Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which is responsible for coronavirus disease 2019 (COVID‐19), is known to cause severe respiratory infections with occasional accompanying pleural effusion (PE), pericardial effusion (PCE), or peritoneal effusion (PTE). The effect of COVID‐19 on effusion cytology is not yet known. This study aimed to examine the cytomorphologic features and workup of effusion fluids in patients with active COVID‐19 infection versus those in recovery.
Methods
PE (n = 15), PCE (n = 1), and PTE samples (n = 20) from hospitalized patients with a SARS‐CoV‐2 infection (from June 1, 2020, to December 30, 2020) were reviewed. Effusion fluids with metastatic carcinoma were excluded. Differential cell counts, cytomorphology, and relevant immunostains for effusion fluids were retrospectively evaluated and compared between patients with active infection (positive on a SARS‐CoV‐2 nucleic acid amplification test [NAAT] within 2 months; n = 23) and those in the recovery phase from COVID‐19 (negative on a SARS‐CoV‐2 NAAT for >2 months; n = 13).
Results
The cytology diagnoses were negative for malignancy (n = 31), atypical (n = 4), and suspicious for malignancy (n = 1). Active infection cases showed more atypical mesothelial cells than recovery cases (P < .05); some had enlarged nuclei, prominent nucleoli, occasional multinucleation, and bizarre nuclei. Immunostains were performed more often in active infection cases than recovery cases (47.8% vs 7.7%; P < .05). Differential cell counts (available for 28 cases) showed no significant differences between the active infection and recovery groups.
Conclusions
This study found atypical and bizarre mesothelial cells more often in effusions of cases with active COVID‐19 infection in comparison with patients in recovery. It is important for cytopathologists to become familiar with the cytomorphologic effects of SARS‐CoV‐2 on effusion cytology so that these cases can be properly triaged.
Keywords: atypical mesothelial cells, coronavirus disease 2019 (COVID‐19), effusion cytology, fluid, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)
Short abstract
This study examined the cytomorphologic features and work‐up of effusion fluids in patients with active COVID‐19 infection versus those in recovery, and found atypical and bizarre mesothelial cells to be present more often in effusions of cases with active COVID‐19 infection than those from patients in recovery. It is important for cytopathologists to become familiar with the cytomorphologic effects of SARS‐CoV‐2 on effusion cytology.
Introduction
Coronavirus disease 2019 (COVID‐19) is a pandemic infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which is a predominantly respiratory disease. COVID‐19 infection can cause pathologic changes in multiple organs that range from mild to severe manifestations. The most common severe presentation of the disease includes diffuse alveolar damage with the formation of hyaline membranes, pneumocyte syncytia and multinucleated giant cells (early stage), and interstitial fibrosis (late stage) in the lungs. 1 , 2 , 3 Less common manifestations include diffuse lymphocytic myocarditis in the heart, 4 acute hepatitis with predominant portal inflammation and ductal/lobular cholestasis in the liver, 5 and maternal vascular malperfusion features in the placenta. 6 Cytomorphologic changes in bronchoalveolar lavage specimens were recently summarized by Canini et al 7 as increased neutrophil counts and multinucleated giant cells with occasional nuclear clearing and pseudo‐inclusions in type II pneumocytes.
COVID‐19 is occasionally complicated with body cavity effusions, including pleural effusion (PE), pericardial effusion (PCE), and peritoneal effusion (PTE). 8 , 9 , 10 , 11 COVID‐19 patients with extensive body cavity effusions usually present with more severe inflammation and poorer clinical outcomes. 8 , 10 , 11 Therefore, effusions may be a complementary risk factor for critical COVID‐19 infections. 10 , 11 A recent study has shown that body cavity effusions in COVID‐19 patients tend to be transudative with lymphohistiocytic inflammation and commonly exhibit hemophagocytosis. 12 However, the effect of COVID‐19 infection on mesothelial changes in effusion fluid cytology is not yet known. Therefore, in this study, we aimed to explore the essential cytomorphologic features in mesothelial cells and the workup of effusion fluid specimens from patients with active COVID‐19 infection versus those in the recovery phase.
Materials and Methods
Study Design and Data Collection
Our protocol was approved by our institution's institutional review board. A search was conducted in our laboratory information system for effusion cytology cases from hospitalized patients who had at least 1 positive SARS‐CoV‐2 nucleic acid amplification test (NAAT) between June 1, 2020, and December 30, 2021. Cytology slides of PE (n = 15), PCE (n = 1), and PTE samples (n = 20) were reviewed by a board‐certified cytopathologist. Cytologic features, including the presence of atypical mesothelial cells and multinucleated giant cells, the aggregation of histocytes, the types of inflammation (acute, chronic, or both), and the severity of inflammation (none, mild, moderate, or severe), as well as the presence of immunohistochemical workup were tabulated. Effusion fluid cases with metastatic carcinoma were excluded because of potential overlapping cytomorphologic features of malignant cells with reactive atypia in the mesothelial cells. Differential cell count data were evaluated and compared between the patients with active infection (SARS‐CoV‐2 NAAT–positive within 2 months; n = 23) and the patients in the recovery phase of COVID‐19 (SARS‐CoV‐2 NAAT–negative for >2 months; n = 13).
Specimen Procession and Immunocytochemistry Stains
All effusion fluid cytology specimen samples consisted of a Diff‐Quik–stained smear, a ThinPrep liquid‐based and Papanicolaou‐stained slide, and a hematoxylin‐eosin–stained cell block slide. Cell blocks were prepared from fresh or refrigerated aliquots of effusion fluid specimens via the plasma thrombin clot procedure, fixed in 10% neutral buffered formalin, and processed as biopsy specimens. Immunohistochemical stains on cell blocks were performed for clinical care as needed.
The total number of nucleated cells was counted with an automated hematology cell counter (model XN 9000; Sysmex), and this was followed by a manual differential cell count after cytospinning and Wright‐Giemsa staining.
Statistical Analysis
A P value < .05 was considered to indicate statistical significance. Continuous variables are presented as means and standard deviations or as medians and quartiles (I‐III). Categorical data are expressed as frequencies and percentages. Differential cell counts of effusion fluids were compared between patients with active infection and patients in recovery with the Mann‐Whitney U test. Differences in cytomorphologic features in effusion fluids were studied with the χ2 test to compare the 2 groups. Statistical analysis was performed with IBM SPSS Statistics (version 21).
Results
Nonmalignant effusion fluid cases of hospitalized patients (23 with active COVID‐19 infection and 13 in the recovery phase) were included in the study (15 PEs, 1 PCEs, and 20 PTEs; Table 1). The groups of patients with active infection and patients in recovery were similar in terms of age and sex (Table 1). Cytology diagnoses were negative for malignancy (n = 31), atypical (n = 4), or suspicious for malignancy (n = 1). Patients with active infection versus those in recovery showed no statistically significant difference in their distribution of cytology diagnoses (P > .05; Table 1).
TABLE 1.
Effusion Cytology for Patients With an Active COVID‐19 Infection Versus Those in Recovery
| SARS‐CoV‐2, COVID‐19 | Active Infection (n = 23) | Recovery Phase (n = 13) | P a |
|---|---|---|---|
| Gender (male:female), No. | 13:10 | 5:8 | .298 |
| Age, mean ± SD, y | 60.3 ± 13.1 | 60.7± 16.1 | .552 b |
| Duration of infection, mean ± SD, mo c | 0.60 ± 0.13 | 5.4 ± 0.93 | N/A |
| Cytologic diagnosis, No. (%) | 23 | 13 | .261 |
| Negative for malignancy | 18 (78.3) | 13 (100) | |
| Atypical | 4 (17.4) | 0 (0) | |
| Suspicious for malignancy | 1 (4.3) | 0 (0) | |
| Immunostains ordered, n/N (%) | 11/23 (47.8) | 1/13 (7.7) | .014 |
| Mesothelial cells, n/N (%) | |||
| Atypical mesothelial cells | 17/23 (73.9) | 7/13 (53.8) | .005 |
| Multinucleated mesothelial cells | 13/23 (56.5) | 3/13 (23.1) | .029 |
| Cellularity of mesothelial cells, No. (%) | .086 | ||
| Low | 11 (47.8) | 6 (46.2) | |
| Moderate | 4 (17.4) | 6 (46.2) | |
| High | 8 (34.8) | 1 (7.7) | |
| Mesothelial cell distribution | Clusters and single cells | Clusters and single cells | N/A |
| Inflammation, n/N (%) | .699 | ||
| Acute | 5/23 (21.7) | 2/13 (15.4) | |
| Chronic | 12/23 (52.2) | 9/13 (69.2) | |
| Both | 6/23 (26.1) | 2/13 (15.4) | |
| Severity of inflammation, n/N (%) | .855 | ||
| Mild | 7/23 (30.4) | 3/13 (23.1) | |
| Moderate | 12/23 (52.2) | 8/13 (61.5) | |
| Severe | 4/23 (17.4) | 2/13 (15.4) | |
| Aggregation of macrophages, n/N (%) | 15/23 (65.2) | 6/13 (46.2) | .346 |
Abbreviations: COVID‐19, coronavirus disease 2019; N/A, not applicable; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Pearson χ2 test.
Student t test.
Duration from the last positive SARS‐CoV‐2 nucleic acid amplification test to the date on which the specimen was collected.
Effusion cytology samples from those with active infection showed increased cellularity and atypia in mesothelial cells. Atypical mesothelial cells forming large 3‐dimensional clusters and exhibiting enlarged hyperchromatic nuclei with increased nuclear to cytoplasmic ratios and prominent nucleoli along with occasional multinucleation (Fig. 1) were more often seen in patients with active infection than those in the recovery phase. The cytomorphologic parameters are summarized in Table 1. The cases of active infection showed more atypical mesothelial cells than the recovery cases (P < .05; Table 1) with more frequent multinucleation (Fig. 2) and bizarre nuclei (P < .01; Fig. 2 and Table 1).
Figure 1.
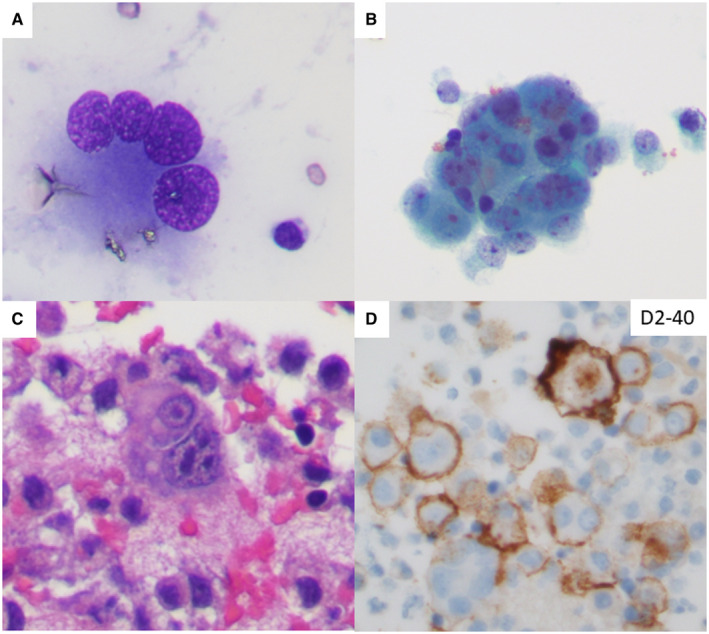
Pleural effusion cytology of a 46‐year‐old female with an active severe acute respiratory syndrome coronavirus 2 infection showing (A) atypical mesothelial cells with multinucleation (Diff‐Quik stain) and (B,C) bizarre nuclei and prominent nucleoli (Papanicolaou stain and cell block with H & E stain, respectively). (D) An immunohistochemistry workup performed on the cell block section confirmed the cytology diagnosis of negative for malignancy and the presence of reactive mesothelial cells (D2‐40 immunostain).
Figure 2.
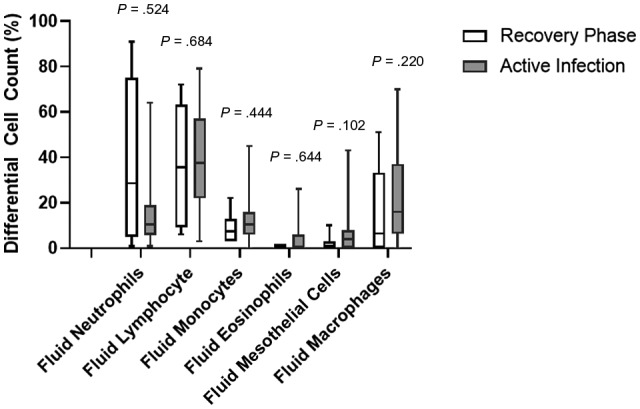
Box plots of differential cell counts in the effusion fluids of patients with an active infection and those in the recovery phase of a coronavirus disease 2019 infection. Boxes represent the 25th and 75th percentiles, lines inside the boxes are medians, small squares represent means, and crosses indicates 1st and 99th percentiles. Medians of differential cell counts between active infection and recovery phase cases are compared with the Mann‐Whitney U test; P values are indicated on top of each pair.
A routine panel of calretinin, D2‐40, MOC‐31, BerEP4, and/or organ system differential markers was ordered in some effusion cytology cases to include or exclude malignancy (adenocarcinoma) from the differential diagnosis. Immunohistochemical stains were performed more often in active infection cases than recovery cases (47.8% vs 7.7%; P < .05). If mesothelioma is clinically suspected, BAP1 staining may be performed to distinguish between reactive cells (BAP1 retained) and malignant cells (BAP1 lost). However, in this data set, none of the cases warranted BAP1 staining.
Differential Cell Counts in Effusion Fluids
Twenty‐eight of the effusion fluid cases had concurrent differential cell count data available. Active infection cases were mostly exudates with a median lymphocyte count of 37.5% and a macrophage count of 20.0%, whereas the recovery cases were predominantly exudates with a median lymphocyte count of 35.5% followed by a neutrophil count of 28.5%. No significant difference in the differential cell count was observed between active infection cases and recovery cases (P > .05; Fig. 2).
Discussion
Patients with severe COVID‐19 infections have an increased incidence of body cavity effusions. 8 Therefore, we hypothesized that the severity of the COVID‐19 infection may be correlated with cytologic changes in effusion cytology samples. We aimed to detect whether patients with active infection displayed increased cytomorphologic changes in mesothelial cells in comparison with patients in the recovery phase. Atypical mesothelial cells in effusion cytology samples from patients with active COVID‐19 infection displayed large 3‐dimensional clusters with enlarged hyperchromatic nuclei, increased nuclear to cytoplasmic ratios, and prominent nucleoli along with occasional multinucleation (Fig. 1). These findings were diminished or partially resolved in the effusions of patients in the recovery phase when the SARS‐CoV‐2 NAAT became negative. Multinucleation and syncytia are common morphologic findings in the cells of various organs of COVID‐19–infected patients. Pneumocyte syncytia and multinucleated giant cells in the lungs have been found in the acute phase of COVID‐19 infections. 1 , 2 , 3 Recent studies examining the bronchoalveolar lavage cytology of COVID‐19–infected patients revealed multinucleated giant cells with occasional nuclear clearing and pseudo‐inclusions in type II pneumocytes. 7 Our study represents the first report of mesothelial cell atypia with bizarre nuclei and multinucleation in effusion cytology samples of patients with active COVID‐19 infections.
Immunohistochemical stains were ordered by the cytopathologists during the cytologic workup for certain cases to rule out malignancy. Immunostains were ordered more often in samples from patients with active COVID‐19 infections because of the increased level of cytologic atypia and bizarre nuclear forms seen in those cases. In our study, all cases were found to be negative for malignancy after 6 months of follow‐up. Reports examining the workflow of effusion cytology have shown that cytopathologists perform immunostains in the workup of 6.7% to 22% of effusion cytology cases; this depends on pathologists' years of experience and the clinical background of the patient population. 13 , 14 In our institution, immunostains are ordered at a rate similar to rates in previous reports. Moreover, immunostains performed on the effusion fluids of COVID‐19 patients in recovery were also ordered at a rate similar to that for the general cases. However, in the effusion fluids of patients with active COVID‐19 infection, there was a significant increase in the number of cases with immunohistochemistry workup, which also correlated with increased cytological atypia in those specimens. One of the effusion fluid cases was signed out as suspicious for malignancy because severe atypical cells were present only on the ThinPrep and not on the cell block. After 6 months of follow‐up, no metastatic malignancy of the pleura was identified in this patient. These findings indicate that severe cytologic and nuclear atypia in the effusion fluid may mimic malignancy. However, none of the cases were stained for BAP1 because of a lack of suspicion for mesothelioma morphologically and/or clinically at the time of the cytologic diagnosis. Understanding the cytomorphologic changes in effusion cytology samples is critical to the cytologic diagnosis and ultimately in the clinical management of patients with COVID‐19 infections.
Lymphocyte‐rich effusions are most commonly caused by metastatic malignancy, which is followed by infection, cardiac failure, inflammatory pleuritis, malignant mesothelioma, and hematological malignancy. 15 Effusion fluids of COVID‐19 patients were lymphocyte‐predominant in both active infection and recovery cases, and this was consistent with the behavior of immune cells in effusion fluids associated with infection. Interestingly, although no significant difference was present in the differential cell count between the active infection and recovery cases, the neutrophil cell count was higher in the recovery phase cases. The neutrophil count is an important feature to diagnose effusions associated with acute infectious processes, such as bacterial pneumonia and lung abscess. 16 However, in patients with active COVID‐19 infection, neutrophil counts are low in effusions but increase when the COVID‐19 NAAT test turns negative. These findings are compatible with a few previous case reports describing effusion fluids in COVID‐19 patients, 17 , 18 and they indicate that there may be a physiologic process during the COVID‐19 infection different from other infections resulting in body cavity effusion.
In summary, our study found atypical and bizarre mesothelial cells more often in effusions of cases with active COVID‐19 infection in comparison with patients in recovery. Because of the mild to severe nuclear atypia observed in cases with active COVID‐19 infection, a diagnosis of malignancy may be considered, and this may explain the increased immunohistochemical workups observed for the cases with active infection. Our description of cytomorphologic changes in effusion fluid specimens of COVID‐19 patients may help cytopathologists to better understand and manage effusion cytology cases and ultimately improve diagnostic triage for patients with COVID‐19.
Funding Support
No funding.
Conflict of Interest Disclosures
The authors have no disclosures.
Author Contributions
Rong Xia: Conceptualization, methodology, investigation, data curation, project administration, writing–original draft, writing–review and editing, and visualization. Lawrence Hsu Lin: Conceptualization, data curation, methodology, and writing–review and editing. Wei Sun: Conceptualization, data curation, methodology, writing–review and editing, project administration, and supervision. Andre L. Moreira: Investigation, data curation, and writing–review and editing. Aylin Simsir: Conceptualization, methodology, writing–review and editing, and project administration. Tamar C. Brandler: Conceptualization, investigation, methodology, data curation, writing–review and editing, supervision, project administration, and visualization.
Xia R, Lin LH, Sun W, Moreira A, Simsir A, Brandler TC. Effusion fluid cytology and COVID‐19 infection. Cancer Cytopathol.2022. 10.1002/cncy.22545
References
- 1. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700‐704. doi: 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borczuk AC, Salvatore SP, Seshan SV, et al. COVID‐19 pulmonary pathology: a multi‐institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33:2156‐2168. doi: 10.1038/s41379-020-00661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rendeiro AF, Ravichandran H, Bram Y, et al. The spatial landscape of lung pathology during COVID‐19 progression. Nature. 2021;593:564‐569. doi: 10.1038/s41586-021-03475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basso C, Leone O, Rizzo S, et al. Pathological features of COVID‐19–associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827‐3835. doi: 10.1093/eurheartj/ehaa664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lagana SM, Kudose S, Iuga AC, et al. Hepatic pathology in patients dying of COVID‐19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147‐2155. doi: 10.1038/s41379-020-00649-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID‐19. Am J Clin Pathol. 2020;154:23‐32. doi: 10.1093/ajcp/aqaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canini V, Bono F, Calzavacca P, et al. Cytopathology of bronchoalveolar lavages in COVID‐19 pneumonia: a pilot study. Cancer Cytopathol. 2021;129:632‐641. doi: 10.1002/cncy.22422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID‐19 pneumonia. Invest Radiol. 2020;55:327‐331. doi: 10.1097/rli.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. doi: 10.1056/nejmoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morparia K, Park MJ, Kalyanaraman M, McQueen D, Bergel M, Phatak T. Abdominal imaging findings in critically ill children with multisystem inflammatory syndrome associated with COVID‐19. Pediatr Infect Dis J. 2021;40:e82‐e83. doi: 10.1097/inf.0000000000002967 [DOI] [PubMed] [Google Scholar]
- 11. Blumfield E, Levin TL, Kurian J, Lee EY, Liszewski MC. Imaging findings in multisystem inflammatory syndrome in children (MIS‐C) associated with coronavirus disease (COVID‐19). AJR Am J Roentgenol. 2021;216:507‐517. doi: 10.2214/ajr.20.24032 [DOI] [PubMed] [Google Scholar]
- 12. Cantley RL, Hrycaj S, Konopka K, Chan MP, Huang T, Pantanowitz L. Cytologic findings in effusions from patients with SARS‐CoV‐2 infection. J Am Soc Cytopathol. 2021;10:261‐269. doi: 10.1016/j.jasc.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alshaikh S, Lapadat R, Atieh MK, et al. The utilization and utility of immunostains in body fluid cytology. Cancer Cytopathol. 2020;128:384‐391. doi: 10.1002/cncy.22256 [DOI] [PubMed] [Google Scholar]
- 14. Valerio E, Nunes W, Cardoso J, et al. A 2‐year retrospective study on pleural effusions: a cancer centre experience. Cytopathology. 2019;30:607‐613. doi: 10.1111/cyt.12755 [DOI] [PubMed] [Google Scholar]
- 15. Dixon G, Bhatnagar R, Zahan‐Evans N, et al. A prospective study to evaluate a diagnostic algorithm for the use of fluid lymphocyte subset analysis in undiagnosed unilateral pleural effusions. Respiration. 2018;95:98‐105. doi: 10.1159/000481290 [DOI] [PubMed] [Google Scholar]
- 16. Ferreiro L, Lado‐Baleato O, Suarez‐Antelo J, et al. Diagnosis of infectious pleural effusion using predictive models based on pleural fluid biomarkers. Ann Thorac Med. 2019;14:254‐263. doi: 10.4103/atm.atm_77_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee PH, Tan HL, Chia YW, Ling LM. A case of acute bacterial pericarditis in a COVID‐19 patient. Singapore Med J. Published online March 12, 2021. doi: 10.11622/smedj.2021022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chong WH, Huggins JT, Chopra A. Characteristics of pleural effusion in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pneumonia. Am J Med Sci. 2021;361:281‐284. doi: 10.1016/j.amjms.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


