Abstract
From December 2019, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has spread rapidly, leading to a global pandemic. Little is known about possible relationships between SARS‐CoV‐2 and other viruses in the respiratory system affecting patient prognosis and outcomes. This study aims to characterize respiratory virome profiles in association with SARS‐CoV‐2 infection and disease severity, through the analysis in 89 nasopharyngeal swabs collected in a patient's cohort from the Campania region (Southern Italy). Results show coinfections with viral species belonging to Coronaviridae, Retroviridae, Herpesviridae, Poxviridae, Pneumoviridae, Pandoraviridae, and Anelloviridae families and only 2% of the cases (2/89) identified respiratory viruses.
Keywords: coinfections, metagenomics analysis, respiratory virome, SARS‐CoV‐2
Key Points
-
•
The severe acute respiratory syndrome coronavirus 2 infection is considered a major global threat that is still spreading around the world.
-
•
Nasopharyngeal swabs samples were collected from the Campania region cohort of 89 Covid‐19 patients.
-
•
Descriptive analysis of respiratory virome was carried out with the HOME‐BIO pipeline, that performed viral taxonomy profiling.
-
•
It detected coinfections with viral species belonging to Coronaviridae, Retroviridae, Herpesviridae, Poxviridae, Pneumoviridae, Pandoraviridae, and Anelloviridae family.
-
•
Only 2% of the cases (2/89) identified respiratory viruses.
1. INTRODUCTION
In December 2019, a few cases of “pneumonia of unknown aetiology” were reported in Wuhan (Hubei region, China). 1 On January 7, 2020, a new coronavirus (CoV), highly related to bats’ SARS‐like virus, was isolated and identified. Not even a decade since the Middle East respiratory syndrome‐related CoV and 15 years after the severe acute respiratory syndrome coronavirus 1 (SARS‐CoV‐1) epidemic, another menace, posing a new unimaginable challenge. 2
Since the advent of this pandemic, in October 2021, more than 240 million confirmed cases and over 4.9 million deaths have been reported (World Health Organization data), and a variable case‐fatality rate estimated to be slightly below 3%, influenced by several factors such as patient's age and comorbidities, healthcare setting, geography, and epidemic phase.
Most of the infected individuals have mild clinical symptoms, while only about 20% of positive patients progress to severe disease. During COVID‐19 treatment, many factors have been shown to affect patient prognosis and one of those is respiratory coinfections with other viruses. 3 The COVID‐19 outbreak occurred first during the winter season, with a high incidence of other respiratory viruses such as influenza viruses. Indeed, SARS‐CoV‐2 coinfections with entero/rhinovirus, human metapneumovirus, respiratory syncytial virus (RSV), other coronaviruses (non‐SARS‐CoV‐2), and influenza A virus have been reported in several studies. 3 , 4 In these situations, infected patients are more prone to develop acute respiratory distress syndrome, which makes their management more challenging. 5 Still, little is known about the impact of coinfections in COVID‐19 patients and a proper virome profiling of SARS‐CoV‐2 infection sites is lacking.
Our study is a descriptive analysis aiming at the identification of SARS‐CoV‐2 and other viral coinfections, to assess the possible association of such coinfections with disease severity. Here, nasopharyngeal swabs samples were collected from a Campania region (Southern Italy) cohort of 89 patients that have been diagnosed with COVID‐19. If and how SARS‐CoV‐2 infections can influence the composition of the upper respiratory tract remains unclear. Therefore, the differentiation between SARS‐CoV‐2 single‐infection and SARS‐CoV‐2 coinfection with other pathogens, and in particular other viruses, is of huge importance for clinical management.
2. METHODS
2.1. Samples cohort
The cohort of SARS‐CoV‐2 infection cases from the Campania region (Southern Italy) consists of 89 patients. Nasopharyngeal swabs were collected during the three main COVID‐19 waves in Italy: first wave (March–May 2020); second wave (September–November 2020); and third wave (January–February 2021). Infections were then confirmed through a positive molecular test. In total, 27 positive cases from the first period were included in this study, as well as 43 from the second and 19 from the third period. Forty‐six percent of patients were female (n = 41) and 54% were male (n = 48) with a median age (interquartile range) of 55 years, ranging from 3 to 99 years (ethical approved number 1316, November 23, 2020). Patients were distributed on the basis of symptom severity as previously described 6 into nonsevere (total: n = 49; asymptomatic: n = 26; and mild: n = 13 cases), moderate (n = 6), severe (n = 10 included 3 deceased), and unknown groups (n = 34). Patient data are summarized in Table 1.
Table 1.
Epidemiological features of the 89 cohort members between the three collection periods
| Mar–May 2020 (n = 27) | Sep–Nov 2020 (n = 43) | Jan–Feb 2021 (n = 19) | |
|---|---|---|---|
| Age (years) | |||
| 0–20 | 3 (11%) | 3 (7%) | 5 (26%) |
| 21–40 | 4 (15%) | 8 (19%) | 1 (5%) |
| 41–60 | 4 (15%) | 15 (35%) | – |
| 61–80 | 10 (37%) | 13 (30%) | 6 (32%) |
| >80 | 3 (11%) | 4 (9%) | 7 (37%) |
| Unknown | 3 (11%) | – | |
| Gender | |||
| Male (%) | 16 (59%) | 27 (63%) | 5 (26%) |
| Female (%) | 8 (30%) | 16 (37%) | 14 (74%) |
| Unknown | 3 (11%) | – | – |
| Disease severity (%) | |||
| Asymptomatic | 11 (41%) | 15 (35%) | – |
| Mild | 7 (26%) | 6 (14%) | – |
| Moderate | 2 (7%) | – | 4 (21%) |
| Severe | 5 (2 dead) (19%) | 4 (1 dead) (9%) | 1 (5%) |
| Unknown | 2 (7%) | 18 (42%) | 14 (74%) |
Note: The values shown in this table are expressed in the format of number (percentage).
2.2. Library preparation, sequencing, and bioinformatics analysis
RNA was extracted from 200 µl of 89 nasopharyngeal swabs using ELITeInGenius fully automated system (ELITechGroup) and ELITeInGenius SP RNA cartridge (ELITechGroup), which exploits a magnetic bead technology, eluting in 100 µl. Extracted RNAs were retro‐transcribed using SensiFAST™ cDNA Synthesis Kit (meridian BIOSCIENCE). The viral load of each sample was assessed by real‐time polymerase chain reaction (RT‐PCR), targeting the Sars‐CoV‐2 viral nucleoprotein gene (forward primer: GGGGAACTTCTCCTGCTAGAAT, reverse primer: CAGACATTTTGCTCTCAAGCTG). RNA concentration was quantified using a Qubit RNA HS Assay Kit (Thermo Fisher Scientific). Libraries were made starting from 100 ng of RNA extract and using the TruSeq Stranded Total RNA Kit (Illumina) according to the manufacturer's guidelines. Briefly, RNA was depleted for ribosomal RNA, fragmented, and first‐strand complementary DNA was synthesized. The following synthesis of the second strand was performed using dUTPs instead of dTTP to quench the amplification of the second strand during the PCR amplification step. After adenylation of double‐strand DNA (dsDNA) fragments, indexed adapters were ligated and DNA fragments containing adapter molecules were enriched by 15 cycles of PCR. Final library concentration was assessed using the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific), while library size was verified by Agilent 4200 Tapestation System (Agilent), showing an average size of 400 bp. Equimolar pools of the samples were prepared and sequenced on the NextSeq 500 (Illumina) in 2 × 75paired‐end mode at a final concentration of 1.7 pMol or on NovaSeq 6000 (Illumina) in 2 × 100 bp paired‐end mode at a final concentration of 250 pMol. The sequencing runs generated 57.6 Gbp of data, with 91.8% of passing filter reads and 94.5% of reads with a quality ≥Q30 for NextSeq and 880.7 Gbp of data, with 82.45% of passing filter reads and 93.3% of reads with a quality ≥Q30 for NovaSeq. Raw sequencing data were analyzed with the HOME‐BIO pipeline. 7 Host‐related sequences were filtered out by mapping on the human reference genome (GRCh38.p13 release 37) and viral taxonomy assignation was obtained with default parameters by querying RefSeq complete viral genomes/proteins database. Classification data were then imported in R software (version 3.6.3) and normalized in reads per million (RPM) values (RPM mapped on the viral database).
3. RESULTS AND DISCUSSION
A total of 9.7 billion raw reads were obtained, with an average of 109.4 million reads per sample (range 26 853.188–240 193.698 reads). For the entire dataset, 609 million reads were mapped on the virus database, with an average of 6.8 million viral reads per sample (range 1718–52 613.608 reads). Sequences related to viruses and their targeted natural hosts were identified. Those specific hosts include invertebrates, plants, fungi, protozoa, and bacteria. For further analysis, reads related to bacteria and phages have not been considered in this study. We focused our attention on eukaryotic viruses, for which the reads relative abundance per sample varied from a minimum of 0.01% of the total viral reads.
As expected, SARS‐CoV‐2 (Coronaviridae family), is the most abundant viral species identified with an average RPM of 703 555 (range 1582–993 313 RPM). In addition, six other viral families were detected during the three different waves: Retroviridae, Herpesviridae, Poxviridae, Pneumoviridae, Pandoraviridae, and Anelloviridae (Figure 1A–C and Table 2). The Retroviridae family was identified in 76% (68/89) of the samples, 6 females and 14 males belonging to the first wave, 10 females as well as 22 males from the second, and 8 females and 5 males from the third (Figure 1D). No direct association with the disease severity seems to be revealed. Amongst those 68 patients, 1 was infected by Lentivirus of human immunodeficiency virus‐1 species, and the others by human endogenous retroviruses K (HERV‐K) species. The patient positive for human immunodeficiency virus‐1 was a female infected by Sars‐CoV‐2 during the first wave, which outcome was fatal. It was previously shown that, in HIV patients, the mortality associated with COVID‐19 disease is higher. 8 Regarding the patients infected by HERV‐K, 10 were characterized by severe outcomes (Figure 1D). Souza et al. 9 recently reported (preprint version) that the presence of Retroviridae HERV‐K in the lower respiratory tract of severe COVID‐19 patients is associated with early mortality. These results are pointing out its possible association with disease severity.
Figure 1.
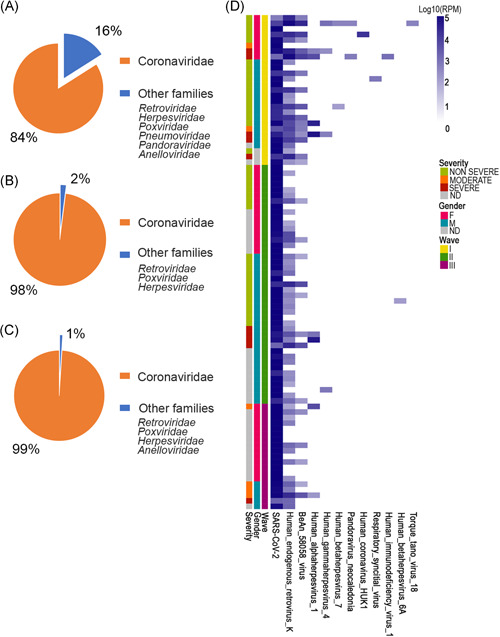
Mean of RPM reads related to detected families among first (A), second (B), and third waves (C). Values are reported as the percentage of all RPM assigned to detected families in the considered period. (D) Heatmap reporting Log10 RPM values of species on entire dataset. RPM, reads per millionl SARS‐COV‐2, severe acute respiratory syndrome coronavirus 2
Table 2.
Overview of the most abundant viruses (family and genus) detected in SARS‐COV‐2 positive samples from three different waves in the Campania Region
| Mar–May 2020 (10 genus from 7 families) | Sep–Nov 2020 (6 genus from 4 families) | Jan–Feb 2021 (5 genus from 5 families) | |||
|---|---|---|---|---|---|
| Family (genus) | Reads number (RPM) | Family (genus) | Reads number (RPM) | Family (genus) | Reads number (RPM) |
|
Coronaviridae (Betacoronavirus) |
517.032 516.869 |
Coronaviridae (Betacoronavirus) |
765.266 764.777 |
Coronaviridae (Betacoronavirus) |
867.010 866.518 |
|
Pneumoviridae (Orthopneumovirus) |
694 1.225 |
– | – | – | – |
|
Retroviridae (Lentivirus, human immunodeficiency virus‐1) |
71.339 86 |
Retroviridae Human endogenous retroviruses |
6.532 6.529 |
Retroviridae Human endogenous retroviruses |
6.010 6.009 |
| Human endogenous retroviruses | 71.233 | ||||
|
Herpesviridae (Simplexvirus) (Lymphocryptovirus) |
19.160 18.539 138 |
Herpesviridae (Simplexvirus) (Roseolovirus) (Lymphocryptovirus) |
6.704 6.623 8 36 |
Herpesviridae (Simplexvirus) |
849 815 |
|
Poxviridae Chordopoxvirinae (subfamily) Orthopoxvirus BeAn58058 virus |
7.793 7.793 |
Poxviridae Orthopoxvirus (BeAn58058virus) |
680 680 |
Poxviridae Orthopoxvirus (BeAn58058virus) |
543 543 |
|
Anelloviridae (Alphatorquevirus) |
28 28 |
– | – |
Anelloviridae Alphatorquevirus |
14 14 |
|
Pandoraviridae (Pandoravirus) |
71 | – | – | – | – |
Note: The viral reads (expressed as a mean of the reads/total samples for each period) identified from samples collected during the first, second, and third waves were classified into 7, 4, and 5 families, respectively.
Abbreviations: RPM, reads per million; SARS‐COV‐2, severe acute respiratory syndrome coronavirus 2.
Another highly represented viral family was the Herpesviridae, which was detected in 21% of the patients (20/89). The specific species found included human‐alfa‐herpesvirus 1 in 9% (8/89) of our samples, human gamma‐herpesvirus 4 (Epstein–Barr virus) in 4.5% (4/89) and human‐betaherpesvirus 6A as well as human‐betaherpesvirus 7A found in, respectively, in 1% and 2.2% (1/89 and 2/89).
In particular, human‐alfa‐herpesvirus 1 was found in five males and in three females distributed along the three different waves. Human gamma‐herpesvirus 4 was found in two males and two females from the first and second waves, while human‐betaherpesvirus 6A was identified in a male from the second wave. Human‐betaherpesvirus 7A was detected in a male and a female during the first sampling campaign. In our data, the human gamma‐herpesvirus 4 was detected in patients with mild to severe/deadly outcomes (Figure 1D).
Human‐alfa‐herpesvirus 1 was also found in samples with severity ranging from nonsevere (n = 1), moderate (n = 2), to severe (n = 5) (Figure 1D). Consequently, it seems that those two species are linked with poorer outcomes in our cohort of COVID‐19 patients. Interestingly, Katz et al. 10 observed that human‐alfa‐herpesvirus 1 reactivation occurs more frequently in COVID‐19 patients than in the normal population. Also, it was already noted, in previous studies, that the human‐alfa‐herpesvirus 1 presence in the lungs of pneumonia patients was associated with worse outcomes. 11 , 12 A reason for Herpesviridae detection in the more severe COVID‐19 cases might be SARS‐CoV‐2 advanced infection association with immunosuppression. 13
Amongst reads attributed to Poxviridae family, BeAn 58058 virus was detected in 32.5% (29/89) of our subjects. Positive samples for the BeAn virus are spread over the three waves (Figure 1D). BeAn 58058 is a zoonotic orthopoxvirus able to infect a wide range of hosts, both wild and domestic animals as well as humans. BeAn 58058 has previously been identified in postmortem Covid‐19 patients as a frequently nonpathogenic detected species. 14
Reads matching to the Anelloviridae family were detected as well and belonged to the Alphatorquevirus genus. In our samples, Torquetenovirus18 was found in a 76‐year‐old female patient, from the first wave, with a nonsevere (mild) outcome (Table 3) and in a 69‐year‐old male (third wave, moderate outcome, Table S2). This was codetected with Herpesviridae species (Figure 1D). It is noteworthy that, even though anelloviruses are not known to be pathogenic, they are considered possible markers of immunosuppression. 15 In our study, Anelloviridae reads were detected at low abundance only in two patients with nonsevere (mild) and moderate outcomes. This result is most likely related to a technical limitation of RNA‐seq. Indeed, it has been shown that RNA sequencing represents a challenge for detection and quantitation of DNA virus, such as the Anelloviridae family, in biological samples as this method was not specifically designed for genomes with such complexity. 16 Furthermore, due to the intrinsic design of the RNA‐seq assays, the low abundance of detected reads relates more to low viral RNA expression levels than to the poor representativeness of these DNA viruses in the samples.
Table 3.
Metagenomic detection of viruses from human nasal‐throat swab samples SARS‐CoV‐2 positive
| Sample ID | Clinical outcome | C t value |
SARS‐CoV‐2 Reads |
Other virus detected (reads) | Sample ID | Clinical outcome | C t value |
SARS‐CoV‐2 Reads |
Other virus detected (reads) |
|---|---|---|---|---|---|---|---|---|---|
| 3_CA44 | Asymptomatic | 33.52 | 1.186 | HERV‐ K113 (105179) | SA49 | Asymptomatic | 27.23 | 128.385 | HERV‐K113 (58450) |
| 4‐CA04 | Mild | 27.62 | 27.068 |
HERV‐ K113 (607115) Human beta herpesvirus7 (773) Human gamma herpesvirus4 (2320) BeAn58058virus (107501) Torquetenovirus18 (773) Pandoravirus (773) |
SA56 | Asymptomatic | 25.90 | 122.347 |
HERV‐K113 (40449) BeAn 58058 virus (8426) |
| 5_COS41 | Dead | 29.35 | 53.429 | HERV‐ K113 (127591) | SA04 | Asymptomatic | 21.2 | 751.085 |
Respiratory syncytial virus (1440) HERV‐K113 (515) |
| 6‐E6‐NA | Severe | 16.79 | 17.4587 |
HERV‐ K113 (12785) Human alpha herpesvirus1 (310554) Human gamma herpesvirus4 (969) |
7‐H6‐NA | Severe | 24.75 | 553.888 |
HERV‐K113 (147339) Human alpha herpesvirus1 (909) |
| SA47 | Asymptomatic | 22.81 | 71.9161 | HERV‐K113 (5650) | SA06 | Severe | 21.685 | 682.398 | HERV‐K113 (35971) |
| 8‐BE14 | Dead | 34.96 | 3.492 |
HERV‐K113 (562281) Human immunodeficiency virus 1 (2328) Human alpha herpesvirus 1 (15133) Pandoravirus (1164) |
SA12 | Moderate | 27.24 | 11.012 | HERV‐K113 (25860) |
| SA68 | Asymptomatic | 25.48 | 104.904 |
HERV‐K113 (53134) BeAn 58058 virus (5136) |
p34‐A7 | Mild | 15.45 | 55.620 | HERV‐K113 (24953) |
| SA73 | Asymptomatic | 22.94 | 704.533 |
HERV‐K113 (18507) Human alpha herpesvirus 1 (156540) |
SA16 | Asymptomatic | 26.33 | 54.533 |
Human coronavirus HKU1 (61377) HERV‐K113 (92600) BeAn 58058 virus (31437) |
Pandoravirus genus, and more specifically the Pandoravirus neocaledonia species, was found in two females from the first wave (Figure 1D). One of those patients had a fatal outcome and human alpha‐herpesvirus 1 was codetected (Figure 1D). The other female, the same one that presented the Torquetenovirus18, had a nonsevere outcome (mild) and presented human gamma‐herpesvirus 4 sequences. In both patients of them were codetected a high number of reads matched the HERV‐K as well as the BeAn 58058 virus (Figure 1D). Pandoraviruses are typical giant viruses of amoebas and are often detected in environmental samples, insects, and simian bushmeat. 17 However, data showed that these giant viruses are present in humans as well when looking into various body parts of both healthy and sick individuals. This kind of virus is found in intensive care units, in patients suffering from pneumonia, and seems to be associated with ventilator use. 18 We, unfortunately, do not know if the patient with a fatal outcome was indeed ventilated.
The Pneumoviridae family reads were found in a young (asymptomatic) child from the first wave (Figure 1D and Table 3). More specifically, they matched on the RSV species. RSV is known to cause bronchiolitis and lower respiratory tract infection in young children that can rarely progress into pneumonia. 19
In addition, we also detected the HKU1 coronavirus (Coronaviridae family) in an 86‐year‐old female from the first wave (Figure 1D and Table 3). Human coronaviruses, such as HKU1, generally cause mild upper‐respiratory tract illness and are responsible for common colds in human adults, however severe lower respiratory tract infections can sometimes occur in elderly people, infants, or immunocompromised patients. It is known to coinfect patients with other respiratory viruses, including other Coronaviridae pathogens. 20
Starting from our descriptive analysis, respiratory viral coinfections seem to be not closely associated with SARS‐CoV‐2 infection or disease severity, period of diagnosis, and gender.
Unlike several other studies reporting influenza virus coinfection with SARS‐CoV‐2, we found no presence of these viruses infection in our samples (in line with the evidence of an unusual global low circulation of influenza viruses during the pandemic period). In the patient cohort, described here, besides asymptomatic RSV detection in an 8‐year‐old child, we also found HKU1 in an elderly female. Both were diagnosed during the first wave before the Italian measures to wear face masks (introduced on April 26, 2020), social distancing, and other measures intended to stop disease spread.
A study conducted by the Icahn School at Mount Sinai, New York, 21 reports that coinfection with other respiratory viruses appears to be rare in patients with SARS‐CoV‐2 infection. Some viruses, such as rhinoviruses, are known to interfere with the ability of other viruses to establish an infection. Hence, in our samples, the lack, or a low presence of other respiratory viruses, can be analyzed in light of these studies. 22 Different mechanisms of the interference have been suggested, including alteration of cell surface viral receptor, cell death, or the host interferon responses. The protective antibody‐driven interferences have also been proposed for the conflict of genetically close viruses such as parainfluenza, metapneumovirus, and RSV. The immune response can be triggered by a virus, through different mechanisms, and their interactions can determine an advantage concerning competition between coinfecting viruses. From these considerations, we can speculate that competitive advantage may play a role in SARS‐CoV‐2 interaction with other respiratory viruses during coinfection, and thus could be one of the reasons why the coinfection rate in SARS‐CoV‐2 patients we analyzed is low. Factors other than viral interference could determine low virus co‐detection rates, such as variations in virus seasonality based on environmental factors and or differences in virus‐host range (e.g., range of cell types, viruses preferentially infect different age groups).
Interestingly, as others have reported before, there seems to be an association between Herpesvididae and SARS‐CoV‐2 infections 8 , 13 (Figure 1D). Nonetheless, in our data, the samples presenting human‐alfa‐herpesvirus 1 and human gamma‐herpesvirus 4 are mostly patients with severe outcomes and is in agreement with other observations. 23 Additionally, we detected the contemporary presence, in two different SARS‐CoV‐2's positive samples (Figure 1D, Table 3, and Table S2), of members of the Herpesviridae and Anelloviridae family. This kind of coinfection is considered worthy of study by the scientific literature. In fact, Mallet et al. 24 analyzed in a recent paper the association of virological markers, as the presence of herpesvirus and anellovirus with clinical outcomes and various immunological parameters to better define the causes and consequences of viral reactivation in 377 patients admitted to the Intensive Care Unit. The concomitant presence of herpesvirus and anellovirus (detected also from us) may have important clinical implications. Between potential coinfector, influencing the SARS‐CoV‐2's disease severity, find in HIV a valuable candidate. HIV and SARS‐CoV‐2 infections were found to be a dire combination 8 and, indeed, the only HIV‐positive patient from our cohort had a fatal outcome (Figure 1D and Table 3). The purpose of this study is to characterize virome composition in COVID‐19 patients.
This study presents some limitations: it is only involving a single COVID‐19 patient cohort from the Campania region including only a single time‐point for each case. Detection of viruses employing supplementary specimen types such as oropharyngeal and broncho‐alveolar lavage fluids could also provide important additional information. Despite the intrinsic exploratory purpose of this study, it lifted up questions about whether some viruses with uncertain pathogenicity could be contributing to symptoms manifestation, complicating clinical outcome, or might be possible biomarkers of infection or host response.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Francesca Rizzo and Gianluigi Franci: Conceptualization. Roberta Astorri, Pasquale Pagliano, and Giorgio Giurato:Software analysis.Teresa Rocco, Ylenia D'Agostino, and Jessica Lamberti:Investigation.Carlo Ferravante, Giuseppina Sanna, Viola Melone, Aldo Manzin, Gianluigi Franci, and Giovanni Pecoraro: Data curation.Giuseppina Sanna and Carlo Ferravante: writing – original draft preparation. Gianluigi Franci, Francesca Rizzo, Giuseppina Sanna, Carlo Ferravante, and Giorgio Giurato: Writing – review and editing. Francesca Rizzo, Gianluigi Franci, Alessandro Weisz, Aldo Manzin, and Massimiliano Galdiero: Supervision.Giorgio Giurato, Massimiliano Galdiero, and Alessandro Weisz: Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
This study was supported by Regione Campania (Grants “GENOMAeSALUTE,” POR Campania FESR 2014/2020, azione 1.5; CUP: B41C17000080007 and “Monitoring the spread and genomic variability of the Covid 19 virus in Campania using NGS technology,” POR Campania FESR 2014/2020, CUP: B14I20001980006), PRIN 2017 “Natural and pharmacological inhibition of the early phase of viral replication (VirSudNet)” N° 2017M8R7N9 and Ministero dell'Istruzione, Università e Ricerca, progr. POC R&I 2014‐2020 and PON R&I 2014‐2020 “Dottorati innovativi con caratterizzazione industriale” XXXV Ciclo (Fellowships DOT1328517, CUP D52G19000580006 to Viola Melone and DOT1318705, CUP: E66C18000940007 to Carlo Ferravante). Teresa Rocco and Ylenia D'Agostino are fellows of Rete Oncologica Campana and Elena Alexandrova is a fellow of Fondazione U. Veronesi. Carlo Ferravante, Jessica Lamberti, and Viola Melone are PhD students of the Research Doctorate in Veterinary Sciences of the University of Napoli “Federico II,” in “Molecular and Translational Oncology and Innovative Medical‐Surgical Technologies,” University of Catanzaro “Magna Graecia” and in “Translational Medicine for Development and Active Ageing” of the University of Salerno. The authors would like to thank Prof. Ivan Gentile, Dr. Nicola S. Moriello, PO Malattie Infettive and Prof. Giuseppe Portella, Dr. Michele Cennamo, PO Patologia Clinica of “Federico II” University Hospital, Napoli; Dr. Angelo Salomone Megna, U.O.C. Malattie Infettive and Dr. Vincenzo Rocco, Dr. Maurizio Fumi, U.O.C. Patologia Clinica of AORN "San Pio" PO G. Rummo, Benevento; Dr. Gregorio Goffredi, Dr. Francesca Marciano, G.O.I. Medicina di Laboratorio e Biologia Molecolare of “Maria Santissima Addolorata” Hospital, Eboli, Salerno; Dr. Paolo Sorrentino, Dr. Carmine Sanseverino, Unità Fegato, UO Malattie infettive, Dr. Maria Landi, Dr. Maria G. Foti, Servizio di Microbiologia e Virologia ‐ A.O.R.N. “San Giuseppe Moscati”, Avellino; Prof. Paolo Maggi, U.O.C. Malattie Infettive e Tropicali—Dr. Maddalena Schioppa, UOSD Genetica e Biologia Molecolare, A.O.R.N. “S. Anna e S. Sebastiano,” Caserta; and Dr. Emilia Vaccaro, U.O.S.D. NAT e Biologia Molecolare, A.O.U. “San Giovanni di Dio e Ruggi d'Aragona,” Salerno for providing nasopharyngeal swab RNA and other information used in this study.
Ferravante C, Sanna G, Melone V, et al. Nasopharyngeal virome analysis of COVID‐19 patients during three different waves in Campania region of Italy. J Med Virol. 2022;94:2275‐2283. 10.1002/jmv.27571
Contributor Information
Francesca Rizzo, Email: frizzo@unisa.it.
Gianluigi Franci, Email: gfranci@unisa.it.
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. 10.1038/s41586-020-2008- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen X, Liao B, Cheng L, et al. The microbial coinfection in COVID‐19. Appl Microbiol Biotechnol. 2020;104(18):7777‐7785. 10.1007/s00253-020-10814-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co‐infection between SARS‐CoV‐2 and other respiratory pathogens. JAMA. 2020;323(20):2085‐2086. 10.1001/jama.2020.6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID‐19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020;71(15):706‐712. 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferravante C, Memoli D, Palumbo D, et al. HOME‐BIO (sHOtgun MEtagenomic analysis of BIOlogical entities): a specific and comprehensive pipeline for metagenomic shotgun sequencing data analysis. BMC Bioinformatics. 2021;22(Suppl 7):106. 10.1186/s12859-021-04004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID‐19 in HIV‐infected individuals: a systematic review and meta‐analysis. Sci Rep. 2021;11(1):6283. 10.1038/s41598-021-85359-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Temerozo J, Fintelman‐Rodrigues N, Santos MC, et al. Human endogenous retrovirus K activation in the lower respiratory tract of severe COVID‐19 patients associates with early mortality. Res Square. 2021. 10.21203/rs.3.rs-514541/v1 [DOI] [Google Scholar]
- 10. Katz J, Yue S, Xue W. Herpes simplex and herpes zoster viruses in COVID‐19 patients. Ir J Med Sci. 2021; 10.1007/s11845-021-02714-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coisel Y, Bousbia S, Forel JM, et al. Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator‐associated pneumonia. PLoS One. 2012;7(12):e51340. 10.1371/journal.pone.0051340 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Al‐Omari A, Aljamaan F, Alhazzani W, Salih S, Arabi Y. Cytomegalovirus infection in immunocompetent critically ill adults: literature review. Ann Intensive Care. 2016;6:110. 10.1186/s13613-016-0207-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Le Balc'h P, Pinceaux K, Pronier C, Seguin P, Tadié JM, Reizine F. Herpes simplex virus and cytomegalovirus reactivations among severe COVID‐19 patients. Crit Care. 2020;24(1):530. 10.1186/s13054-020-03252-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nienhold R, Ciani Y, Koelzer VH, et al. Two distinct immunopathological profiles in autopsy lungs of COVID‐19. Nat Commun. 2020;11(1):5086. 10.1038/s41467-020-18854-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Vlaminck I, Khush KK, Strehl C, et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155(5):1178‐1187. 10.1016/j.cell.2013.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Depledge DP, Mohr I, Wilson AC. Going the distance: optimizing RNA‐Seq strategies for transcriptomic analysis of complex viral genomes. J Virol. 2018;93(1):e01342. 10.1128/JVI.01342-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rolland C, Andreani J, Louazani AC, et al. Discovery and further studies on giant viruses at the IHU Mediterranee infection that modified the perception of the virosphere. Viruses. 2019;11(4):312. 10.3390/v11040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colson P, Fancello L, Gimenez G, et al. Evidence of the megavirome in humans. J Clin Virol. 2013;57(3):191‐200. 10.1016/j.jcv.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 19.Schweitzer JW, Justice NA. Respiratory syncytial virus infection. StatPearls, 2021. https://www.ncbi.nlm.nih.gov/books/NBK459215/ [PubMed]
- 20. Chaung J, Chan D, Pada S, Tambyah PA. Coinfection with COVID‐19 and coronavirus HKU1—the critical need for repeat testing if clinically indicated. J Med Virol. 2020;92(10):1785‐1786. 10.1002/jmv.25890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nowak MD, Sordillo EM, Gitman MR, Paniz Mondolfi AE. Coinfection in SARS‐CoV‐2 infected patients: where are influenza virus and rhinovirus/enterovirus? J Med Virol. 2020;92(10):1699‐1700. 10.1002/jmv.25953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pinky L, Dobrovolny HM. Coinfections of the respiratory tract: viral competition for resources. PLoS One. 2016;11(5):e0155589. 10.1371/journal.pone.0155589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Z, Hu X, Li Z, et al. Effect of SARS‐CoV‐2 infection on the microbial composition of upper airway. Infect Drug Resist. 2020;13:2637‐2640. 10.2147/IDR.S259984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mallet F, Diouf L, Meunier B, et al. Herpes DNAemia and TTV viraemia in intensive care unit critically ill patients: a single‐centre prospective longitudinal study. Front Immunol. 2021;12:698808. 10.3389/fimmu.2021.698808 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.


