Abstract
The pharmacokinetics of an orally administered valine ester of ganciclovir (GCV), valganciclovir (VGC), were studied. These were compared to the pharmacokinetics of oral and intravenous GCV. Twenty-eight liver transplant recipients received, in an open-label random order with a 3- to 7-day washout, each of the following: 1 g of oral GCV three times a day; 450 mg of VGC per os (p.o.) once a day (q.d.); 900 mg of VGC p.o. q.d.; and 5 mg of intravenous (i.v.) GCV per kg of body weight q.d., given over 1 h. GCV and VGC concentrations were measured in blood over 24 h. One-sided equivalence testing was performed to test for noninferiority of 450 mg of VGC relative to oral GCV (two-sided 90% confidence interval [CI] > 80%) and nonsuperiority of 900 mg of VGC relative to i.v. GCV (two-sided 90% CI < 125%). The exposure of 450 mg of VGC (20.56 μg · h/ml) was found to be noninferior to that of oral GCV (20.15 μg · h/ml; 90% CI for relative bioavailability of 95 to 109%), and the exposure of 900 mg of VGC (42.69 μg · h/ml) was found to be nonsuperior to that of i.v. GCV (47.61 μg · h/ml; 90% CI = 83 to 97%). Oral VGC delivers systemic GCV exposure equivalent to that of standard oral GCV (at 450 mg) or i.v. GCV (at 900 mg of VGC). VGC has promise for effective CMV prophylaxis or treatment with once-daily oral dosing in transplant recipients.
Following organ transplantation, a majority of allograft recipients are at risk of developing clinically significant cytomegalovirus (CMV) disease that contributes significantly to both morbidity and mortality (8). The reported incidence of clinically apparent CMV disease in liver transplant recipients ranges from approximately 20 to 60% (10, 13). Ganciclovir (GCV), given intravenously (i.v.) at 5 mg/kg of body weight once daily, or orally as capsules at 1,000 mg three times a day (TID), is the standard drug regimen for both the treatment and prevention of CMV disease in transplant recipients (11, 15). However, i.v. GCV is an inconvenient drug regimen for long-term use, requiring i.v. catheters and frequent home health visits. Although GCV capsules are more convenient, the low relative bioavailability (6%) limits the concentrations in serum and overall drug exposure that can be achieved (10). Administration using divided doses is necessary for oral GCV to maintain adequate GCV exposure. Valacyclovir, the valine ester of acyclovir, requires an even higher dose than oral GCV (2,000 mg four times a day for valacyclovir versus 1,000 mg TID for oral GCV) to achieve efficacy in the prevention of CMV disease posttransplant (10, 14).
Valganciclovir (VGC) is a valine ester of GCV. Following ingestion, the great majority of VGC is converted rapidly to GCV by hydrolysis prior to reaching systemic circulation (12). In human immunodeficiency virus (HIV)-infected patients, the oral bioavailability of VGC is approximately 60%, 10-fold higher than the bioavailability of oral GCV capsules. Studies with HIV-infected individuals have shown that 900 mg of VGC once a day should give a drug exposure, represented as area under the plasma concentration time curve (AUC), similar to that of i.v. GCV at 5 mg/kg/day (12). If VGC provides drug exposure in transplant patients comparable to that achieved with i.v. GCV, it would represent a significant advance in the prevention of CMV disease in transplant recipients.
The goal of this study was to determine the dose of VGC that would provide a drug exposure (AUC) bounded by that of i.v. GCV above and oral GCV below. The GCV doses chosen (5 mg/kg once a day i.v. and 1,000 mg per os [p.o.] TID) represented the highest and lowest drug exposures, respectively, known to provide efficacious and safe prevention of CMV infection and disease posttransplant.
(This study was presented at the 18th Annual Meeting of the American Society of Transplantation, May 1999, Chicago, Ill.)
MATERIALS AND METHODS
This study was conducted at one center in England and six centers in the United States, and the human experimentation guidelines of the U.S. Department of Health and Human Services and/or those of the authors' institutions were followed in the conduct of the clinical research. The protocol was reviewed and approved by the relevant independent review boards at each site. Written informed consent was obtained from each subject prior to enrollment in the study. The study was an open-label, four-way crossover design consisting of seven replications of the four-period William's design. Subjects were randomly assigned to treatment sequences by computer by the study sponsor. There was a 3- to 7-day washout period between treatments. The treatments, interchanged to provide balanced period combinations, were as follows: 3,000 mg of oral GCV (as 250-mg capsules) in three doses (1,000 mg every 6 h) (treatment A); 450 mg of VGC (as one 450-mg tablet p.o.) (treatment B); 900 mg of VGC (as a single dose of two 450-mg tablets p.o.) (treatment C); and 5 mg of i.v. GCV per kg as a single 1-h infusion (treatment D). The sample size was selected on the basis of results for HIV-infected patients where the intra- and intersubject coefficients of variation (CV) were approximately 8.8 and 21% for the AUC, respectively. It was predicted that in liver transplant recipients CV would be greater. Allowing for a 30% increase in variation, 24 evaluable patients would provide a power of 0.8, assuming a significance level of 0.05. A total of 28 patients were studied to allow for unevaluable patients.
Liver transplant recipients who were CMV seropositive and 45 to 180 days posttransplant at entry or who were CMV seronegative and had received an organ from a CMV-seronegative donor and were 21 to 180 days posttransplant were enrolled. Patients were excluded if they were <18 years old, had an estimated creatinine clearance (CLCR) of <50 ml/min, had CMV disease or CMV antigenemia, had received oral or i.v. GCV within 3 days of starting the study to ensure total elimination of any residual GCV, had uncontrolled diarrhea, or were cytopenic. Immunosuppressive drugs were utilized as clinically determined, but doses of cyclosporine and tacrolimus were kept stable during the duration of the study.
A complete medical history, transplant history, and list of medications were obtained, a physical examination and baseline laboratory studies (complete blood count, chemistry, urinalysis) were performed at the screening, and laboratory studies and adverse event monitoring were done the day before each dose or on the following morning prior to dosing and at the termination visit. Any spontaneously reported adverse events were also noted as they occurred. An estimated CLCR was calculated at screening and follow-up visits using the Cockcroft and Gault formula (4). CLCR was measured on each dosing day using the total measured 24-h excreted urine creatinine and serum creatinine concentrations.
The presence of CMV antigenemia was determined using a commercial kit utilizing antibody to the pp65 CMV lower matrix protein (CMV Brite; Biotest Laboratories). A positive test for CMV antigenemia was the finding of one or more positive (fluorescing) cells on duplicate slides. Any patient with a positive CMV test could be withdrawn at the discretion of the investigator.
Pharmacokinetic studies.
Subjects were dosed in a clinical research unit. Each subject received each of the treatments A, B, C, and D in a random order on one of four separate occasions (periods). Subjects reported to the medical facility on the evening before dosing within each period, and laboratory tests, antigenemia testing, and body weight measurement were performed. Subjects fasted from 10 p.m. the night before dosing but were allowed water ad libitum. A cannula inserted into a peripheral vein to collect blood samples was kept patent by flushing it with saline after each collection. Subjects were dosed 10 min after eating a standard breakfast consisting of one bowl of corn flakes with 100 ml of whole milk, two pieces of bacon, two fried eggs, two slices of toast with butter, 100 ml of orange juice, and 150 ml of coffee or tea. All subjects received a light lunch and an evening meal.
Five-milliliter blood samples in EDTA were drawn at the following times: treatment A, predose and 0.5, 1, 2, 3, 4, 6, 6.5, 7, 8, 9, 10, 12, 12.5, 13, 14, 15, 16, 18, and 24 h after the first dose; treatments B, C, and D, predose and 0.083, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 16, and 24 h after the start of the dose. A different sampling schedule was required for treatment A in order to obtain information for all three administered doses, thereby ensuring the accuracy of the calculated exposure. For treatment D, the first four samples (0.083, 0.25, 0.5, and 0.75 h) were drawn during the i.v. infusion, and the 1-h sample was taken at the end of infusion. Blood samples were centrifuged immediately at 2,000 × g for 15 min at 4°C. Plasma was extracted and stored at −70°C until analysis. Urine, after a predose void, was collected over the postdose intervals 0 to 6, 6 to 12, and 12 to 24 h, and at each interval a 10- to 15-ml aliquot was frozen at −20°C. The total urine volume was recorded. Subjects left the medical facility after the 24-h sample of blood and urine was collected. The same study procedure was repeated for each period, with each subject crossing over to the other regimen in their randomly assigned sequence. The washout time between periods was 3 to 7 days.
Plasma samples were analyzed for VGC in treatments B and C, and plasma and urine samples were analyzed for GCV in all treatments. Both VGC and GCV were quantified by a specific high-pressure liquid chromatography column-switching method using UV detection (λ, 254 nm) for VGC and fluorescence detection λ (excitation) and λ (emission), 278 and 380 nm) for GCV (2, 3). The lower quantification limit for the assay of plasma samples for both VGC and GCV was 0.04 μg/ml, while the lower quantification limit for the assay of GCV in urine samples was 1 μg/ml. The quality control ranges across all samples (percent CV and percent accuracy, respectively) were 7.5 to 15.9 and 100.5 to 105.3 for plasma GCV, 4.2 to 6.9 and 94.4 to 95.7 for plasma VGC, and 5.4 to 12.1 and 95.8 to 100 for urine GCV. For the lowest standard (0.04 μg/ml of plasma; 1.0 μg/ml of urine) the values were 6.8 and 96.5 for plasma GCV, 5.4 and 101.3 for plasma VGC, and 17.4 and 94.6 for urine GCV.
Pharmacokinetic parameters.
The pharmacokinetic parameters were derived by noncompartmental methods. Samples below the limit of quantification at the beginning or end of profiles were considered to have a value of 0 μg/ml, while those occurring during the profile were assumed to be missing. The primary parameters for statistical analysis were the GCV AUC extrapolated to infinity (AUC∞) for the comparison of 900 mg of VGC with 5 mg of i.v. GCV per kg and the GCV AUC calculated over 24 h (AUC24) for the comparison of 450 mg of VGC with 1 g of oral GCV TID. For patients on oral GCV, AUC24 was calculated over all three GCV doses.
AUC24 was computed using the linear-trapezoidal method, from time zero to 24 h. When only the 24-h sample was undetectable, the concentration at 16 h and the terminal elimination rate constant (kel) were used to estimate a concentration at 24 h, which was included in the calculation of AUC24. AUC∞, the area from the last sample to infinity, was calculated using kel and combined with the AUC24. AUC∞ was not calculated for treatment A because insufficient samples were taken during the elimination phase due to the divided (TID) dosing. The maximum observed concentration (Cmax) was taken directly from the data for each subject, and time to the maximum observed concentration (Tmax) was calculated. kel was calculated from log-linear regression of the terminal portion of the concentration time profile. Half-life associated with kel (t1/2) was calculated as ln2/kel. Absolute bioavailability was calculated as follows: [AUC∞(p.o.)/dose(p.o.)]/[AUC∞(i.v.)/dose(i.v.)], where the p.o. doses are expressed in GCV equivalents, calculated as follows:
 |
Urinary pharmacokinetic parameters were calculated for GCV only and were as follows: renal clearance (CLR) was calculated from the gradient of a plot of AUCΔt (AUC over the interval Δt) versus AeΔt (amount of GCV excreted in urine over the same time interval Δt), Ae (cumulative amount excreted in the urine) was calculated as ΣAeΔ, and percent dose was the percentage of administered dose which appeared in urine over 24 h. Doses for VGC treatments were expressed as GCV equivalents, and values for oral treatments were adjusted to account for bioavailability.
Statistics.
A sample size of 28 liver transplant recipients was chosen in order to achieve a sample size of at least 24 evaluable subjects. One-sided equivalence testing was performed for the log-transformed primary pharmacokinetic parameters AUC∞ of GCV for the comparison with i.v. GCV and AUC24 of GCV for the comparison with oral GCV. An analysis of variance with the factors subject, period, and treatment was applied to lnAUC using the main-effects model to estimate the least-squares mean differences, the within-subject variance ς, and two-sided 90% confidence limits for the least-squares mean difference. The treatment effect ratio and the confidence limits for the corresponding ratio of means of the untransformed variables were calculated by exponentiation of the least-squares mean differences and the confidence limits for the transformed values, respectively. One-sided equivalence testing was performed to test for noninferiority of treatment B relative to treatment A (two-sided 90% confidence interval [CI] > 80%) and nonsuperiority of treatment C relative to treatment D (two-sided 90% CI < 125%). These are comparisons at the significance level 0.05. No adjustment for multiple testing was used. One-sided equivalence testing was used and justified since the questions of interest were the following: did 450 mg of VGC provide GCV exposure less than that provided by 3 g of GCV p.o., and did 900 mg of VGC provide GCV exposure greater than that provided by 5 mg of i.v. GCV per kg?
RESULTS
Subject disposition.
Thirty-two patients were screened, of which 28 qualified to enter the randomized period of the study. Patient demographics (means ± standard deviations) were as follows: sex, 21 males (75%) and 7 females (25%); age, 47.2 ± 8.3 years; weight, 88.2 ± 18.3 kg; height, 174.7 ± 9.3 cm; CLCR, 92.7 ± 20.8 ml/min; and race, 24 caucasians (86%), 1 black (4%), 2 hispanics (7%), and 1 other (4%).
There were four screen failures: two patients were found to be CMV antigenemia positive at baseline, one patient had a CLCR of less than 50 ml/min, and one patient was not enrolled because the study had been closed. All 28 subjects received corticosteroids, 68% received tacrolimus, 32% received cyclosporine, 50% received azathioprine, and 32% received mycophenolate mofetil. For each patient, individual immunosuppressive therapy did not change during the course of the study. All 28 patients received all four study treatments and completed the study. There was a good correlation between estimated CLCR (Cockcroft and Gault method) and measured CLCR (r2 = 0.6).
VGC pharmacokinetics.
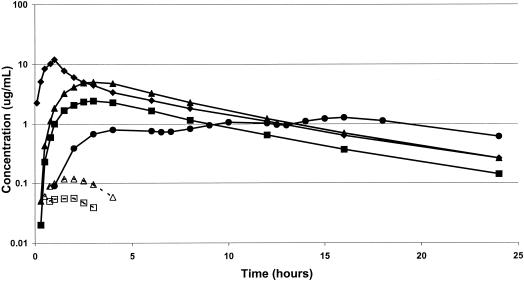
Plasma samples from treatments B and C were analyzed for VGC. Data from three subjects from treatment B and one from treatment C were excluded from the calculation of VGC pharmacokinetic parameters due to unevaluable data, assay interference, or lack of quantifiable VGC. Mean VGC pharmacokinetic parameters are shown in Table 1. VGC was absorbed rapidly, with peak concentrations in plasma occurring 1.5 to 2.0 h after dosing in the presence of food, after which concentrations in plasma declined rapidly, falling below the limit of quantification in most subjects within 3 to 4 h after dosing. In no subject was VGC measurable beyond 6 h (Fig. 1). For many of the subjects (Table 1), it was not possible to obtain reliable estimates of the t1/2 of VGC due to its rapid decline in plasma. Elimination of VGC was rapid, with terminal elimination rate t1/2s of approximately 1.5 h for both the 450- and 900-mg doses.
TABLE 1.
VGC pharmacokinetic parameters following single doses of VGC
| Parameter | Mean value for treatmenta
|
|
|---|---|---|
| 450 mg of VGC (n = 25) | 900 mg of VGC (n = 27) | |
| AUC24 (μg · h/ml)b | 0.179 (58) | 0.435 (41) |
| Cmax (μg/ml) | 0.097 (51) | 0.172 (40) |
| Tmax (h) | 1.8 (55) | 1.9 (56) |
| t1/2 (h) | 1.52 (40)c | 1.59 (46)d |
Values in parentheses are CV (percents).
Samples beyond the last measurable concentration were set to zero for the calculation of AUC24.
n = 11.
n = 13.
FIG. 1.
Mean plasma pharmacokinetic profiles following dosing with i.v. GCV, oral GCV, and VGC. Solid symbols are mean plasma drug concentrations (on a log scale) over the 24-h dosing interval of GCV after i.v. GCV (⧫), oral GCV (●), 450 mg of oral VGC (■), or 900 mg of oral VGC (▴). Open symbols are plasma VGC concentrations after 450 mg of oral VGC (□) and 900 mg of oral VGC (▵). Plasma VGC concentrations were only measured and reported for patients dosed with VGC. A dose-dependent increase in GCV concentration was seen after dosing with VGC. The amount of unmetabolized VGC was substantially less than that of GCV (<3%).
GCV pharmacokinetics.
Data from one subject for treatment D (i.v. GCV) were excluded because the 1-h sample (end of infusion) was taken from the same arm as the infusion. Mean GCV pharmacokinetic parameters for all four treatments are shown in Table 2. Mean concentrations in plasma from i.v. GCV reached a maximum of about 10 μg/ml at the end of infusion, after which a biexponential decline was apparent (Fig. 1). GCV concentrations following administration of VGC appeared in plasma on an average of 0.25 h after dosing and reached a maximum 3.0 h after dosing. Plasma GCV concentrations then declined, with a terminal elimination t1/2 similar to that seen with i.v. GCV. Absorption of GCV from oral GCV capsules was much slower than from VGC tablets, with maximum concentrations following the first dose only being reached 4 to 5 h after dosing. The GCV AUC24 values after oral GCV and dosing with 450 mg of VGC were similar, although concentrations in plasma of GCV rose more rapidly and reached higher levels following administration of VGC than following administration of oral GCV. The GCV AUC values associated with i.v. GCV and 900 mg of VGC were similar, with values being slightly lower in the VGC group and maximum concentrations being higher in the i.v.-GCV group. The terminal elimination GCV t1/2s associated with i.v. GCV and the oral VGC doses were very similar (5.10 to 5.22 h). For i.v. GCV the mean value obtained for total systemic clearance was 157 ml/min (CV, 28%) (1.87 ml/min/kg [CV, 35%]). A steady-state volume of distribution of 52.2 liters (CV, 25%) was seen in these subjects. Because of imprecise measurement of total absorption, such calculations cannot be done for the oral treatment periods.
TABLE 2.
GCV pharmacokinetic parameters following dosing with i.v. GCV, oral GCV, and VGCa
| Parameter | Mean value for treatmenta
|
|||
|---|---|---|---|---|
| Oral GCV (n = 28) | 450 mg of VGC (n = 28) | 900 mg of VGC (n = 28) | i.v. GCV (n = 27) | |
| AUC24 (μg · h/ml) | 20.7 (22) | 21.1 (23) | 41.7 (24) | 48.2 (36) |
| AUC∞ (μg · h/ml) | NC | 22.2 (24) | 43.9 (25) | 50.6 (40) |
| Cmax (μg/ml) | 1.46 (23) | 3.01 (27) | 6.18 (30) | 12.2 (24) |
| Tmax (h) | 14.3b (22) | 3.0 (44) | 2.9 (36) | 1.0 (12) |
| t1/2 (h) | NC | 5.22 (20) | 5.1 (22) | 5.17 (27) |
| Urine clearance (ml/min) | 137 (30) | 126 (31) | 137 (31) | 125 (30) |
| % of dose excreted in urinec | 86.5 (23) | 79.5 (20) | 84.9 (19) | 77.4 (14) |
Values in parentheses are CV (percents). NC, could not be calculated.
Calculated as the maximum concentration seen over all three doses of oral GCV.
Values are adjusted for bioavailability.
Urinary GCV.
CLR could not be calculated in one of the four treatments for five patients due to incomplete urine collections, lost samples, or unevaluable plasma data. Mean values for CLR and percentage of dose excreted in the urine (adjusted for bioavailability) are shown in Table 2. The majority of an absorbed dose of GCV is excreted in the urine over a 24-h period. CLR, of GCV was comparable between the four treatments, with average values ranging from 125 to 137 ml/min (Table 2). For the i.v.-GCV group, for which assessment of total systemic clearance was possible, the majority (80%) of this clearance was accounted for by renal excretion.
AUC equivalence testing.
The results of the primary analysis for the comparison of treatment C (900 mg of VGC) versus treatment D (i.v. GCV) and of treatment B (450 mg of VGC) versus treatment A (3 g of oral GCV) are summarized in Table 3. No period or crossover effect was found (data not shown). Bioavailability of treatment B relative to treatment A was 102% (90% CI, 95 to 109%), while that of treatment C relative to treatment D was 90% (90% CI, 83 to 97%).
TABLE 3.
Comparison of relative bioavailabilitya
| Reference treatment | AUCb estimate (μg · h/ml) | Test treatment | AUC estimate (μg · h/ml) | AUC ratio (90% CI)c |
|---|---|---|---|---|
| i.v. GCV, 5 mg/kgd | 47.61 | VGC, 900 mg | 42.69 | 90 (83, 97) |
| Oral GCV, 1 g TIDe | 20.15 | VGC, 450 mg | 20.56 | 102 (95, 109) |
Reference regions are 0 to 125% for one-sided equivalence of 900 mg of VGC to the reference i.v. GCV and 80% to ∞ for one-sided equivalence of 450 mg of VGC to the reference oral GCV.
AUC derived from back transformation of mean of lnAUC values.
Ratio of test AUC to reference AUC, expressed as a percentage.
Primary parameter AUC∞.
Primary parameter for AUC24.
Absolute bioavailability of GCV.
The estimated absolute bioavailability of oral GCV was 6.3% (95% CI, 5.5 and 7.1%), while the two VGC formulations delivered 60% (95% CI, 56 to 64%) and 59% (95% CI, 55 to 63%) of their doses (GCV equivalents) for treatments B and C, respectively, to the systemic circulation.
Safety results.
The percentages of patients experiencing at least one adverse event were comparable among all four treatments (GCV i.v., 43%; GCV p.o., 43%; 450 mg of VGC, 36%; 900 mg of VGC, 36%). A total of 83 different adverse events were reported during the course of the study, but over 80% were considered by the investigator to be unrelated to study treatment and over 60% were considered to be mild in intensity. The most frequently reported events were headaches, nausea, and diarrhea. Two subjects experienced serious adverse events during the course of the study. These were an anastomotic common bile duct stenosis and a hepatic artery thrombosis, both of which required hospitalization. Neither of these events was considered to be related to the study drug. There were no premature withdrawals from the study due to adverse events. No deaths were recorded during the course of the study or within the 4 weeks following completion.
Abnormal laboratory values were sporadic and not generally considered clinically relevant. Both the frequency and the nature of these abnormalities were comparable across all four treatments. These included lymphopenia, neutropenia, anemia, thrombocytopenia, increased alanine transaminase, alkaline phosphatase levels, and increased serum creatinine. No patient withdrew from the study because of abnormal laboratory values.
DISCUSSION
Although oral GCV is effective in the prevention and treatment of CMV disease, its low oral bioavailability limits the systemic drug exposure achieved to an approximate plasma AUC of 25 μg · h/ml, a level adequate for the prevention of CMV disease in the majority of organ transplant recipients (10). However, higher levels of drug exposure may be required to prevent CMV disease in high-risk allograft recipients (donor+ recipient−) or to treat established CMV infection and disease (10). Higher drug exposure can be achieved with i.v. GCV, but daily i.v. infusions are impractical for delivery of prophylactic GCV over many months and carry the risk of catheter-related morbidity as well (25).
The AUC24 values of GCV following dosing with VGC in this study (21.1 and 41.7 μg · h/ml for the 450- and 900-mg doses, respectively) were higher than the AUC24 values of 12.7 and 24.8 μg · h/ml observed in HIV-infected individuals receiving doses of 450 and 875 mg, respectively (1, 12). Evaluation of the pharmacokinetic parameters suggests that the difference is due to the longer terminal elimination t1/2 (5.1 to 5.22 h) seen in the transplant recipients compared to that in HIV-infected patients (3.66 h). GCV clearance is likewise different: 1.87 ml/min/kg for transplant patients versus 3.39 ml/min/kg in HIV-infected individuals. The etiology of the decreased clearance is probably multifactorial, resulting from the use of nephrotoxic immunosuppressive drugs and from underlying disease.
In the present study of liver transplant recipients, the extent of drug exposure to VGC itself was low. This drug exposure was similar to that seen with VGC administered to HIV-infected individuals, where mean AUC24 values of 0.167 and 0.393 μg · h/ml were seen after oral doses of 450 and 875 mg, respectively (12). The mean Cmax values for VGC were also a fraction (2.8 to 3.2%) of those of GCV, and the mean AUC24 values for VGC were 0.8 to 1.0% of those for GCV.
The absolute bioavailability of oral GCV in this study was 6.7%, in the range of that noted in previously studied transplant recipients (19). The addition of the valine moiety dramatically increases the absorption, by approximately a factor of 10. The mechanism of the increased bioavailability most likely involves a peptide-mediated active transport, as has been shown for valacyclovir (9, 21). The relative bioavailability of 900 mg of VGC provided drug exposure equivalent to that provided by a 5-mg/kg dose of i.v. GCV, and the dose of 450 mg of VGC provided an exposure equivalent to that provided by oral GCV given as 1,000 mg TID. Since the GCV exposure following a 900-mg dose of VGC is not greater than that following an i.v. dose of GCV, the safety of VGC (at either dose) should be at least as good as seen with i.v. GCV in transplant patients. And since the GCV exposure following a 450-mg dose of VGC is not less than that following a 1,000-mg TID dose of oral GCV, the efficacy of VGC (at either dose) should be no less than that seen with oral GCV in transplant patients.
The single doses of VGC were generally well tolerated in these liver transplant recipients and were not associated with any unexpected adverse events. The ultimate safety of VGC can only be determined in larger chronic-dosing studies. Since the GCV exposure falls between those of oral GCV and i.v. GCV, it is expected that the safety profile should be similar.
An alternative to primary prevention of CMV is preemptive therapy. In this approach, patients are monitored for signs of CMV infection and/or disease by various methods, including antigenemia and detection of viral DNA by PCR (16). Only patients who demonstrate viral replication are then treated with GCV. Although such an approach may reduce the number of patients who receive GCV, the optimal management of such patients when viral replication is detected is not clear. A short course of i.v. GCV has been used, but this is associated with a rate of recurrence of CMV viremia of almost 25% (17). Oral GCV, even at high doses, may not provide adequate drug levels to suppress viral replication. In a recent study, dosing with 2 g TID for 2 weeks followed by 1 g TID for 4 weeks failed to clear antigenemia in 20% of patients (20).
Although oral GCV has been shown to reduce asymptomatic infection in most CMV risk groups, the rate of subclinical infection may remain quite high, up to 60% among those at highest risk in one study (10). Recent data suggest that CMV infection is associated with chronic rejection of kidney transplants (22), restenosis of cardiac atherosclerosis (23), and transplant atherosclerosis in heart transplants (24). Better suppression of subclinical disease achievable with greater GCV exposure provided by VGC may translate into reduced vascular damage or chronic rejection.
A concern with the use of oral GCV is that the level of drug exposure achieved may result in the selection of GCV-resistant CMV (5, 6). The high levels achievable with VGC may eliminate these problems. Lastly, established CMV infection or disease may be treatable with oral dosing, eliminating the need for long-term i.v. access with its associated morbidity. This may be particularly relevant to children, in whom doses of oral GCV needed for prevention were nearly three times those used in adults in order to achieve adequate levels (7, 18).
In conclusion, the addition of a valine ester to GCV produces a molecule with dramatically increased oral bioavailability and delivery of GCV. Doses of 450 or 900 mg of VGC provide exposures similar to those achieved with oral and i.v. GCV, respectively. This raises the possibility of highly effective prevention and treatment of CMV disease in organ transplant recipients with a simple once-a-day oral dose regimen.
ACKNOWLEDGMENTS
This study was supported by Roche Pharmaceuticals and in part by PHS MO1RR750 (to M.D.P.). As noted, several of the authors are employees or contractors of the study sponsor.
REFERENCES
- 1.Brown F, Banken L, Saywell K, Arum I. Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers. Clin Pharmacokinet. 1999;37:167–176. doi: 10.2165/00003088-199937020-00005. [DOI] [PubMed] [Google Scholar]
- 2.Chan R, LaFargue J, Reeve R, Tam Y, Tarnowski T. An HPLC method for the determination of diastereomeric prodrug RS-79070-004 in human plasma. J Pharm Biomed Anal. 1999;21:647–656. doi: 10.1016/s0731-7085(99)00160-0. [DOI] [PubMed] [Google Scholar]
- 3.Chu F, Kiang C-H, Sung M-L, Huang B, Reeve R L, Tarnowski T. A rapid, sensitive HPLC method for the determination of ganciclovir in human plasma and serum. J Pharm Biomed Anal. 1999;21:657–667. doi: 10.1016/s0731-7085(99)00161-2. [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 5.Drew W L, Stempien M J, Andrews J, Shadman A, Tan S J, Miner R, Buhles W. Cytomegalovirus (CMV) resistance in patients with CMV retinitis and AIDS treated with oral or intravenous ganciclovir. J Infect Dis. 1999;179:1352–1355. doi: 10.1086/314747. [DOI] [PubMed] [Google Scholar]
- 6.Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clin Microbiol Rev. 1999;12:286–297. doi: 10.1128/cmr.12.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filler G, Lampe D, von Bredow M A. Prophylactic oral ganciclovir after renal transplantation—dosing and pharmacokinetics. Pediatr Nephrol. 1998;12:6–9. doi: 10.1007/s004670050391. [DOI] [PubMed] [Google Scholar]
- 8.Fox J C, Kidd I M, Griffiths P D, Sweny P, Emery V C. Longitudinal analysis of cytomegalovirus load in renal transplant recipients using a quantitative polymerase chain reaction: correlation with disease. J Gen Virol. 1995;76:309–319. doi: 10.1099/0022-1317-76-2-309. [DOI] [PubMed] [Google Scholar]
- 9.Ganapathy M E, Huang W, Wang H, Ganapathy V, Leibach F H. Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998;19:470–475. doi: 10.1006/bbrc.1998.8628. [DOI] [PubMed] [Google Scholar]
- 10.Gane E, Saliba F, Valdecasas G, O'Grady J, Behrend M, Pescovitz M D, Pruett T, Hockerstedt K, Broelsch C, Mino-Fugarlos G, Houssin D, Davis C, Henry M, Erhard J, Kam I, Walker M G, Lyman S, King P, Robinson C A. Efficacy and safety of oral ganciclovir in the prevention of CMV disease in liver transplant recipients: results of a multicenter, multinational clinical trial. Lancet. 1997;350:1729–1733. doi: 10.1016/s0140-6736(97)05535-9. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths P D. Progress in the clinical management of herpesvirus infections. Antivir Chem Chemother. 1995;6:191–209. [Google Scholar]
- 12.Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol. 1999;39:800–804. doi: 10.1177/00912709922008452. [DOI] [PubMed] [Google Scholar]
- 13.Kanj S S, Sharara A I, Pa P A C, Hamilton J D. Cytomegalovirus infection following liver transplantation: review of the literature. Clin Infect Dis. 1996;22:537–549. doi: 10.1093/clinids/22.3.537. [DOI] [PubMed] [Google Scholar]
- 14.Lowance D, Neumayer H H, Legendre C M, Squifflet J P, Kovarik J, Brennan P J, Norman D, Mendez R, Keating M R, Coggon G L, Crisp A, Lee I C International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N Engl J Med. 1999;340:1462–1470. doi: 10.1056/NEJM199905133401903. [DOI] [PubMed] [Google Scholar]
- 15.Noble S, Faulds D. Ganciclovir: an update on its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs. 1998;56:115–146. doi: 10.2165/00003495-199856010-00012. [DOI] [PubMed] [Google Scholar]
- 16.Patel R, Syndman D R, Rubin R H, Ho M, Pescovitz M, Martin M, Paya C V. Cytomegalovirus prophylaxis in solid organ transplant recipients. Transplantation. 1996;61:1279–1289. doi: 10.1097/00007890-199605150-00001. [DOI] [PubMed] [Google Scholar]
- 17.Paterson D L, Stapelfeldt W H, Wagener M M, Gayowski T, Marino I R, Singh N. Intraoperative hypothermia is an independent risk factor for early cytomegalovirus infection in liver transplant recipients. Transplantation. 1999;67:1151–1155. doi: 10.1097/00007890-199904270-00011. [DOI] [PubMed] [Google Scholar]
- 18.Pescovitz M D, Brook B, Leapman S B, Milgrom M L, Filo R S. Oral ganciclovir in pediatric transplant recipients: a pharmacokinetic study. Clin Transplant. 1997;11:613–617. [PubMed] [Google Scholar]
- 19.Pescovitz M D, Pruett T L, Gonwa T, Brook B, McGory R, Wicker K, Griffy K, Robinson C A, Jung D. Oral ganciclovir dosing in transplant recipients and dialysis patients based on renal function. Transplantation. 1998;66:1104–1107. doi: 10.1097/00007890-199810270-00023. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Yu V L, Gayowski T, Marino I R. Changes in the level of CMV antigenemia (pp65) in liver transplant recipients receiving oral ganciclovir as CMV prophylaxis. Transplantation. 1999;67:S98. [Google Scholar]
- 21.Sinko P J, Balimane P V. Carrier-mediated intestinal absorption of valacyclovir, the l-valyl ester prodrug of acyclovir. 1. Interactions with peptides, organic anions and organic cations in rats. Biopharm Drug Dispos. 1998;19:209–217. doi: 10.1002/(sici)1099-081x(199805)19:4<209::aid-bdd93>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Solez K, Vincenti F, Filo R S. Histopathologic findings from 2-year protocol biopsies from a U.S. multicenter kidney transplant trial comparing tacrolimus versus cyclosporine: a report of the FK506 Kidney Transplant Study Group. Transplantation. 1998;66:1726–1740. doi: 10.1097/00007890-199812270-00029. [DOI] [PubMed] [Google Scholar]
- 23.Speir E, Modali R, Huang E S, Leon M B, Shawl F, Finkel T, Epstein S E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994;265:391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- 24.Valantine H A, Gao S Z, Menon S G, Renlund D G, Hunt S A, Oyer P, Stinson E B, Brown B W, Jr, Merigan T C, Schroeder J S. Impact of prophylactic immediate posttransplant ganciclovir on development of transplant atherosclerosis: a post hoc analysis of a randomized, placebo-controlled study. Circulation. 1999;100:61–66. doi: 10.1161/01.cir.100.1.61. [DOI] [PubMed] [Google Scholar]
- 25.Winston D J, Wirin D, Shaked A, Busuttil R W. Randomised comparison of ganciclovir and high-dose acyclovir for long-term cytomegalovirus prophylaxis in liver-transplant recipients. Lancet. 1995;346:69–74. doi: 10.1016/s0140-6736(95)92110-9. [DOI] [PubMed] [Google Scholar]