Abstract
Coronavirus disease 2019 (COVID‐19) has caused a global pandemic that continues to cause numerous deaths to date. Four vaccines have been approved by the Food and Drug Administration as of July 2021 to prevent the transmission of COVID‐19: Pfizer, Moderna, AstraZeneca, and Janssen. These vaccines have shown great efficacy and safety profile. One side effect that has been widely reported is post‐COVID‐19 vaccination lymphadenopathy. Due to the mimicry of the lymphadenopathy for metastases in some oncologic patients, there have been reports of patients who underwent biopsies that showed pathologic confirmation of benign reactive lymphadenopathy secondary to the COVID‐19 vaccine. Therefore, understanding the incidence of lymphadenopathy post‐COVID‐19 vaccinations will help guide radiologists and oncologists in their management of patients, both present oncologic patients, and patients with concerns over their newly presenting lymphadenopathy. A systematic literature search was performed using several databases to identify relevant studies that reported lymphadenopathy post‐COVID‐19 vaccination. Our results revealed that several cases have been detected in patients undergoing follow‐up fluorodeoxyglucose (FDG)‐positron emission tomography‐computerized tomography scans where lymph nodes ipsilateral to the vaccine injection site show increased uptake of FDG. Thus, knowledge of the incidence of lymphadenopathy may help avoid unnecessary biopsies, interventions, and changes in management for patients, especially oncologic patients who are at risk for malignancies.
Keywords: AztraZeneca, coronavirus, COVID‐19, COVID‐19 vacccine, fluorodeoxyglucose (FDG) PET‐CT scan, Janssen, lymphadenopathy, Moderna, Pfizer
Highlights
Lymphadenopathy has been widely reported post‐COVID‐19 vaccination including patients undergoing follow‐up FDG‐PET‐CT scans which showed increased FDG uptake
Care must be taken before suspecting lymph node metastasis or deciding for lymphadenectomy following COVID‐19 vaccination
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a respiratory disease caused by a newly discovered coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in 2019. It was discovered in Wuhan city in China after documenting unknown etiology pneumonia cases by the end of December of 2019. 1 Afterward, the World Health Organization stated on 11th March 2020 that COVID‐19 is a “public health emergency of international concern.” 2
SARS‐CoV‐2 is a positive‐sense single‐stranded RNA virus, that can infect animals and humans. 3 Among positive‐stranded RNA viruses, SARS‐CoV‐2 has the largest reported replicating RNA molecules. 4 SARS‐CoV‐2 invades the host cells by binding to angiotensin‐converting enzyme 2 and mostly radiates through the respiratory tract. 5 SARS‐CoV‐2 may infect individuals of all age groups. However, there is a higher risk of infection in people aged above 60 years, as well as those with chronic diseases such as chronic respiratory disease, diabetes, and cardiovascular diseases. 6 Complications of the virus can lead to an uncontrolled inflammatory response, resulting in pneumonia and acute respiratory distress syndrome.
1.1. Development of the COVID‐19 vaccines
In December 2020, Pfizer and BioNTech released the first messenger RNA (mRNA)‐based vaccine targeted against COVID‐19 for use in United Kingdom (UK) and United States (USA). Their vaccine BNT162b2 was authorized for emergency use only. 7 The vaccine consists of lipid nanoparticle (LNP)‐formulation encapsulated mRNA, given through the intramuscular route, in 2 doses separated by 21 days. The vaccine works by stimulating CD4+ and CD8+ T cells' responses and high neutralizing antibody titers. 8 The European Medicines Agency recommended the vaccine for authorization in the EU on December 21, 2020. 9
Moderna's vaccine mRNA‐1273 was authorized by the US Food and Drug Administration (FDA) for emergency use in December 2020. 10 Moderna's vaccine was the second COVID‐19 vaccine recommended for emergency use in the EU by the European Medicines Agency. The vaccine is made of prefusion stabilized S protein mRNA encapsulated in LNP. It is given via the intramuscular route, in 2 doses separated by 28 days. It has been shown that repeated vaccine doses stimulate neutralizing antibodies and CD4+ and CD8+ T cell responses. 8
Oxford/AstraZeneca's COVID‐19 vaccine AZD1222 is a Chimpanzee adenovirus vector expressing the spike protein on its surface. 8 It is given intramuscularly, in 2 doses 8–12 weeks apart. 11 It was approved in the UK for emergency use in December 2020 12 while the European Medicines Agency recommended the COVID‐19 vaccine by AstraZeneca for authorization in the EU in January 2021. 13
The Johnson & Johnson/Janssen company made the Ad26.CoV2.S vaccine. The vaccine contains a recombinant, replication‐incompetent adenovirus type 26 that presents the SARS‐CoV‐2 spike protein on its surface. 14 It was approved for emergency use by the FDA in February 2021. 14 The World Health Organization Strategic Advisory Group of Experts recommended the use of Ad26.CoV2.S vaccine against COVID‐19 in March 2021 as a single intramuscular dose. 15
According to the COVID‐19 vaccinations tracker by the New York Times, up to June 2021, Oxford‐AstraZeneca is being used in 177 countries, while Pfizer‐BioNTech is being used in 104, Johnson & Johnson in 25, and Moderna in 54. 16 Based on the data in “Our World In Data” on 7th June 2021, 20.9% of the world population has received at least one dose of a COVID‐19 vaccine. 17 Several studies were conducted to assess the efficacy/effectiveness and safety of the COVID‐19 vaccines. Pfizer vaccine was found to be 95% efficient while Moderna vaccine trials showed an efficiency rate of 94.1% in all ages. The AstraZeneca vaccine has shown 70.4% efficacy following 2 doses. 18
1.2. COVID‐19 reported postvaccination events
The US FDA Center for Biologics Evaluation and Research published a protocol on Background Rates of Adverse Events of Special Interest (AESIs) for COVID‐19 vaccine safety monitoring. Many side effects were listed as the outcome for the general population including acute myocardial infarction, anaphylaxis, appendicitis, Bell's palsy, deep vein thrombosis, disseminated intravascular coagulation, encephalomyelitis, Guillain‐Barre syndrome, hemorrhagic and nonhemorrhagic stroke, immune thrombocytopenia, myocarditis/pericarditis, narcolepsy, pulmonary embolism, and transverse myelitis. 19 In addition to the above adverse events, many studies reported the occurrence of lymphadenopathy post‐COVID‐19 vaccination.
1.3. Postvaccination lymphadenopathy
Lymphadenopathy can be defined as any type of inconsistency or abnormality in lymph nodes. The abnormality can lay within the firmness, size, or number of lymph nodes in a particular section of the body. Lymphadenopathies can result from a number of causes and can vary depending on the location, including bacterial, parasitic, and viral infections. Studies have shown that unilateral lymphadenopathy has been highly associated with vaccines such as the influenza vaccine, BCG vaccine, and HPV vaccine. 20
Postvaccination lymphadenopathy may be falsely attributed to oncological disorders in patients who are diagnosed with cancer, in remission, or those who are at high risk for developing malignancies. Therefore, it is necessary to be familiar with the possible post‐COVID‐19 vaccine lymphadenopathy to avoid unnecessary stressful diagnostic procedures including imaging and invasive biopsies. 21 This review compiles data about the events of lymphadenopathy reported post‐COVID‐19 vaccination and the recommendations to avoid any clinical and/or diagnostic complications.
2. METHODS
A comprehensive search was conducted to target any studies about vaccines against COVID‐19. The following databases were searched in April 2021 (see Appendix S1): PubMed, Medline (Ovid, 1946–April 2021), Embase (Ovid, 1974–2021), Scopus, Web of Science, Science Direct, MedRxiv, and Lens.org. All searches were limited by year to 2020 and 2021 (or current date). During the screening phase, the studies reporting any lymphadenopathy post‐COVID‐19 vaccination were selected. No restrictions were made about the country, age, or gender. Any duplicated articles were removed. Any articles that did not include primary data, such as reviews were excluded from the study. Studies that were not in English were also excluded. Title and abstract as well as full‐text screening were conducted by two different reviewers for each study using Covidence and disagreements were resolved by consensus. Demographic and clinical data of patients reported in each study (wherever data were available) were extracted independently by two different reviewers using Covidence and disagreements were resolved by consensus. Extracted data included age, sex, comorbidities, treatment/interventions and clinical progress. Categorical variables were expressed as percentages while continuous variables were expressed as mean standard deviation or range of results. Data were extracted from each study by two different reviewers.
3. RESULTS
Figure 1 shows the results of database search and screening. The flow diagram summarizes the details of our protocol. After removing the duplicates, a total of 16 308 studies were retrieved for screening. After removing the studies deemed irrelevant to our topic, 209 studies were selected for full‐text screening with only 37 studies that met the inclusion criteria being included. A total of 172 studies were excluded as 115 studies were irrelevant to the data of interest, 34 had no primary data, 13 were duplicates, 6 were ongoing trials, 3 were not in English and 1 used animal models.
Figure 1.
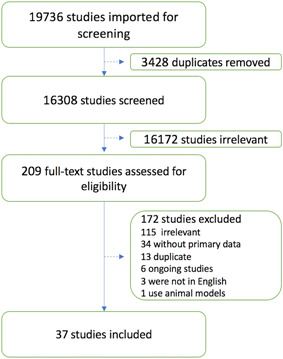
Screening and study selection protocol
3.1. Types of studies
Tables S1 and S2 summarize the types of the included studies. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 The results from our search yielded 24 case series/reports, 7 cohort studies without control (Table S1), 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 3 cohort studies with controls, and 3 randomized control trials (RCT) (Table S2). 52 , 53 , 54 , 55 , 56 , 57 Two of the cohort studies without control were conducted in the USA and two in Israel. The other three were conducted in Germany, South Korea, and the Czech Republic. As for the cohort studies with control, two were also conducted in the USA and one in Israel. Two of the RCTs were conducted mainly in the USA while the study conducted by Polack et al. 52 was a multinational study that had 152 total sites including Argentina (1), Brazil (2), South Africa (4), Germany (6), Turkey (9), USA (130). Out of the 24 case series/reports, 11 were from the USA, 4 from the UK, 2 from Israel, 2 from Italy, 2 from Canada, and 1 from each of Ireland, Germany, and Spain.
3.2. Demographic and clinical data
Tables S1 and S2 summarize the demographic and clinical data extracted from the included studies. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 Table 1 summarizes the findings of the studies without control including cohort studies, case series, and case reports. The table sums the cases and groups the studies by vaccine type and study type. Figure 2 illustrates the total number of lymphadenopathy cases reported in all the included studies. A total of 6022 cases were reported, the majority of which were part of the Moderna vaccine safety trials. 54 , 55 Eighty‐three cases were included from the case series out of which 75 were females (F) as compared to only 8 males (M). Of the 13 case reports, 6 cases of lymphadenopathy developed in females and 7 in males. In cohort studies with controls, a total of 1544 (62.6% F, 15.8% M, 21.5% NR) individuals developed lymphadenopathy after taking the COVID‐19 vaccines. The data reported by McMurry et al., 2021 was not compiled with these numbers as they reported person‐day rather than individuals who developed lymphadenopathy. McMurry et al. 57 reported an incidence ratio (cases per 1000 person‐days) of 0.65 seven days after taking the first dose of a COVID‐19 vaccine and 0.42 after taking the second dose. Interestingly, in the majority of studies where gender was reported, an appreciably greater incidence can be observed in females over males.
Table 1.
Number of lymphadenopathy cases, demographics, and dose after which symptoms developed for each vaccine type observed in case reports, cases series, and cohort studies without control
| Type of vaccine | Type of study | Number of cases (%) | Gender | Age range | Which dose | References | |
|---|---|---|---|---|---|---|---|
| Pfizer | Case reports and case series | 58 (NA) | 10 M | 25–75+ | 30 1st | Özütemiz et al. 22 | |
| Xu & Lu 23 | |||||||
| 22 2nd | Lu 24 | ||||||
| 5 Both | Smith & Yang 26 | ||||||
| Granata et al. 27 | |||||||
| 48 F | 1 NR | Hanneman et al. 28 | |||||
| Hiller et al. 21 | |||||||
| Mehta et al. 29 | |||||||
| Avner et al. 30 | |||||||
| Finnegan et al. 32 | |||||||
| Cellina et al. 33 | |||||||
| Dominguez et al. 34 | |||||||
| Edler et al. 35 | |||||||
| Fernández‐Prada et al. 37 | |||||||
| Pfizer | Cohort study | 478 (24.5%) | 106 M | 19–95 | 65 1st | Riad et al. 25 | |
| 62 F | 235 2nd | Bernstine et al. 31 | |||||
| 310 NR | 178 NR | Eifer et al. 36 | |||||
| Moderna | Case reports and case series | 5 (NA) | 1 M | 35–68 | 5 1st | Mehta et al. 29 | |
| 4 F | Fernández‐Prada et al. 37 | ||||||
| Ulaner & Giuliano 38 | |||||||
| Washington et al. 39 | |||||||
| Johnson et al. 40 | |||||||
| Moderna | Cohort study | 18 (4.17%) | NR | 18–80 | 18 NR | Kadali et al. 41 | |
| Pfizer or Modernaa | Case reports and case series | 26 (NA) | 25 F/1 M | 28–70 | 1st: 21 | Mortazavi 42 | |
| 2nd: 4 | Ahn et al. 44 | ||||||
| NR: 1 | |||||||
| Pfizer or Modernaa | Cohort study | 98 (1.9%) | NR | 22–89 | 2nd: 7 | Ahamad et al. 43 | |
| NR: 91 | Geisen et al. 51 | ||||||
| AstraZeneca | Case reports and case series | 3 (NA) | 2 M/1 F | 70–76 | NR: 3 | Nawwar et al. 45 | |
| Nawwar et al. 46 | |||||||
| Nawwar et al. 47 | |||||||
| AstraZeneca | Cohort study | 14 (1%) | NR | 24.71–46.97 | 1st: 14 | Kim et al. 48 | |
| NR (which COVID‐19 vaccine) | Case reports and case series | 4 (NA) | 1 M/3 F | 47–71 | 1st: 1 | Johnson et al. 40 | |
| NR: 3 | Mitchell et al. 49 | ||||||
| Moghimi et al. 50 | |||||||
Abbreviations: COVID‐19, coronavirus disease 2019; F, female; M, male; NA, not applicable; NR, not reported.
Numbers are not separated in the study.
Figure 2.
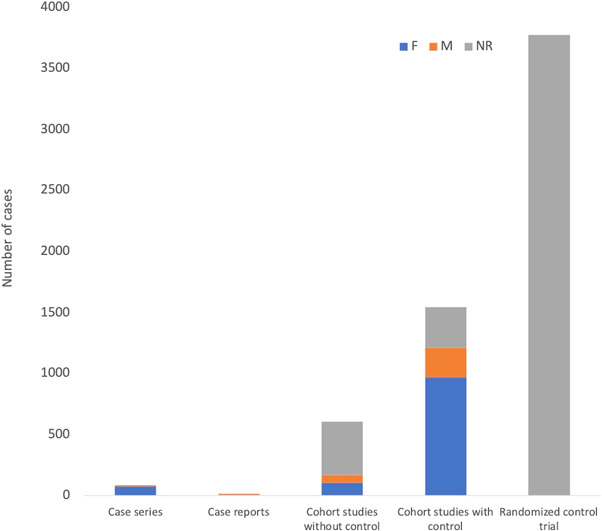
Total number of lymphadenopathy cases reported following any COVID‐19 vaccine and their genders separated based on study type. A total of 6022 cases were observed from the studies included. Out of the 83 lymphadenopathy events reported in the case series, 75 were females (90.4%). In cohort studies with controls, a total of 1544 (62.6% F, 15.8% M, 21.5% NR) individuals developed lymphadenopathy after taking the COVID‐19 vaccines. COVID‐19, coronavirus disease 2019; F, Female; M, Male; NR, not reported
Figure 3 presents the total lymphadenopathy cases observed after each vaccine type. Lymphadenopathy was observed in 932 cases after the Pfizer vaccine. The Moderna vaccine has the most cases (3733). 54 , 55 Only 5 cases were reported to develop lymphadenopathy after taking the Moderna vaccine in case reports and series. Furthermore, 17 cases of lymphadenopathy were seen after the AstraZeneca vaccine as reported by the included studies. Some of the studies did not report which specific vaccine was taken for each case. Three hundred seventy‐one cases of lymphadenopathy were reported in cohort studies in which participants were given either the Pfizer or Moderna vaccine. In the studies where the vaccine taken was either Moderna, Pfizer, or Janssen, lymphadenopathy developed in 965 patients. In 4 cases, no information regarding the vaccine type was reported.
Figure 3.
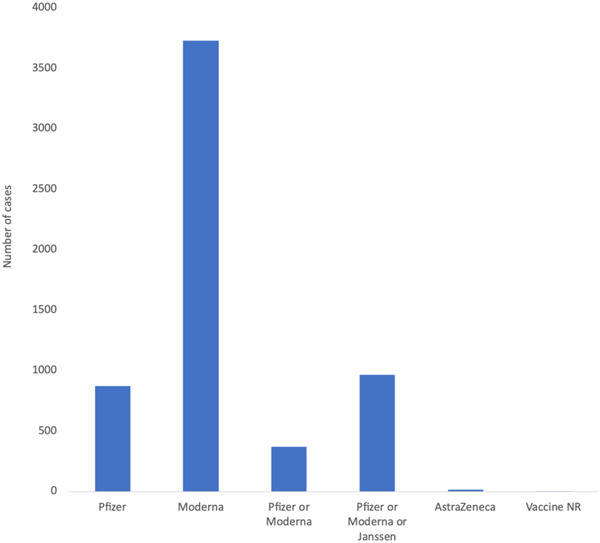
Number of lymphadenopathy cases reported following each type of COVID‐19 vaccine. The highest number of cases were reported after taking the Moderna vaccine as reported by the included studies including clinical trials. COVID‐19, coronavirus disease 2019
3.3. Rate of lymphadenopathy in the cohort studies and clinical trials
Tables 1 and 2 summarize the summed percentages of individuals who developed lymphadenopathy out of the total individuals in cohort studies without control, cohort studies with control, and RTCs. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57
Table 2.
Number of lymphadenopathy cases, demographics, and dose after which symptoms developed following taking each vaccine type as reported in the randomized control trials (RTCs) and cohort studies with control
| Type of vaccine | Type of study | Number of cases (%) | Gender | Age range | Which dose | References |
|---|---|---|---|---|---|---|
| Pfizer | RTC | 64 (0.3%) | NR | 16–91 | 1st | Polack et al. 52 |
| Pfizer | Cohort study with control | 332 (45.6%) | 315 M | 57.6–76.5 | 126 1st | Cohen et al. 53 |
| 413 F | 206 2nd | |||||
| Moderna | RTC | 3710 (23.8%) | 8062 M | 18–95 | 1581 1st | Baden et al. 54 |
| 7519 F | 2129 2nd | Chu et al. 55 | ||||
| Pfizer or Modernaa | Cohort study with control | 247 (0.8%) | 133 F | NR | 189 1st | Venkatakrishnan et al. 56 |
| 114 M | 58 2nd | |||||
| Pfizer or Modernaa | Cohort study with control | Units reported as cases/person daysb | NR | NR | 152 1st | McMurry et al. 57 |
| 1st shot: | 50 2nd | |||||
| −7 days post: 78/216 571 (0.36%) | ||||||
| −14 days post: 93/432 225 (0.22%) | ||||||
| −21 days post: 152/647 178 (0.23%) | ||||||
| 2nd shot: | ||||||
| − 7 days post: 34/118 741 (0.29%) | ||||||
| −14 days post: 33/237 349 (0.14%) | ||||||
| −21 days post: 50/355 769 (0.14%) | ||||||
| Pfizer or Moderna or Janssena | Cohort study with control | 965 (2.8%) | 131 M | NR | NR | Venkatakrishnan et al. 56 |
| 834 F |
Abbreviations: COVID‐19, coronavirus disease 2019; F, Female; M, Male; NR, not reported.
Numbers are not separated in the study. For the study by McMurry et al., the data were extracted from the preprint where numbers were not yet separated for Pfizer and Moderna.
Cases/person days: Number of lymphadenopathy cases over an estimate of the actual time‐at‐risk in days that all persons contributed to the study. The study only reported this value as it looked into ED notes at a specific time period rather than a specific cohort of people.
Summed data from 3 cohort studies without control showed a 24.5% incidence of lymphadenopathy in individuals taking the Pfizer vaccine (Table 1). 25 , 31 , 36 Figure 4 separates the studies based on the type of subjects as 3 cohort studies included only oncologic patients. Two of the 3 compiled cohort studies had only subjects with malignancy and the individual rate of lymphadenopathy in such studies was 45% and 25.8% as reported by Eifer et al. 36 and Brenstine et al. 31 respectively. Riad et al. 25 reported that 16.2% of the cohort with a normal population (not specifically oncologic) that received Pfizer vaccine developed lymphadenopathy (Figure 4A). Approximately 4.2% of the individuals who took the Moderna vaccine developed lymphadenopathy in one cohort study. 41 In two cohort studies in which participants took either the Pfizer or Moderna vaccine (unspecified), 1.9% of the individuals developed lymphadenopathy. 43 Additionally, in another cohort 1.0% of the individuals who took the AstraZeneca vaccine developed lymphadenopathy 48 (Figure 4A).
Figure 4.
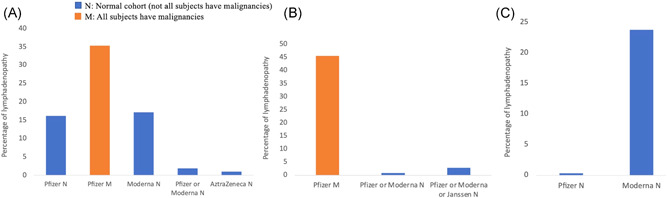
Percentage of individuals who developed lymphadenopathy post‐COVID‐19 vaccination in the cohort studies, and randomized controlled trials (RCTs) separated by vaccine type and the type of population. (A) Percentage of lymphadenopathy post‐COVID‐19 vaccination in the cohort studies without control. The 2 cohort studies that included only subjects with malignancies reported an average higher rates of lymphadenopathy (35.4%) postvaccination as compared to the normal cohort (16.2%). (B) Percentage of lymphadenopathy post‐COVID‐19 vaccination in the cohort studies with control. Only one cohort study with control reported lymphadenopathy following Pfizer vaccination. A high rate (45.6%) was reported by Cohen et al. 53 as compared to the other studies with control which could be attributed to the inclusion of only subjects with malignancies. (C) Percentage of lymphadenopathy post‐COVID‐19 vaccination in RCTs. The Pfizer RCT reported a rate of 0.3% while 23.8% of the participants developed lymphadenopathy in the Moderna RCT. COVID‐19, coronavirus disease 2019
In the cohort studies with control groups, lymphadenopathy was seen in 45.6%, 0.8%, and 2.8% of the participants who took the Pfizer vaccine, Pfizer or Moderna vaccine (unspecified), and Pfizer or Moderna or Janssen vaccine (unspecified), respectively. Important to note that the study reporting a 45.6% lymphadenopathy recruited subjects who had a known malignancy and used a full‐body fluorodeoxyglucose (FDG)‐positron emission tomography (PET)‐computerized tomography (CT) to assess for lymphadenopathy (Figure 4B). 53 The Pfizer RCT revealed a 0.3% development of lymphadenopathy in 18 860 participants while 23.8% of the participants developed lymphadenopathy in the Moderna RCT. Table 2 reports the findings from cohort studies with control and RCTs (Figure 4C).
The overall rate of lymphadenopathy in all the cohort studies with and without controls including the clinical trials is 13.51%.
3.4. Lymphadenopathy following COVID‐19 vaccination in patients with malignancies
Out of the 6022 cases of lymphadenopathy that have been in this review, 693 had confirmed malignancies. The majority of the cases especially those reported by the population studies were not specified for the presence or absence of malignancies (Figure 5). Only 191 lymphadenopathy cases were reported not to have any malignancies out of which 18 underwent diagnostic tests such as CT, FDG‐PET scan, magnetic resonance imaging (MRI), ultrasound (US), and/or fine‐needle aspiration (FNA) biopsy (TableS3). Furthermore, all the 693 lymphadenopathy cases with malignancies were shown to have positive results on FDG‐PET or other PET‐CT tracers.
Figure 5.
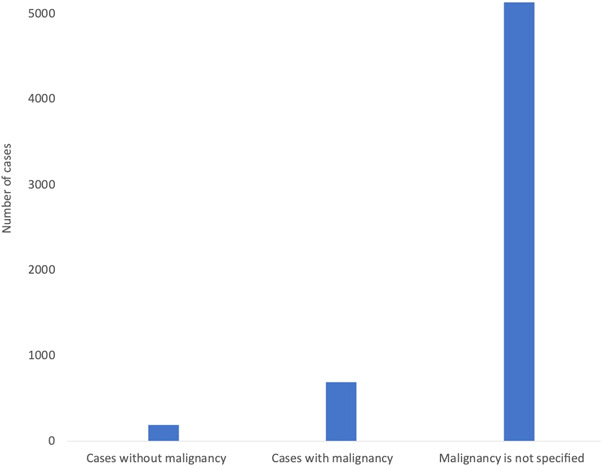
Number of lymphadenopathy cases reported following COVID‐19 vaccination separated based on the presence or absence of malignancy as reported by the 37 included studies. Out of the 191 reported cases without malignancy, 18 underwent different diagnostic tests to assess the condition including FDG‐PET‐CT, MRI, US, and/or FNA biopsy. Malignancy was reported for 693 cases who all got positive FDG‐PET‐CT or other PET‐CT tracer results. Out of the 6022 reported lymphadenopathy cases in this review, malignancy and/or diagnostic tests were not specified for 5138 cases. COVID‐19, coronavirus disease 2019; CT, computerized tomography; FDG‐PET, fluorodeoxyglucose‐positron emission tomography; FNA, fine‐needle aspiration; MRI, magnetic resonance imaging; US, ultrasound.
4. DISCUSSION
Lymphadenopathy as a side effect of vaccination is neither a new phenomenon nor isolated to COVID‐19 vaccines. This presentation is considered common with the human papillomavirus vaccine, as well as H1N1 vaccinations. 53 , 58 , 59 Of those, lymphadenopathy was seen in the ipsilateral lymph nodes; specifically in axillary, supraclavicular, and infraclavicular lymph nodes. 21
4.1. How may vaccines cause lymphadenopathy?
The exact mechanism of how the COVID‐19 vaccines may cause lymphadenopathy is still not clear. It is hypothesized that the increased immune response following vaccination causes a localized inflammatory response in the area surrounding the vaccination site. Immune cells in the nearby lymph nodes may proliferate as they become exposed to the vaccine antigen. This hyperplasia in response to the vaccine may cause lymphadenopathy to develop. Lymphadenopathy reactions have been reported after several other vaccines including measles, anthrax, smallpox, H1N1 and seasonal influenza, Bacille Calmette‐Guerin, and human papillomavirus vaccines. 59 , 60 , 61 , 62 , 63 , 64 , 65 Lymphadenopathy seems to be a reaction common to most vaccines rather than specifically to those of COVID‐19. Hence, the mechanism in which lymphadenopathy occurs may be similar in all vaccine types.
4.2. Lymphadenopathy following COVID‐19 vaccination
Our results signify that lymphadenopathy may occur following COVID‐19 vaccination. A total of 6022 individuals developed lymphadenopathy post‐COVID‐19 vaccination as reported by different types of studies including 24 case reports/series, 10 cohort studies, and 2 RCTs. Although some of the studies reported higher rates of lymphadenopathy in the COVID‐19 vaccinated groups compared to the control groups, only Cohen et al., 53 established a significant association between Pfizer vaccination and lymphadenopathy (45.6%) compared with the unvaccinated group (7.6%) (p value <0.01). Furthermore, the rate of lymphadenopathy was significantly higher after the second dose as compared to the first dose. However, this cohort is not representative of the entire population as only patients with malignancies who may be more susceptible were included. Baden et al., 54 Polack et al., 52 and Chu et al. 55 reported a higher incidence of lymphadenopathy post‐COVID‐19 vaccine in comparison to placebo. However, the association has not been statically determined. 52 , 54 , 55 Surprisingly, McMurry et al., 57 reported a significantly lower rate of lymphadenopathy in the vaccinated group as compared to the nonvaccinated control group. This contradicts the findings of increased lymphadenopathy observed after vaccination in most other studies. This might be attributed to the selection process of the participants as anyone who visited the emergency department (ED) was used as a subject and was classified as either vaccinated or unvaccinated. It was, therefore, suggested that those vaccinated may have been warned about the side effects and were less likely to present to the ED. Relatively high rates of lymphadenopathy were reported in 3 cohort studies 45.6% (Cohen et al.), 53 45% (Eifer et al.), 36 and 25.8% (Bernsite et al.). 31 This could be attributed to the nature of the cohorts which had only subjects with malignancies. The overall rate of lymphadenopathy in the included cohort studies and RTCs is 13.15%. However, this overall rate is impacted by the high lymphadenopathy rate in the cohorts with oncologic subjects. The average rate of lymphadenopathy in the 3 studies with only oncologic patients is 38.8% which is higher than the average rate in the normal cohorts (6.65%).
4.3. Was lymphadenopathy reported following COVID‐19 vaccination during the clinical trials?
It is common that some side effects do not become apparent until after the drug or vaccine is released to the public. Looking into the COVID‐19 safety clinical trials, lymphadenopathy was reported as a side effect after taking the Moderna or Pfizer vaccines. The RCT reported by Chu et al. 55 showed the development of lymphadenopathy in 11% of the participants after the first dose of the Moderna vaccine and 9% after the second dose in the 18–55‐year‐old cohort. In the control group who had received saline instead, 4% developed lymphadenopathy after the first dose and 1% did after the second dose. Similarly, Baden et al. 54 reported lymphadenopathy in 10.2% of the Moderna vaccinated group after the 1st dose and in 14.2% after the 2nd dose as compared to 4.8% after the 1st dose and 3.9% after the 2nd dose in the control group. 54 Such rates are higher as compared to the rates of lymphadenopathy following Pfizer vaccination as reported by the safety trials.
In the Pfizer vaccine safety trials, only 64 vaccine recipients (0.3%) as compared to 6 placebo recipients (<0.1%) reported lymphadenopathy. 52 , 54 While there were reports of lymphadenopathy in the clinical trials, they were few specifically in the Pfizer ones. The AstraZeneca vaccine phase 3 clinical trials did not report any cases of lymphadenopathy. 18 Similarly, no cases of lymphadenopathy were reported in the Janssen safety trial. 66 Interestingly, however, some cases were described in case reports and cohort studies after each of these vaccines as well. 45 , 46 , 47 , 48 , 56 The unprecedented circumstances and emergent need for COVID‐19 vaccines have led to fast approvals for widespread use. Although extensive and thorough clinical trials have been carried out, due to the rapid turnover, the emergence of some unexpected side effects that were not reported or underreported during the clinical trials may occur.
The Janssen and AstraZeneca COVID‐19 vaccines, however, did not report any case of lymphadenopathy in their phase 3 trials. 18 , 66 It is unclear if the absence of any cases is due to none occurring or if participants were not examined for lymphadenopathy. Nevertheless, 14 cases in a cohort study and 3 case reports described lymphadenopathy occurring after the AstraZeneca vaccine. 45 , 46 , 47 , 48
4.4. Lymphadenopathy in patients with malignancies post‐COVID‐19 vaccination
Hypermetabolic lymphadenopathy describes an abnormal lymph node that is metabolizing at an increased rate, which is demonstrated using an FDG‐PET‐CT scan. FDG‐PET‐CT scan is a medical imaging tool that uses radiotracers to detect metabolically active lesions within the body. Whole‐body FDG‐PET‐CT is a standard practice to examine cancer patients to evaluate the progress of the disease. However, FDG uptake can be also detected in inflammatory and infectious lesions which can also be caused by vaccination. 31 Our included case reports and case series revealed the emergence of much post‐COVID‐19 vaccination lymphadenopathy especially in patients undergoing follow‐up FDG‐PET‐CT where lymph nodes ipsilateral to the vaccine injection site show increased uptake of FDG. 21 , 23 , 28 For example, Smith and Yang reported a case of benign hypermetabolic axillary lymph nodes following Pfizer/BioNTech vaccination near the injection site, most likely due to a vaccine‐elicited immune response. 26 Other resources refer to vaccine‐related lymphadenopathy to be associated with sonographic and clinical features, such as being more common in oncological patients who may be experiencing an impact on the accuracy of their diagnostic tests. 22 Out of the 6022 reported cases of lymphadenopathy in our included studies, 693 had confirmed malignancies as reported by several case reports/series or cohort studies with or without control. All subjects in the studies conducted by Cohen et al., 53 Eifer et al., 36 and Bernstine et al., 31 had known malignancies and were assessed for lymphadenopathy using FDG‐PET‐CT or other PET‐CT tracers. The studies reported relatively high rates of FDG‐PET‐CT positive results within the cohorts. It was observed that cancer patients started to undergo FDG‐PET‐CT hypermetabolic axillary lymph nodes and a focal hypermetabolic region in the ipsilateral deltoid muscle following Pfizer vaccination especially after the second dose in the cohort study conducted by Bernstine et al. 31 Similarly, Eifer et al. 36 reported that a high proportion of cancer patients showed ipsilateral lymph node axillary uptake following Pfizer vaccination. Unlike the studies conducted by Bernstine et al. 31 and Eifer et al. 36 which did not include control groups, Cohen et al., 53 conducted a cohort study that recruited vaccinated cancer patients and compared them with a control unvaccinated group. Statistical analysis revealed that the rate of occurrence of lymph nodes with benign metabolic hyperactivity was significantly higher in the Pfizer vaccinated group as compared to the control group. Furthermore, the rate was also significantly higher after the second dose as compared to the first dose. The same study reported that it was not always possible to differentiate between the benign and malignant nodal involvement especially when the vaccine was administrated on the same side as the tumor expected nodal drainage. The study, therefore, recommended that patients with breast cancer, axillary lymphoma, and malignancy of the upper limb should not be vaccinated in the arm next to the tumor expected nodal drainage. 53 Furthermore, 18 cases who developed lymphadenopathy following COVID‐19 vaccination conducted different tests such as FDG‐PET‐CT scan, MRI, US, and/or FNA biopsy without having any malignancies. 21 , 22 , 28 , 33 , 34 , 37 , 49 In a recent study, Placke et al., 67 reported 8 patients (with melanoma or Merkel cell carcinoma) who were misdiagnosed with lymph nodes metastases and underwent lymph node excision following COVID‐19 vaccination. 68 Therefore, care must be taken before suspecting lymph node metastasis or deciding for lymphadenectomy following COVID‐19 vaccination. Awareness of the incidence of lymphadenopathy post‐COVID‐19 vaccinations will help guide radiologists and oncologists in their management of patients, both present oncologic patients, and patients with concerns over their newly presenting lymphadenopathy. 27
4.5. What is the outcome of the lymphadenopathy post‐COVID‐19 vaccination?
The studies reported the spontaneous resolution of lymphadenopathy post‐COVID‐19 vaccination. The reported duration until complete resolution varied among studies. Some studies reported a maximum duration of 10 days, with most resolutions occurring around the second day of symptoms. 25 , 52 , 54 , 55 However, durations of up to 32 days with a resolution still ongoing have been reported by some case reports and case series. 21 , 37
4.6. Are females more affected?
One cross‐sectional survey‐based study found that lymphadenopathy as a side effect of the COVID‐19 vaccine had a higher frequency among females in comparison to males. 25 However 88% of the subjects in this study were females, and thus the study population may not have had a wider scope on the male subjects. Although noting the prevalence of this side effect on females versus males was not the main objective for many of the included studies, one cohort study focused on investigating the female to male differences in adverse effects of the COVID‐19 vaccine. 56 It was reported that females were more likely to experience a wider range of adverse effects than males such as nausea, fever, and vomiting. The difference was explained by the enhanced immune reactogenicity in females as shown by reviews of vaccine‐induced hormonal immunity. This enhanced reaction results in more immunity to infectious diseases but also in a higher rate of adverse effects. 68 It was suggested that the interaction between the flu vaccine and estrogen may boost immunity which may apply to COVID‐19 vaccines. 69 However, the same study reported that lymphadenopathy was more common in males than in females. 56 Therefore, further investigations are required to determine whether lymphadenopathy post‐COVID‐19 vaccination has a higher prevalence in either sex.
5. CONCLUSION AND RECOMMENDATIONS
Our results revealed that lymphadenopathy following COVID‐19 vaccinations may be occurring more often than previously thought. However, the majority of cases have been benign with no major adverse effects occurring as a result of the lymphadenopathy. Therefore, it is important to recognize that postvaccination lymphadenopathy may not pose significant harm to the vaccinated individuals and is not a reason to withhold vaccinations. However, lymph node enlargements following COVID‐19 vaccination is expected to be increasingly observed in the near future especially those that could be suspicious for malignancy during follow‐up of tumor patients with imaging techniques. 67 It is, therefore, especially important to consider postvaccination lymphadenopathy in patients who undergo regular tests such as FDG‐PET‐CT or MRI as results may be misinterpreted. Clinicians must be aware of such possible transient detection of hypermetabolic regional lymph nodes following COVID‐19 vaccination. 70 Several authors of the included studies recommend that vaccination information must be included in the medical history of patients who are being imaged. 39 , 40 Patients are encouraged to always communicate their vaccination history to their oncologist, radiologist, and other medical staff treating them. 67 Other recommendations specific to patients with any kind of malignancy include taking the vaccine shots on the arm contralateral from the limb with expected lymphatic drainage of the malignancy if possible. 53 This may help minimize the need for repeated imaging and more invasive procedures such as biopsies due to inconclusive scans. It is encouraged that imaging, such as mammography, should be carried out before or 4–12 weeks following vaccination in line with the Society of Breast Imaging's recommendations. 71 It has been noted that the lymphadenopathy may last for over 5 weeks after taking the vaccine. 28 With that being said, it is important that patients get assessed by a doctor if they develop any lymphadenopathy after taking the COVID‐19 vaccine especially if worrying features are present such as prolonged course, widespread lymphadenopathy, and/or signs of infection.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors whose names appear on the submission made substantial contributions to the conception or design of the work, screening of the studies, data extraction, and/or drafting the manuscript. All authors critically reviewed the manuscript and approved the version to be published.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGEMENTS
We would like to thank Weill Cornell Medicine‐Qatar for its continuous support.
Bshesh K, Khan W, Vattoth AL, et al. Lymphadenopathy post‐COVID‐19 vaccination with increased FDG uptake may be falsely attributed to oncological disorders: a systematic review. J Med Virol. 2022;94:1833‐1845. 10.1002/jmv.27599
Khalifa Bshesh, Wafa Khan, and Ahamed Lazim Vattoth equally contributed to this work as co‐first authors.
Emmad Janjua, Areej Nauman, Muna Almasri, Ateeque Mohamed Ali, Vinutha Ramadorai, Beshr Mushannen, Mai Al‐Subaie, Ibrahim Mohammed, Mais Hammoud, Pradipta Paul, Haya Alkaabi, and Aliyaa Haji equally contributed to this work.
[Correction added on 25 February 2022, after first online publication: Table 2 footnote and reference McMurry et al. have been updated.]
Contributor Information
Pradipta Paul, Email: prp4005@qatar-med.cornell.edu.
Dalia Zakaria, Email: dez2003@qatar-med.cornell.edu.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. World Health Organization . Wuhan 2019 Novel Coronavirus—2019‐nCoV. Mater Methods. 2020;10(January):1‐5. 10.13070/mm.en.10.2867 [DOI] [Google Scholar]
- 2. World Health Organization . “Public Health Emergency of International Concern (PHEIC),”. WHO; 2020:1‐10. [Google Scholar]
- 3. El‐Sahly HM, Atmar RL, Glezen WP, Greenberg SB. Spectrum of clinical illness in hospitalized patients with ‘common cold’ virus infections. Clin Infect Dis. 2000;31(1):96‐100. 10.1086/313937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo Y‐R, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—an update on the status. Mil Med Res. 2020;7(1):1‐10. 10.1186/S40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization , “Coronavirus disease (COVID‐19) Situation Report‐141 Situation in numbers (by WHO Region),” 2020.
- 7. Food US, Administration Drug, “Pfizer‐BioNTech COVID‐19 Vaccine FDA.” https://www.fda.gov/emergency‐preparedness‐and‐response/coronavirus‐disease‐2019‐covid‐19/pfizer‐biontech‐covid‐19‐vaccine/ (accessed Jul. 26, 2021).
- 8. Chung JY, Thone MN, Kwon YJ. COVID‐19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1‐25. 10.1016/J.ADDR.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. European Medicines Agency , “EMA recommends first COVID‐19 vaccine for authorisation in the EU European Medicines Agency.” https://www.ema.europa.eu/en/news/ema‐recommends‐first‐covid‐19‐vaccine‐authorisation‐eu (accessed Jul. 26, 2021).
- 10. Moderna , “Moderna Announces FDA Authorization of Moderna COVID‐19 Vaccine in U.S. Moderna, Inc.” https://investors.modernatx.com/news‐releases/news‐release‐details/moderna‐announces‐fda‐authorization‐moderna‐covid‐19‐vaccine‐us/ (accessed Jul. 26, 2021).
- 11. World Health Organization , “The Oxford/AstraZeneca COVID‐19 vaccine: what you need to know.” https://www.who.int/news‐room/feature‐stories/detail/the‐oxford‐astrazeneca‐covid‐19‐vaccine‐what‐you‐need‐to‐know?gclid=CjwKCAjwwqaGBhBKEiwAMk‐FtGgd2hPGq6‐b4dXWoqVVymWpnzub9EonfFgtM3jGIrfe83zQRjL7YhoCLLQQAvD_BwE (accessed Jul. 26, 2021).
- 12. AstraZeneca , “AstraZeneca's COVID‐19 vaccine authorised for emergency supply in the UK,” Dec. 30, 2020. https://www.astrazeneca.com/media‐centre/press‐releases/2020/astrazenecas‐covid‐19‐vaccine‐authorised‐in‐uk.html (accessed Jul. 26, 2021).
- 13. European Medicine Agency , “EMA recommends COVID‐19 Vaccine AstraZeneca for authorisation in the EU European Medicines Agency.” https://www.ema.europa.eu/en/news/ema‐recommends‐covid‐19‐vaccine‐astrazeneca‐authorisation‐eu (accessed Jul. 26, 2021).
- 14. Janssen , “Fact sheet for recipients and caregivers emergency use authorization (EUA) of the janssen COVID‐19 vaccine to prevent coronavirus disease 2019 (COVID‐19) in individuals 18 years of age and older,” 2021, Accessed: Jul. 26, 2021. [Online]. Available: www.janssencovid19vaccine.com.
- 15. World Health Organization . 2021. https://www.who.int/news‐room/feature‐stories/detail/the‐j‐j‐covid‐19‐vaccine‐what‐you‐need‐to‐know?gclid=CjwKCAjwwqaGBhBKEiwAMk‐FtEZPx8YGtVyK_N5ODMftfVT28PLw‐275t4or2w7WcQkxmafG8zD8CBoCpu4QAvD_BwE
- 16. Holder J, “Covid World Vaccination Tracker ‐ The New York Times.” https://www.nytimes.com/interactive/2021/world/covid‐vaccinations‐tracker.html (accessed Jul. 26, 2021).
- 17. Mathieu E, et al., “A global database of COVID‐19 vaccinations,” Nat. Hum. Behav., 2021, 10.1038/S41562-021-01122-8. [DOI] [PubMed]
- 18. Voysey M, Clemens S, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK,”. Lancet. 2021;397(10269):99‐111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. U.S. Food and Drug Administration , “CBER Surveillance Program Background Rates of Adverse Events of Special Interest for COVID‐19 Vaccine Safety Monitoring Protocol,” 2021, Accessed: Jul. 27, 2021. [Online]. Available: https://www.bestinitiative.org/wp‐.
- 20. Tu W, Gierada DS, Joe BN. COVID‐19 vaccination‐related lymphadenopathy: what to be aware of. Radiol Imaging Cancer, 3(3):e210038. 10.1148/RYCAN.2021210038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiller N, Goldberg SN, Cohen‐Cymberknoh M, Vainstein V, Simanovsky N. Lymphadenopathy associated with the COVID‐19 vaccine. Cureus. 2021;13(2):13524. 10.7759/CUREUS.13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID‐19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology, 300:E296‐E300. 10.1148/RADIOL.2021210275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu G, Lu Y. COVID‐19 mRNA vaccination‐induced lymphadenopathy mimics lymphoma progression on FDG PET/CT. Clin Nucl Med. 2021;46(4):353‐354. 10.1097/RLU.0000000000003597 [DOI] [PubMed] [Google Scholar]
- 24. Lu Y, “DOTATATE ‐Avid Bilateral Axilla and Subpectoral Lymphadenopathy Induced From COVID‐19 mRNA Vaccination Visualized on PET/CT,” Clin. Nucl. Med., 2021, [Online]. Available: https://journals.lww.com/nuclearmed/Fulltext/9000/DOTATATE__Avid_Bilateral_Axilla_and_Subpectoral.96112.aspx. [DOI] [PMC free article] [PubMed]
- 25. Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID‐19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10(7):1428. 10.3390/JCM10071428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith MV, Yang M. Reactive axillary lymphadenopathy to COVID‐19 vaccination on 18 F‐FDG PET/CT. J Nucl Med Technol, 49(3):286‐287. 10.2967/JNMT.121.262008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Granata V, Fusco R, Setola SV, et al. Lymphadenopathy after BNT162b2 Covid‐19 Vaccine: preliminary ultrasound findings. Biol. 2021;10(3):214. 10.3390/BIOLOGY10030214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanneman K, Iwanochko RM, Thavendiranathan P. Evolution of lymphadenopathy at PET/MRI after COVID‐19 vaccination. Radiology, 299(3):E282. 10.1148/RADIOL.2021210386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehta N, Sales RM, Babagbemi K, et al. Unilateral axillary adenopathy in the setting of COVID‐19 vaccine. Clin Imaging. 2021;75:12‐15. 10.1016/J.CLINIMAG.2021.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Avner M, Orevi M, Caplan N, Popovtzer A, Lotem M, Cohen JE. COVID‐19 vaccine as a cause for unilateral lymphadenopathy detected by 18F‐FDG PET/CT in a patient affected by melanoma. Eur J Nucl Med Mol Imaging. 2021;48(8):2659‐2660. 10.1007/S00259-021-05278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernstine H, Priss M, Anati T, et al. Axillary lymph nodes hypermetabolism after BNT162b2 mRNA COVID‐19 vaccination in cancer patients undergoing 18F‐FDG PET/CT: a cohort study. Clin Nucl Med. 2021;46(5):396‐401. 10.1097/RLU.0000000000003648 [DOI] [PubMed] [Google Scholar]
- 32. Finnegan J, Govender P, Torreggiani WC. Re: unilateral axillary adenopathy in the setting of COVID‐19 vaccine, clinical imaging, January 2021. Clin Imaging. 2021;75:171‐172. 10.1016/J.CLINIMAG.2021.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cellina M, Irmici G, Carrafiello G. Unilateral Axillary Lymphadenopathy After Coronavirus Disease (COVID‐19) Vaccination. AJR Am J Roentgenol. 2021;216(5):W27. 10.2214/AJR.21.25683 [DOI] [PubMed] [Google Scholar]
- 34. Dominguez JL, Eberhardt SC, Revels JW. Unilateral axillary lymphadenopathy following COVID‐19 vaccination: a case report and imaging findings. Radiol. Case Reports. 2021;16(7):1660‐1664. 10.1016/J.RADCR.2021.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edler C, Klein A, Schröder AS, Sperhake JP, Ondruschka B. Deaths associated with newly launched SARS‐CoV‐2 vaccination (Comirnaty®). Leg Med. 2021;51:101895. 10.1016/J.LEGALMED.2021.101895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eifer M, Tau N, Alhoubani Y, et al. Covid‐19 mRNA vaccination: age and immune status and its association with axillary lymph node PET/CT uptake. J Nucl Med. 2021;63(1):134‐139. 10.2967/JNUMED.121.262194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernández‐Prada M, Rivero‐Calle I, Calvache‐González A, Martinón‐Torres F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID‐19 may be related to vaccine injection technique, Spain, January and February 2021. Euro Surveill. 2021;26(10):2100193. 10.2807/1560-7917.ES.2021.26.10.2100193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ulaner GA, Giuliano P. 18F‐FDG‐avid lymph nodes after COVID‐19 vaccination on 18F‐FDG PET/CT. Clin Nucl Med. 2021;46(5):433‐434. 10.1097/RLU.0000000000003633 [DOI] [PubMed] [Google Scholar]
- 39. Washington T, Bryan R, Clemow C. Adenopathy following COVID‐19 vaccination. Reviews and Commentary Images in Radiology, 299(3):E280‐E281. 10.1148/RADIOL.2021210236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson BJ, Van Abel K, Ma D, Johnson DR. FDG avid axillary lymph nodes after COVID‐19 vaccination. J Nucl Med. 2021; 62(10):1483–1484. 10.2967/JNUMED.121.262108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kadali R, Janagama R, Peruru S, et al. Non‐life‐threatening adverse effects with COVID‐19 mRNA‐1273 vaccine: A randomized, cross‐sectional study on healthcare workers with detailed self‐reported symptoms. J Med Virol. 2021;93(7):4420‐4429. 10.1002/JMV.26996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mortazavi S, “Coronavirus Disease (COVID‐19) Vaccination Associated Axillary Adenopathy: Imaging Findings and Follow‐Up Recommendations in 23 Women,” Feb. 2021, 10.2214/AJR.21.25651 [DOI] [PubMed]
- 43. Ahamad MM, Aktar S, Uddin MJ, et al. Adverse effects of COVID‐19 vaccination: machine learning and statistical approach to identify and classify incidences of morbidity and post‐vaccination reactogenicity. medRxiv. 2021:2021.04.16.21255618. 10.1101/2021.04.16.21255618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahn RW, Mootz AR, Brewington CC, Abbara S. Axillary lymphadenopathy after mRNA COVID‐19 vaccination. Radiol Cardiothoracic Imaging. 2021;3(1):210008. 10.1148/RYCT.2021210008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nawwar AA, Searle J, Hagan I, Lyburn ID. COVID‐19 vaccination induced axillary nodal uptake on [18F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(8):2655‐2656. 10.1007/S00259-021-05274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nawwar AA, Searle J, Hopkins R, Lyburn ID, “False‐Positive Axillary Lymph Nodes on FDG PET/CT Resulting From COVID‐19 Immunization,” Clin. Nucl. Med., 2021, [Online]. Available: https://journals.lww.com/nuclearmed/Fulltext/9000/False_Positive_Axillary_Lymph_Nodes_on_FDG_PET_CT.96078.aspx. [DOI] [PMC free article] [PubMed]
- 47. Nawwar AA, Searle J, Singh R, Lyburn ID. Oxford‐AstraZeneca COVID‐19 vaccination induced lymphadenopathy on [18F]Choline PET/CT—not only an FDG finding. Eur J Nucl Med Mol. 2021;48(8):2657‐2658. 10.1007/S00259-021-05279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim S‐H, Wi YM, Yun SY, et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV‐19 or BNT162b2 mRNA COVID‐19 vaccination: a single center experience. J Korean Med Sci. 2021;36(14):1‐8. 10.3346/JKMS.2021.36.E107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mitchell OR, Dave R, Bekker J, Brennan PA. Supraclavicular lymphadenopathy following COVID‐19 vaccination: an increasing presentation to the two‐week wait neck lump clinic? Br J Oral Maxillofac Surg. 2021;59(3):384‐385. 10.1016/J.BJOMS.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moghimi S, Wilson D, Martineau P. FDG PET findings post‐COVID vaccinations: signs of the times? Clin Nucl Med. 2021;46(5):437‐438. 10.1097/RLU.0000000000003636 [DOI] [PubMed] [Google Scholar]
- 51. Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti‐SARS‐CoV‐2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80(10):1306‐1311. 10.1136/ANNRHEUMDIS-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19vaccine. N Engl J Med. 2020;383:2603‐2615. 10.1056/NEJMOA2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cohen D, Krauthammer SH, Wolf I, Even‐Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid‐19 vaccine: incidence assessed by [18F]FDG PET‐CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48(no. 6):1854‐1863. 10.1007/S00259-021-05314-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2020;384:403‐416. 10.1056/NEJMOA2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA‐1273 SARS‐CoV‐2 vaccine. Vaccine. 2021;39(no. 20):2791‐2799. 10.1016/J.VACCINE.2021.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Venkatakrishnan A, Kumar‐M P, Silvert E, et al. Female‐male differences in COVID vaccine adverse events have precedence in seasonal flu shots: a potential link to sex‐associated baseline gene expression patterns. medRxiv. 2021:2021.04.01.21254798. 10.1101/2021.04.01.21254798 [DOI] [Google Scholar]
- 57. McMurry R, Lenehan P, Awasthi S, et al. Real‐time analysis of a mass vaccination effort confirms the safety of FDA‐authorized mRNA COVID‐19 vaccines. Med (N Y). 2021. Aug 13;2(8):965‐978.e5. 10.1016/j.medj.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Coates EE, Costner PJ, Nason MC, et al. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papillomaviruses. Clin Nucl Med. 2017;42(no. 5):329‐334. 10.1097/RLU.0000000000001603 [DOI] [PubMed] [Google Scholar]
- 59. Burger IA, Husmann L, Hany TF, Schmid DT, Schaefer NG. “Incidence and intensity of F‐18 FDG uptake after vaccination with H1N1 vaccine,”. Clin Nucl Med. 2011;36(no. 10):848‐853. 10.1097/RLU.0B013E3182177322 [DOI] [PubMed] [Google Scholar]
- 60. Dorfman RF, Herweg JC. Live, attenuated measles virus vaccine: inguinal lymphadenopathy complicating administration. JAMA. 1966;198(no. 3):320‐321. 10.1001/JAMA.1966.03110160148051 [DOI] [PubMed] [Google Scholar]
- 61. Pittman PR, Gibbs PH, Cannon TL, Friedlander AM. Anthrax vaccine: short‐term safety experience in humans. VaccineDec 2001;20(no. 5–6):972‐978. 10.1016/S0264-410X(01)00387-5 [DOI] [PubMed] [Google Scholar]
- 62. Casey CG, Iskander JK, Roper MH, et al. adverse events associated with smallpox vaccination in the United States, January‐October 2003. JAMA. 2005;294(no. 21):2734‐2743. 10.1001/JAMA.294.21.2734 [DOI] [PubMed] [Google Scholar]
- 63. Thomassen A, Nielsen AL, Gerke O, Johansen A, Petersen H. Duration of 18F‐FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38(5):894‐898. 10.1007/S00259-011-1729-9 [DOI] [PubMed] [Google Scholar]
- 64. Pereira MP, Flores P, Neto AS. Neck and supraclavicular lymphadenopathy secondary to 9‐valent human papillomavirus vaccination. BMJ Case Rep. 2019;12(no. 11). 10.1136/BCR-2019-231582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marais BJ, Wright CA, Schaaf HS, et al. Tuberculous lymphadenitis as a cause of persistent cervical lymphadenopathy in children from a tuberculosis‐endemic area, Pediatr Infect Dis J. 25, (no. 2), 2006:142‐146. 10.1097/01.INF.0000199259.04970.D1 [DOI] [PubMed] [Google Scholar]
- 66. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384(23):2187‐2201. 10.1056/NEJMOA2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Placke JM, Reis H, Hadaschik E, et al. Coronavirus disease 2019 vaccine mimics lymph node metastases in patients undergoing skin cancer follow‐up: a monocentre study. Eur J Cancer. 2021;154:167‐174. 10.1016/J.EJCA.2021.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine‐induced humoral immunity. Semin Immunopathol. 2018;41(no. 2):239‐249. 10.1007/S00281-018-0726-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26(no. 29–30):3551‐3555. 10.1016/J.VACCINE.2008.04.054 [DOI] [PubMed] [Google Scholar]
- 70. Prieto PA, Mannava K, Sahasrabudhe DM, Prieto A, Sahasrabudhe DM. COVID‐19 mRNA vaccine‐related adenopathy mimicking metastatic melanoma A B. Lancet Oncol. 2021;22:281. 10.1016/S1470-2045(21)00197-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grimm L, et al., “SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID‐19 Vaccination Society of Breast Imaging Patient Care and Delivery Committee,” Accessed: Jul. 26, 2021. [Online]. Available: https://www.cdc.gov/vaccines/covid‐19/info‐by
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


