala J, Van Damme P, Oehen S. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine. 2000;19:877‐885. [DOI] [PubMed] [Google Scholar]
- 23. Jin P, Li J, Pan H, Wu Y, Zhu F. Immunological surrogate endpoints of COVID‐2019 vaccines: the evidence we have versus the evidence we need. Signal Transduct Target Ther. 2021;6:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371, 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gorvett Z. How effective is a single vaccine dose against Covid‐19? Accessed March 5, 2021. https://www.bbc.com/future/article/20210114-covid-19-how-effective-is-a-single-vaccine-dose
- 26. Rigby S, Science PA. Pfizer vaccine: Single dose '90 per cent effective after 21 days. Accessed March 5, 2021. https://www.sciencefocus.com/news/pfizer-vaccine-single-dose-90-per-cent-effective-after-21-days/
- 27. Bartsch SM, O'Shea KJ, Ferguson MC, et al. Vaccine efficacy needed for a COVID‐19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med. 2020;59:493‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al‐Tawfiq JA. Asymptomatic coronavirus infection: MERS‐CoV and SARS‐CoV‐2 (COVID‐19). Travel Med Infect Dis. 2020;35:101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu X, Yang R. COVID‐19 transmission through asymptomatic carriers is a challenge to containment. VInfluenza Other Respir Viruses. 2020;14:474‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bleier BS, Ramanathan M Jr., Lane AP. COVID‐19 vaccines may not prevent nasal SARS‐CoV‐2 infection and asymptomatic transmission. Otolaryngol Head Neck Surg. 2021;164:305‐307. [DOI] [PubMed] [Google Scholar]
- 31. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin D‐Y, Zeng D, Mehrotra DV, Corey L, Gilbert PB. Evaluating the Efficacy of COVID‐19 vaccines. Clin Infect Dis. 2020;73:1540‐1544. 10.1093/cid/ciaa1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iwasaki A. What reinfections mean for COVID‐19. Lancet Infect Dis. 2021;21:3‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hall V, Foulkes S, Charlett A, et al. Do antibody positive healthcare workers have lower SARS‐CoV‐2 infection rates than antibody negative healthcare workers? Large multi‐centre prospective cohort study (the SIREN study) June to November 2020: England. bioRxiv. 2021. 10.1101/2021.01.13.21249642 [DOI]
- 35. Abu‐Raddad LJ, Chemaitelly H, Coyle P, et al. SARS‐CoV‐2 reinfection in a cohort of 43,000 antibody‐positive individuals followed for up to 35 weeks. bioRxiv. 2021. 10.1101/2021.01.15.21249731 [DOI] [Google Scholar]
- 36. Momplaisir F, Haynes N, Nkwihoreze H, Nelson M, Werner RM, Jemmott J. Understanding drivers of COVID‐19 vaccine hesitancy among Blacks. Clin Infect Dis. 2021;73:1784‐1789. 10.1093/cid/ciab102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuhn M, Campillos M, Letunic I, Jensen LJ, Bork P. A side effect resource to capture phenotypic effects of drugs | Molecular Systems Biology. Mol Syst Biol. 2010;6:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Information for UK recipients on COVID 19 Vaccine AstraZeneca. Accessed February 26, 2021. https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-uk-recipients-on-covid-19-vaccine-astrazeneca
- 39.Pfizer‐BioNTech COVID‐19 Vaccine EUA Fact Sheet for Recipients and Caregivers.
- 40. Vojdani A, Kharrazian D. Potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akinosoglou K, Tzivaki I, Marangos M. Covid‐19 vaccine and autoimmunity: awakening the sleeping dragon. Clin Immunol. 2021;226:108721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldman M, Hermans C. Thrombotic thrombocytopenia associated with COVID‐19 infection or vaccination: Possible paths to platelet factor 4 autoimmunity. PLoS Med. 2021;18:e1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thakkar A, Pradhan K, Jindal S, et al. Patterns of seroconversion for SARS‐CoV‐2 IgG in patients with malignant disease and association with anticancer therapy. Nature. Cancer. 2021;2:392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti‐SARS‐CoV‐2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gresham LM, Marzario B, Dutz J, Kirchhof MG. An evidence‐based guide to SARS‐CoV‐2 vaccination of patients on immunotherapies in dermatology. J Am Acad Dermatol. 2021;84:1652‐1666. 10.1016/j.jaad.2021.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS‐CoV‐2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Randall T, Sam C, Tartar A, et al. More than 1.04 billion shots given: Covid‐19 Tracker. In: Bloomberg‐2020. Accessed April 27, 2021. http://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/
- 48. Ritchie H, Ortiz‐Ospina E, Beltekian D, et al. Coronavirus Pandemic (COVID‐19). Our World in Data. 2020.
- 49. Mathieu E, Ritchie H, Ortiz‐Ospina E, et al. A global database of COVID‐19 vaccinations. Nat Hum Behav. 2021;5:947‐953. [DOI] [PubMed] [Google Scholar]
- 50.'Vaccine nationalism' threatens global plan to distribute COVID‐19 shots fairly. 2020. Accessed April 27, 2021. http://www.sciencemag.org/news/2020/07/vaccine-nationalism-threatens-global-plan-distribute-covid-19-shots-fairly
- 51.Fair allocation mechanism for COVID‐19 vaccines through the COVAX Facility. Accessed April 27, 2021. https://www.who.int/publications/m/item/fair-allocation-mechanism-for-covid-19-vaccines-through-the-covax-facility
- 52. World Health Organization . WHO SAGE values framework for the allocation and prioritization of COVID‐19 vaccination. September 14, 2020.
- 53.WHO SAGE values framework for the allocation and prioritization of COVID‐19 vaccination. 2020. Accessed June 27, 2021. https://www.who.int/publications/i/item/who-sage-values-framework-for-the-allocation-and-prioritization-of-covid-19-vaccination
- 54. Kupferschmidt K. Despite obstacles, WHO unveils plan to distribute vaccine. Science. 2020;369:1553. [DOI] [PubMed] [Google Scholar]
- 55.Our World in Data. Accessed November 19, 2021. https://ourworldindata.org/covid-vaccinations
- 56. Rzymski P, Camargo CA, Fal A, et al. COVID‐19 vaccine boosters: the good, the bad, and the ugly. Vaccines. 2021;9:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novavax COVID‐19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial. Accessed April 20, 2021. https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3
- 58. Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA‐1273 SARS‐CoV‐2 Vaccine. N Engl J Med. 2020;384:403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bharat biotech‐vaccines & bio‐therapeutics manufacturer in India. Accessed July 26, 2021. https://www.bharatbiotech.com/
- 60. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Voysey M, Costa Clemens SA, Madhi SA, et al. Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sadoff J, Le Gars M, Shukarev G, et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid‐19 Vaccine. N Engl J Med. 2021;384:1824‐1835. 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhu F‐C, Guan X‐H, Li Y‐H, et al. Immunogenicity and safety of a recombinant adenovirus type‐5‐vectored COVID‐19 vaccine in healthy adults aged 18 years or older: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet. 2020;396:479‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kyriakidis NC, López‐Cortés A, González EV, Grimaldos AB, Prado EO. SARS‐CoV‐2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zimmer C, Corum J, Wee S‐L. Coronavirus Vaccine Tracker. The New York Times. 2021.
- 66. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tande AJ, Pollock BD, Shah ND, et al. Impact of the COVID‐19 vaccine on asymptomatic infection among patients undergoing pre‐procedural COVID‐19 molecular screening. Clin Infect Dis. 2021. 10.1093/cid/ciab229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS‐CoV‐2 variants. N Engl J Med. 2021;384:2212‐2218. 10.1056/NEJMoa2105000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peck KM, Lauring AS. Complexities of viral mutation rates. J Virol. 2018;92:e01031‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Boivin S, Cusack S, Ruigrok RWH, Hart DJ. Influenza A virus polymerase: structural insights into replication and host adaptation mechanisms. J Biol Chem. 2010;285:28411‐28417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Denison MR, Graham RL, Donaldson EF, Eckerle LD, Baric RS. Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol. 2011;8:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Le TT, Cramer JP, Chen R, Mayhew S, Nrdd J. Evolution of the COVID‐19 vaccine development landscape. 2020. [DOI] [PubMed]
- 73. Guruprasad L. Human SARS CoV‐2 spike protein mutations. Proteins. 2021;89:569‐576. 10.1002/prot.26042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bos R, Rutten L, van der Lubbe JEM, et al. Ad26 vector‐based COVID‐19 vaccine encoding a prefusion‐stabilized SARS‐CoV‐2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Corbett KS, Edwards DK, Leist SR, et al. SARS‐CoV‐2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and Immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383:2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chand M, Hopkins S, Dabrera G, et al. Investigation of novel SARS‐COV‐2 variant: variant of Concern 202012/01 (PDF). Public Health England. 2020. Accessed March 19, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/947048/Technical_Briefing_VOC_SH_NJL2_SH2.pdf
- 78. Tegally H, Wilkinson E, Giovanetti M, et al. Emergence and rapid spread of a new severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) lineage with multiple spike mutations in South Africa. medRxiv. 2020.
- 79.World Health Organization Tracking SARS‐CoV‐2 variants. Accessed November 19, 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 80. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nie J, Li Q, Wu J, et al. Quantification of SARS‐CoV‐2 neutralizing antibody by a pseudotyped virus‐based assay. Nat Protoc. 2020;15:3699‐3715. [DOI] [PubMed] [Google Scholar]
- 82. Muik A, Wallisch AK, Sänger B, et al. Neutralization of SARS‐CoV‐2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine‐elicited human sera. Science. 2021;371:1152‐1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Collier DA, De Marco A, Ferreira I, et al. Sensitivity of SARS‐CoV‐2 B.1.1.7 to mRNA vaccine‐elicited antibodies. Nature. 2021;593:136‐141. 10.1038/s41586-021-03412-7 [DOI] [PubMed] [Google Scholar]
- 84. Shen X, Tang H, McDanal C, et al. SARS‐CoV‐2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29:529‐539.e3. 10.1016/j.chom.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu K, Werner AP, Koch M, et al. Serum neutralizing activity elicited by mRNA‐1273 vaccine ‐ preliminary report. N Engl J Med. 2021;384:1468‐1470. 10.1056/NEJMc2102179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dejnirattisai W, Zhou D, Supasa P, et al. Antibody evasion by the Brazilian P.1 strain of SARS‐CoV‐2. bioRxiv 2021.03.12.435194. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Edara V‐V, Pinsky BA, Suthar MS, et al. Infection and vaccine‐induced neutralizing‐antibody responses to the SARS‐CoV‐2 B.1.617 Variants. N Engl J Med. 2021;385:664‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang J, Xiao T, Cai Y, et al. Membrane fusion and immune evasion by the spike protein of SARS‐CoV‐2 Delta variant. Science. 2021:eabl9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID‐19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kato H, Miyakawa K, Ohtake N, et al. Vaccine‐induced humoral and cellular immunity against SARS‐CoV‐2 at 6 months post BNT162b2 vaccination. bioRxiv. 2021. 10.1101/2021.10.30.21265693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Woldemeskel BA, Garliss CC, Blankson JN. mRNA vaccine‐elicited SARS‐CoV‐2‐specific T cells persist at 6 months and recognize the delta variant. Clin Infect Dis. 2021. 10.1093/cid/ciab915 [DOI] [PubMed] [Google Scholar]
- 92. Bar‐On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid‐19 in Israel. N Engl J Med. 2021;385:1393‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Falsey AR, Frenck RW Jr., Walsh EE, et al. 2021) SARS‐CoV‐2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS‐CoV‐2 variant B.1.351 from natural and vaccine induced sera. Cell. 2021;184:2348‐2361. 10.1016/j.cell.2021.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV‐19 Covid‐19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885‐1898. 10.1056/NEJMoa2102214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mahase E. Covid‐19: where are we on vaccines and variants? BMJ. 2021;372:n597. [DOI] [PubMed] [Google Scholar]
- 97. Thomas SJ, Moreira ED Jr., Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine through 6 months. N Engl J Med. 2021;385:1761‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lasek‐Nesselquist E, Lapierre P, Schneider E, George KS, Pata J. The localized rise of a B.1.526 SARS‐CoV‐2 variant containing an E484K mutation in New York State. medRxiv. 2021. [Google Scholar]
- 99.European Centre for Disease Prevention and Control SARS‐CoV‐2 variants of concern as of 18 November 2021. Accessed November 18, 2021. https://www.ecdc.europa.eu/en/covid-19/variants-concern
- 100. Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS‐CoV‐2 in an immunocompromised host. N Engl J Med. 2020;383:2291‐2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Truong TT, Ryutov A, Pandey U, et al. Persistent SARS‐CoV‐2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv. 2021. 10.1101/2021.02.27.21252099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS‐CoV‐2 lineage B.1.1.7 in England. Science. 2021:eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wise J. Covid‐19: The E484K mutation and the risks it poses. BMJ. 2021;372:n359. [DOI] [PubMed] [Google Scholar]
- 104. Tay MZ, Wiehe K, Pollara J. Antibody‐dependent cellular phagocytosis in antiviral immune responses. Front Immunol. 2019;10:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gómez Román VR, Murray JC, Weiner LM. Chapter 1 ‐ Antibody‐dependent cellular cytotoxicity (ADCC). In: Ackerman ME, Nimmerjahn F, eds. Antibody Fc. Academic Press; 2014:1‐27. [Google Scholar]
- 106. Balfour H. First patients receive Moderna's modified COVID‐19 vaccine candidates. 2021. Accessed March 17, 2021. https://www.europeanpharmaceuticalreview.com/news/147162/first-patients-receive-modernas-modified-covid-19-vaccine-candidates/
- 107. Dror AA, Eisenbach N, Taiber S, et al. Vaccine hesitancy: the next challenge in the fight against COVID‐19. Eur J Epidemiol. 2020;35:775‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lurie N, Saville M, Hatchett R, Halton J. 2020) Developing Covid‐19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969‐1973. [DOI] [PubMed] [Google Scholar]
- 109. Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586:516‐527. [DOI] [PubMed] [Google Scholar]
- 110. Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID‐19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337‐348. [DOI] [PubMed] [Google Scholar]
- 111. Kennedy DA, Read AF. Why does drug resistance readily evolve but vaccine resistance does not? Proc Biol Sci. 2017;284, 10.1098/rspb.2016.2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kennedy DA, Read AF. Monitor for COVID‐19 vaccine resistance evolution during clinical trials. PLoS Biol. 2020;18:e3001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kennedy DA, Read AF. Why the evolution of vaccine resistance is less of a concern than the evolution of drug resistance. Proc Natl Acad Sci U S A. 2018;115:12878‐12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yeh T‐Y, Contreras GP. Full vaccination against COVID‐19 suppresses SARS‐CoV‐2 delta variant and spike gene mutation frequencies and generates purifying selection pressure. bioRxiv. 2021. 10.1101/2021.08.08.21261768 [DOI] [Google Scholar]
- 115. Persad G, Emanuel EJ. The ethics of COVID‐19 immunity‐based licenses ("Immunity Passports"). JAMA. 2020;323:2241‐2242. [DOI] [PubMed] [Google Scholar]
- 116.Vaccine Ethics. BBC.
- 117.The logic of China's vaccine diplomacy. Accessed July 26, 2021. https://thediplomat.com/2021/03/the-logic-of-chinas-vaccine-diplomacy/
- 118. Fernandes N. Economic effects of coronavirus outbreak (COVID‐19) on the world economy. 2020. 10.2139/ssrn.3557504 [DOI]
- 119.Draft landscape and tracker of COVID‐19 candidate vaccines. Accessed March 2, 2021. https://www.who.int/publications/m/item/draft-landscape-of-COVID-19-candidate-vaccines
- 120. Li Q, Wu J, Nie J, et al. The impact of mutations in SARS‐CoV‐2 spike on viral infectivity and antigenicity. Cell. 2020;182:1284‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request


