Abstract
The COVID‐19 pandemic has affected cancer care worldwide. This study aimed to estimate the long‐term impacts of cancer care disruptions on cancer mortality in Canada using a microsimulation model. The model simulates cancer incidence and survival using cancer incidence, stage at diagnosis and survival data from the Canadian Cancer Registry. We modeled reported declines in cancer diagnoses and treatments recorded in provincial administrative datasets in March 2020 to June 2021. Based on the literature, we assumed that diagnostic and treatment delays lead to a 6% higher rate of cancer death per 4‐week delay. After June 2021, we assessed scenarios where cancer treatment capacity returned to prepandemic levels, or to 10% higher or lower than prepandemic levels. Results are the median predictions of 10 stochastic simulations. The model predicts that cancer care disruptions during the COVID‐19 pandemic could lead to 21 247 (2.0%) more cancer deaths in Canada in 2020 to 2030, assuming treatment capacity is recovered to 2019 prepandemic levels in 2021. This represents 355 172 life years lost expected due to pandemic‐related diagnostic and treatment delays. The largest number of expected excess cancer deaths was predicted for breast, lung and colorectal cancers, and in the provinces of Ontario, Québec and British Columbia. Diagnostic and treatment capacity in 2021 onward highly influenced the number of cancer deaths over the next decade. Cancer care disruptions during the COVID‐19 pandemic could lead to significant life loss; however, most of these could be mitigated by increasing diagnostic and treatment capacity in the short‐term to address the service backlog.
Keywords: cancer mortality, COVID‐19, decision model, time to diagnosis, time to treatment
What's new?
The COVID‐19 pandemic has interfered with people getting timely cancer diagnosis and treatment, due to fears of infection as well as limitations in healthcare capacity. Here, the authors created a model to predict the effect of these disruptions on long‐term cancer mortality. The model predicted a 2% increase in cancer deaths over the next 10 years. However, they also show that by increasing the capacity of cancer care services by at least 10% to tackle the backlog, most excess deaths could be avoided.
Abbreviations
- CIHI
Canadian Institute of Health Information
- CT
computerized tomography
- MRI
magnetic resonance imaging
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has led to severe disruptions in healthcare delivery worldwide. Cancer patients are at particularly high risk of negative outcomes from delays in diagnosis and treatment. A meta‐analysis estimated that each 4‐week delay in cancer surgery increases the rate of mortality by 6% to 8% across several major cancer sites. 1 The pandemic has impacted the entire cancer care trajectory due to changes in healthcare seeking behavior and health system capacity. 2 Many individuals have delayed consulting for cancer‐related symptoms due to access barriers, or fears of infection with coronavirus in a healthcare setting. 3 , 4 Healthcare staffing shortages may occur due to redeployment to the pandemic response, leave due to coronavirus infection, burnout or increased childcare responsibilities. Key cancer diagnostic tests, such as endoscopies, colonoscopies, mammographies, computerized tomography (CT) scans and magnetic resonance imaging (MRI) scans, have declined, 5 , 6 either due to fewer patient referrals or delays in accessing these services. Many cancer surgeries and other treatments were postponed or delayed during the pandemic. 2
In Canada, both public health and cancer services are publicly funded and provincially managed. In March 2020, most provinces declared a state of public health emergency, which led to cancelation and postponement of many cancer treatments, screenings and routine healthcare visits. Provincial health agencies eventually issued directives and practice guidelines for cancer care provision during the pandemic. 7 Many included criteria for prioritization and triage of cancer patients in case of healthcare disruptions, and specified cancer treatments should be maintained as a high priority service. Cancer treatments gradually resumed over the next few months, 8 and our discussions with officials from cancer agencies indicated that most perceived cancer service provision had returned to normal over the course of 2020. However, fewer cancer treatments were performed in 2020, 8 suggesting many cancer cases experienced delays in their diagnosis and treatment due to the pandemic.
Our objective was to predict the long‐term impact of pandemic‐related cancer diagnostic and treatment delays on cancer mortality in Canada by cancer site, age, sex and province. We also examined factors which could mitigate or increase the expected cancer mortality over the next decade.
2. METHODS
We built an individual‐level stochastic microsimulation model in C++. The model simulates the health trajectories of cancer cases over time and was designed to capture the impacts of diagnostic and treatment delays on cancer incidence and mortality (Figure 1).
FIGURE 1.
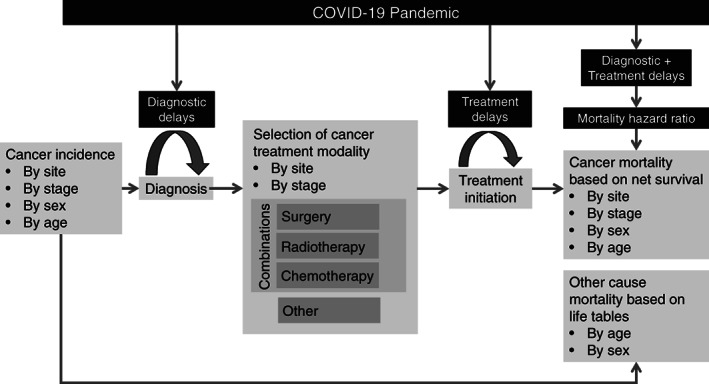
Model conceptual diagram
2.1. Modeled outcomes
The main model outcome was predicted excess cancer deaths caused by the pandemic, which were calculated as the difference in the number of cancer deaths between pandemic scenarios and a no pandemic counterfactual scenario. Relative mortality increases were calculated as the number of cancer deaths in pandemic scenarios divided by the number of cancer deaths in a no pandemic counterfactual scenario. Life years lost were calculated as the difference between a cancer case's observed death date and their expected death date without pandemic‐related care delays.
2.2. Model structure
The model's main features are described below; a more detailed description of parameters and assumptions can be found in Supporting information published elsewhere. 9
2.3. Cancer incidence
We modeled the incidence of 25 cancer sites (bladder, brain, breast, central nervous system, cervix, colorectal, esophagus, Hodgkin lymphoma, kidney and renal pelvis, larynx, leukemia, liver, lung, melanoma, multiple myeloma, non‐Hodgkin lymphoma, oral, ovary, pancreas, prostate, stomach, testes, thyroid, uterus and all other cancers) based on 2015 to 2017 Canadian incidence rates 10 and population size by sex and age. 11 Cancers were assigned an overall TNM stage at diagnosis based on the distribution reported by provincial cancer registries. 12
2.4. Survival
Two death dates were sampled for each cancer case: their expected cancer death date and their expected death date from other causes. The expected cancer death date was sampled from a net cancer survival function depending on sex, age, cancer site and stage. 13 , 14 , 15 The expected death date from other causes was sampled from 2017 to 2019 Canadian life tables by sex, 16 adjusted for seasonality using a sinusoidal function fitted to weekly death counts. A cancer case's actual death date was the earliest of these two expected death dates. If the expected cancer death date was earlier than the expected death date from other causes, then we assumed that the person died from cancer.
2.5. Treatment
Four cancer treatment modalities were included in the model: surgery, radiotherapy, chemotherapy and other. Patients could receive combinations of surgery, radiotherapy and chemotherapy. Patients in the other category were those who did not receive any of these three treatments and received other care. We were unable to identify a nationally representative data set for the distribution of cancer treatments in Canada. We based treatment distributions on expert opinion from an Expert Advisory Group of 31 oncologists and surgeons, supplemented with data on treatment distributions from England and the United States. 17 , 18 The experts used a survey tool to validate whether the treatment distributions from England and the United States were applicable to Canada based on their experience. If they judged treatment probabilities to differ, they were asked to quantify the probability of receiving each treatment for a given cancer site by stage. We combined the probability distributions elicited from experts using an equal weights linear opinion pool. 19
2.6. Prepandemic diagnostic and treatment intervals
We defined the treatment interval as the time between the diagnosis date and the start date of curative or palliative treatment. The treatment interval was explicitly modeled: upon diagnosis, each cancer case was assigned a treatment interval sampled from a Weibull distribution, fitted to prepandemic reported times to cancer treatment. 6 , 20 We defined the diagnostic interval as the time between the date a patient first notices cancer symptoms and the date of diagnosis. This definition included the patient interval 21 (time between noticing symptoms and presenting to healthcare) in the diagnostic interval, because declines in cancer diagnoses were due to patients both taking longer to present to healthcare as well as a reduction in diagnostic activities during the pandemic. This diagnostic interval was not explicitly modeled; however, the date of diagnosis could be delayed as described below, implicitly increasing the diagnostic interval.
2.7. Pandemic diagnostic and treatment delays
We modeled the pandemic's impact on healthcare delivery as a relative change in the monthly number of diagnoses and treatments compared to the numbers expected had there been no pandemic. This relative change set the maximum diagnostic and treatment capacity each month. Diagnoses and cancer treatments scheduled to occur which exceeded the monthly system capacity were placed on a backlog. During each time step, the model would attempt to clear the backlog by randomly selecting from the list of undiagnosed cases which were diagnosed, and selecting from the list of diagnosed untreated cases which received treatment, up to the maximum capacity. The backlogs increased during time steps where diagnostic and treatment volumes were below expected levels. Because selection of cases on the backlog was random, some cancer cases did not experience any delays, while other cases could experience significant diagnostic and treatment delays.
2.7.1. Surgeries
Relative changes in surgeries were based on the volume of surgeries per month in 2020 to 2021 relative to the same month in 2019. Data for surgeries were extracted for all provinces, except Québec, from the Canadian Institute of Health Information (CIHI) web portal up to February 2021, and supplementary data were provided for Québec by the Ministère de la santé et des services sociaux (MSSS) up to March 2021. The estimated relative changes in cancer surgeries are shown in Figure 2A. Declines in cancer surgeries roughly coincided with peaks in COVID‐19 hospitalizations in Canada (Figure 2B). 22 Most provinces experienced a third infection wave around April 2021; we therefore assumed that the trends observed in January‐February 2021 would be repeated in March‐April 2021.
FIGURE 2.
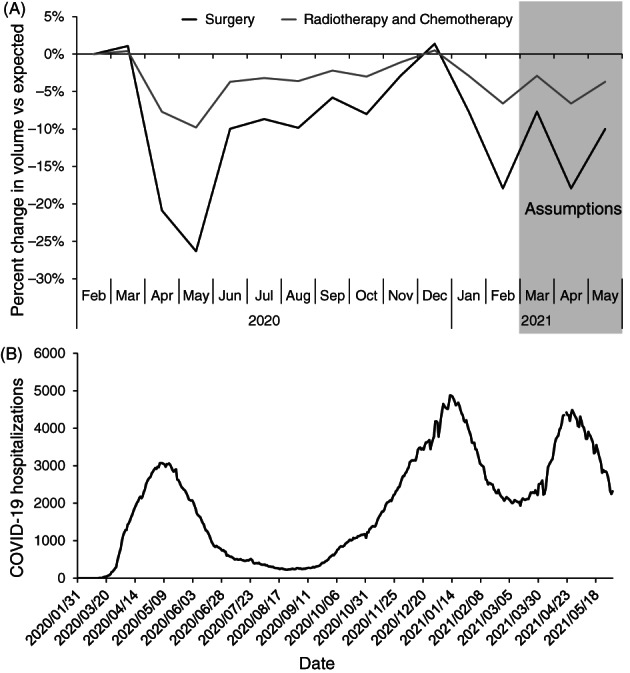
Impact of the COVID‐19 pandemic on cancer treatments and hospitals in Canada. (A) Modeled percent change in cancer treatments for Canada as a whole. The percent change in surgeries is based on data on the volume of cancer surgeries in 2020 to 2021 relative to the same month in 2019, using data from the Canadian Institute of Health Information portal extracted on 28 May 2021. The percent change in radiotherapies is based on the yearly volume of radiotherapies in 2020 relative to 2019 reported by the Canadian Institute of Health Information, 6 rescaled by month using the surgery data. Chemotherapies were assumed to follow the same changes as radiotherapies. (B) Number of people hospitalized for COVID‐19 in Canada from February 2020 to May 2021. Data compiled by Radio‐Canada extracted on 1 June 2021. 22
2.7.2. Radiotherapies and chemotherapies
There was a small −2.5% yearly percent change in the number of radiotherapies in Canada in 2020 compared to 2019 reported to CIHI. 6 We rescaled this percent change per month in the model so that the largest declines occurred in Spring, with recovery in the Summer and Fall. Changes in radiotherapies were only modeled for Québec, Ontario, New Brunswick and Prince Edward Island, as other provinces did not report declines in radiotherapies. 6 There was no data for chemotherapies; we assumed these would experience the same monthly percent changes as radiotherapies.
2.7.3. Diagnoses
We used 2019 to 2021 monthly pathology report data provided by the MSSS as a proxy for new diagnoses in Québec. This province reported a similar overall percent change in pathology reports as in cancer surgeries over 2020 to 2021. Based on this, we assumed for other provinces that the monthly decline in new diagnoses would be the same as the reported monthly decline in cancer surgeries (Figure 2A).
2.8. Effects of delays on cancer mortality
The diagnostic delay was the difference between the date the cancer would have been diagnosed without the pandemic and the actual diagnosis date because of the diagnostic backlog. The treatment delay was the difference between the initially scheduled treatment date and the actual treatment date because of the treatment backlog. A meta‐analysis estimated that each 4‐week delay in cancer surgery leads to a 1.06 to 1.08 hazard ratio increase in mortality across several major cancer sites. 1 Hazard ratios for radiotherapy and systemic therapy delays were more variable, but included similar values in their confidence intervals. Based on this, we assumed that all cancer sites and all treatment modalities would have a 1.06 times higher cancer mortality hazard rate per 4‐week treatment delay. We assumed the same hazard ratio would apply to a 4‐week diagnostic delay. The relationship between time and mortality rates was assumed to be log‐linear, with each 4‐week delay increasing the mortality rate multiplicatively. The effects were also multiplicative across treatments. Consequently, patients with longer delays and who require multiple treatments were those most likely to experience higher mortality.
For each patient experiencing pandemic‐related delays, the model resampled a new cancer death date for them based on their cancer survival function given their diagnostic and treatment delays. Cancer cases whose resampled cancer death dates were earlier than their original expected death dates were reassigned the earlier death date; the rest retain their original expected death date. This reflects an assumption that delays are either detrimental or have no effect on cancer survival.
We also integrated a probability of a stage shift at diagnosis for breast, lung, colorectal and ovarian cancers due to diagnostic delays. 23 However, the probability of stage shifts was very small in the literature, leading to very few predicted stage shifts in the model. Because the risk of stage shifts is likely underestimated in the literature due to confounding by indication, 24 and consequently underestimated in the model, the diagnostic delay hazard ratio was the parameter largely used to account for pathways through which later diagnosis would lead to worse outcomes, including potential stage shifts.
2.9. Pandemic scenarios
In the base case pandemic scenario, we assumed there would be declines in diagnoses and treatments from March 2020 to May 2021 (Figure 2A), and that, starting June 2021, treatment capacity would return to normal prepandemic levels and diagnostic capacity would increase by 15% due to increased diagnostic activities and increased patient interactions with the healthcare system. In sensitivity analyses, we evaluated more pessimistic scenarios, where treatment capacity remains 10% to 20% below prepandemic levels throughout 2021, and more optimistic scenarios, where treatment capacity is increased over normal capacity after June 2021 to decrease the treatment backlog. We also varied the mortality hazard ratio associated with a 4‐week delay due to the high uncertainty regarding the effect of delays on cancer mortality. Simulations were run until the end of 2030; this cutoff date was determined based on the results of simulations, which showed that cancer mortality returned to expected levels by this date in all scenarios. Results are the median (minimum − maximum interval) of 10 stochastic simulations per scenario.
2.10. Validation
The model reproduces net cancer survival by cancer site in Canada and the prepandemic (2017‐2019) number of cancer deaths reported per year by province (Figures S6‐S8). 9
3. RESULTS
Figure 3 shows the median predicted monthly number of cancer incidence and deaths in Canada for all cancer sites combined between 2017 and 2030. Cancer incidence and deaths are predicted to increase over time even in a no‐pandemic counterfactual due to population aging and increasing population size. In all pandemic scenarios, a temporary decline in cancer incidence is expected during 2020 to 2021 due to pandemic‐related declines in new diagnoses. Most missed diagnoses are expected to be caught up later, however, as diagnostic capacity recovers and patients return to normal healthcare seeking behaviors. The model predicted an increase in cancer deaths in pandemic scenarios starting from 2020 due to pandemic‐related diagnostic and treatment delays.
FIGURE 3.
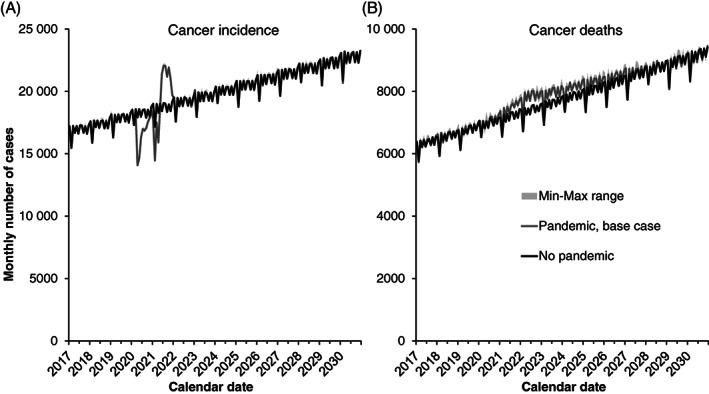
Predicted monthly (A) cancer incidence and (B) cancer deaths for all cancer sites combined, Canada. Lines are the median of 10 stochastic simulations for each scenario, and shaded areas represent the minimum‐maximum range. The pandemic scenario assumes that treatment capacity changes occur starting in June 2021, and that each 4‐week delay in cancer care increases the rate of cancer mortality by 6% (hazard ratio of 1.06)
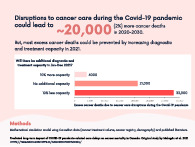
In terms of excess cancer mortality, 21 247 (18 108‐26 136) cumulative excess cancer deaths were predicted between 2020 and 2030 for Canada as a whole in the base case scenario due to pandemic‐related delays (Figure 4). This constituted a 2.0% (1.7%‐2.5%) increase over expected cancer mortality over this time period, and 355 172 (348 434‐401 887) life years lost. The year with the most excess cancer mortality was predicted to be 2022, with cancer mortality being 6% higher than expected that year. Excess cancer mortality from pandemic‐related delays was predicted to last up to 2027, after which the yearly number of cancer deaths would return to expected levels.
FIGURE 4.
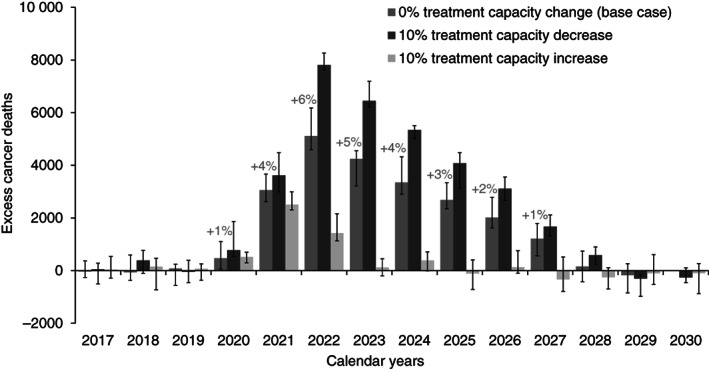
Predicted yearly excess cancer deaths compared to those expected without the pandemic for all cancer sites combined, Canada. Results are the median and error bars are the minimum and maximum of 10 stochastic simulations for the base case scenario (recovery in June 2021), and scenarios with ±10% treatment capacity over prepandemic levels starting June 2021. Percentages indicate the yearly median relative increase in cancer deaths over expected
The above results from the base case scenario assume that prepandemic cancer treatment capacity levels are recovered by June 2021. However, predicted excess cancer mortality was sensitive to assumed treatment capacity levels in 2021 and after (Figure 4). Continued pandemic‐related reductions in treatment capacity would substantially increase excess cancer mortality, while increasing treatment capacity would substantially decrease excess cancer mortality. If capacity for all treatments remains 10% lower than normal throughout 2021, then 33 262 (31 381‐35 077) cumulative excess cancer deaths were predicted between 2020 and 2030. If treatment capacity were increased by 10% over normal, only 4210 (2719‐5675) excess cancer deaths were predicted between 2020 and 2030; this is equivalent to preventing 80% of excess cancer mortality predicted in the base case. Future treatment capacity strongly influenced results because more cancers are expected to be diagnosed and require treatment in 2021/2022 than in 2020 due to diagnostic delays.
Cumulative excess cancer deaths between 2020 and 2030 predicted in the base case scenario by sex, age, province and cancer site are presented in Table 1. The relative mortality increase was predicted to be highest in cases diagnosed under age 45, but the highest absolute life years lost were predicted to occur in cases diagnosed between ages 55 and 74. Most excess cancer deaths were predicted to occur in the most populous provinces of Ontario, Québec, Alberta and British Columbia. In general, cancer sites with poor expected survival (pancreas, esophagus, lung) were predicted to experience lower relative mortality increases, though lung cancer accounted for many absolute excess deaths due to its high incidence.
TABLE 1.
Predicted cumulative excess cancer mortality and life years lost by sex, age, province and site for Canada 2020 to 2030 in the base case scenario
| Excess deaths (N) | Relative mortality increase (%) | Life lost (years) | |
|---|---|---|---|
| Overall | 21 247 (18 108; 26 136) | 2.0% (1.7%; 2.5%) | 355 172 (348 434; 401 887) |
| Sex | |||
| Female | 11 346 (9538; 13 790) | 2.3% (1.9%; 2.8%) | 201 697 (198 282; 228 997) |
| Male | 10 714 (8974; 12 750) | 1.9% (1.6%; 2.3%) | 154 817 (147 470; 172 890) |
| Age (y) | |||
| 0‐14 | 80 (31; 156) | 3.8% (1.5%; 7.4%) | 4204 (3619; 5515) |
| 15‐44 | 1268 (952; 1432) | 4.1% (3.1%; 4.7%) | 44 147 (40 736; 49 529) |
| 45‐54 | 2148 (1216; 2703) | 3.8% (2.2%; 4.8%) | 48 897 (48 109; 56 258) |
| 55‐64 | 4419 (3790; 5346) | 2.6% (2.3%; 3.2%) | 89 272 (86 444; 98 177) |
| 65‐74 | 6504 (5516; 9098) | 2.1% (1.7%; 2.9%) | 99 876 (97 134; 113 519) |
| 75‐84 | 5452 (4134; 6150) | 1.6% (1.3%; 1.9%) | 57 699 (55 733; 64 278) |
| 85+ | 1861 (1126; 2592) | 1.2% (0.7%; 1.6%) | 13 332 (12 798; 14 611) |
| Province a | |||
| Alberta | 1978 (1319; 2429) | 2.0% (1.3%; 2.4%) | 32 349 (28 550; 38 439) |
| British Columbia | 2832 (2392; 3994) | 2.0% (1.7%; 2.9%) | 46 257 (40 135; 50 096) |
| Manitoba | 957 (662; 1185) | 2.9% (2.0%; 3.6%) | 14 040 (9686; 16 035) |
| New Brunswick | 274 (−22; 764) | 1.0% (−0.1%; 2.9%) | 4318 (2141; 7370) |
| Newfoundland & Labrador | 446 (197; 983) | 2.4% (1.1%; 5.4%) | 6397 (4430; 8950) |
| Nova Scotia | 810 (356; 936) | 2.4% (1.1%; 2.8%) | 9879 (4084; 11 435) |
| Ontario | 8794 (8622; 11 408) | 2.1% (2.1%; 2.7%) | 159 287 (144 342; 177 073) |
| Prince Edward Island | 68 (−42; 232) | 1.3% (−0.8%; 4.5%) | 2419 (1537; 3214) |
| Québec | 2782 (1953; 3912) | 1.0% (0.7%; 1.4%) | 45 196 (41 857; 52 860) |
| Saskatchewan | 558 (224; 810) | 1.9% (0.8%; 2.8%) | 10 687 (8314; 13 504) |
| Site | |||
| Bladder | 1464 (1257; 1983) | 3.9% (3.4%; 5.3%) | 17 455 (16 754; 19 500) |
| Brain | 606 (469; 927) | 2.2% (1.7%; 3.4%) | 16 944 (14 570; 17 769) |
| Breast | 3116 (2927; 3846) | 5.9% (5.5%; 7.2%) | 61 354 (59 311; 69 418) |
| Central nervous system | 58 (32; 104) | 8.2% (4.6%; 14.5%) | 1403 (1085; 1908) |
| Cervix | 267 (132; 469) | 4.4% (2.2%; 7.8%) | 6340 (5541; 7966) |
| Colorectal | 4305 (3789; 4676) | 4.1% (3.6%; 4.4%) | 62 968 (61 535; 70 464) |
| Esophagus | 225 (10; 620) | 0.9% (0.0%; 2.5%) | 4566 (4336; 5043) |
| Hodgkin lymphoma | 129 (73; 218) | 5.0% (2.8%; 8.5%) | 1995 (1751; 2452) |
| Kidney and renal pelvis | 608 (470; 784) | 2.4% (1.9%; 3.1%) | 9822 (9008; 11 399) |
| Larynx | 278 (76; 364) | 4.4% (1.2%; 5.7%) | 3148 (2682; 3494) |
| Leukemia | 462 (114; 1164) | 1.2% (0.3%; 3.1%) | 9339 (8923; 11 260) |
| Liver | 332 (73; 522) | 1.2% (0.3%; 1.9%) | 4785 (4480; 5270) |
| Lung | 3082 (2202; 3208) | 1.1% (0.8%; 1.2%) | 42 472 (40 881; 46 379) |
| Melanoma | 589 (467; 763) | 4.2% (3.3%; 5.4%) | 12 720 (11 509; 14 214) |
| Multiple myeloma | 434 (86; 572) | 1.6% (0.3%; 2.1%) | 5342 (4801; 5889) |
| Non‐Hodgkin lymphoma | 1566 (1438; 1839) | 3.0% (2.7%; 3.5%) | 22 811 (21 946; 26 646) |
| Oral | 1720 (1354; 2004) | 6.2% (4.9%; 7.3%) | 28 167 (26 101; 31 159) |
| Ovary | 623 (367; 776) | 3.0% (1.8%; 3.7%) | 11 720 (11 108; 12 821) |
| Pancreas | 145 (−234; 318) | 0.2% (−0.4%; 0.5%) | 5126 (4460; 5795) |
| Prostate | 314 (148; 676) | 1.1% (0.5%; 2.4%) | 4773 (4274; 5369) |
| Stomach | 584 (126; 948) | 1.8% (0.4%; 2.9%) | 8671 (7745; 8920) |
| Testis | 66 (10; 120) | 8.4% (1.2%; 15.2%) | 1796 (1374; 2211) |
| Thyroid | 61 (−36; 144) | 2.6% (−1.5%; 6.1%) | 1657 (1450; 1839) |
| Uterus | 848 (642; 1020) | 5.4% (4.1%; 6.5%) | 14 878 (13 639; 16 879) |
| Other sites b | 0 | 0.0% | 0 |
Note: Results are the median (minimum‐maximum) of model predictions.
Each jurisdiction modeled separately, so overall Canada results do not equal sum of provincial results.
Other sites were assumed not to experience delays due to lack of data on treatment modalities for other sites.
In sensitivity analyses, we varied the effect of delays on the mortality hazard ratio, stratified by site (Table 2). Unsurprisingly, higher hazard ratios led to higher expected excess mortality than in the base case scenario. The time frame for this excess mortality remained the same as in the base case (2020‐2027). Further results stratified by province are also available through an interactive web application at https://tmalagon.shinyapps.io/CancerCOVID19Model/.
TABLE 2.
Predicted cumulative excess cancer mortality by site, assuming different hazard ratios (HRs) for the effect of a 4‐week delay on cancer mortality, for Canada 2020 to 2030
| Excess deaths (N) | Relative mortality increase (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR 1.03 | HR 1.06 a | HR 1.1 | HR 1.2 | HR 1.5 | HR 1.03 | HR 1.06 a | HR 1.1 | HR 1.2 | HR 1.5 | |
| Overall | 12 606 | 21 247 | 31 178 | 42 987 | 59 577 | 1.2% | 2.0% | 2.9% | 4.0% | 5.6% |
| By site | ||||||||||
| Bladder | 956 | 1464 | 2235 | 3232 | 4706 | 2.5% | 3.9% | 6.0% | 8.6% | 12.5% |
| Brain | 488 | 606 | 824 | 970 | 1084 | 1.8% | 2.2% | 3.0% | 3.5% | 4.0% |
| Breast | 1953 | 3116 | 4735 | 6932 | 10 478 | 3.7% | 5.9% | 8.9% | 13.0% | 19.7% |
| Central nervous system | 22 | 58 | 94 | 102 | 176 | 3.1% | 8.2% | 13.2% | 14.2% | 24.6% |
| Cervix | 183 | 267 | 307 | 498 | 728 | 3.0% | 4.4% | 5.1% | 8.3% | 12.1% |
| Colorectal | 2400 | 4305 | 5896 | 8624 | 12 143 | 2.3% | 4.1% | 5.6% | 8.2% | 11.5% |
| Esophagus | 118 | 225 | 300 | 442 | 522 | 0.5% | 0.9% | 1.2% | 1.8% | 2.1% |
| Hodgkin lymphoma | 82 | 129 | 166 | 260 | 362 | 3.2% | 5.0% | 6.4% | 10.1% | 14.1% |
| Kidney and renal pelvis | 348 | 608 | 866 | 1304 | 1960 | 1.4% | 2.4% | 3.4% | 5.1% | 7.7% |
| Larynx | 139 | 278 | 354 | 506 | 632 | 2.2% | 4.4% | 5.6% | 8.0% | 9.9% |
| Leukemia | 206 | 462 | 832 | 1198 | 1686 | 0.5% | 1.2% | 2.2% | 3.1% | 4.4% |
| Liver | 294 | 332 | 604 | 554 | 764 | 1.1% | 1.2% | 2.3% | 2.1% | 2.9% |
| Lung | 1574 | 3082 | 3791 | 5116 | 6405 | 0.6% | 1.1% | 1.4% | 1.9% | 2.3% |
| Melanoma | 304 | 589 | 944 | 1428 | 2110 | 2.2% | 4.2% | 6.7% | 10.1% | 14.9% |
| Multiple myeloma | 344 | 434 | 560 | 756 | 1026 | 1.3% | 1.6% | 2.1% | 2.8% | 3.8% |
| Non‐Hodgkin lymphoma | 927 | 1566 | 2159 | 3216 | 4406 | 1.8% | 3.0% | 4.1% | 6.1% | 8.4% |
| Oral | 1164 | 1720 | 2440 | 3159 | 4087 | 4.2% | 6.2% | 8.9% | 11.5% | 14.8% |
| Ovary | 332 | 623 | 882 | 1226 | 1326 | 1.6% | 3.0% | 4.3% | 5.9% | 6.4% |
| Pancreas | 70 | 145 | 304 | 346 | 560 | 0.1% | 0.2% | 0.5% | 0.5% | 0.9% |
| Prostate | 356 | 314 | 502 | 702 | 1076 | 1.3% | 1.1% | 1.8% | 2.5% | 3.9% |
| Stomach | 353 | 584 | 667 | 923 | 1242 | 1.1% | 1.8% | 2.0% | 2.8% | 3.8% |
| Testis | 28 | 66 | 89 | 142 | 177 | 3.6% | 8.4% | 11.3% | 17.9% | 22.4% |
| Thyroid | 40 | 61 | 132 | 196 | 256 | 1.7% | 2.6% | 5.6% | 8.4% | 10.9% |
| Uterus | 528 | 848 | 1108 | 1768 | 2619 | 3.4% | 5.4% | 7.1% | 11.3% | 16.7% |
Note: Results are the median of model predictions.
Abbreviation: HR, hazard ratio.
Base case scenario.
4. DISCUSSION
This modeling study predicted that cancer care disruptions during the COVID‐19 pandemic would lead to important increases in cancer mortality in Canada. Increased cancer mortality is expected to span over several years, with the highest excess cancer mortality expected in 2022. Increasing cancer diagnostic and treatment capacity in the postpandemic era by ≥10% over normal levels was predicted to avert a substantial amount of this excess mortality.
Cancer sites with moderate to high net survival and in younger patients were those predicted to experience the highest relative mortality increases. This may be because cancers with higher survival are those which stand to lose the most from delays; many cancers with low net survival such as pancreatic and esophageal cancers would not be expected to survive very long even with normal wait‐times. Treatment modality also influenced results by cancer site, because most treatment delays were expected for surgeries. For example, prostate cancers were predicted to be less impacted by the pandemic because a high proportion of prostate cancers are expected to be managed with active surveillance, which was assumed not to be affected by treatment delays. Interestingly, provinces that reported higher declines in cancer treatments were not necessarily those predicted to experience the highest relative increases in cancer mortality. This suggests that age structure and cancer case mix also influence the expected population‐level excess cancer mortality.
Increasing cancer treatment capacity in the postpandemic era by ≥10% over prepandemic levels was predicted to avert most predicted excess cancer deaths caused by the pandemic. This occurs for two reasons: (a) increasing treatment capacity helps clear treatment backlogs accumulated during the pandemic and (b) cancer treatment demand is predicted to increase in the future due to cancer diagnoses which were missed or delayed during the pandemic, but which are expected to eventually be diagnosed and require treatment. In the model, these delayed diagnoses are added to the treatment backlog and increase treatment wait times for all cancers once diagnosed, unless treatment capacity is increased to meet demand. While we did not assess the duration of time that would be necessary to maintain increased treatment capacity to reduce cancer mortality, it is likely that the period of increased needs will span the period of time required to diagnose and treat the cancers which were missed during 2020 to 2021. Our results in Figure 3 suggest the period of increased treatment needs could span until at least the end of 2022. This is consistent with another study which estimated that it could take between 46 and 145 weeks to clear the accumulated pandemic surgical backlog assuming a +10% surge capacity in Ontario, Canada. 25 Some provincial governments have announced investments to address diagnostic and treatment backlogs, but it is unclear whether these will be sufficient to prevent excess cancer mortality. Some provinces also experienced their worst COVID‐19 pandemic wave in the Fall of 2021, potentially leading to further decreases in healthcare capacity. Ongoing healthcare worker staffing shortages across Canada may also limit increases in diagnostic and treatment capacity.
Much evidence suggests an important part of the observed decline in cancer treatments is likely attributable to cancer diagnostic delays, and not necessarily treatment delays. Cancer surgical wait‐times returned to normal and even improved in Canada during 2020; however, the cumulative number of surgeries was lower than in previous years. 5 , 6 Fewer pathology reports, MRI scans and CT scans have been performed than in previous years. 5 , 6 Many diagnostic procedures and cancer screenings such as colonoscopies and mammographies were interrupted or slowed due to the pandemic. 5 , 26 This suggests there is a backlog of undiagnosed cancers that have yet to be identified for treatment, either due to decreased patient interactions with the healthcare system or due to delays in the diagnostic pathway. An important part of the model‐predicted excess mortality is attributable to these delayed diagnoses which will eventually require treatment. These delayed diagnoses may not yet be apparent in observed treatment backlogs and waitlists, but should be accounted for in the planning for future healthcare needs, especially as many are likely to present with more advanced cancers.
Diagnostic and treatment delays are hypothesized to lead to worse cancer survival due to tumor progression, leading to later stage at diagnosis and treatment. We were unable to predict the impact of delays on stage shifts in the model due to a lack of quality data on the quantitative relationship between time and the risk of stage progression. Much of the literature on this topic is biased by a wait time paradox, a type of confounding by indication where cancers with worse survival have shorter diagnostic and treatment intervals due to being prioritized in cancer care pathways. 23 , 24 , 27 However, there is concern that early stage cancers which went undiagnosed during the pandemic may eventually present at later stages. 28 Later stages at diagnosis with worse survival are expected to account for part of the future cancer mortality increase. As evidence on this topic accrues, we plan to further integrate the probability of stage shifts in the model when better data becomes available.
An important limitation of our model is that we were not able to integrate triaging of cases and treatment substitutions during the pandemic. Many provinces implemented clinical protocols to prioritize the treatment of the most severe cancer cases and to provide alternative treatments in order to minimize the impact of treatment delays. 7 Given the paucity of detailed data on how these policies were implemented on the ground, it was not possible to model the impact of these policies.
The causal effect of cancer care trajectory delays on cancer mortality is the most uncertain parameter in our model. Research on this topic has been challenging due to the wait time paradox. 27 We based the effect of delays in the model on results from a high‐quality systematic review that accounted for the wait time paradox. 1 The effects of delays in our model were similar to those assumed by other models. By our calculation, Hartman et al assumed mortality hazard ratios between 1.03 and 1.2 per 4‐week treatment delay based on data from 5 million patients in the United States National Cancer Database 29 ; Sud et al assumed mortality hazard ratios between 1.09 and 1.17 per 4‐week diagnostic delay based on their own literature review. 30 We have chosen a hazard ratio on the lower side of the range of estimates (1.06) in order to provide more conservative predictions of the pandemic's impact on cancer mortality. However, studies have found many cancer sites and treatment modalities with larger hazard ratios, 1 , 29 , 30 suggesting the impact of pandemic‐related care delays could be even larger than in our base case. Because the true causal effect of delays remains highly uncertain by cancer site, we provided estimates by cancer site assuming different effects of delays (Table 2).
Predictions of the impact of the COVID‐19 pandemic on cancer deaths have varied across models. 29 , 30 , 31 , 32 While it is difficult to directly compare models due to structural differences, we believe assumptions regarding the extent and duration of disruptions to cancer‐related healthcare greatly influence differences in predictions. Due to the difficulty of obtaining real‐time healthcare data, previous models have mostly examined the impact of hypothetical 3‐ to 24‐month pandemic disruptions to cancer care. For example, we predicted fewer excess breast cancer deaths than Maringe et al, who predicted a 8% to 10% increase over 5 years in England, 32 but we predicted more excess breast cancer deaths than Alagoz et al, who predicted a 0.5% increase between 2020 and 2030 in the United States. 31 We believe these differences are partly due to differing assumptions regarding the extent of disruptions to cancer care in different settings. Our predictions are based on empirical data on the volume of cancer‐related procedures, which suggest that while cancer care provision did rapidly recover in Canada, there have been long‐lasting disruptions to the number of cancer surgeries and diagnoses performed since March 2020. While our model does not include the effects of screening, pandemic‐related disruptions to screening are also likely to lead to further excess cancer mortality for breast, colorectal and cervical cancers. 33 , 34
Different countries and jurisdictions have been impacted differently by the pandemic, depending on implemented public health measures and the resilience of their healthcare systems. In Canada, cancer treatments were generally prioritized over many other types of healthcare, so the declines in cancer care procedures were not as large as they could have been. However, we believe there are some lessons that are broadly generalizable across settings. While our predicted numbers are specific to Canada, we believe our predictions and those of other models are indicative of the magnitude of the effect that pandemic‐related healthcare disruptions could be expected to cause in many countries with similar COVID‐19 epidemic profiles. Most importantly, our findings highlight the potential impact the COVID‐19 pandemic could have on non‐COVID‐19 health outcomes such as cancer. Averting excess deaths from treatment delays requires additional treatment capacity after disruptions, which may be difficult to achieve if health systems continue to experience strains due to an ongoing pandemic.
CONFLICT OF INTEREST
Talía Malagón, Jean H. E. Yong and Parker Tope have no conflicts of interest to disclose. Eduardo L. Franco reports grants to his institution from CIHR to assist the conduct of the study; he also reports the following disclosures about activities unrelated to the present paper: personal fees from Merck; a patent related to the discovery “DNA methylation markers for early detection of cervical cancer,” registered at the Office of Innovation and Partnerships, McGill University, Montreal, Quebec, Canada. Wilson H. Miller Jr. reports grants to his institution from Merck, CIHR, Cancer Research Society, Terry Fox Research Institute, Samuel Waxman Cancer Research Foundation and CCSRI; consulting fees from Merck, BMS, Roche, GSK, Novartis, Amgen, Mylan, EMD Serono; honoraria from McGill university, Jewish General Hospital, BMS, Merck, Roche, GSK, Novartis, Amgen, Mylan, EMD Serono; payments for participation on an advisory board from BMS, Merck, Roche, Novartis, Amgen, GSK; and payments to his institution for participation in a clinical trial within the past 2 years from BMS, Novartis, GSK, Roche, AstraZeneca, Methylgene, MedImmune, Bayer, Amgen, Merck, Incyte, Pfizer, Astellas, Genetech, Ocellaris Pharma, MIMIC, Exelixis, Roche, Alkermes.
AUTHOR CONTRIBUTIONS
Talía Malagón conceived and programmed the model, ran simulations, performed analyses and wrote the first draft of the manuscript. Jean H. E. Yong helped parameterize the model and validated model assumptions for analyses. Wilson H. Miller Jr. provided clinical input for model parameterization and assumptions. Parker Tope constructed the expert treatment validation survey, extracted data for model parameters and performed literature reviews. Eduardo L. Franco was the principal investigator for the study, obtained funding and provided team supervision and advice. All authors read, provided feedback and approved the final protocol and manuscript.
ETHICS STATEMENT
This research did not involve human participants or biological materials, and used only publicly available aggregate information. This research therefore did not require ethical approval, in accordance with the Tri‐Council Policy Statement: Ethical Conduct for Research Involving Humans (second edition).
ACKNOWLEDGMENTS
We would like to thank Larry Ellison from Statistics Canada for providing data on net cancer survival in Canada; James Jung from the Canadian Partnership Against Cancer for extracting data from the Canadian Institute for Health Information Portal; Elba Gomez Navas from the Canadian Partnership Against Cancer for developing the companion web application for model results; Annie Bourassa, Christiane Bertrand and Joëlle Sarra‐Bournet from the Québec Ministère de la santé et des services sociaux for providing data on cancer surgeries, radiotherapies and pathology reports in Québec. Parts of this material are based on data and information compiled and provided by Cancer Care Ontario. However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of Cancer Care Ontario.
McGill Task Force on the Impact of COVID‐19 on Cancer Control and Care: (Core Group) Wilson Miller, Eduardo Franco, Rami Ali, Mariam El‐Zein, Eliya Farah, Farzin Khosrow‐Khavar, Caroline Lambert, Talía Malagón, Luca Petruccelli, Gayle Shinder; (Members Serving an Advisory Role) Armen Aprikian, Jamil Asselah, Sarit Assouline, Hitesh Bhanabhai, Gerald Batist, Christine Bouchard, Matthew Cheng, Fabio Cury, Sinziana Dumitra, Arielle Elkrief, Melissa Henry, Carmen Loiselle, Antoine Loutfi, Christine Maheu, Ari Meguerditchian, Sarkis Meterissian, Michael Pollak, Shannon Salvador, Donald Sheppard, Erin Strumpf, Stephanie Wong; (Expert Oncology Practice Advisory Group) Joanne Alfieri, Armen Aprikian, Boris Bahoric, Gerald Batist, Marylise Boutros, Chantal Cassis, Fabio Cury, Marc David, Sinziana Dumitra, Lorenzo Ferri, Carolyn Freeman, Michael Hier, Tarek Hijal, Sender Liberman, Victor McPherson, Ari Meguerditchian, Sarkis Meterissian, Wilson Miller, Victoria Mandilaras, Alex Mlynarek, Thierry Muanza, Michael Palumbo, Valerie Panet‐Raymond, Kevin Petrecca, Gizelle Propadi, Shannon Salvador, Sonia Skamene, Khalil Sultanem, Robert Turcotte, Te Vuong, Stephanie Wong.
Malagón T, Yong JHE, Tope P, Miller WH Jr., Franco EL, McGill Task Force on the Impact of COVID‐19 on Cancer Control and Care . Predicted long‐term impact of COVID‐19 pandemic‐related care delays on cancer mortality in Canada. Int. J. Cancer. 2022;150(8):1244‐1254. doi: 10.1002/ijc.33884
Members of “McGill Task Force on the Impact of COVID‐19 on Cancer Control and Care” are listed in the Appendix.
Funding informationThis work was supported by the Canadian Institutes of Health Research (operating grant VR5‐172666 and foundation grant 143347 to Eduardo L. Franco). The model simulations were run using the supercomputer Béluga from École de technologie supérieure, managed by Calcul Québec (www.calculquebec.ca/) and Compute Canada (www.computecanada.ca). The operation of this supercomputer is funded by the Canada Foundation for Innovation (CFI), Ministère de l'Économie, des Sciences et de l'Innovation du Québec (MESI) and the Fonds de recherche du Québec ‐ Nature et technologies (FRQ‐NT). The companion web application was developed with in‐kind support from the Canadian Partnership Against Cancer.
The funders played no role in the writing of the manuscript, the collection/analysis of the data or the decision to submit it for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
DATA AVAILABILITY STATEMENT
The data collected to inform model parameters are reported in Supporting information available at https://doi.org/10.5683/SP2/REMSZ6. The results from model simulations reported in this study are publicly available as R objects at the McGill University Dataverse (https://doi.org/10.5683/SP2/AQHVJB). A public web application to further explore results by province is available at https://tmalagon.shinyapps.io/CancerCOVID19Model/. Further details and other data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta‐analysis. BMJ. 2020;371:m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID‐19 pandemic on cancer care. Nat Cancer. 2020;1:565‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petrova D, Pollán M, Rodriguez‐Barranco M, Garrido D, Borrás JM, Sánchez M‐J. Anticipated help‐seeking for cancer symptoms before and after the coronavirus pandemic: results from the Onco‐barometer population survey in Spain. Br J Cancer. 2021;124:2017‐2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quinn‐Scoggins H, Cannings‐John R, Moriarty Y, Quinn‐Scoggins Harriet, Cannings‐John Rebecca, Moriarty Yvonne, Whitelock Victoria, Whitaker Katriina L., Grozeva Detelina, Hughes Jacqueline, Townson Julia, Osborne Kirstie, Goddard Mark, McCutchan Grace, Waller Jo, Robling Michael, Hepburn Julie, Moore Graham, Gjini Ardiana, Brain Kate, Quinn‐Scoggins Harriet, Cannings‐John Rebecca, Moriarty Yvonne, Whitelock Victoria, Whitaker Katriina L., Grozeva Detelina, Hughes Jacqueline, Townson Julia, Osborne Kirstie, Goddard Mark, McCutchan Grace, Waller Jo, Robling Michael, Hepburn Julie, Moore Graham, Gjini Ardiana & Brain Kate et al. The impact of COVID‐19 on cancer symptom experience and help‐seeking behaviour in the United Kingdom: a cross‐sectional population survey. Available at SSRN: https://ssrn.com/abstract=3793564 or 10.2139/ssrn.3793564 [DOI]
- 5. Ministère de la Santé et des Services sociaux . Analyse des répercussions de la pandémie de la COVID‐19 sur les soins et services en cancérologie au Québec. 2021. https://publications.msss.gouv.qc.ca/msss/document-002878/. Accessed June 21, 2021.
- 6. Canadian Institute for Health Information . Wait times for priority procedures in Canada — data table. 2021. https://www.cihi.ca/en/wait-times-for-priority-procedures-in-canada. Accessed July 19, 2021.
- 7. Farah E, Ali R, Tope P, El‐Zein M, Franco EL, McGill Task Force on Covid‐and Cancer . A review of Canadian cancer‐related clinical practice guidelines and resources during the COVID‐19 pandemic. Curr Oncol. 2021;28:1020‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canadian Institute for Health Information . COVID‐19's effect on hospital care services. 2021. https://www.cihi.ca/en/covid-19-resources/impact-of-covid-19-on-canadas-health-care-systems/covid-19s-effect-on-hospital. Accessed July 19, 2021.
- 9. Malagón T. Model technical documentation. 2021. Publisher, V1. 10.5683/SP2/REMSZ6 [DOI]
- 10. Statistics Canada . Table 13‐10‐0111‐01 Number and rates of new cases of primary cancer, by cancer type, age group and sex. 2021. doi: 10.25318/1310011101-eng. [DOI]
- 11. Statistics Canada . Table 17‐10‐0005‐01, Population estimates on July 1st, by age and sex. 2021. doi: 10.25318/1710000501-eng. [DOI]
- 12. Canadian Cancer Society's Advisory Committee on Cancer Statistics Canadian Cancer Statistics: a 2018 special report on cancer incidence by stage in Canada. Toronto, ON: Canadian Cancer Society. 2018. Available at: cancer.ca/Canadian‐Cancer‐Statistics‐2018‐EN.pdf (accessed 2021‐11‐30).
- 13. Canadian Cancer Society's Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2019. Toronto, ON: Canadian Cancer Society; 2019. http://cancer.ca/Canadian-Cancer-Statistics-2019-EN [Google Scholar]
- 14. Ellison LF. Progress in net cancer survival in Canada over 20 years. Health Rep. 2018;29:10‐18. [PubMed] [Google Scholar]
- 15. CCO SEER*Stat Package ‐ Release 11 ‐ OCR (Dec. 2018). Pop Est Summary (Statistics Canada, Ontario Ministry of Finance). Spring 2018 based on the 2011 Census.
- 16. Statistics Canada . Life tables, Canada, provinces and territories. Catalogue no. 84‐537‐X. 2020. https://www150.statcan.gc.ca/n1/en/catalogue/84-537-X. Accessed March 22, 2021.
- 17. Public Health England, Cancer Research UK . Chemotherapy, radiotherapy and surgical tumour resections in England 2013–2016. 2020. https://www.cancerdata.nhs.uk/treatments. Accessed April 22, 2021.
- 18. American Cancer Society . Cancer Treatment & Survivorship Facts & Figures 2019–2021. Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- 19. Clemen RT, Winkler RL. Combining probability distributions from experts in risk analysis. Risk Anal. 1999;19:187‐203. [DOI] [PubMed] [Google Scholar]
- 20. Ministère de la Santé et des Services Sociaux . Bulletin national de performance en cancérologie ‐ Automne 2018. Gouvernement du Québec. 2018. https://publications.msss.gouv.qc.ca/msss/fichiers/2018/18-902-07W.pdf. Accessed April 15, 2021.
- 21. Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106:1262‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radio Canada . Évolution de la COVID‐19. 2021. https://ici.radio-canada.ca/info/2020/coronavirus-covid-19-pandemie-cas-carte-maladie-symptomes-propagation/. Accessed June 1, 2021.
- 23. Tørring ML, Falborg AZ, Jensen H, et al. Advanced‐stage cancer and time to diagnosis: an International Cancer Benchmarking Partnership (ICBP) cross‐sectional study. Eur J Cancer Care. 2019;28:e13100. [DOI] [PubMed] [Google Scholar]
- 24. Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112(suppl 1):S92‐S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang J, Vahid S, Eberg M, et al. Clearing the surgical backlog caused by COVID‐19 in Ontario: a time series modelling study. Can Med Assoc J. 2020;192:E1347‐E1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walker MJ, Meggetto O, Gao J, et al. Measuring the impact of the COVID‐19 pandemic on organized cancer screening and diagnostic follow‐up care in Ontario, Canada: a provincial, population‐based study. Prev Med. 2021;151:106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crawford SC, Davis JA, Siddiqui NA, et al. The waiting time paradox: population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. BMJ. 2002;325:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Limb M. Covid‐19: early stage cancer diagnoses fell by third in first lockdown. BMJ. 2021;373:n1179. [DOI] [PubMed] [Google Scholar]
- 29. Hartman HE, Sun Y, Devasia TP, et al. Integrated survival estimates for cancer treatment delay among adults with cancer during the COVID‐19 pandemic. JAMA Oncol. 2020;6:1881‐1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sud A, Torr B, Jones ME, et al. Effect of delays in the 2‐week‐wait cancer referral pathway during the COVID‐19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alagoz O, Lowry KP, Kurian AW, et al. Impact of the COVID‐19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst. 2021;113:1484‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21:1023‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith MA, Burger EA, Castanon A, et al. Impact of disruptions and recovery for established cervical screening programs across a range of high‐income country program designs, using COVID‐19 as an example: a modelled analysis. Prev Med. 2021;151:106623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yong JH, Mainprize JG, Yaffe MJ, et al. The impact of episodic screening interruption: COVID‐19 and population‐based cancer screening in Canada. J Med Screen. 2021;28:100‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data collected to inform model parameters are reported in Supporting information available at https://doi.org/10.5683/SP2/REMSZ6. The results from model simulations reported in this study are publicly available as R objects at the McGill University Dataverse (https://doi.org/10.5683/SP2/AQHVJB). A public web application to further explore results by province is available at https://tmalagon.shinyapps.io/CancerCOVID19Model/. Further details and other data that support the findings of this study are available from the corresponding author upon request.


