Abstract
Red blood cell distribution width (RDW) was frequently assessed in COVID‐19 infection and reported to be associated with adverse outcomes. However, there was no consensus regarding the optimal cutoff value for RDW. Records of 98 patients with COVID‐19 from the First People's Hospital of Jingzhou were reviewed. They were divided into two groups according to the cutoff value for RDW on admission by receiver operator characteristic curve analysis: ≤11.5% (n = 50) and >11.5% (n = 48). The association of RDW with the severity and outcomes of COVID‐19 was analyzed. The receiver operating characteristic curve indicated that the RDW was a good discrimination factor for identifying COVID‐19 severity (area under the curve = 0.728, 95% CI: 0.626–0.830, p < 0.001). Patients with RDW > 11.5% more frequently suffered from critical COVID‐19 than those with RDW ≤ 11.5% (62.5% vs. 26.0%, p < 0.001). Multivariate logistic regression analysis showed RDW to be an independent predictor for critical illness due to COVID‐19 (OR = 2.40, 95% CI: 1.27−4.55, p = 0.007). A similar result was obtained when we included RDW > 11.5% into another model instead of RDW as a continuous variable (OR = 5.41, 95% CI: 1.53−19.10, p = 0.009). RDW, as an inexpensive and routinely measured parameter, showed promise as a predictor for critical illness in patients with COVID‐19 infection. RDW > 11.5% could be the optimal cutoff to discriminate critical COVID‐19 infection.
Keywords: COVID‐19, infection, red blood cell distribution width, respiratory tract
Highlights
-
•
RDW is an inexpensive and routinely measured parameter that was independently associated with the disease severity of COVID‐19 infection.
-
•
RDW > 11.5% could be the optimal cutoff to discriminate critical COVID‐19 infection and might be helpful in clinical practice to identify critical cases at an early stage.
-
•
Future studies should focus on elucidating the underlying mechanism of the association between RDW and the severity of illness in COVID 19 infection.
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- NO
nitrate oxide
- RAAS
renin−angiotensin−aldosterone system
- RBC
red blood cell
- RDW
red blood cell distribution width
- ROC
receiver operator characteristic
- RT‐PCR
reverse‐transcriptase polymerase chain reaction
- SARS‐COV2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2) causes coronavirus disease 2019 (COVID‐19), a pandemic that has affected more than 4 500 000 individuals and caused nearly 300 000 deaths worldwide as of mid‐May 2020. It has been reported that 14% of patients with COVID‐19 had severe disease, of which 5% progressed to critically ill with multiorgan failure, and the mortality rate in the critically ill group was 49%. 1 Moreover, the duration from the development of initial symptoms to the onset of critical illness is 10 days. 2 The delayed onset of critical illness in patients with COVID‐19 and high mortality rate among them emphasize the importance of identifying an early predictive biomarker.
Red blood cell distribution width (RDW) is a routinely measured laboratory parameter that reflects the variation of red blood cell (RBC) volume. 3 RDW is influenced by imbalanced physiological conditions, including oxidative stress, tissue hypoxia, neuro‐humoral over‐activity, endothelial dysfunction, and inflammation status, which plays an important role in COVID‐19 infection. 4 , 5 Clinically, elevated RDW has been demonstrated to be associated with adverse outcomes in several clinical conditions, 3 , 6 , 7 including COVID‐19 infection. 8 , 9 Nevertheless, the optimal cut‐off value for RDW in predicting adverse outcomes in COVID‐19 was inconsistent among studies. 9 Therefore, we conducted the present study to further investigate the relationship between RDW and COVID‐19 severity, and to explore the optimal cut‐off value for RDW.
2. MATERIALS AND METHODS
Patients older than 18 years old that were admitted at the First People's Hospital of Jingzhou from January 2020 to March 2020, and with a laboratory‐confirmed diagnosis of COVID‐19 infection were consecutively included. A laboratory‐confirmed diagnosis of COVID‐19 was determined by a positive result on a reverse‐transcriptase polymerase chain reaction assay of a specimen collected on a nasopharyngeal swab according to the WHO interim guideline. 10 Patients lacking laboratory results on RDW were excluded. The study was approved by the Ethics Committee of the First People's Hospital of Jingzhou, with a waiver of informed consent because of the urgency of the epidemic (L20200208).
2.1. Data collection and biochemical assays
Demographic and clinical data were obtained retrospectively from electronic medical records in The First People's Hospital of Jingzhou and assimilated by three researchers (Wang, Li, and Geng). Data were transferred to other team members in Guangdong Provincial People's Hospital (Guangzhou, China) for statistical analyses. The electronic medical records, nursing records, laboratory results, and imaging findings of all enrolled patients were reviewed. Specifically, laboratory results consisted of complete blood count (CBC) with differential (including RDW), electrolyte, C‐reactive protein (CRP), procalcitonin, serum creatinine, estimated glomerular filtration rate (eGFR), alanine transaminase (ALT), creatine kinase, creatine kinase‐MB, lactate dehydrogenase, and d‐dimer.
All blood samples were collected routinely on admission and analyzed within 4 h of collection. The CBC with differential, including RDW, was performed using an Automated Hematology Analyzer (sx500i, sx2800 and sx9000; Sysmex Corporation).
2.2. Definition
The degree of severity of COVID‐19 was defined during hospitalization based on the fifth version of the National Health Commission Guideline on the Management of Novel Coronavirus Pneumonia. Critical COVID‐19 was defined by one of the following criteria: indication of respiratory failure or mechanical ventilation, shock, and organ dysfunction requiring ICU admission. Anemia was defined as hemoglobin level <120 g/L in men and <110 g/L in women.
2.3. Statistical analysis
Statistical analyses were undertaken using SPSS 24.0 (IBM). Continuous variables are expressed as the mean ± standard deviation (SD), median values, interquartile ranges, or simple ranges. Categorical variables are summarized as counts and percentages. Continuous data with a normal distribution were compared using the Student's t test. Data with a non‐normal distribution were compared using the Wilcoxon rank‐sum test and presented as the median and interquartile range. Categorical data were compared using the χ2 test or Fisher's exact test. Receiver operator characteristic (ROC) curve analysis was performed to evaluate the predictive value of RDW for critical COVID‐19. In addition, the optimal cutoff value was calculated via Youden's J statistic, which is the result of highest sensitivity and specificity. The optimal cutoff value was the point that the Youden function reaches its maximum value. Univariate logistic regression analysis was conducted to assess the risk factors for critical COVID‐19, and the indicators with pvalue < 0.05 were entered into a multivariate logistic regression for further analysis. p < 0.05 was considered significant.
3. RESULTS
A total of 98 patients with laboratory‐confirmed COVID‐19 were included in this study. The mean age was 56 ± 17 years, 48 (49.0%) patients were male, and 43 (43.9%) patients suffered from critical COVID‐19. An ROC curve analysis was conducted to determine the predictive value of RDW on admission for COVID‐19 severity. The optimal cutoff value was 11.5%, with relatively high sensitivity and specificity (area under the curve = 0.728, 95% confidence interval (CI): 0.626–0.830, p < 0.001, Figure 1).
Figure 1.
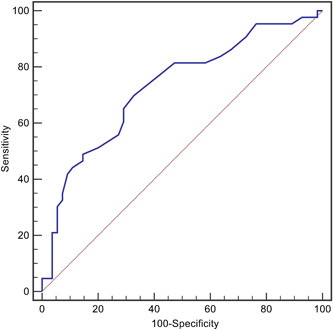
The receiver operating characteristic curves (ROC) for red blood cell distribution width (RDW) in predicting the severity of COVID‐19
The subjects were classified into two groups according to the cutoff of RDW ≤ 11.5% (n = 50) and >11.5% (n = 48). Clinical information was compared between the two groups (Table 1). There was no significant difference in baseline clinical characteristics between the groups. During the hospitalizations, six (6.1%) patients died.
Table 1.
Baseline clinical characteristics of included patients stratified by RDW
| RDW > 11.5% (n = 48) | RDW ≤ 11.5% (n = 50) | p value | |
|---|---|---|---|
| Age, years | 58.1 ± 14.8 | 54.4 ± 18.8 | 0.289 |
| Gender, female, n (%) | 23 (47.9) | 27 (54.0) | 0.547 |
| Concomitant disorders, n (%) | |||
| Hypertension | 13 (27.1) | 14 (28.0) | 0.919 |
| Diabetes | 2 (4.2) | 2 (4.0) | 1.000 |
| SBP, mmHg | 134.0 ± 24.2 | 133.4 ± 22.9 | 0.902 |
| DBP, mmHg | 82.3 ± 15.6 | 78.2 ± 12.3 | 0.155 |
| Heart rate, bpm | 89.6 ± 14.7 | 89.5 ± 17.8 | 0.980 |
| CRP, mg/L | 10.1 (1.5, 24.1) | 16.4 (4.6, 49.3) | 0.059 |
| Serum creatinine, μmol/L | 63.0 (51.9, 76.2) | 65.5 (52.8, 83.7) | 0.293 |
| eGFR, ml/min/1.73 m2 | 110.2 ± 44.1 | 120.8 ± 39.3 | 0.224 |
| ALT, U/L | 16.0 (10.0, 27.0) | 23.0 (13.0, 43.0) | 0.053 |
| WBC, ×10⁹/L | 6.7 ± 4.5 | 6.0 ± 3.8 | 0.415 |
| Hemoglobin, g/L | 116.9 ± 21.0 | 121.6 ± 15.6 | 0.207 |
| Anemia, n (%) | 22 (45.8) | 15 (30.0) | 0.106 |
| Platelet count, ×10⁹/L | 174.7 ± 75.5 | 162.4 ± 73.6 | 0.415 |
| Creatine kinase, U/L | 70.5 (51.0, 98.5) | 55.5 (36.0, 106.0) | 0.183 |
| Creatine kinase‐MB, U/L | 12.0 (10.0, 17.0) | 11.0 (9.0, 15.5) | 0.456 |
| d‐dimer, mg/L | 0.8 (0.3, 2.2) | 0.5 (0.2, 0.9) | 0.039 |
| Critical cases, n (%) | 30 (62.5) | 13 (26.0) | <0.001 |
| Treatment | |||
| Antibiotic therapy | 37 (77.1) | 46 (92.0) | 0.040 |
| Glucocorticoid therapy | 27 (56.3) | 21 (42.0) | 0.158 |
| Interferon therapy | 22 (45.8) | 14 (28.0) | 0.067 |
| Hospital stay, days | 25 (20, 29) | 25.0 (19.0, 32.8) | 0.536 |
| In‐hospital death | 4 (8.3) | 2 (4.0) | 0.636 |
Abbreviations: ALT, alanine transaminase; CRP, C‐reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; RDW, red cell distribution width; SBP, systolic blood pressure; WBC, white blood cell.
Univariate survival analysis indicated that RDW (OR = 2.07; 95% CI: 1.31−3.29; p = 0.002) was associated with COVID‐19 severity. Other significant variables included age, CRP, eGFR <90 ml/min/1.73 m2, ALT, WBC, anemia, and d‐dimer. These variables were included in the multivariate analysis (Table 2). After adjusting for these variables, RDW remained as an independent predictor for COVID‐19 severity (adjusted OR = 2.40, 95% CI: 1.27−4.55, p = 0.007). A similar result was observed when we included RDW > 11.5% instead of as a continuous variable in Model 2 (adjusted OR = 5.41, 95% CI: 1.53−19.10, p = 0.009).
Table 2.
Multivariate logistic regression analysis for the severity of COVID‐19
| Clinical variables | OR | 95% CI | p value |
|---|---|---|---|
| Model 1 | |||
| RDW | 2.40 | 1.27, 4.55 | 0.007 |
| Age | 1.09 | 1.03, 1.16 | 0.003 |
| CRP | 1.00 | 0.98, 1.02 | 0.873 |
| eGFR<90 ml/min/1.73 m2 | 1.37 | 0.26, 7.16 | 0.711 |
| ALT | 1.02 | 1.00, 1.04 | 0.066 |
| WBC | 1.02 | 0.82, 1.26 | 0.880 |
| Anemia | 0.67 | 0.17, 2.69 | 0.575 |
| d‐dimer | 1.28 | 0.98, 1.68 | 0.067 |
| Model 2 | |||
| RDW > 11.5% | 5.41 | 1.53, 19.10 | 0.009 |
| Age | 1.08 | 1.02, 1.14 | 0.010 |
| CRP | 1.00 | 0.98, 1.01 | 0.659 |
| eGFR<90 ml/min/1.73 m2 | 2.12 | 0.42, 10.81 | 0.365 |
| ALT | 1.02 | 1.00, 1.04 | 0.105 |
| WBC | 1.04 | 0.84, 1.30 | 0.701 |
| Anemia | 0.80 | 0.20, 3.22 | 0.750 |
| d‐dimer | 1.31 | 1.01, 1.71 | 0.041 |
Abbreviations: ALT, alanine transaminase; CI, confidence interval; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; OR, odds ratio; RDW, red cell distribution width;WBC, white blood cell.
4. DISCUSSION
Our study showed that RDW could serve as a predictor for critical illness in patients with COVID‐19 infection. In addition, RDW > 11.5% was an optimal cutoff to discriminate critical COVID‐19 infection.
RDW quantifies the heterogeneity of circulating RBCs and has been used to differentiate the causes of anemia. RDW was found in previous studies to be a robust predictor for all‐cause mortality in critically ill or ICU patients. 11 , 12 , 13 As for COVID‐19 infection, the significance of RDW in predicting adverse outcomes remained. 14 The present study further verified a significant association between RDW and disease severity in patients with COVID‐19, whereas hemoglobin was not an independent predictor when RDW was included in the model. As RDW is a simple, inexpensive, and widely available test, these data may have significant clinical implications for determining potential critical disease in patients with COVID‐19 during the ongoing pandemic.
Currently, there was no consensus on the optimal cutoff of RDW. There were several studies that defined ≥14.5% as the abnormally elevated RDW, and have demonstrated good predictive performance in terms of unfavorable clinical outcomes. 15 , 16 , 17 , 18 However, some studies found that cutoff values from different centers might vary—ranging from 12.85% to 14.35%. 19 , 20 , 21 , 22 The cutoff value generated in our study population was 11.5%, which was close to Wang et al. 21 Whether the minor difference between the cutoff values of our study and Wang et al.'s study was significant required further investigations.
Although the underlying pathophysiological mechanism of these strong associations remains unclear, several explanations may account for this phenomenon. Increased RDW may reflect overall inflammatory status, oxidative stress, or arterial underfilling. 3 Anemia was a common finding in patients with COVID‐19. Prevalence of anemia was significantly higher in patients who died from COVID‐19 than survivors. 2 In our univariate analysis, anemia was also a risk factor for COVID‐19 severity. In addition, hemoglobin level was found to be negatively correlated with RDW. However, in the multivariate analysis, RDW was an independent predictor for COVID‐19 severity even after adjusting anemia, indicating other potential explanations for this effect.
Accumulating evidence suggested that patients with severe COVID‐19 might have hyperinflammatory status with a cytokine storm. 4 , 23 Systemic inflammation accompanied by cytokine release has a negative impact on bone marrow function and iron metabolism, and inflammatory cytokines suppress erythrocyte maturation, accentuated with sepsis, allowing newer, larger reticulocytes to enter the circulation, which is associated with RDW increase. 24 , 25 , 26 , 27 The role of oxidative stress during infection is not fully elucidated, but free radicals have been shown to protect against invading microorganisms. 28 Reactive oxygen species such as nitrate oxide (NO), peroxynitrite, and superoxide radicals have been associated with endothelial damage. 29 Pathological findings in patients with COVID‐19 supported the presence of endothelial cell damage and endotheliitis possibly due to direct viral infection and diffuse endothelial inflammation. 30 Oxidative stress could directly damage RBCs and decrease their survival, which might lead to anisocytosis and increased RDW. 31 , 32 , 33 Furthermore, selenium, as a component of the antioxidant defense system, was negatively correlated with RDW. 34 Higher RDW might reflect severe oxidative stress.
Finally, elevated RDW was found to be associated with activation of the renin−angiotensin−aldosterone system (RAAS). 35 Angiotensin‐converting enzyme 2 (ACE2), an enzyme that physiologically counters RAAS activation, is the functional receptor for SARS‐CoV‐2. Furthermore, SARS‐CoV‐2 not only enters through ACE2 but also subsequently downregulates ACE2 expression such that the enzyme is unable to exert protective effects in organs. 36 It has been postulated that unabated angiotensin II activity may be responsible for organ injury in COVID‐19. 37 , 38 These factors may therefore possibly explain the higher RDW level in patients with critical COVID‐19. Future studies are needed to further clarify the underlying mechanism.
4.1. Limitation
This study had several limitations. Firstly, nearly half of the enrolled subjects in the study had critical COVID‐19. This high proportion of critical disease in the study subjects could be attributed to the fact that our hospital is mainly responsible for admitting and caring for critical patients with COVID‐19 in Jingzhou. Secondly, we did not include data on reticulocyte count, erythropoietin level, and hemolysis‐related measurement, which might be of value to account for RDW results. Thirdly, this is a retrospective observational study that was conducted at a single‐center hospital with a limited sample size, thereby necessitating larger prospective cohort studies to further validate the optimal cutoff value of RDW as well as its clinical application.
4.2. Conclusion
Among patients with COVID‐19 infection, RDW, as a simple, inexpensive, and routinely measured laboratory parameter, was independently associated with disease severity. RDW > 11.5% could be the optimal cutoff to discriminate critical COVID‐19 infection and might be helpful in clinical practice to identify critical cases at an early stage. Future studies should focus on elucidating the underlying mechanism of this association.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Shou‐hong Wang, Heng Geng, and Tie‐he Qin contributed to the conception and design of the study. Zhong‐hua Wang, Bing‐qi Fu, Ying‐wen Lin, Xue‐biao Wei, Fei Li, Wei‐xin Guo, Hui‐qing Yuan, You‐wan Liao contributed to the acquisition, analysis, and interpretation of data. Zhong‐hua Wang, Bing‐qi Fu, and Ying‐wen Lin drafted the manuscript. All authors critically revised the manuscript and gave final approval for publication.
ACKNOWLEDGMENTS
This study was supported by grants from the Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (20211001). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Wang Z‐h, Fu B‐q, Lin Y‐w, et al. Red blood cell distribution width: a severity indicator in patients with COVID‐19. J Med Virol. 2022;94:2133‐2138. 10.1002/jmv.27602
Zhong‐hua Wang, Bing‐qi Fu, and Ying‐wen Lin are considered co‐first authors.
Contributor Information
Tie‐he Qin, Email: qintiehe@163.com.
Fei Li, Email: 2037282869@qq.com.
Shou‐hong Wang, Email: gdwangshouhong@163.com.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared at reasonable request to the corresponding author.
REFERENCES
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salvagno GL, Sanchis‐Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86‐105. [DOI] [PubMed] [Google Scholar]
- 4. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loprinzi PD. Comparative evaluation of red blood cell distribution width and high sensitivity C‐reactive protein in predicting all‐cause mortality and coronary heart disease mortality. Int J Cardiol. 2016;223:72‐73. [DOI] [PubMed] [Google Scholar]
- 6. Han YQ, Zhang L, Yan L, et al. Red blood cell distribution width predicts long‐term outcomes in sepsis patients admitted to the intensive care unit. Clin Chim Acta. 2018;487:112‐116. [DOI] [PubMed] [Google Scholar]
- 7. Soohoo M, Molnar MZ, Ujszaszi A, et al. Red blood cell distribution width and mortality and hospitalizations in peritoneal dialysis patients. Nephrol Dial Transplant. 2019;34(12):2111‐2118. [DOI] [PubMed] [Google Scholar]
- 8. Lee JJ, Montazerin SM, Jamil A, et al. Association between red blood cell distribution width and mortality and severity among patients with COVID‐19: a systematic review and meta‐analysis. J Med Virol. 2021;93(4):2513‐2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarkar S, Kannan S, Khanna P, Singh AK. Role of red blood cell distribution width, as a prognostic indicator in COVID‐19: a systematic review and meta‐analysis. Rev Med Virol. 2021:e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected: interim guidance. World Health Organization. March 13, 2020. Accessed February 28, 2021. https://apps.who.int/iris/handle/10665/331446
- 11. Havens JM, Seshadri AJ, Salim A, Christopher KB. Red cell distribution width predicts out of hospital outcomes in critically ill emergency general surgery patients. Trauma Surg Acute Care Open. 2018;3(1):e000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schepens T, De Dooy JJ, Verbrugghe W, Jorens PG. Red cell distribution width (RDW) as a biomarker for respiratory failure in a pediatric ICU. J Inflamm. 2017;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all‐cause mortality in critically ill patients. Crit Care Med. 2011;39(8):1913‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID‐19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henry BM, Benoit JL, Benoit S, et al. Red blood cell distribution width (RDW) predicts COVID‐19 severity: a prospective, observational study from the Cincinnati SARS‐CoV‐2 Emergency Department Cohort. Diagnostics. 2020;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foy BH, Carlson JCT, Reinertsen E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS‐CoV‐2 infection. JAMA Netw Open. 2020;3(9):e2022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karampitsakos T, Akinosoglou K, Papaioannou O, et al. Increased red cell distribution width is associated with disease severity in hospitalized adults with SARS‐CoV‐2 infection: an observational multicentric study. Front Med. 2020;7:616292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaufmann CC, Ahmed A, Brunner U, et al. Red cell distribution width upon hospital admission predicts short‐term mortality in hospitalized patients with COVID‐19: a single‐center experience. Front Med. 2021;8:652707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lorente L, Martín MM, Argueso M, et al. Association between red blood cell distribution width and mortality of COVID‐19 patients. Anaesth Crit Care Pain Med. 2021;40(1):100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banon T, Wortsman J, Ben Moshe S, et al. Evaluating red blood cell distribution width from community blood tests as a predictor of hospitalization and mortality in adults with SARS‐CoV‐2: a cohort study. Ann Med. 2021;53(1):1410‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang C, Zhang H, Cao X, et al. Red cell distribution width (RDW): a prognostic indicator of severe COVID‐19. Ann Transl Med. 2020;8(19):1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellan M, Azzolina D, Hayden E, et al. Simple parameters from complete blood count predict in‐hospital mortality in COVID‐19. Dis Markers. 2021;2021:8863053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103(16):2055‐2059. [DOI] [PubMed] [Google Scholar]
- 25. Rogiers P, Zhang H, Leeman M, et al. Erythropoietin response is blunted in critically ill patients. Intensive Care Med. 1997;23(2):159‐162. [DOI] [PubMed] [Google Scholar]
- 26. Chiari MM, Bagnoli R, De Luca PD, Monti M, Rampoldi E, Cunietti E. Influence of acute inflammation on iron and nutritional status indexes in older inpatients. J Am Geriatr Soc. 1995;43(7):767‐771. [DOI] [PubMed] [Google Scholar]
- 27. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011‐1023. [DOI] [PubMed] [Google Scholar]
- 28. Pohanka M. Role of oxidative stress in infectious diseases. A review. Folia Microbiol. 2013;58(6):503‐513. [DOI] [PubMed] [Google Scholar]
- 29. Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc Natl Acad Sci USA. 2018;115(23):5839‐5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7(2):113‐116. [DOI] [PubMed] [Google Scholar]
- 32. Friedman JS, Lopez MF, Fleming MD, et al. SOD2‐deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood. 2004;104(8):2565‐2573. [DOI] [PubMed] [Google Scholar]
- 33. Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10(11):1923‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women's Health and Aging Study I. Clin Nutr. 2010;29(5):600‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kato H, Ishida J, Imagawa S, et al. Enhanced erythropoiesis mediated by activation of the renin−angiotensin system via angiotensin II type 1a receptor. FASEB J. 2005;19(14):2023‐2025. 10.1096/fj.05-3820fje [DOI] [PubMed] [Google Scholar]
- 36. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin−angiotensin−aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382(17):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev Res. 2020;81(5):537‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China: Life Sci. 2020;63(3):364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared at reasonable request to the corresponding author.


