Abstract
In March, people living with HIV infection (PLWH) were included in the risk category of fragile people for severe COVID‐19 receiving priority access to vaccination with BNT162b2 vaccine. The aim of the study was to evaluate the immunogenicity and safety of the two doses regimen. The antibodies titer for severe acute respiratory syndrome‐related coronavirus‐2 (SARS‐CoV‐2) was evaluated after 21 days since the first administration (Time 1), 1 (Time 2), and 3 (Time 3) months post‐vaccination. Information regarding virological and immunological conditions at baseline, previous SARS‐CoV‐2 state of infection, other immunodeficiencies, current antiretroviral therapy (ART), comorbidities, and severe adverse events (SAE) to vaccination was collected. Six hundred and ninety‐seven patients were tested for quantitative anti‐spike antibodies at Time 1, 577 patients had a second detection at Time 2, and 491 patients had the third detection. Baseline characteristics of the study population are reported in Table 1. At the time of vaccine administration, all patients were on ART (except one long‐term nonprogressor); 632 (90.7%) patients had undetectable HIV‐RNA; 12 (1.7%) patients were immunosuppressed due to chemotherapy or other immunosuppressive drugs; 345 (49.5%) patients had at least one COVID‐19 related comorbidity and 155 (22.2%) had two or more comorbidities. No SAEs were reported. Final serological results are available for 694 patients after the first dose, 577 and 491 after the second and third ones, respectively; positive titer (values ≥ 50 AU/ml) was demonstrated in 653 (94.1%), 576 (99.8%), 484 (98.6%) patients, respectively. Only one patient was a nonresponder after completing vaccination, who was a newly diagnosed one for HIV infection. All vaccinations were well tolerated, with no SAEs. BNT162b2 mRNA vaccine was immunogenic and safe in PLWH.
Keywords: anti‐S1RBD, COVID‐19, immunogenicity, PLWH, safety, SARS‐CoV‐2
Key points
-
•
The aim of the study was to evaluate the immunogenicity and safety of the two doses regimen in people with HIV infection.
-
•
The severe acute respiratory syndrome‐related coronavirus‐2 anti‐spike antibodies titer was evaluated after 21 days since the first administration (in 697 patients) and 1 (in 577 patients) and 3 months (in 491 patients) post‐vaccination.
-
•
Positive titer (values ≥ 50 AU/ml) was demonstrated in 653 (94.1%), 576 (99.8%), and 484 (98.6%) patients, respectively.
-
•
Only one patient was a nonresponder after completing vaccination, who was a newly diagnosed one for HIV infection.
-
•
All vaccinations were well tolerated, with no severe adverse events.
-
•
BNT162b2 mRNA vaccine was immunogenic and safe in people living with HIV infection, regardless of CD+ cell count.
1. INTRODUCTION
After about 2 years of the severe acute respiratory syndrome‐related coronavirus‐2 (SARS‐CoV‐2) pandemic, information about the epidemiology and the outcome of COVID‐19 in people living with HIV infection (PLWH) are currently evolving. The last global consensus disclosed by WHO shows HIV infection as an independent risk factor that is associated with a higher risk of death compared to the HIV‐negative population. 1 Moreover, as older age represents a determinant for a severe outcome of COVID‐19, it must be considered in PLWH getting old as an additional risk factor.
Therefore, a world priority is to reduce the susceptible population through immunization, particularly for people with underlying risk factors. In Italy, access to SARS‐CoV‐2 vaccination has been extended to the population at risk for severe outcomes, including PLWH, starting from March 2021. However, clinical trials before the marketing of these vaccines have not been tested in a large HIV population and therefore, evidence of immunogenicity and safety in PLWH are lacking.
Currently, it has been demonstrated that people with any immune deficiency may have a suboptimal vaccination response and shorter protection compared to the general population. 2 , 3 , 4 Data on the clinical effectiveness of common vaccines in PLWH are still partial and often difficult to compare as they are based on single experiences, different baseline features, and limited samples. Data appears to confirm a less protective response to common vaccines in HIV‐infected patients, compared to the general population. 4 Immunogenicity levels are largely related to CD4+ cell count, viral load (VL), and disease stage. 5 Concerning anti‐SARS‐CoV‐2 vaccination in PLWH, some data have been recently published. Overall, the initial experience appears to be positive in terms of safety and immunogenicity in a limited follow‐up. 6 , 7
Another critical element is represented by the identification of more accurate timing to proceed with the administration of a vaccine in PLWH, especially for those people who need to start antiretroviral therapy (ART). It is widely accepted by the scientific community that the reconstitution of the immune system induced by ART can increase the immunogenicity of vaccinations in relation to the increase of CD4+ cell count. However, this recommendation is in open conflict with the strategy of early immunization for SARS‐CoV‐2 of the susceptible population in a pandemic era. 8
Since March 21, 2021, the Italian government issued recommendations on the vaccination of PLWH, using the BNT162b2 vaccine. The Pfizer‐BioNTech BNT162b2 mRNA vaccine has been tested for safety and efficacy in a multinational randomized placebo‐controlled trial with more than 40 000 participants, 9 of which 196 were PLWH. This study demonstrates the immunogenicity and safety of the vaccine over a median of 2 months.
In our retrospective observational monocentric study, we describe the experience of an Italian reference HIV/AIDS center, with particular insights into immunologic response to BNT162b2 vaccine in a larger cohort of PLWH after 3 months from complete vaccination. Moreover, we collected data about vaccine safety after the first and second administrations. Lastly, we assessed the correlation between clinical data and anti‐receptor binding domain (RBD) title at three time points.
2. METHODS
Over 1000 outpatients HIV infected are followed in the Clinic of Infectious Diseases, University of Bari. All outpatients aged ≥18 years, who received vaccination between April 14 and May 14, 2021 in our Center, in collaboration with the Section of Hygiene, and with a follow‐up of at least 3 months were enrolled in this retrospective observational study. Patients who had recovered from SARS‐CoV‐2 within 3 months or had an active infection at the time of the vaccination (as shown by positive PCR on respiratory swabs or for history) were excluded.
Sociodemographic details, medical history, clinical and laboratory data, in particular regarding HIV status, and co‐morbidities were extracted from a computerized database and collected.
Potential contraindications were evaluated before the administration of BNT162b2 by medical interview.
Safety of BNT162b2 mRNA vaccine was evaluated at the end of the study. All clinical adverse events (AE) were monitored and recorded in a survey collected 3 months after vaccination, by means of focused questions regarding any sign or symptom, which had occurred after each administration. In particular, items explored were local pain at the site of injection, fatigue, myalgia, fever, headache, lymphadenopathy, allergic rash, and other non‐common AE. Any AE was characterized by grade of severity (according to the Common Terminology Criteria for Adverse Events, version 6.0, 2020).
Immunogenicity rate was evaluated with a chemiluminescent microparticle immunoassay from Abbott, that detects IgG antibodies against receptor binding domain (IgG anti‐RBD) of SARS‐CoV‐2 spike protein after 21 days from the first administration (Time 1), 1 month (Time 2), and 3 months after the end of complete vaccination (Time 3), as shown in Figure 1. Values ≥ 50 AU/ml were considered positive with high sensitivity and specificity, near 100%. 9
Figure 1.
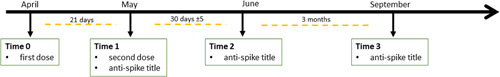
Planning of vaccination and outpatient controls
HIV VL was determined with RT‐PCR, where <20 copies/ml is considered undetectable.
CD4+ and CD8+ T‐cell counts were determined by flow cytometry analysis in peripheral blood.
Continuous variables were presented as means and standard deviation or as geometric means and 95% CI. Categorical variables (age, sex, hypertension, diabetes, dyslipidaemia, cardiac, and/or vascular disease, chronic obstructive pulmonary disease) were presented as N (%).
The correlation between IgG title and clinical data (CD4+ cell count, CD8 cell count, CD4/CD8 ratio, and VL) were analyzed using multivariate linear regression.
Moreover, occasional viral blips were noticed after the complete vaccination, during planned outpatient control due to HIV infection.
3. RESULTS
In the study period, a total of 697 participants were enrolled, with an age ranging between 19 and 79 years, 521 were male (74.7%). The distribution is described in Table 1. Among enrolled patients, 12 people had an immunodeficiency due to causes other than HIV (recent chemotherapy and immunosuppressant therapy), the 49.5% presented at least one comorbidity, while 22.2% had two or more. Only eight patients (1.1%) had a previously documented SARS‐CoV‐2 infection, however, they received two standard doses of vaccine.
Table 1.
Anamnestic and clinical characteristics at baseline
| Total 697 patients | |
|---|---|
| Age, years median (range) | 53 (19−79) |
| Males, N (%) | 521 (74.7) |
| Immunodeficiency not HIV‐related, N (%) | 12 (1.7) |
| With comorbidity, N (%) | 345 (49.5) |
| Dislipidemia, N (%) | 158 (45.8) |
| Hypertension, N (%) | 113 (32.7) |
| Diabetes, N (%) | 45 (13.0) |
| Cardiac and/or vascular disease, N (%) | 24 (6.9) |
| COBD, N (%) | 5 (1.4) |
| Two or more comorbidities, N (%) | 155 (22.2) |
| Previous documented SARS‐CoV‐2 infection, N (%) | 8 (1.1) |
Abbreviations: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
Median years of HIV infection was 10 (0−32); HIV‐RNA was not detectable in 632 patients (90.7%), 696 (99.8%) were on treatment with ART; the only patient not currently treated as a long‐term non‐progressor. The median range of CD4+ cells% was 35 (0−59.2), 14 of the participants had a count of CD4+ cell/µl <200 at the time of vaccination; the median range of NADIR was 224 cell/µl (Table 2).
Table 2.
Viral and immunological characteristics at baseline
| HIV‐related clinical data HIV related clinical data | Total 697 patients |
|---|---|
| Years of HIV infection, years median (range) | 10 (0−32) |
| HIV‐RNA not detectable,a N (%) | 632 (90.7) |
| On ART,a N (%) | 696 (99.8) |
| CD4+ cells%,a median (range) | 35 (0−59.2) |
| CD4 cell/µl < 200,a N (%) | 14 (2.0) |
| CD+ count cell/µl NADIR, median (range) | 224 (0−997) |
| History of CD4 cell/µl < 200, N (%) | 299 (42.9) |
Abbreviation: ART, antiretroviral therapy.
At time of vaccination.
We collected and analyzed 694 serum samples at Time 1, 578 at Time 2, and 491 at Time 3. The results of IgG anti‐RBD titer were 578.0 (5.4–117 245.4), 7582.0 (44.7 to >200 000.0), and 1305.9 (9.8–109 881.6), respectively (Figure 2). In percentage terms, PLWHs with a protective titer were 94.1%, 99.8%, and 98.6% at Time 1, Time 2, and Time 3, respectively, as shown in Figure 3.
Figure 2.
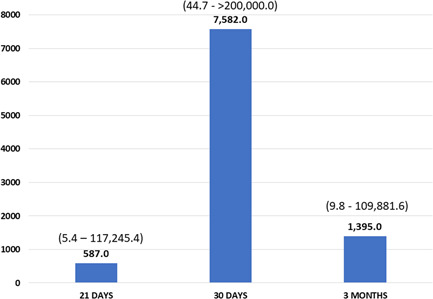
IgG antibodies against‐receptor binding domain title median at Time 1, Time 2, and Time 3
Figure 3.
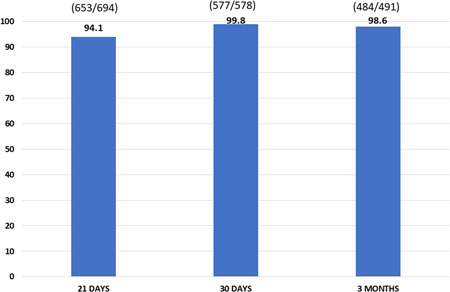
Patients % with protective title for severe acute respiratory syndrome‐related coronavirus‐2 at Time 1, Time 2, and Time 3
AE after the first dose was recorded in 47.3% of the cases, while a percentage of 47.0% was found after the second dose, for a total of 61.1% AEs overall (Table 3). The most frequent symptoms were pain at the injection site, fatigue, and myalgia. Systemic symptoms were more prevalent after the second dose than after the first one. A total of 269 AE were recorded with an equal distribution between the first and second dose (208 vs. 207). None of the patients developed a serious AE, except one patient who developed lower extremity deep venous thrombosis after approximately 24 h of administration. Patient clinical data are described in Table 4.
Table 3.
Safety and AEs distribution
| Total population 440 patients | N | First dose | Second dose |
|---|---|---|---|
| Any AE, N (%) | 269 (61.1) | 208 (47.3) | 207 (47.0) |
| Local pain, N (%) | 228 (51.8) | 174 (39.5) | 170 (38.6) |
| Fatigue, N (%) | 120 (27.3) | 63 (14.3) | 94 (21.4) |
| Myalgia, N (%) | 50 (11.4) | 22 (5.0) | 45 (10.2) |
| Fever, N (%) | 41 (9.3) | 14 (3.2) | 37 (8.4) |
| Headache, N (%) | 11 (2.5) | 6 (1.4) | 7 (1.6) |
| Lymphoadenopathy, N (%) | 3 (0.7) | 2 (0.5) | 2 (0.5) |
| Rash, N (%) | 2 (0.5) | 1 (0.2) | 2 (0.5) |
| Other, N (%) | 3 (0.7) | 0 (0.0) | 3 (0.7) |
Abbreviation: AE, adverse event.
Table 4.
Clinical data of patients who developed DVT after the first dose of BNT162b2 mRNA vaccine
| Sex | Female |
| Age | 35 years old |
| Immunodeficiency | No |
| Year of HIV diagnosis | 2013 |
| CDC '93 classification | A2 |
| Allergy to drugs | No |
| Osteopenia | Yes |
| Total cholesterol | 209 mg/dl |
| LDL | 116 mg/dl |
| HDL | 75 mg/dl |
| Triglyceride | 92 mg/dl |
| ART | DTG/ABC/3TC |
| Other therapy | Drospirenone, ethinylestradiol, cholecalciferol |
| HIV‐RNA, cp/ml | Not detectable |
| Previous SARS‐CoV‐2 infection | No |
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; DTG, dolutegravir; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; 3TC, lamivudine.
After 3 months, a non‐protective title was observed in seven patients, whose clinical features are described in Table 5. One of them was already nonresponsive after the first month (Patient 1), six other PLWH lost protective title within the following 3 months (Patient 2–7). Moreover, three had high levels of HIV‐RNA and a low count of CD4, one of the three was a new diagnosis, four had a non‐detectable viremia, and a count of CD4 > 200 cell/µl, all of them were on ART at the time of vaccination.
Table 5.
Clinical and viroimmunological data of all people living with HIV infection who had IgG antibodies against receptor binding domain title for severe acute respiratory syndrome‐related coronavirus‐2 < 50 AU/ml
| Pt1 | Pt2 | Pt3 | Pt4 | Pt5 | Pt6 | Pt7 | |
|---|---|---|---|---|---|---|---|
| Age, years | 49 | 42 | 60 | 62 | 38 | 56 | 54 |
| HIV‐RNA cp/ml | 385 468 | 534 | Not detectable | Not detectable | 729 | Not detectable | Not detectable |
| CD4 cell/μl | 76 | 160 | 428 | 461 | 175 | 332 | 772 |
| CD8 cell/μl | 279 | 1314 | 274 | 2007 | 506 | 679 | 845 |
| CD4/CD8 ratio | 0.3 | 0.1 | 1.6 | 0.2 | 0.3 | 0.5 | 0.9 |
| Immunodeficiency not HIV‐related | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comorbidities | No | Hypertension | No | Diabetes | No | No | Hypertension |
| On ART at the time of vaccination | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
HIV‐RNA was tested before vaccination in 697 patients; 65 (9.3%) of them had detectable HIV‐RNA at baseline. After the second dose of vaccine, HIV‐RNA was tested for in 228 participants. These records were collected during routine outpatient checks for HIV infection: 41 (16.7%) of them were HIV‐RNA positive of which 20 (8.9%) patients were previously undetectable (VL < 20 cp/ml) and referred high adherence to ART. These positive VL were all viral blip (VL < 200 cp/ml), were registered after 30 days (± 20 days) and then controlled after 1−3 months: 17 patients of those not detectable without therapeutic changes, one patient was a long‐term nonprogressor, another patient had VL persistently detectable (VL 40 cp/ml) and one patient was not tested.
4. DISCUSSION
This study describes the immunogenicity and safety of the BNT162b2 mRNA vaccine and clinical characteristics of PLWH in follow‐up in our Clinic, before and after completing vaccination for SARS‐CoV‐2. In our setting, the BNT162b2 vaccine was found to be immunogenic and safe for all patients we vaccinated, with an immunogenicity rate even higher than expected if compared to previous studies. 6 , 7 There are at least two possible explanations to this finding. The first one is that the enrolled population appears to be apparently younger, compared to the entire population with HIV infection in follow‐up in our Center. Indeed, elderly PLWH were previously included in risk categories with priority for vaccination, because of their age, in accordance with the Italian government policy. Therefore, they completed vaccination in the previous months elsewhere, and for that reason they were not included in the study.
Moreover, our study population included mainly stable and heterogeneous PLWH, on ART and with mostly undetectable VL and current high CD4+ cell count. In our cohort, only one patient, a long‐term nonprogressor, was not on ART with low VL and >200 CD4+ cell/µl; although, the patient showed a valid immunological response to vaccination (anti‐RBD IgG 1778.7 AU/m at 30 days after the second dose).
A secondary aim of the study was to investigate the possible correlation between patient characteristics and response to the BNT162b2 mRNA vaccine. However no statistically significant correlation was observed between antibodies title for anti‐RBD IgG and immunological and virologic characteristics measured following the first and second administration, as well as 3 months after the second vaccination. Although in other studies a reduced (IgG positive with neutralizing antibodies title negative 10 ) or delayed (e.g., 2 months 11 ) antibody production was found in PLWH with <200 cell/µl after SARS‐CoV‐2 infection, in our study BNT162b2 vaccine was found immunogenic for 98.6% of PLWH enrolled, including patients with CD4+ cell count <200 cell/µl. Moreover, humoral response to SARS‐CoV‐2 mRNA vaccination in patients with other immunodeficiencies (e.g., cancer, rheumatic disease) has been analyzed in other studies 12 , 13 and a high rate of anti‐RBD antibody production was found, slightly less compared with our entire cohort. The possible explanation is that enrolled people in our study were mainly immunocompetent, only 2.0% had <200 CD+ cell/µl and 1.7% any immunodepression not HIV‐related at the time of vaccination.
Another study involving 78 PWLH (Qualitative assessment of anti‐SARS‐CoV‐2 spike protein immunogenicity [QUASI] after COVID‐19 vaccination in older people living with HIV, Tuan) noted that patients on an Integrase Strand Transfer Inhibitor‐based antiretroviral regimen might present a higher rate of seroconversion after the first dose of COVID‐19 vaccine and that a CD4 count <500 cells/μl could be associated with lower rates of seroconversion after the first dose of COVID‐19 vaccine, but both associations did not reach statistical significance. 14 Even our study failed to show statistically significant differences analyzing the same clinical characteristics including the CD4 level. ART, however, was not evaluated.
Jedicke et al. (humoral immune response following prime and boost BNT162b2 vaccination in people living with HIV on ART) evaluated the anti‐S IgG and neutralizing antibody responses after BNT162b2 in a cohort of 88 PLWH patients, who received the prime dose and 52 patients who received the boost. They found that those titers were significantly lower in PLWH having a CD4:CD8 T‐cell ratio < 0.5. 15 A similar difference was observed when patients were categorized in groups with CD4 cell counts above and below 25%, suggesting that CD4 T‐cell immunity is associated with humoral vaccine‐induced immunity in the early phase after priming. Our study did not find any correlation between the CD4:CD8 T‐cell ratio or CD4 cell counts and the immune response to the vaccine, describing high rates of immunogenicity, regardless of CD4 cell count.
Finally, a study by Woldemeskel et al. 16 compared titers of SARS‐CoV‐2 spike binding antibodies in healthy donors and PLWH vaccinated with the BNT162b2 mRNA vaccine, with no significant difference. Even if the study is limited by the small number of participants, both cohorts (12 PLWH and 17 healthy donors) had similar levels of neutralizing antibodies to the vaccine strain spike protein and spike proteins from variants of concern. Furthermore, given that the BNT162b2 vaccine induces a lower antibody titer in older individuals, 16 these results are interesting as the PLWH study participants were older than the healthy donors. This study, differently from the above‐mentioned papers,14,15 enrolled a cohort with a higher presence of women, allowing us to conclude that the BNT162b2 vaccine will lead to similar protection from COVID‐19 in men and women living with HIV. In our study, 74.7% of enrolled people were male, and it was not possible to find a correlation between immunogenicity and gender, due to a not homogeneous distribution of the population.
Most studies evaluated SARS‐CoV‐2 vaccination in people who were aviraemic while on ART 6 , 7 , 13 and they found mRNA vaccine immunogenic in PLWH with a range of 86% for ChAdOx1 nCoV‐19 vaccine—97.2% for BNT162b2 mRNA vaccine. In our cohort 9.3% of enrolled people were viraemic at the time of vaccination and received two doses of the vaccine.
At 30 days the only nonresponder patient was a newly diagnosed with HIV, on ART for only 2 weeks, and still presenting a detectable HIV‐RNA, in which immunological reconstitution was not achieved. In the other six people who lost their protective title, it was not possible to evidence a common characteristic that could explain a not effective humoral response. Moreover, none of them had any documented immunodeficiency. However, it should be noted that 5/6 patients had <500 CD4 + cell/µl, and two referenced low adherence to ART.
Viral blips were detected one month following anti‐SARS‐CoV‐2 vaccination in previously aviraemic patients. Transient positive viremia is described in the literature after other vaccination (eg., following influenza vaccination) 17 and this does not imply a viral failure. A similar VL increase was found in another study in a smaller group of patients, 6 but no clinical consequences were associated with viral blips, in agreement with our results. However, virological control in newly vaccinated outpatients should be postponed because it would not be possible to relate positive VL to a failure of ART or to vaccine‐related immunological activation.
The BNT162b2 vaccine was safe for PLWH. None of the patients developed a serious AE, except one patient who developed lower extremity deep venous thrombosis after approximately 24 h of administration, without predisposing conditions or coagulopathy. No patients developed immediate hypersensitivity objective reactions, although, the rate of mild/moderate AEs was collected after the end of complete vaccination and data collection was lower than that reported in other clinical trials. 18 , 19 This difference was probably due to the different ways in which AE was monitored: simultaneously with administration in other trials and at the end of the study in our setting.
During follow‐up and during the period between two doses of vaccine, SARS‐CoV‐2 infections were not found in the vaccinated population.
The main limitations of this study include lack of a control group, heterogeneity of study population in sex, age, and immunological state and limited follow‐up. On the contrary, our study is characterized by a larger cohort study group with a follow‐up, that, although limited, reaches a more extended time point, compared with other studies. 6 , 7
5. CONCLUSIONS
In conclusion, this prospective study demonstrates the immunogenicity and safety of the BNT162b2 mRNA vaccine in a cohort of PLWH. Protective titers were recorded in 94.1%, 99.8%, and 98.6% at Time 1 (post first dose of vaccine), Time 2 (post‐second dose of vaccine), and Time 3 (after 3 months from complete vaccination). Positive VL and CD+ cell counts did not appear to have affected immunogenicity. Despite these optimistic findings, it should be further monitored if protective antibodies titer will be found after more time since vaccination to further characterize data on the durability of protection in PLWH. According to other authors, 4 we suggest including a larger HIV‐infected population in future clinical trials to test immunogenicity and safety of vaccines. We found that global priority to achieve the highest proportion of protected people is achievable and practicable in PLWH, regardless of their immunological and virological state.
CONFLICT OF INTERESTS
The author declares that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Study design: Eugenio Milano, Silvio Tafuri, Annalisa Saracino. Data collection: Eugenio Milano, Aurelia Ricciardi, Raffaella Casciaro, Elisabetta Pallara, Elda De Vita. Data analysis: DFB, Angela Maria Vittoria Larocca. First draft writing: Eugenio Milano, Aurelia Ricciardi, Raffaella Casciaro, Elisabetta Pallara, Elda De Vita. Supervision: Davide F. Bavaro. Final approval: All authors.
Milano E, Ricciardi A, Casciaro R, et al. Immunogenicity and safety of the BNT162b2 COVID‐19 mRNA vaccine in PLWH: A monocentric study in Bari, Italy. J Med Virol. 2022;94:2230‐2236. 10.1002/jmv.27629
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.WHO. Clinical features and prognostic factors of COVID‐19 in people living with HIV hospitalized with suspected or confirmed SARS‐CoV‐2 infection. July 15, 2021.
- 2. Geretti AM, Brook G, Cameron C, et al. British HIV Association guidelines on the use of vaccines in HIV‐positive adults 2015. HIV Med. 2016;17(Suppl 3):s2‐s81. 10.1111/hiv.12424 [DOI] [PubMed] [Google Scholar]
- 3. Guaraldi G, Marcotullio S, Maserati R, et al. The management of geriatric and frail HIV patients. A 2017 update from the Italian guidelines for the use of antiretroviral agents and the diagnostic clinical management of HIV‐1 infected persons. J Frailty Aging. 2019;8(1):10‐16. 10.14283/jfa.2018.42 [DOI] [PubMed] [Google Scholar]
- 4. El Chaer F, El Sahly HM. Vaccination in the adult patient infected with HIV: a review of vaccine efficacy and immunogenicity. Am J Med. 2019;132(4):437‐446. 10.1016/j.amjmed.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 5. Menson EN, Mellado MJ, Bamford A, et al. Guidance on vaccination of HIV‐infected children in Europe. HIV Med. 2012;13(6):333‐336. 10.1111/j.1468-1293.2011.00982.x [DOI] [PubMed] [Google Scholar]
- 6. Levy I, Wieder‐Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in people living with HIV‐1. Clin Microbiol Infect. 2021;S1198‐743X(21):00423‐00427. 10.1016/j.cmi.2021.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madhi SA, Koen AL, Izu A, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 (AZD1222) vaccine against SARS‐CoV‐2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double‐blind, placebo‐controlled, phase 1B/2 A trial. Lancet HIV. 2021;8:e568‐e580. 10.1016/S2352-3018(21)00157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. EACS Guidelines , Version 10.1. 2020.
- 9. Manalac J, Yee J, Calayag K, et al. Evaluation of Abbott anti‐SARS‐CoV‐2 CMIA IgG and Euroimmun ELISA IgG/IgA assays in a clinical lab. Clin Chim Acta. 2020;510:687‐690. 10.1016/j.cca.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mondi A, Cimini E, Colavita F, et al. COVID‐19 in people living with HIV: clinical implications of dynamics of the immune response to SARS‐CoV‐2. J Med Virol. 2021;93(3):1796‐1804. 10.1002/jmv.26556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang M, Luo L, Bu H, Xia H. One case of coronavirus disease 2019 (COVID‐19) in a patient co‐infected by HIV with a low CD4+ T‐cell count. Int J Infect Dis. 2020;96:148‐150. 10.1016/j.ijid.2020.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruddy JA, Connolly CM, Boyarsky BJ, et al. High antibody response to two‐dose SARS‐CoV‐2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80(10):1351‐1352. 10.1136/annrheumdis-2021-220656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palich R, Veyri M, Marot S, et al. Weak immunogenicity after a single dose of SARS‐CoV‐2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021;32(8):1051‐1053. 10.1016/j.annonc.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tuan JJ, Zapata H, Critch‐Gilfillan T, et al. Qualitative assessment of anti‐SARS‐CoV‐2 spike protein immunogenicity (QUASI) after COVID‐19 vaccination in older people living with HIV. HIV Med. 2021;23:178‐185. 10.1111/hiv.13188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jedicke N, Stankov MV, Cossmann A, et al. Humoral immune response following prime and boost BNT162b2 vaccination in people living with HIV on antiretroviral therapy. HIV Med. 2021. 10.1111/hiv.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woldemeskel BA, Karaba AH, Garliss CC, et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with HIV. Clin Infect Dis. 2021:ciab648. 10.1093/cid/ciab648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Günthard HF, Wong JK, Spina CA, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent anti‐retroviral therapy. J Infect Dis. 2000;181:522e31‐531e31. [DOI] [PubMed] [Google Scholar]
- 18. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


