Abstract
Coronavirus disease 2019 (COVID‐19) infection in elderly patients is more aggressive and treatments have shown limited efficacy. Our objective is to describe the clinical course and to analyze the prognostic factors associated with a higher risk of mortality of a cohort of patients older than 80 years. In addition, we assess the efficacy of immunosuppressive treatments in this population. We analyzed the data from 163 patients older than 80 years admitted to our institution for COVID‐19, during March and April 2020. A Lasso regression model and subsequent multivariate Cox regression were performed to select variables predictive of death. We evaluated the efficacy of immunomodulatory therapy in three cohorts using adjusted survival analysis. The mortality rate was 43%. The mean age was 85.2 years. The disease was considered severe in 76.1% of the cases. Lasso regression and multivariate Cox regression indicated that factors correlated with hospital mortality were: age (hazard ratio [HR] 1.12, 95% confidence interval [CI]: 1.03–1.22), alcohol consumption (HR 3.15, 95% CI: 1.27–7.84), CRP > 10 mg/dL (HR 2.67, 95% CI: 1.36–5.24), and oxygen support with Venturi Mask (HR 6.37, 95% CI: 2.18–18.62) or reservoir (HR 7.87, 95% CI: 3.37–18.38). Previous treatment with antiplatelets was the only protective factor (HR 0.47, 95% CI: 0.23–0.96). In the adjusted treatment efficacy analysis, we found benefit in the combined use of tocilizumab (TCZ) and corticosteroids (CS) (HR 0.09, 95% CI: 0.01–0.74) compared to standard treatment, with no benefit of CS alone (HR 0.95, 95% CI: 0.53–1.71). Hospitalized elderly patients suffer from a severe and often fatal form of COVID‐19 disease. In this regard, several parameters might identify high‐risk patients upon admission. Combined use of TCZ and CS could improve survival.
Keywords: corticosteroids, COVID‐19, elderly, prognosis, tocilizumab
Highlights
The COVID‐19 disease in the elderly population (> 80 years) is severe in most cases.
We analyze which parameters at admission may be useful to better stratify the risk of mortality. Oxygen demand on admission is the most accurate parameter to determine prognosis.
A combined use of corticosteroids and tocilizumab may improve overall survival versus corticosteroids alone or standard of care.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), is a major cause of acute respiratory syndrome and mortality. 1 Initially data from China showed a total case‐fatality rate of 2.3%, but in patients, over 80 years this rate may be as high as 14.8%. 2 Spain has been one of the most severely affected European countries reaching up to three times the usual capacity of intensive care units (ICUs). 3 Elderly patients and nursing homes were especially hit by the pandemic. According to the Spanish National Surveillance Net, the 80‐years and older population showed the highest hospitalization −44.7%‐ and mortality −21.8%‐ rates. 4
COVID‐19 in elderly patients is a matter of great importance for its morbi‐mortality. Until now, several reports have tried to establish risk factors for poor outcomes, but few studies have focused on the elderly population 5 , 6 Furthermore, these patients are underrepresented in clinical trials and the specific use of some treatments should be confirmed in this group of patients. More evidence is needed about SARS‐CoV‐2 infection in this group of patients.
In this study, we aim to: (a) describe clinical characteristics as well as outcomes of patients over 80 years admitted for COVID‐19; (b) identify prognostic factors for risk of death; and (c) assess the efficacy of immunosuppressive treatments ‐ tocilizumab (TCZ) and corticosteroids (CS) ‐ applied to this population.
2. METHODS
2.1. Study design
We design a single‐center, retrospective observational study that included all patients older than 80 years, admitted into our institution between March 1 and April 30, 2020, with a diagnosis of COVID‐19 and followed up until hospital discharge. Due to the difficulties to provide adequate follow‐up in that pandemic scenario, patients referred to other hospitals from our Emergency Department were ultimately excluded. The Fuenlabrada University Hospital's local Ethics Committee approved this study (APR 20/26).
2.2. Procedures and study variables
Diagnosis of confirmed cases was performed by real‐time reverse transcription‐polymerase chain reaction (PCR) from nasopharyngeal swab samples (platform Roche Cobas Z 480). During the period of greatest healthcare overload, there was no availability of PCR tests for all patients. According to hospital protocols, patients with high clinical suspicion (bilateral pneumonia, lymphopenia, and close contact with positive cases) were defined and managed as probable cases. Probable cases were included in statistical analysis.
The following variables were recorded from electronic medical records.
Baseline characteristics and comorbidities at the time of initial contact: age, sex, former or current smoker, previous alcohol consumption, hypertension, diabetes mellitus (DM), dyslipidemia, obesity (defined as body mass index (BMI) > 3 kg/m2), chronic kidney disease (CKD) (glomerular filtration rate <60 ml/min), heart disease (including arrhythmias, ischemic disease, heart failure, and valvular disease), respiratory pathology (chronic obstructive pulmonary disease, asthma, chronic respiratory insufficiency, and use of previous mechanical noninvasive ventilation devices), venous thromboembolic disease (VTE), cirrhosis, neurological conditions (dementia and neurodegenerative disorders, previous stroke, or epilepsy), anemia, history of malignancy (including hematological and solid cancer), autoimmune disease, and autonomy for activities of daily living.
Previous treatments: antiplatelets, anticoagulants, angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptors antagonists type II (ARA‐II), nonsteroidal anti‐inflammatory drugs (NSAIDs), inhaled corticosteroids, and immunosuppressive therapies.
Symptoms and clinical signs on admission: fever (temperature over 37.8°C), cough, dyspnea, chest pain, digestive symptoms, confusion, blood pressure, heart rate, tachypnea, and mental status.
Laboratory parameters: lactate dehydrogenase (LDH), C‐reactive protein (CRP), procalcitonin, d‐dimer (DD), creatinine, glomerular filtration rate (GFR), urea, sodium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma‐glutamyl transpeptidase (GGT), albumin, white cell blood count, neutrophils, lymphocytes, hemoglobin, platelets. Severity of disease on admission: Classified as mild, moderate, or severe according to recommendations from WHO guidelines. Critical cases were re‐classified into the severe group. 7 We recorded additional information about oxygen supplementation on entry, and radiological findings on entry.
Treatments received during the course of the hospital stay: Antibiotics, chloroquine/hydroxychloroquine, ritonavir/lopinavir, interferon beta 1b, CS, TCZ, and heparin (prophylactic or intermediate‐high doses). Due to its retrospective observational design, the dose of CS administered was not predefined in advance. Our institutional protocol contemplated the possibility of CS use in case of rapidly progressive respiratory failure or elevation of inflammatory parameters (such as CRP > 10 mg/dL), despite antiviral or antimalarial treatment. Although the institutional protocol recommended doses of 20 mg dexamethasone for 5–10 days or 250/500 mg methylprednisolone pulses for 3–5 days, the final dose was individually decided by the treating physician. Data were collected on daily and total doses received, in mg equivalent to prednisone. The use of TCZ (in 2 doses of 600 mg on consecutive days) was restricted during part of the study due to stock‐outs. When available, it was used with the same criteria as CS, generally in the absence of response to them.
Length of stay, complications during hospitalization (bacterial infection, kidney and liver worsening, heart failure, thrombosis, bleeding, confusional syndrome, and blood transfusion), and final outcomes (survival or death).
2.3. Outcomes
The main outcome was mortality for both analyses: prognostic risk factors and efficacy of treatments. Patients were followed up until discharge or death.
2.4. Statistical analysis
The descriptive variables were analyzed using means and standard deviations (Student's t‐test) as well as counts and percentage (χ 2 tests) where appropriate. Given the multitude of baseline variables, we report univariate Cox regression results and chose the least absolute shrinkage and selection operator (Lasso) binary logistic regression model to select and report variables jointly predictive of death. The selected variables were entered into a multivariate Cox regression analysis. To facilitate model interpretation, we transformed the laboratory data continuous variable into bivariate. Cut‐off levels were determined according to previous reports 8 or reference values from our laboratory.
In a subsequent step, we evaluated the efficacy of the treatments by creating three patient cohorts: (1) patients without immunomodulatory therapy, (2) patients who received only CS, and (3) patients who received CS and TCZ, and compared them using crude Kaplan–Meier survival curves and adjusted survival analysis for age, sex, the presence of hypertension, diabetes mellitus, previous heart disease, severity of disease, and initial supplemental oxygen requirements on admission. This analysis was not time updated but uses the time from hospitalizations to death or discharge, regardless of when the treatment was administered.
Statistical analysis was performed with R 4.0.1 and the level of statistical significance considered in all univariate analyses and the Kaplan–Meier survival curves was p = 0.05.
3. RESULTS
During the first wave of the COVID‐19 pandemic, a total of 1700 patients were admitted to our center. Of them, 163 (9.5%) were over 80 years old and were included in the study.
Baseline characteristics, comorbidities, and previous treatments are presented in Table 1, comparing survivors (93 cases) and deceased (70 cases, 43% of total). Seventy‐five (46%) of patients were female. The median age was 85.2 years. None of these patients were admitted to the ICU.
Table 1.
Differences in baseline characteristics between survivors and deceased groups
| Overall | Survivors | Dead | ||
|---|---|---|---|---|
| Baseline characteristics | (n = 163) | (n = 93) | (n = 70) | p |
| Age (mean, year (sd)) | 85.23 (4.11) | 85.18 (4.19) | 85.29 (4.03) | 0.875 |
| Sex (female, %) | 75 (46.0%) | 47 (50.5%) | 28 (40.0) | 0.239 |
| Comorbidities, n (%) | ||||
| Smoker | 59 (36.9) | 29 (31.5) | 30 (44.1) | 0.142 |
| Alcohol consumption | 11 (6.7) | 3 (3.2) | 8 (11.4) | 0.08 |
| Hypertension | 134 (82.2) | 78 (83.9) | 56 (80.0) | 0.665 |
| Diabetes mellitus | 58 (35.6) | 32 (34.4) | 26 (37.1) | 0.845 |
| Obesity | 51 (35.4) | 31 (37.3) | 20 (32.8) | 0.697 |
| Chronic kidney disease | 67 (44.1) | 31 (35.2) | 36 (56.2) | 0.016 |
| Heart disease | 83 (50.9) | 44 (47.3) | 39 (55.7) | 0.366 |
| Respiratory disease | 50 (30.7) | 24 (25.8) | 26 (37.1) | 0.167 |
| Previous thrombosis | 20 (12.3) | 11 (11.8) | 9 (12.9) | 1 |
| Cirrhosis | 2 (1.2) | 1 (1.1) | 1 (1.4) | 1 |
| Neurological disease | 62 (38.0) | 35 (37.6) | 27 (38.6) | 1 |
| Anemia | 44 (27.5) | 27 (30.0) | 17 (24.3) | 0.532 |
| Malignancy | 47 (28.8) | 24 (25.8) | 23 (32.9) | 0.419 |
| Autoimmune disease | 11 (6.7) | 4 (4.3) | 7 (10.0) | 0.263 |
| Dependency | 61 (37.7) | 31 (33.7) | 30 (42.9) | 0.304 |
| Previous treatments, n (%) | ||||
| Antiplatelets | 51 (31.7) | 34 (36.6) | 17 (25.0) | 0.166 |
| Anticoagulants | 53 (32.7) | 27 (29.0) | 26 (37.7) | 0.322 |
| ACEI‐ARAII | 91 (55.8) | 54 (58.1) | 37 (52.9) | 0.615 |
| NSAID | 5 (3.1) | 2 (2.2) | 3 (4.3) | 0.743 |
| Inhaled corticosteroids | 19 (11.7) | 12 (12.9) | 7 (10.1) | 0.77 |
| Immunosupresive drugs | 19 (11.8) | 6 (6.5) | 13 (18.8) | 0.031 |
Note: p‐values represent the comparison between survivors and dead groups.
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARAII, angiotensin receptors antagonists type II; NSAIDs, nonsteroidal anti‐inflammatory drugs.
The most frequent comorbidity was hypertension in 82.2% of cases, followed by heart disease 50.9%, CKD 44.1%, neurological conditions 38%, former and current smoker 36.9%‐, DM 35.6%, obesity 35.4%, and respiratory diseases 30.7%. CKD was more frequent among patients that died (56.2% vs. 35.2%, p = 0.02) while the rest of the variables did not show relevant differences. ACEIs and ARAII were frequently used among patients (91 cases, 55.8%) without differences between both groups. Regarding the remaining drugs, they had similar rates of use, except for immunosuppressive agents, which were more frequently used among patients who eventually died (18.8% vs. 6.5%, p = 0.03).
3.1. Symptoms, clinical signs, and laboratory findings at admission
Data about symptoms, clinical signs, laboratory, and radiological findings, and severity of disease upon admission are shown in Table 2.
Table 2.
Differences in clinical signs, symptoms, and laboratory values on admission
| Overall | Survivors | Dead | ||
|---|---|---|---|---|
| (n = 163) | (n = 93) | (n = 70) | p | |
| Symptoms on admission, n (%) | ||||
| Fever | 97 (59.5) | 51 (54.3) | 46 (66.7) | 0.111 |
| Cough | 111 (69.4) | 68 (73.1) | 43 (64.2) | 0.3 |
| Dyspnea | 102 (63.4) | 53 (57.0) | 49 (72.1) | 0.073 |
| Chest pain | 7 (4.5) | 5 (5.5) | 2 (3.1) | 0.759 |
| Digestive symptoms | 31 (19.0) | 24 (25.8) | 7 (10.0) | 0.019 |
| Confusion | 25 (15.9) | 9 (9.8) | 16 (24.6) | 0.023 |
| Clinical signs on admission | ||||
| Temperature (mean, °C [SD]) | 36.63 (1.09) | 36.56 (1.11) | 36.75 (1.06) | 0.306 |
| Systolic BP (mean, mmHg [SD]) | 130.62 (25.00) | 131.45 (24.92) | 129.49 (25.25) | 0.628 |
| Diastolic BP (mean, mmHg [SD]) | 71.52 (13.18) | 71.92 (12.71) | 70.96 (13.86) | 0.649 |
| Heart rate (mean, bpm [SD]) | 90.18 (20.88) | 86.58 (14.00) | 95.06 (26.97) | 0.011 |
| Tachypnea (n, %) | 66 (44.3) | 24 (27.9) | 42 (66.7) | <0.001 |
| Mental status (n, %) | <0.001 | |||
| Normal | 123 (78.8) | 80 (88.9) | 43 (65.2) | |
| Altered | 26 (16.7) | 10 (11.1) | 16 (24.2) | |
| Coma | 7 (4.5) | 0 (0.0) | 7 (10.6) | |
| Laboratory findings | ||||
| LDH (mean, U/L [SD]) | 352.19 (420.20) | 272.91 (120.99) | 461.21 (619.11) | 0.017 |
| CRP (mean, mg/dL [SD]) | 11.10 (9.17) | 8.81 (8.26) | 14.35 (9.47) | <0.001 |
| Procalcitonin (mean, ng/ml [SD]) | 1.55 (8.29) | 1.62 (10.80) | 1.46 (3.72) | 0.923 |
| d‐Dimer (mean, mg/L [SD]) | 4.55 (15.78) | 5.02 (18.83) | 3.90 (10.30) | 0.685 |
| Creatinine (mean, mg/dL [SD]) | 1.32 (0.79) | 1.20 (0.76) | 1.49 (0.81) | 0.022 |
| GFR (mean, ml/min/1.73 m2 [SD]) | 53.53 (23.05) | 58.85 (22.13) | 46.78 (22.61) | 0.003 |
| Urea (mean, mg/dL [SD]) | 70.14 (46.39) | 62.59 (46.31) | 79.63 (45.37) | 0.105 |
| Sodium (mean, mmol/L [SD]) | 138.30 (7.01) | 138.32 (6.35) | 138.28 (7.95) | 0.975 |
| AST (mean, U/L ([SD]) | 43.95 (34.08) | 36.56 (27.86) | 55.26 (39.68) | 0.012 |
| ALT (mean, U/L [SD]) | 29.85 (34.12) | 25.76 (24.58) | 35.39 (43.51) | 0.1 |
| GGT (mean, U/L [SD]) | 72.40 (94.92) | 54.65 (71.68) | 95.05 (114.88) | 0.015 |
| Albumin (g/dL, mean [SD]) | 3.31 (0.45) | 3.27 (0.42) | 3.36 (0.48) | 0.381 |
| White cell blood count (mean, 109/9 L [SD]) | 10.38 (18.96) | 8.66 (4.66) | 12.70 (28.50) | 0.181 |
| Neutrophils (mean, 109/L [SD]) | 8.00 (17.25) | 6.38 (3.88) | 10.17 (26.00) | 0.168 |
| Lymphocytes (mean, 109/L [SD]) | 1.30 (1.43) | 1.32 (0.96) | 1.29 (1.90) | 0.92 |
| Hemoglobin (mean, g/dL [SD]) | 12.91 (2.18) | 12.86 (2.10) | 12.98 (2.29) | 0.726 |
| Platelets (mean, 109/L [SD]) | 198.85 (95.22) | 211.38 (100.95) | 181.94 (84.71) | 0.051 |
| Severity of illness | ||||
| Severity classification | 0.001 | |||
| Mild | 11 (6.7) | 8 (8.6) | 3 (4.3) | |
| Moderate | 28 (17.2) | 24 (25.8) | 4 (5.7) | |
| Severe | 124 (76.1) | 61 (65.6) | 63 (90.0) | |
| Oxygen support at entry | <0.001 | |||
| No support | 78 (47.9) | 60 (64.5) | 18 (25.7) | |
| Nasal ducts | 43 (26.4) | 27 (29.0) | 16 (22.9) | |
| VentiMask | 13 (8.0) | 3 (3.2) | 10 (14.3) | |
| Reservoir | 29 (17.8) | 3 (3.2) | 26 (37.1) | |
| Radiology findings | 0.03 | |||
| No pneumonia | 22 (13.5) | 16 (17.2) | 6 (8.6) | |
| Unilateral pneumonia | 41 (25.2) | 28 (30.1) | 13 (18.6) | |
| Bilateral pneumonia | 100 (61.3) | 49 (52.7) | 51 (72.9) | |
Note: p‐values represent a comparison between survivors and dead groups.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure; CRP, C‐reactive protein; GFR, glomerular filtration rate; GGT, gamma‐glutamyl transpeptidase; LDH, lactate dehydrogenase; SD, standard deviation.
Cough and dyspnea were the most frequent symptoms (69.4% and 63.4%), while fever was present in 40.1% of all cases. Notably, digestive symptoms were more frequent in the survival group (25.8% vs. 10%). Tachypnea and tachycardia were more frequent in dead patients (66.7% vs. 27.9% and 95 bpm vs. 86.5 bpm, respectively). In addition, these patients presented more frequently altered mental status (confusion in 24.2% vs. 11.1% and coma in 10.6% vs. 0%).
The mean values of analytical parameters are also shown in Table 2. The dead group had higher levels of LDH (461.2 vs. 272.9 U/L), CRP (14.35 vs. 8.81 mg/dL), creatinine (1.49 vs. 1.2 mg/dL), AST (55.26 vs. 36.56 U/L), GGT (95.05 vs. 54.65 U/L), white blood cell count (12.7 vs. 8.66 × 109/L), neutrophils (10.17 vs. 6.38 × 109/L) with decreased levels of GFR (46.78 vs. 58.85 ml/min/1.73 m2) and platelets (181.94 vs. 211.39 × 103).
The first assessment of the severity of the disease was clearly different between the groups. 90% of all patients who died had a severe disease on admission (65.6% among survivors). Furthermore, important differences were found in the need for oxygen support upon admission. The group of survivors had more cases with no support (64.5 vs. 25.7%) while a high number of dead patients required a reservoir oxygen mask (37.1% vs. 3.2%). When considering the 29 cases that required a reservoir in the emergency department, only 3 of 29 eventually survived. Regarding the radiological patterns, those patients who died presented more often with bilateral pneumonia (72.9% vs. 52.7%), while in the survivors' group unilateral pneumonia (30.1% vs. 18.6%) and no pneumonia (17.2% vs. 8.6%) were more common.
3.2. Length of stay, treatments, and complications
In relation to the length of stay, treatments and complications suffered during hospitalization are shown in Table 3. The time from the onset of self‐reported symptoms to emergency department admission was 5.73 days in general, slightly shorter among the patients who died (4.89 vs. 6.32 days).
Table 3.
Differences in time to admission, mean stay, treatments received, and complications
| Variable | Overall | Survivors | Dead | |
|---|---|---|---|---|
| (n = 163) | (n = 93) | (n = 70) | p | |
| Time symptoms to admission (mean, days [SD]) | 5.73 (5.95) | 6.32 (6.46) | 4.89 (5.07) | 0.138 |
| Mean stay (mean, days [SD]) | 9.45 (7.12) | 12.08 (7.56) | 6.04 (4.69) | <0.001 |
| Treatments (n, %) | ||||
| Antibiotics | 149 (90.9) | 87 (92.6) | 62 (88.6) | 0.382 |
| Chloroquine | 33 (20.2) | 18 (19.4) | 15 (21.4%) | 0.744 |
| Hydroxychloroquine | 124 (75.6) | 77 (81.9) | 47 (67.1) | 0.029 |
| Lopinavir/ritonavir | 127 (77.4) | 72 (76.6) | 55 (78.6) | 0.765 |
| Interferon | 4 (2.4) | 2 (2.1) | 2 (2.9) | 0.765 |
| Corticosteroids | 86 (52.4) | 39 (41.5) | 47 (67.1) | 0.001 |
| Total dose (mg PDN, [SD]) | 508.91 (708.53) | 475.78 (773.72) | 553.4 (612.8) | 0.489 |
| Mean dose (mg PDN, [SD]) | 172.96 (112.17) | 118.31 (63.92) | 217.36 (123.4) | <0.001 |
| Tocilizumab | 11 (6.7) | 10 (10.8) | 1 (1.4) | 0.025 |
| Heparin | 56 (34.4) | 38 (40.9) | 18 (25.7) | 0.044 |
| Complications | ||||
| Bacterial infection | 14 (8.5) | 8 (8.5) | 6 (8.6) | 0.989 |
| Kidney injury | 36 (22) | 16 (17) | 20 (28.6) | 0.077 |
| Liver injury | 5 (3) | 1 (1.1) | 4 (5.7) | 0.087 |
| Heart failure | 20 (12.2) | 8 (8.5) | 12 (17.1) | 0.095 |
| Thrombosis | 4 (2.4) | 4 (4.3) | 0 | 0.137 |
| Bleeding | 4 (2.4) | 1 (1.1) | 3 (4.3) | 0.314 |
| Confusional syndrome | 24 (14.6) | 12 (12.8) | 12 (17.1) | 0.433 |
| Transfusion | 7 (4.3) | 6 (6.4) | 1 (1.4) | 0.240 |
Note: p‐values represent the comparison between survivors and dead groups.
Abbreviations: PDN, prednisone; SD, standard deviation.
Most of the patients received antibiotics (90.9% at least one dose), chloroquine/hydroxychloroquine (95.8%), and lopinavir/ritonavir (77.4%) following the established institutional protocol. Corticosteroids (expressed in equivalent doses to mg of prednisone) were used in 52.4% of cases, with greater frequency in patients who died (67.1% vs. 41.5%) at higher cumulative (553 vs. 475 mg), and medium daily doses (217 vs. 118 mg). In addition, tocilizumab was used in 11 patients (in nine with simultaneous corticosteroid treatment) mainly in the survivors' group. Heparin either in intermediate or therapeutic doses was prescribed in 34.4% of patients.
Among the complications, acute kidney failure was the most prevalent, in 22% of the cases; followed by confusional syndrome in 14.6%. Bacterial infections were documented in 8.5%, similarly among groups.
3.3. Uni and multivariate analysis for risk factors
We first performed a univariate Cox regression analysis to identify prognostic factors associated with death. In a first step, we included 55 variables relatives to comorbidities, previous treatments, symptoms on admission, laboratory data, and severity of the disease. Results with correspondent hazard ratio (HR) and confidence intervals (CI) are presented in Table 4. In the univariate analysis, the factors associated with poor outcomes were: alcohol consumption, chronic kidney disease, previous immunosuppressive drugs, dyspnea, confusion, tachypnea, altered mental status, increased LDH, CRP, PCT, creatinine or AST, decreased GFR, lymphopenia, and the type of oxygen support. Digestive symptoms were the only factor with a protective role.
Table 4.
Univariate Cox regression for prognostic factors associated with death
| Univariate Cox analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prognostic factors | HR (95% CI) | p‐value | Prognostic factors | HR (95% CI) | p‐value | Prognostic factors | HR (95% CI) | p‐value | Prognostic factors | HR (95% CI) | p‐value |
| Age | 1.02 (0.97–1.08) | 0.457 | Antiplatelets | 0.62 (0.36–1.09) | 0.112 | Mental status (n, %) | Neutrophils > 7.5 × 103/L | 1.3 (0.8–2.13) | 0.304 | ||
| Sex (female) | 0.88 (0.54–1.41) | 0.612 | Anticoagulants | 1.25 (0.76–2.03) | 0.380 | Normal | 1 | Lymphocytes <1 × 103/L | 1.96 (1.2–3.19) | 0.008 | |
| Smoker | 1.43 (0.89–2.31) | 0.153 | ACEIs‐ARAII | 0.96 (0.6–1.54) | 0.882 | Altered | 2.18 (1.25–3,88) | 0.010 | Hemoglobin < 13 g/dL | 0.87 (0.54–1.4) | 0.578 |
| Alcohol consumption | 2.24 (1.07–4.68) | 0.036 | NSAIDs | 1.62 (0.51–5.19) | 0.428 | Coma | 6.82 (3.03–15.33) | <0.001 | Platelets <150 × 103/L | 1.29 (0.8–2.1) | 0.295 |
| Hypertension | 0.87 (0.48–1.56) | 0.669 | Inhaled corticosteroids | 0.75 (0.34–1.63) | 0.467 | LDH > 250 U/L | 3.2 (1.5–6.86) | 0.003 | Severity classification | ||
| Diabetes | 0.93 (0.57–1.52) | 0.784 | Immunosupresive drugs | 1.95 (1.06–3.57) | 0.036 | CRP > 10 mg/dL | 2.35 (1.43–3.85) | 0.001 | Mild | 1 | |
| Obesity | 0.98 (0.57–1.67) | 0.930 | Fever | 1.4 (0.85–2.32) | 0.187 | Procalcitonin > 0.5 ng/ml | 1.97 (1.06–3.64) | 0.042 | Moderate | 0.4 (0.09–1.78) | 0.231 |
| Chronic kidney disease | 1.83 (1.17–3) | 0.019 | Cough | 0.85 (0.51–1.4) | 0.519 | d‐Dimer > 0.5 mg/L | 1.17 (0.56–2.48) | 0.681 | Severe | 1.67 (0.53–5.36) | 0.394 |
| Cardiopathy | 1.42 (0.89–2.27) | 0.160 | Dyspnea | 2.16 (1.26–3.69) | 0.016 | Creatinine > 1.17 mg/dL | 1.97 (1.06–3.64) | 0.004 | Oxygen support | ||
| Neumopathy | 1.32 (0.81–2.15) | 0.275 | Chest pain | 0.75 (0.18–3.1) | 0.711 | GFR < 60 ml/min/1.73 m2 | 2.05 (1.15–3.67) | 0.017 | No support | 1 | |
| Previous thrombosis | 0.99 (0.49–2) | 0.986 | Digestive symptoms | 0.43 (0.2–0.94) | 0.040 | Urea > 49 mg/dL | 1.59 (0.76–3.33) | 0.224 | Nasal ducts | 1.72 (0.87–3.36) | 0.123 |
| Cirrhosis | 1.56 (0.21–11.3) | 0.685 | Confusion | 2.12 (1.24–3.74) | 0.011 | Sodium > 145 mmol/L | 1.4 (0.6–3.27) | 0.321 | VentiMask | 7.31 (3.35–15.94) | <0.001 |
| Neurological disease | 1.09 (0.67–1.77) | 0.726 | Temperature | 1.12 (0.9–1.4) | 0.333 | AST > 50 U/L | 3.4 (1.7–6.75) | 0.001 | Reservoir | 6.31 (3.45–11.55) | <0.001 |
| Anemia | 0.76 (0.44–1.32) | 0.344 | Systolic BP | 1 (0.99–1) | 0.838 | ALT > 50 U/L | 1.4 (0.66–2.96) | 0.390 | Radiology findings | ||
| Malignancy | 1.18 (0.71–1.93) | 0.530 | Diastolic BP | 1 (0.97–1.01) | 0.486 | GGT > 85 U/L | 1.63 (0.9–2.94) | 0.112 | No pneumonia | 1 | |
| Autoimmune disease | 2.03 (0.92–4.46) | 0.083 | Heart rate | 1.02 (1.01–1.04) | <0.001 | Albumin <3.5 g/dL | 0.7 (0.36–1.37) | 0.226 | Unilateral pneumonia | 1.08 (0.41–2.84) | 0.882 |
| Dependency | 1.29 (0.80–2.07) | 0.296 | Tachypnea (n, %) | 3.36 (1.99–5.69) | <0.001 | WBC > 11.6 × 103/L | 1.24 (0.71–2.18) | 0.640 | Bilateral pneumonia | 2.03 (0.87–4.77) | 0.091 |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ALT, alanine aminotransferase; ARAII, angiotensin receptors antagonists type II; AST, aspartate aminotransferase; BP, blood pressure; CI, confidence interval; CRP, C‐reactive protein; GFR, glomerular filtration rate; GGT, gamma‐glutamyl transpeptidase; HR, hazard ratio; LDH, lactate dehydrogenase; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Due to an imbalanced proportion between baseline variables and the number of patients, we then performed a Lasso shrinkage regression for variable selection. After controlling for the most accurate model (Figure 1), 19 variables were selected and entered into a multivariate Cox regression analysis. NSAIDs were excluded for creating instability in the model, due to a low frequency of use. The results of the multivariate analysis are shown in Table 5. Finally the variables that remained as independent prognostic factors for mortality were: age (HR 1.12, 95% CI: 1.03–1.22), alcohol consumption (HR 3.15, 95% CI: 1.27–7.84), CRP > 10 mg/dL (HR 2.67, 95% CI: 1.36–5.24), and oxygen support with Venturi Mask (HR 6.37, 95% CI: 2.18–18.62), or reservoir (HR 7.87, 95% CI: 3.37–18.38). Previous treatment with antiplatelets was the only beneficial factor for survival (HR 0.47, 95% CI: 0.23–0.96).
Figure 1.
The selection of the variables by Lasso regression was controlled by lambda to select the most accurate model
Table 5.
Multivariate Cox regression for prognostic factors associated with death
| Multivariate Cox analysis | |||
|---|---|---|---|
| Prognostic factors | HR | 95% CI | p‐value |
| Age | 1.12 | 1.03–1.22 | 0.012 |
| Alcohol consumption | 3.15 | 1.27–7.84 | 0.013 |
| Antiplatelets | 0.47 | 0.23–0.96 | 0.037 |
| CRP > 10 mg/dL | 2.67 | 1.36–5.24 | 0.004 |
| Platelets < 150×10^3/L | 2.29 | 1.21–4.31 | 0.11 |
| Oxygen support on admission: | |||
| No support | 1 | ||
| Nasal ducts | 1.78 | 0.72–4.37 | 0.209 |
| Venturi Mask | 6.37 | 2.18–18.62 | 0.001 |
| Reservoir | 7.87 | 3.37–18.38 | <0.001 |
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; HR, hazard ratio;.
3.4. Efficacy of treatments: Corticosteroids and tocilizumab
We assessed the efficacy of treatments with survival analysis of three groups: no immunosuppressive therapy (76 patients), CS alone (76 patients), or in combination with TCZ (9 patients).
No statistically significant differences were noted between groups regarding age, sex, and comorbidities. The proportions of severity and oxygen support in the groups receiving CS alone or combined with TCZ were higher than in the group of patients who did not receive either of these drugs. No statistically significant differences were observed when comparing the groups treated with immunomodulatory therapy. The analysis controlled for potential baseline confounding effects such as age, sex, comorbidities, the severity of disease, and the need for supportive oxygen at admission.
The final Kaplan‐Meier curve for the survival of each group is presented in Figure 2. The adjusted estimates for treatment efficacy were: (a) non immunomodulatory therapy (reference treatment HR = 1), (b) patients receiving CS (HR 0.95, 95% CI: 0.53–1.71), and (c) patients receiving CS and TCZ (HR 0.09, 95% CI: 0.01–0.74).
Figure 2.
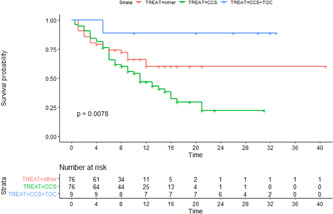
Unadjusted Kaplan–Meier curve for survival of each treatment group. CCS, corticosteroids; TOC, tocilizumab
4. DISCUSSION
In this study we present the characteristics and outcomes of 163 elderly patients admitted to our institution during the first wave of the pandemic in Spain. Population over 80 years represent the largest group by age (23.8% of the cases confirmed until the end of May 2020). 4 Advanced age is a well‐known independent factor for mortality and the overall mortality rate in this group ranges between 15%–35%. 2
In our hospital, the mortality rate for those over 80 years of age was 43%. During the greatest hospital overload, mild cases were transferred to other hospitals, and severe/critical cases represented 76% of our series. The need for ICU was assessed on a case‐by‐case basis, although none of the patients were considered for admission to ICU, due to previous comorbidities and the decision algorithms to rationalize resources in a context of high demand. These reasons may explain higher mortality compared with other cohorts 5 , 9
In the elderly population, COVID‐19 infection evolves rapidly to death in many cases. Once hospitalized, the time to death in our study was half of the time to recovery. Our analysis tries to clarify which symptoms, vital signs, laboratory data, and radiological findings on admission may be useful to better identify patients of high risk. The multivariate model regression finally selected age, alcohol consumption, elevated CRP, low platelets, and oxygen supplementation as the most important factors related to the risk of death. Previous treatment with antiplatelets was considered a protective factor. The results of our model are strongly concordant with existing literature.
Elderly patients suffer from several of the comorbidities that have been already associated with COVID‐19 risk mortality: cardiovascular disease, DM, hypertension, respiratory disease, cancer, CKD, obesity, smoking, and alcoholism 10 , 11 , 12 Dementia, which is a frequent condition in elderly patients, has also been related to mortality in specific studies in older people. 5 In our cohort, these conditions were highly represented and the univariate analysis showed a risk increase in most of them. The subsequent Lasso‐Cox regression selected age itself and alcohol consumption as relevant factors for poor outcomes. A remarkable result was obtained with the beneficial role of previous antiplatelets use. Larger cohorts have already associated antiplatelets with better in‐hospital outcomes 13 , 14 We may hypothesize that antiplatelets reduce the COVID‐19 related thrombosis risk. But the number of events, predominantly in the survivors' groups, is too small to analyze properly this issue. Previous treatment with anticoagulants did not have the same protective effect.
Regarding the prognostic role of laboratory markers, previous reports have emphasized the value of lymphopenia, thrombocytopenia, elevated LDH, CRP or d‐dimer 5 , 8 , 15 Our model finally selected CRP and platelets count as the most important parameters which may guide the therapeutic decision algorithms.
The most consistent risk factor in our study was the type of oxygen supplementation on admission. Hypoxemia or dyspnea have already been linked to poor outcomes by other authors 5 , 16 The risk of death with Venturi mask or reservoir increased by six or seven times, respectively. WHO proposed a classification for COVID‐19 cases into mild, moderate, severe, and critical. But considering the high proportion of elderly patients with the last forms, oxygen demand on admission may be a more accurate form to stratify the risk in this population.
Our study has some limitations in evaluating the efficacy of treatments, mainly due to its retrospective observational design, dose variability in therapies, and the small number of patients treated with the combined treatment. At the time these patients were treated, no treatment had shown an improvement in survival. 17 Most received antimalarials and lopinavir/ritonavir, which were later shown to be ineffective and excluded for the analysis 18 , 19 Remdesivir was not available so no patient received it.
According to the postulated theories about a cytokine storm causing the lung damage 20 , 21 CS and an interleukine‐6 blocker (TCZ) were prescribed in some cases. CS has subsequently been validated in later studies as a mortality‐reducing treatment in clinical trials in patients requiring oxygen therapy, 22 while some controversies remain about TCZ. 23 , 24 , 25 Some studies propose an extra benefit for TCZ when using it in addition to CS, rather than separately. 26
We found of special interest the role of immunosuppressive treatments in this special population. Aging itself is a predisposing condition to cytokine dysregulation and hyperinflammatory states, which may explain the higher mortality rates in the elderly. 27 , 28 Interleukine‐6 is considered a hallmark of inflammatory changes related to aging. 29 Despite a small number of patients treated with the combination of TCZ and CS, the adjusted model showed statistically significant benefit in terms of survival. The results should be interpreted with caution due to a wide confidence interval.
The evolution of those who received only CS seems worse than those who did not receive any treatment, but after the adjustment for severity, the survival curves become similar. It should be noted that in our institutional protocol, CS and TCZ were started under certain severity criteria but TCZ availability was limited for some periods of time. This fact justifies a more widespread use of corticosteroids. Very high doses were often prescribed in a setting of lack of evidence or availability of other treatments. Consequently, the proportion of seriously ill patients was higher in the corticosteroids‐treated group. Despite the adjustment of the model for severity, the bias caused may have been sufficient to explain this result. We must also consider a harmful influence of the dose used as the dose shown to be beneficial in the studies is significantly lower than that received by our patients.
In conclusion, in this study, we extensively describe the clinical evolution of a cohort of elderly patients over 80 years of age with COVID‐19 and highlight the most relevant prognostic factors. Bearing in mind the high mortality rate and the concerns about effective therapeutic interventions, it is necessary to know the prognostic tools that allow better adaptation of medical care. Combined treatment with TCZ and CS may have a potential role in reducing mortality even in the elderly population.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests. The main author corroborates on behalf of Dr. Stefan Walter that no conflicts of interest affected his work in this study.
AUTHORS CONTRIBUTIONS
All authors contributed to data collection, analysis, and writing of the final version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Stefan Walter for his work, and deeply regret his passing before the final publication of this manuscript. The authors would like to thank Dr. Miguel Ángel Canales Albendea (Department of Hematology, Hospital Universitario La Paz, Madrid, Spain) for their helpful comments on previous drafts of this manuscript. No funding was received for this project.
Duarte‐Millán MA, Mesa‐Plaza N, Guerrero‐Santillán M, et al. Prognostic factors and combined use of tocilizumab and corticosteroids in a Spanish cohort of elderly COVID‐19 patients. J Med Virol. 2022;94:1540‐1549. 10.1002/jmv.27488
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Harvard Dataverse, V1 at doi:10.7910/DVN/YUAPGS, reference number UNF:6:I3kmpBRlT4FMbJUR9ng3tw==.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA ‐ J Am Med Assoc. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3. Condes E, Arribas JR. Impact of COVID‐19 on Madrid hospital system. Enferm Infec Microbiol Clin. 2020;39:256‐257. 10.1016/j.eimc.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covid‐19 cases notified to the Epidemiological Net of Survaillance in Spain. Accessed May 9, 2020. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/InformesCOVID-19.aspx
- 5. Covino M, De Matteis G, Santoro M, et al. Clinical characteristics and prognostic factors in COVID‐19 patients aged ≥80 years. Geriatr Gerontol Int. 2020;20(March):1‐5. 10.1111/ggi.13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80(6):639‐645. 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical management of COVID‐19 patients: Clinical guidance. January 2021.
- 8. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mostaza JM, García‐Iglesias F, González‐Alegre T, et al. Clinical course and prognostic factors of COVID‐19 infection in an elderly hospitalized population. Arch Gerontol Geriatr. 2020;91(July):104204. 10.1016/j.archger.2020.104204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID‐19) infection. Int Urol Nephrol. 2020;52:1193‐1194. 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhalla S, Sharma B, Smith D, et al. Unhealthy alcohol use is an independent risk factor for increased COVID‐19 disease severity: observational cross‐sectional study. JMIR Public Health Surveill. 2021;7:33022. 10.2196/33022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santoro F, Nuñez‐Gil IJ, Vitale E, et al. Antiplatelet therapy and outcome in COVID‐19: the Health Outcome Predictive Evaluation Registry. Heart Br Card Soc. Published online October 5, 2021. 10.1136/heartjnl-2021-319552 [DOI] [PMC free article] [PubMed]
- 14. Sisinni A, Rossi L, Battista A, et al. Pre‐admission acetylsalicylic acid therapy and impact on in‐hospital outcome in COVID‐19 patients: the ASA‐CARE study. Int J Cardiol. Published online October 4, 2021. 10.1016/j.ijcard.2021.09.058 [DOI] [PMC free article] [PubMed]
- 15. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA ‐ J Am Med Assoc. 2020;323:1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80(6):639‐645. 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA ‐ J Am Med Assoc. 2020;2019:1824‐1836. 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 18. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild‐to‐moderate Covid‐19. N Engl J Med. 2020;383:2041‐2052. 10.1056/nejmoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe covid‐19. N Engl J Med. 2020;382(19):1787‐1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033‐1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. RECOVERY Collaborative G, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. 10.1056/nejmoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guaraldi G, Meschiari M, Cozzi‐Lepri A, et al. Tocilizumab in patients with severe COVID‐19: a retrospective cohort study. Lancet Rheumatol. 2020;2:591. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salama C, Han J, Yau L, et al. Tocilizumab in nonventilated patients hospitalized with Covid‐19 pneumonia. medRxiv. 2020;384(650):20‐30. 10.1056/NEJMoa2030340 [DOI] [Google Scholar]
- 25. Stone JH, Frigault MJ, Serling‐Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid‐19. N Engl J Med. 2020;383:2333‐2344. 10.1056/nejmoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Remap‐Cap I, Gordon AC, Mouncey PR, et al. Interleukin‐6 receptor antagonists in critically ill patients with Covid‐19. N Engl J Med. 2021;384(16):1491‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akbar AN, Gilroy DW. Aging immunity may exacerbate COVID‐19. Science. 2020;369(6501):256‐257. 10.1126/science.abb0762 [DOI] [PubMed] [Google Scholar]
- 28. Saghazadeh A, Rezaei N. Expert Review of Clinical Immunology Immune‐epidemiological parameters of the novel coronavirus: a perspective. Expert Rev Clin Immunol. 2020;00(00):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID‐19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65(October 2020):101205. 10.1016/j.arr.2020.101205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Harvard Dataverse, V1 at doi:10.7910/DVN/YUAPGS, reference number UNF:6:I3kmpBRlT4FMbJUR9ng3tw==.


