Abstract
To assess the clinical efficacy and safety of neutralizing monoclonal antibodies (mABs) for outpatients with coronavirus disease 2019 (COVID‐19). PubMed, Embase, Web of Science, Cochrane Library, ClinicalTrials.gov, and World Health Organization International Clinical Trials Registry Platform (ICTRP) databases were searched from inception to July 19, 2021. Only randomized controlled trials (RCTs) that assessed the clinical efficacy and safety of neutralizing mABs in the treatment of COVID‐19 outpatients were included. The Cochrane risk‐of‐bias tool was used to assess the quality of the included RCTs. The primary outcome was the risk of COVID‐19‐related hospitalization or emergency department (ED) visits. The secondary outcomes were the risk of death and adverse events (AEs). Five articles were included, in which 3309 patients received neutralizing mAB and 2397 patients received a placebo. A significantly lower rate of hospitalization or ED visits was observed among patients who received neutralizing mABs than those who received a placebo (1.7% vs. 6.5%, odds ratios (OR): 0.26; 95% confidence interval (CI): 0.19–0.36; I 2 = 0%). In addition, the rate of hospitalization was significantly lower in the patients who received neutralizing mABs than in the control group (OR: 0.24; 95% CI: 0.17−0.34; I 2 = 0%). The mortality rate was also significantly lower in the patients who received neutralizing mABs than in the control group (OR: 0.16; 95% CI: 0.05−0.58; I 2 = 3%). Neutralizing mABs were associated with a similar risk of any AE (OR: 0.81; 95% CI: 0.64–1.01; I 2 = 52%) and a lower risk of serious AEs (OR: 0.37; 97% CI: 0.19–0.72; I 2 = 45%) compared with a placebo. Neutralizing mABs can help reduce the risk of hospitalization or ED visits in COVID‐19 outpatients. For these patients, neutralizing mABs are safe and not associated with a higher risk of AEs than a placebo.
Keywords: COVID‐19, emergency department, hospitalization, neutralizing monoclonal antibody, safety
Highlights
Neutralizing monoclonal antibodies (mABs) decreased risk of hospitalization or emergence department visits in coronavirus disease 2019 (COVID‐19) outpatients.
Neutralizing mABs reduced mortality in COVID‐19 outpatients.
Neutralizing mABs had no increased risk of any adverse events (AEs) and lower risk of serious AEs.
1. INTRODUCTION
On March 11, 2020, the World Health Organization (WHO) declared that coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was a global pandemic due to the rapidly increasing number of infected people worldwide. 1 As of August 12, 2021, there have been more than 203 million confirmed cases of COVID‐19, including more than 4.3 million deaths globally. 2 Although the newly developed vaccines can provide effective protection against SARS‐CoV‐2 infection, 3 many new COVID‐19 cases have been reported recently. Therefore, the increasing number of COVID‐19 patients remains a critical public health concern. The clinical spectrum of COVID‐19 can range from asymptomatic status, acute respiratory disease, pneumonia, to acute respiratory distress syndrome. 4 , 5 , 6 Currently, the recommended treatment options for COVID‐19 patients depend on the stage and severity of the disease. 5 , 6 , 7 For hospitalized COVID‐19 patients, antiviral agents such as remdesivir is suggested, however, anti‐inflammatory agents such as corticosteroids and anti‐interleukin‐6 are recommended for patients requiring high‐flow oxygen/noninvasive ventilation therapy with evidence of clinical progression or increased markers of inflammation. 7 , 8 , 9
In addition to patients with severe to critical COVID‐19, a significant number of patients are classified as having mild or moderate illness, some of whom may progress to severe illness or require hospitalization, particularly those with older age, multiple comorbidities, obesity, or immunocompromised status. 6 Therefore, disease progression or hospitalization in patients with mild or moderate COVID‐19 is another important issue. To address this issue, neutralizing monoclonal antibodies (mABs) including bamlanivimab monotherapy, a combination of bamlanivimab plus etesevimab, and a combination of casirivimab plus imdevimab have been proposed and developed for the treatment of nonhospitalized patients with mild to moderate COVID‐19. 10 These neutralizing mABs can interact with the surface spike glycoprotein of SARS‐CoV‐2 thereby preventing viral attachment and infectivity, and they have shown potent in vivo efficacy with marked reductions in viral loads in the upper and lower respiratory tracts in animal studies. 11 , 12 Recently, several randomized controlled trials (RCTs) have been conducted to assess the clinical efficacy of neutralizing mABs for COVID‐19 patients, and they have shown promising results. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 We conducted this systematic review and meta‐analysis of RCTs to provide robust and up‐to‐date evidence of the clinical efficacy and safety of neutralizing mABs for COVID‐19 outpatients.
2. METHODS
2.1. Study search and selection
We searched PubMed, Embase, Web of Science, and Cochrane Library for relevant articles from their inception to July 19, 2021. The following search terms were used: COVID‐19 (including COVID‐19, coronavirus infections, corona virus, corona infection, and SARS‐CoV‐2) and neutralizing mABs (including neutralizing mABs, etesevimab, bamlanivimab, casirivimab, imdevimab, JS016, TY027, BRII‐196, BRII‐198, ABBV‐47D11, STI‐1499, MW33, HFB30132A, ADM03820, HLX70, DZIF‐10c, STI‐2020, BGB DXP593, SCTA01, AZD8895, AZD1061, CT‐P59, VIR‐7831, and GSK4182136). Only RCTs that assessed the clinical efficacy and safety of neutralizing mABs in the treatment of patients with mild or moderate COVID‐19 were included. Searches of ClinicalTrials.gov and WHO International Clinical Trials Registry Platform were also performed for registered trials (Table S1). We also manually searched for additional eligible articles from the reference lists of relevant articles and preprint server of medRxiv. Studies were included if they met the following criteria: (1) nonhospitalized patients with mild to moderate COVID‐19 infection; (2) age ≥ 18 years; (3) used a neutralizing mAB as the intervention; (4) used a placebo or standard of care as the comparator; (5) was designed as an RCT; and (6) reported clinical efficacy and risk of adverse events (AEs) as study outcomes. Reviews or meta‐analysis studies, studies without adequate data for outcome analysis, non‐RCTs, post‐hoc analysis studies, and poster or conference abstracts were excluded.
Two authors (C.‐C. Lai and C.‐H. Chen) screened and identified publications independently to avoid bias. A third author (C.‐Y. Wang) was consulted and made the final decision in cases of disagreement over the same publication. The following data were extracted separately by two authors from each included study: year of publication, study design, the regimen of the neutralizing mABs, clinical outcomes, and risk of AEs. A third author was consulted and discussed if the extracted data was inconsistent. This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐analyses guidelines. 21 The protocol of the systematic review and meta‐analysis was registered at PROSPERO (CRD42021268377).
2.2. Outcome measurements
The primary outcome was the risk of COVID‐19‐related hospitalization or emergency department (ED) visits. The secondary outcomes were the risk of death and the risk of AEs.
2.3. Data analysis
The Cochrane risk‐of‐bias tool 22 was used to assess the quality of the included RCTs and their associated risk of bias. Two reviewers subjectively reviewed all included studies and rated them as being “low risk,” “high risk,” or “unclear” independently. Any disagreement was resolved by a third reviewer who made the final decision. Statistical analyses were performed using Review Manager (version 5.3; Nordic Cochrane Centre). The degree of heterogeneity was evaluated using Q statistics generated from the χ 2 test, and the I 2 measure was used to assess statistical heterogeneity. Heterogeneity was defined as significant when p < 0.10 or I 2 > 50%. A fixed‐effects model was used when the data were homogeneous, and a random‐effects model was used when the data were heterogeneous. The pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for outcome analysis. The sensitivity analysis was performed using a leave‐one‐out approach. The subgroup analysis including different neutralizing mABs regimens and high risk patients for hospitalization were also conducted.
3. RESULTS
3.1. Study selection
The search of the online databases yielded a total of 405 studies, of which 170 duplicate studies were excluded. In addition, 186 studies were judged to be irrelevant after screening the titles, abstracts, and publications with no full text available. Furthermore, 43 studies were excluded after the full texts of 49 articles were screened. Finally, five articles 14 , 15 , 16 , 19 , 20 were included in this meta‐analysis (Figure 1). Unpublished or ongoing studies are summarized in Table S2.
Figure 1.
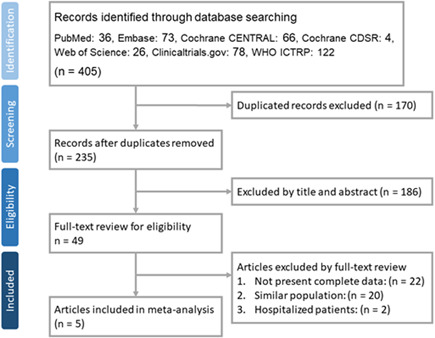
Flow diagram of study selection. ICTRP, International Clinical Trials Registry Platform; WHO, World Health Organization
3.2. Study characteristics
All of the articles were multicenter studies, and three were multinational studies 16 , 19 , 20 (Table 1). Three articles 15 , 19 , 20 focused on adult patients, and the other two articles 14 , 16 included both adult and adolescent patients. Two articles 14 , 15 used bamlanivimab‐containing regimens either alone or in combination with etesevimab as the intervention, and two articles used a combination of casirivimab and imdevimab as the study medication. 16 , 20 In addition, one article used the experimental drug sotrovimab. 19 Four articles 14 , 15 , 19 , 20 included mild or moderate COVID‐19 patients, and one 16 included asymptomatic COVID‐19 patients. Overall, 3309 patients received neutralizing mAB and 2397 patients received placebo. For the risk of bias, none of the five articles described how the random sequence was generated. Otherwise, most of the included studies had a low risk of bias in each domain, although all the studies had an unclear risk of selection bias (Figure 2).
Table 1.
Characteristics of the included studies
| Author, year | Study design | Study site | Study period | Inclusion criteria | Intervention | No. of study patients a | |
|---|---|---|---|---|---|---|---|
| Study group | Control group | ||||||
| Chen et al., 2021 15 | Randomized, double‐blind, placebo‐controlled, phase 2 trial | 41 centers in the US | From June 17 through August 21, 2020 | Mild to moderate COVID‐19 adult outpatients | Bamlanivimab | 309 | 143 |
| Dougan et al., 2021 14 | Randomized, double‐blind, placebo‐controlled, phase 3 trial | Multiple centers in the US | Between December 8, 2020 and January 20, 2021 | Mild to moderate COVID‐19 adults and adolescents | Bamlanivimab and etesevimab | 518 | 517 |
| Gupta et al., 2021 19 | Randomized, double‐blind, placebo‐controlled, phase 3 trial | 91 sites in the US, Austria, Brazil, Canada, Peru, Spain, and the UK | Between August 27, 2020 and April 8, 2021 | Nonhospitalized patients with symptomatic COVID‐19 and at risk of disease progression | Sotrovimab | 291 | 292 |
| O'Brien et al., 2022 16 | Randomized, double‐blind, placebo‐controlled, phase 3 trial | 112 sites in the US, Romania, and Moldova | Between July 13, 2020 and January 28, 2021 | Asymptomatic COVID‐19 adults and adolescents | Casirivimab and imdevimab | 100 | 104 |
| Weinreich et al., 2021 20 | Randomized, double‐blind, placebo‐controlled, phase 3 trial | 115 sites in the US, Chile, Mexico, and Romania | Between September 24, 2020 and January 17, 2021 | COVID‐19 outpatients with one or more risk factor for severe disease | Casirivimab with imdevimab | 2091 | 1341 |
Intention‐to‐treat population.
Figure 2.
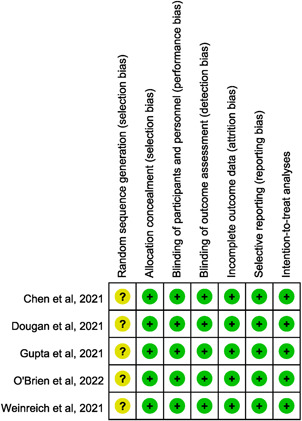
Summary of the risk of bias in each domain
3.3. Primary outcome
The rate of COVID‐19‐related hospitalization or ED visits in the patients who received neutralizing mABs was only 1.7% (57/3309), which was much lower than that of the controls who received a placebo (6.5%, 155/2397). A significant difference in the rate of hospitalization or ED visits was observed between the patients who received neutralizing mABs and those who received a placebo (OR: 0.26; 95% CI: 0.19−0.36; I 2 = 0%, Figure 3). This difference remained significant in the leave‐one‐out sensitivity test, in which individual studies were randomly excluded. In the subgroup analysis, neutralizing mAB treatment was associated with a lower risk of hospitalization or ED visits than a placebo among high‐risk patients (OR: 0.26; 95% CI: 0.18−0.37; I 2 = 0%). In addition, the patients who received mono‐ or combination therapy with neutralizing mABs had a lower risk of hospitalization or ED visits than those who received a placebo (monotherapy: OR: 0.21; 95% CI: 0.11−0.43; I 2 = 0%; combination therapy: OR: 0.27; 95% CI: 0.19−0.39; I 2 = 0%). Furthermore, the patients who received bamlanivimab‐containing regimens as either monotherapy or in combination had a lower risk of hospitalization or ED visits than those who received a placebo (OR: 0.28; 95% CI: 0.15−0.50; I 2 = 0%). A similar trend was found in the comparison between the patients who received a combination of casirivimab and imdevimab and those who received a placebo (OR: 0.27; 95% CI: 0.18−0.40; I 2 = 0%). Regarding the risk of requiring hospitalization, the rate of hospitalization remained significantly lower in the patients who received neutralizing mABs than in those who received a placebo (OR: 0.24; 95% CI: 0.17−0.34; I 2 = 0%).
Figure 3.
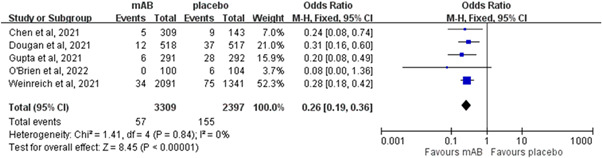
Forest plot of the comparison of coronavirus disease‐19‐related hospitalization or emergency department visit rates between neutralizing monoclonal antibodies (mABs) and placebo. CI, confidence interval
3.4. Secondary outcomes
Two of 3209 patients who received neutralizing mABs died compared to 14 of 3041 patients in the control group. The mortality rate was significantly lower in the neutralizing mABs group than in the control group (OR: 0.16; 95% CI: 0.05−0.58; I 2 = 3%) in the pooled analysis of four RCTs 14 , 15 , 19 , 20 which reported mortality as an outcome (Figure 4). Regarding the risk of AEs, neutralizing mABs were associated with a similar risk of any AE as the control group (OR: 0.81; 95% CI: 0.64–1.01; I 2 = 52%, Figure 5). This similarity persisted regardless of the severity of the AEs (mild AEs: OR: 1.10; 95% CI: 0.76–1.59; I 2 = 0%; moderate AEs: OR: 0.78; 95% CI: 0.32–1.89; I 2 = 72%; severe AEs: OR: 1.57; 95% CI: 0.57–4.35; I 2 = 0%). In contrast, the risk of serious AEs was lower in the study group than in the control group (OR: 0.37; 95% CI: 0.19–0.72; I 2 = 45%, Figure 5). Moreover, no significant differences were noted between those who received neutralizing mABs and those who received a placebo in the risk of nausea (OR: 1.17; 95% CI: 0.51–2.67; I 2 = 0%), vomiting (OR: 0.77; 95% CI: 0.24–2.45; I 2 = 0%), diarrhea (OR: 0.71; 95% CI: 0.29–1.71; I 2 = 0%), dizziness (OR: 1.46; 95% CI: 0.54–3.91; I 2 = 0%), headache (OR: 0.81; 95% CI: 0.23–2.92; I 2 = 0%), rash (OR: 1.65; 95% CI: 0.50–5.52; I 2 = 0%), or pruritis (OR: 3.42; 95% CI: 0.60–19.55; I 2 = 0%).
Figure 4.
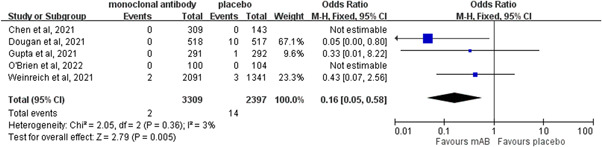
Forest plot of the comparison of the risk of death between neutralizing monoclonal antibodies (mABs) and placebo. CI, confidence interval
Figure 5.
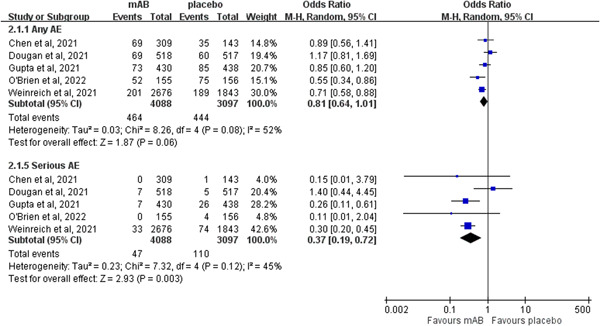
Forest plot of the comparison of the risk of any adverse event (AE) and serious AEs between neutralizing monoclonal antibodies (mABs) and placebo. CI, confidence interval
4. DISCUSSION
In this meta‐analysis, five articles 14 , 15 , 16 , 19 , 20 were reviewed to assess the clinical efficacy and safety of neutralizing mABs, including bamlanivimab, a combination of bamlanivimab and etesevimab, a combination of casirivimab and imdevimab, and sotrovimab in the treatment of COVID‐19 outpatients. The most important finding of this study is that neutralizing mABs could help prevent hospitalization or ED visits among COVID‐19 patients, as supported by the following evidence. First, the rate of COVID‐19‐related hospitalization or ED visits was significantly lower among the COVID‐19 patients who received neutralizing mABs than in those who received a placebo. Second, the lower rate of hospitalization or ED visits in those who received neutralizing mABs remained unchanged in the sensitivity test and subgroup analyses of patients at high risk and different regimens of neutralizing mABs. Third, neutralizing mABs were associated with a lower risk of COVID‐19‐related hospitalization than a placebo. Finally, the risk of death was significantly lower among those receiving neutralizing mABs than the control group. These findings are consistent with those of real‐world studies and observational cohort studies. 23 , 24 , 25 , 26 In a retrospective case−control study, Kumar et al., reported a significantly lower 30 day hospitalization rate among patients who received bamlanivimab (7.3% vs. 20.0%, RR: 0.37; 95% CI: 0.21−0.64; p < 0.001), and the number needed to treat was eight. 23 Another retrospective cohort study by Piccicacco et al., included high‐risk outpatients, and their results demonstrated that patients treated with either bamlanivimab or casirivimab and imdevimab had a lower risk of hospitalization or ED visits than the control group (13.5% vs. 40.5%, OR: 0.23; 95% CI: 0.14−0.38; p < 0.001). In addition, the mortality rate was lower in the neutralizing mAB group than in the control group (0% vs. 3.5%, p = 0.02). 24 Even more, for patients with mild to moderate COVID‐19 from the Delta variant, a propensity matched models also demonstrated that neutralizing mABs treatment using casirivimab and imdevimab, or sotrovimab was associated with reduced risk of hospitalization or death compared to no treatment (RR: 0.40; 95% CI: 0.28–0.57). 25 In contrast to the recent meta‐analysis of four studies investigating the individual effect of each neutralizing mABs for nonhospitalized patients, no consistent results were found in each comparison. 27 The present meta‐analysis including five RCTs and conducting the overall effect based on the pooled analysis of all included studies demonstrated the benefit of adding neutralizing mABs for nonhospitalized patients. In summary, these findings indicate that neutralizing mABs can effectively prevent hospitalization or ED visits in COVID‐19 outpatients.
These clinical benefits of neutralizing mABs are consistent with the reported improvements in virological outcomes in the included studies. 14 , 15 , 16 For patients who received a 2800 mg dose of bamlanivimab, Chen et al., reported a significant difference compared to those who received a placebo in the decrease in SARS‐CoV‐2 viral load from baseline (difference: −0.53; 95% CI: −0.98 to −0.08; p = 0.02). 15 In addition, for patients who received bamlanivimab plus etesevimab, Dougan et al., reported a greater reduction from baseline in the log viral load compared to those who received a placebo (difference: −1.20; 95% CI: −1.46 to −0.94; p < 0.001). 14 Moreover, O'Brien et al., reported that a combination of casirivimab and imdevimab significantly reduced the duration of a high viral load in the overall study population (39.7% reduction vs. placebo; 48 vs. 82 total weeks; p = 0.0010). 16 Taken together, these findings indicate that neutralizing mABs can accelerate the reduction of SARS‐CoV‐2 viral load and help reduce the risk of further hospitalization.
Finally, we assessed safety issues associated with neutralizing mABs. The analysis showed that neutralizing mABs were not associated with a higher risk of AEs than a placebo, including any AE and serious AEs. Moreover, no significant difference was observed between neutralizing mABs and the comparators for specific AEs including nausea, vomiting, diarrhea, dizziness, headache, rash, and pruritis. These findings indicate that neutralizing mABs are safe for the treatment of COVID‐19 patients.
This meta‐analysis had several limitations. First, the numbers of studies and patients were small, especially for each neutralizing mAB. Second, because of the lack of available data, we could not evaluate the effect of neutralizing mABs according to different SARS‐CoV‐2 variants, especially for Omicron (B.1.1.529). One study 28 using SARS‐CoV‐2 virus‐like particles (VLP) found that no activity was detected for casirivimab or imdevimab either against Omicron VLPs. Moreover, casirivimab was able to neutralize OmC3 but not OmC1 and imdevimab was able to neutralize OmC1 but not OmC3. All these findings suggested that the failure of these mABs to neutralize Omicron S could be due to the six mutations within the Omicron RBD (K417N, N440K, G446S, G496S, Q498R, and N501Y). 28 Another study using an artificial intelligence model predicted that the efficacy of several neutralizing mABs, such as bamlanivimab and etesevimab, casirivimab and imdevimab against Omicron might largely diminish but the impact of Omicron on the activity of sotrovimab could be mild. 29 The similar findings that bamlanivimab and etesevimab, casirivimab and imdevimab completely lost neutralizing activity against Omicron whereas sotrovimab was only minimally affected, were also reported in an in vitro study in both Vero‐TMPRSS2 and Vero‐hACE2‐TMPRSS2 cells. 30 Third, the risk of mutations leading to neutralizing mAB resistance remains a serious concern, particularly for bamlanivimab. 15 , 31 , 32 , 33 In one study 33 based on the post‐hoc analysis of ACTIV‐2/A5401 trial, Choudhary et al., reported that the emergence of resistance with bamlanivimab treatment could be dependent on the neutralizing mAB's dose and the emergent of drug resistance mutations can adversely affect both the virologic and clinical efficacy of antiviral drugs. Fourth, this study only focused on the usefulness of neutralizing mABs in COVID‐19 outpatients, however available information about their clinical efficacy in COVID‐19 in patients is limited. 17 , 34 Further studies are warranted to clarify these issues. Finally, many ongoing studies are currently investigating the efficacy of neutralizing mABs, 31 , 32 and more evidence will be available in the near feature.
In conclusion, neutralizing mABs can help reduce the risk of hospitalization or ED visits for COVID‐19 outpatients. For these patients, neutralizing mABs are safe and not associated with a higher risk of AEs than a placebo. However, there could be a new serious concern about the diminished activities of most of these neutralizing mABs against the new SARS‐CoV‐2 variant—Omicron.
AUTHOR CONTRIBUTIONS
Study concept and design: Wei‐Ting Lin, Chih‐Cheng Lai, Cheng‐Yi Wang, Chao‐Hsien Chen. Acquisition of data: Chih‐Cheng Lai, Chao‐Hsien Chen, Cheng‐Yi Wang. Analysis and interpretation of data: All. Drafting of the manuscript: Wei‐Ting Lin, Shun‐Hsing Hung, Chih‐Cheng Lai, Chao‐Hsien Chen. Critical revision of the manuscript for important intellectual content: All. Statistical analysis: Shun‐Hsing Hung, Chih‐Cheng Lai, Cheng‐Yi Wang, Chao‐Hsien Chen. Approval of final manuscript: All.
CONFLICT OF INTERESTS
The author declares that there are no conflicts of interest.
Supporting information
Supporting information.
Supporting information.
Lin W‐T, Hung S‐H, Lai C‐C, Wang C‐Y, Chen C‐H. The impact of neutralizing monoclonal antibodies on the outcomes of COVID‐19 outpatients: a systematic review and meta‐analysis of randomized controlled trials. J Med Virol. 2022;94:2222‐2229. 10.1002/jmv.27623
Wei‐Ting Lin and Shun‐Hsing Hung contributed equally to this study.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO coronavirus (COVID‐19) dashboard; 2021. Accessed July 15, 2021. https://covid19whoint/
- 3. Lai CC, Chen IT, Chao CM, Lee PI, Ko WC, Hsueh PR. COVID‐19 vaccines: concerns beyond protective efficacy and safety. Expert Rev Vaccines. 2021;20:1‐13. [DOI] [PubMed] [Google Scholar]
- 4. Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berlin DA, Gulick RM, Martinez FJ. Severe Covid‐19. N Engl J Med. 2020;383(25):2451‐2460. [DOI] [PubMed] [Google Scholar]
- 6. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020;383(18):1757‐1766. [DOI] [PubMed] [Google Scholar]
- 7. National Institutes of Health . Coronavirus disease 2019 (COVID‐19) treatment guidelines; 2021. Accessed August 15, 2021. https://wwwcovid19treatmentguidelinesnihgov/ [PubMed]
- 8. Domingo P, Mur I, Mateo GM, et al. Association between administration of IL‐6 antagonists and mortality among patients hospitalized for COVID‐19: a meta‐analysis. JAMA. 2021;326(6):499‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324(13):1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID‐19. Nat Rev Immunol. 2021;21(6):382‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baum A, Ajithdoss D, Copin R, et al. REGN‐COV2 antibodies prevent and treat SARS‐CoV‐2 infection in rhesus macaques and hamsters. Science. 2020;370(6520):1110‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones BE, Brown‐Augsburger PL, Corbett KS, et al. The neutralizing antibody, LY‐CoV555, protects against SARS‐CoV‐2 infection in nonhuman primates. Sci Transl Med. 2021;13(593):eabf1906. 10.1126/scitranslmed.abf1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganesh R, Pawlowski CF, O'Horo JC, et al. Intravenous bamlanivimab use associates with reduced hospitalization in high‐risk patients with mild to moderate COVID‐19. J Clin Invest. 2021;131(19):e151697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid‐19. N Engl J Med. 2021.385(15):1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen P, Nirula A, Heller B, et al. SARS‐CoV‐2 neutralizing antibody LY‐CoV555 in outpatients with Covid‐19. N Engl J Med. 2021;384(3):229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Brien MP, Forleo‐Neto E, Sarkar N, et al. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID‐19 in early asymptomatic SARS‐CoV‐2 infection: a randomized clinical trial. JAMA. 2022.327(5):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lundgren JD, Grund B, Barkauskas CE, et al. A neutralizing monoclonal antibody for hospitalized patients with Covid‐19. N Engl J Med. 2021;384(10):905‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID‐19: a randomized clinical trial. JAMA. 2021;325(7):632‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta AK, Rojas YG, Juarez E, et al. Early treatment for Covid‐19 with SARS‐CoV‐2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941‐1950. [DOI] [PubMed] [Google Scholar]
- 20. Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN‐COV antibody combination and outcomes in outpatients with Covid‐19. N Engl J Med. 2021;385(23):e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar RN, Wu EL, Stosor V, et al. Real‐world experience of bamlanivimab for COVID‐19: a case‐control study. Clin Infect Dis. 2021;74(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piccicacco N, Zeitler K, Montero J, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high‐risk outpatients. Open Forum Infect Dis. 2021;8(7):ofab292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang DT, McCreary EK, Bariola JR, et al. Effectiveness of casirivimab and imdevimab, and sotrovimab during Delta variant surge: a prospective cohort study and comparative effectiveness randomized trial. medRxiv. 2021. 10.1101/2021.12.23.21268244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCreary EK, Bariola JR, Wadas RJ, et al. Association of subcutaneous or intravenous route of administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in COVID‐19. medRxiv. 2021. 10.1101/2021.11.30.21266756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kreuzberger N, Hirsch C, Chai KL, et al. SARS‐CoV‐2‐neutralising monoclonal antibodies for treatment of COVID‐19. Cochrane Database Syst Rev. 2021;9(9):Cd013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Syed AM, Ciling A, Khalid MM, et al. Omicron mutations enhance infectivity and reduce antibody neutralization of SARS‐CoV‐2 virus‐like particles. medRxiv. 2021. 10.1101/2021.12.20.21268048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen J, Wang R, Gilby NB, Wei GW. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022.62(2):412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. VanBlargan L, Errico J & Halfmann P et al. An infectious SARS‐CoV‐2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Viruses. 2021;13(4):628. 10.3390/v13040628 [DOI] [PMC free article] [PubMed]
- 31. Hurt AC, Wheatley AK. Neutralizing antibody therapeutics for COVID‐19. Viruses. 2021;13(4):628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corti D, Purcell LA, Snell G, Veesler D. Tackling COVID‐19 with neutralizing monoclonal antibodies. Cell. 2021;184(12):3086‐3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choudhary MC, Chew KW, Deo R, et al. Emergence of SARS‐CoV‐2 resistance with monoclonal antibody therapy. medRxiv. 2021. 10.1101/2021.09.03.21263105 [DOI] [Google Scholar]
- 34. RECOVERY Collaborative Group . Casirivimab and imdevimab in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. medRxiv. 2021;2006(2015):21258542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


