To the editor,
We read with interest the autoimmune hepatitis (AIH) case following COVID‐19 vaccination[ 1 ] and the comment by Drs. Mungmunpuntipantip and Wiwanitkit.[ 2 ] COVID‐19 vaccine could also act as a trigger in the disease course of AIH. We report a case of AIH exacerbation following inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac).
The patient is a 57‐year‐old Asian female without medical history. She developed choluria and acholic stools 2 weeks after the first dose of CoronaVac but did not seek medical advice and received the second dose CoronaVac after 3 weeks. Two days later, she developed generalized pruritus and deep scleral and sublingual icterus with markedly elevated total bilirubin (283.8 μmol/L), alanine aminotransferase (974 U/L), aspartate aminotransferase (819 U/L), alkaline phosphatase (212 U/L), and gamma‐glutamyltransferase (238 U/L). She reported no consumption of alcohol or traditional medicine.
Viral serologic tests showed negative hepatitis A/B/C/D/E virus, HIV, cytomegalovirus, Epstein‐Barr virus, and herpes simplex virus. Total IgG was slightly elevated (17.44 g/L; normal range, 8.6–17.4 g/L) with positive antinuclear antibodies (1:640, homogeneous pattern), anti–Sjögren syndrome antigen A, anti–major centromere autoantigen B, and weakly positive anti–Sjögren syndrome antigen B. The antimitochondrial, antimitochondrial‐M2, anti–smooth muscle, anti–liver‐kidney microsomal, anti–liver cytosolic, anti–soluble liver antigen, anti‐glycoprotein‐210, and anti‐SP100 antibodies were all negative.
Contrasted CT and MRI showed no malignancy and biliary lithiasis or dilation. Liver biopsy revealed established fibrosis (Stage 2) and active hepatitis (Grade 2) with moderate to severe interface necroinflammation, severe lobular lymphocytic/lymphoplasmocytic infiltration, hepatic rosette formation, and a dense lymphoid infiltrate (Figure 1A–F). Both her revised original (20 points) and the simplified (7 points) score for AIH suggested a definite diagnosis of AIH. She had excellent responses to treatment (ursodeoxycholic acid and a tapering course of methylprednisolone overlapped with azathioprine) and no relapse during 5‐month follow‐up (Figure 1G).
FIGURE 1.
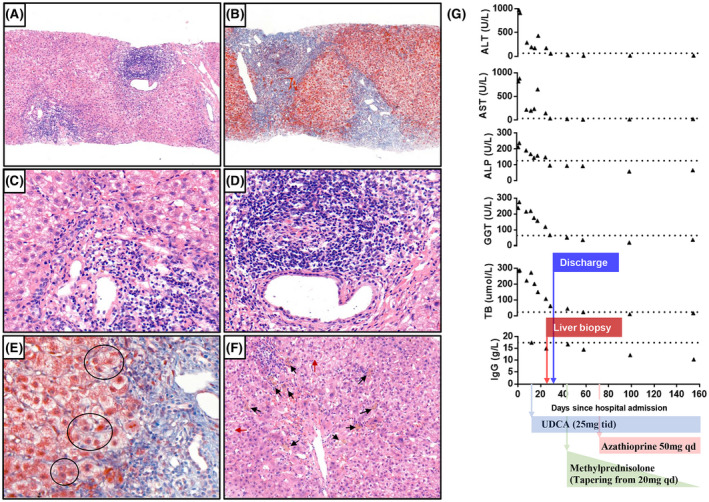
Histological findings and evolution of laboratory tests. At low‐magnification (×100), hematoxylin and eosin staining shows moderate to severe interface hepatitis with a dense lymphoid infiltrate (A), and Masson staining shows the formation of fibrous septa (B). At high magnification (×400) with hematoxylin and eosin stain, the interface necroinflammation consists primarily of lymphocytes with plasma cells (C); the dense periductal lymphocyte infiltrate (D) and the hepatic rosette formation (black circle) were well observed with Masson stain (E). Feathery degeneration of hepatocyte (red arrow) and hepatic cholestasis (black arrow) were also observed (F) (×200 hematoxylin and eosin stain). (G) Trends of liver function tests, total bilirubin, and total IgG levels over time. Dashed lines are the respective lower limit of the normal range of each test. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyltransferase; TB, total bilirubin; UDCA, ursodeoxycholic acid
Several cases of AIH following COVID‐19 vaccine have been reported,[ 3 ] but unlike these cases, the presence of Stage‐2 fibrosis in our case is against the hypothesis of vaccine‐induced AIH onset but suggests vaccine‐induced AIH exacerbation. The vaccination unmasks the undiagnosed AIH and triggers the disease flare. Although CoronaVac vaccination in patients with autoimmune rheumatic diseases is generally safe,[ 4 ] our case suggests the need for data in patients with AIH.
CONFLICT OF INTEREST
Nothing to report.
AUTHOR CONTRIBUTIONS
Zhujun Cao wrote the draft of the manuscript. Qing Xie and Honglian Gui revised the manuscript. Honglian Gui was involved in the clinical care of the patient. Zhujun Cao, Zike Sheng, Honglian Gui, Haiguang Xin, and Qing Xie were involved in the clinical care of the patient during hospitalization. All authors contributed to and approved the final manuscript.
Funding information
Supported by the Shanghai Municipal Key Clinical Specialty (shslczdzk01103).
REFERENCES
- 1. Palla P, Vergadis C, Sakellariou S, Androutsakos T. Letter to the editor: Autoimmune hepatitis after COVID‐19 vaccination. A rare adverse effect? Hepatology. 2021. 10.1002/hep.32156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mungmunpuntipantip R, Wiwanitkit V. Lettter to the editor on “Autoimmune hepatitis after COVID‐19 vaccination.” Hepatology. 2021. 10.1002/hep.32249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bril F. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID‐19) vaccine: one or even several swallows do not make a summer. J Hepatol. 2021;75:1256–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Medeiros‐Ribeiro AC, Aikawa NE, Saad CGS, Yuki EFN, Pedrosa T, Fusco SRG, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744–51. [DOI] [PubMed] [Google Scholar]


