Abstract
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) induces the production of proinflammatory cytokines, which results in a cytokine storm, and immune‐modulators like Mycobacterium indicus pranii (MIP) might ameliorate coronavirus disease of 2019 (COVID‐19) related cytokine storm. Therefore, the present study evaluates whether MIP offers an advantage in the treatment of severe COVID‐19 patients infected with SARS‐CoV‐2. A prospective MIP cohort study was conducted in chest disease hospitals in Srinagar, Jammu and Kashmir, India. In the present prospective, randomized clinical study, critically severe COVID‐19 patients were divided into two groups, the MIP group (n = 105) and the best standard treatment (BST) group (n = 210). Procalcitonin, ferritin, high‐sensitive C‐reactive protein, D‐dimer levels, and interleukin levels on 5th‐day posttreatment were significantly reduced in the MIP group compared to the BST group. Compared to the BST group, 105 consecutive patients with severe COVID‐19 in the MIP group reported early weaning off ventilation, resolution of chest architecture (computed tomography [CT] scan), a significant increase in SpO2 levels, and decreased mortality with a hazard ratio: 0.234 (95% confidence interval: 0.264–2.31) (p = 0.001). MIP restored SpO2, immune/inflammatory response, normalized lung abnormalities (chest CT scan), and reduced mortality without any serious complications. However, there is a need for placebo‐controlled double‐blind and controlled clinical trials to confirm the efficacy.
Keywords: COVID‐19, interleukins, mechanical ventilation, Mycobacterium indicus pranii, proinflammatory response and oxygen saturation
Highlights
Mycobacterium indicus pranii (MIP) at a dose of 0.1 ml resulted in a significant reduction in the stay of patients in the intensive care unit and mortality in severe coronavirus disease of 2019 (COVID‐19) patients.
The MIP was found stronger with respect to normalization of lung architecture (computed tomography scan), a significant increase in oxygen saturation, reduction/weaning off ventilation.
The concomitant improvement in biological and clinical parameters in the present offers indirect evidence of the beneficial/effective role of MIP in COVID‐19 patients.
Furthermore, we could not find any adverse effect during the hospitalization of COVID‐19 patients.
Abbreviations
- ALP
alkaline phosphatase
- BST
best standard treatment
- COVID‐19
coronavirus disease of 2019
- CT
computed tomography
- FDA
Food and Drug Administration
- IL
interleukin
- IQR
interquartile range
- MIP
Mycobacterium indicus pranii
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- SGOT
serum glutamic oxaloacetic transaminase
- SGPT
serum glutamic‐pyruvic transaminase
- TLC
total leukocyte count
1. INTRODUCTION
The global pandemic of coronavirus disease of 2019 (COVID‐19) originated from Wuhan, China, in December 2019, which engulfed the whole continent within a span of 4 months. 1 At the time of preparation of this draft (June 12, 2021), the total number of 176 112 919 cases are recorded so far with 3 802 235 deaths, furthermore, at present most severely affected countries include USA, India, and Brazil. 2 , 3 , 4 Throughout the world, more than 300 clinical trials have been conducted in different parts of the world using a wide range of antiviral drugs and other classes of drugs against COVID‐19, but till now, an effective therapy against COVID‐19 has not been developed. Currently, COVID‐19 is managed by a diverse set of therapeutic regimens, which includes plasma therapy, traditional medicines, and various repurposed pharmacological drugs. 5 In spite of these attempts, Food and Drug Administration (FDA) has officially approved only nucleoside analog remdesivir, but its effectiveness is still a debate. 6 Furthermore, there are reports of the beneficial role of traditional medicines and phytomedicine against different types of viruses. Owing to their antioxidant, immune‐modulatory and anti‐inflammatory activity researchers have hypothesized regarding the beneficial role of traditional cum phytomedicine against COVID‐19. 7 Throughout the world, as of now, there are more than 80 vaccines under clinical trials, and many of them have been approved for use. Similarly, off‐target vaccine‐like BCG vaccines are being evaluated for their protective role in COVID‐19 patients. Although some vaccine trials have reported very promising results in preclinical stages and have postulated that these candidate vaccines can provide effective protection against severe forms of COVID‐19. 8 As there is every chance that the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) virus can mutate and change antigenically hence search for the vaccine will be a never lasting attempt. SARS COV‐2 have a similar pathogenetic pathway like that of Middle East respiratory syndrome coronavirus and SARS‐CoV‐1, which involves hyper‐activation of inflammatory pathways, which results in cytokine storm and consequently multiple organ failure. 9 , 10 SARS‐CoV‐2 is believed to bind with toll‐like receptors and causes activation of the inflammasome and henceforth production of proinflammatory cytokines. 11 Plethora of studies have proposed immune deregulation as an independent risk factor for negative outcomes in COVID‐19, henceforth various immune modulators have been proposed to have a beneficial role in COVID‐19 or might be helpful in inhibiting the severe progression of diseases caused by SARS‐CoV‐2. 3 But the majority of these immune modulators are yet to be evaluated in actual clinical conditions.
In India, owing to the dramatic increase in the number of cases and unavailability of specific therapy against COVID‐19, the Indian government proposed Mycobacterium indicus pranii (MIP; commercially available as Sepsivac), which is prepared from heat‐killed MIP (formerly known as Mycobacterium W), will be tested on 50 COVID‐19 patients at two centers in India. 9 MIP is an easily cultivatable and nonpathogenic microorganism grouped with other beneficial Mycobacterium (M. fortuitum, M. smegmatis, and M. vaccae) in Runyon Group IV. Furthermore, MIP was extensively evaluated for its biochemical and immunological properties and has been successfully used as an immune modulator in a wide spectrum of diseases. 12 MIP was earlier developed for the treatment of sepsis caused by gram‐negative bacteria and has been already approved for a phase‐III clinical trial in India. 13 MIP was used as a vaccine for leprosy warts caused by human papillomavirus 14 , 15 and as a prophylactic drug against pulmonary tuberculosis. 16 The rationale of using MIP is based on the analogy that in both gram‐negative sepsis and severe progression of COVID‐19, immune‐dysregulation mediated by cytokine storm plays an important role in disease progression and mortality. Recently a study has found that candidate drug offers protection against COVID‐19 via upregulation of adaptive natural killer cells and regulates the generation of robust cytokines, which include interleukin‐2 (IL‐2) and interferon‐γ (IFN‐γ). 13 , 16 To further support this, in situ studies have found differential expression of cytokine genes in the presence of MIP, and these studies have attributed differential expression of cytokines to modulation of immune response evoked by MIP. 17 Immunomodulatory action of MIP has been shown to be mediated by upregulation of Th1 immune response and simultaneously downregulation of Th2 immune response which results in upregulation of inhibitor Κappa Kinase‐α and ‐β in patients infected with pulmonary form of tuberculosis. 18 Elsewhere, in Human Immunodeficiency Virus patients, MIP in combination with antiretroviral therapy resulted in increased expression of the CD4 T‐cells. To summarize the immune modulator role of MIP, it may be postulated that MIP evokes a balanced immune cum inflammatory response which helps in restoration of the tissue microenvironment and keeps check on cytokine storm. An added advantage of using MIP in developing countries like India includes drugs being easily available as cheap preparations and is not associated with any serious side effects associated with its use. 19 Accordingly, these findings led us to hypothesize the beneficial role of MIPin severely ill COVID‐19 patients. Therefore, the present clinical study was conducted to gather initial experience of evaluating the role of MIP in severely ill COVID‐19 patients.
2. METHOD
The institutional ethical committee approved the study (CDSCO U/P No: EC/NEW/INST/2020/7452/16 Dated: September 26, 2020, under protocol title: role of immunomodulator MIP in COVID‐19). In the present study, we identified risk factors for severe progression of diseases and death as an outcome of the disease and subsequently evaluated the role of MIP therapy in severely ill COVID‐19 patients based on changes in these risk factors.
2.1. Identification of factors for severe progression of the disease
From March 23, 2020, to December 15, 2020, a total of 1894 cases of COVID‐19 were admitted to CD hospital Srinagar, Jammu and Kashmir. Patients were divided as per the severity of disease; henceforth severely ill patients were used for identification of risk factors for the severe outcome of COVID‐19 disease as per guidelines of Chinese management guidelines for COVID‐19 (version 6.0) and WHO Interim Guidelines. 20 , 21 For identification of risk factors for the severe outcome of disease, we compared inflammatory and immune response in these patients by correlation matrix analysis, and the parameters that showed significant correlation with days for hospitalization and number of days under high/medium flow invasive/mechanical ventilator support were identified. Henceforth role MIP therapy was evaluated in severely ill COVID‐19 patients based on changes in these identified risk factors.
2.2. Patient selection (inclusion/exclusion criteria)
A study was conducted in chest disease hospitals in Srinagar, Jammu and Kashmir. Inclusion criteria for patients in both groups were defined as (i) confirmed COVID‐19 infection as per real‐time reverse transcription (RT‐PCR), (ii) severely ill COVID‐19 patients, (iii) computed tomography (CT) scan abnormalities indicative of COVID‐19 (multiple ground‐glass abnormalities), (iv) patients that need mechanical oxygen intubation, (v) age above 18 years, (vi) pulmonary function defined as oxygen saturation 93% or below as per Chinese management guidelines for COVID‐19 version 6.0). Written consent was obtained from patients once at the time of admission and once at the time of instituting the treatment. Patients undergoing treatment with MIP were made aware of their treatment by medical staff. 12 , 14 Exclusion criteria were defined as (i) patients died within 48 h of hospitalization, (ii) patients suffering from other pulmonary morbidities, (iii) patients below 18 years of age, (iv) patients having more than two associated comorbidities, (iv) patients where written consent could not be obtained.
2.3. Study design
A prospective cohort study (interventional) was conducted on patients treated with MIP and was compared in terms of all‐cause mortality, inflammatory and immune response (risk factors identified) with patients treated with best standard treatment (BST) group/comparator group. To ensure strict control on baseline characteristics, MIP recipients were retrospectively matched by propensity‐score matched at 1:2 ratios (MIP: BST) to comparator patients admitted from March 3, 2020, to November 15, 2020. After establishing matching, BST patients records were retrospectively chart reviewed by a team of experts who were unaware of patient information in the MIP group. For patients whose baseline characteristics were not available in the system database, their records were retrieved manually.
2.4. Treatment
2.4.1. MAP
Patients in the MIP group received MIP at a dosage of 0.1 ml intramuscular three times a day at three different sites for 3 consecutive days in addition to BST used and approved at chest disease hospital Srinagar, Jammu and Kashmir, India. Each dose of 0.1 ml MIP (Sepsivac; Cadila Pharmaceutical) contains 0.5 × 109 heat‐killed MIP 0.9% sodium chloride, and 0.01% thimerosal (as preservative).
2.4.2. BST
In the comparator group, patients received standard/symptomatic therapy, which comprised of Ivermectin 12 mg twice daily for 3 days, doxycycline 200 mg stat followed by 100 mg twice daily for 4 days, and azithromycin 500 mg once daily for 5 days.
2.5. Patient
Patient information was obtained from the hospital master data file and medical records of patients. Variables considered of clinical relevance in the present study included Immune, inflammatory, all‐cause mortality, the need for ventilator support. In both groups above, parameters were considered at the time of initiation/administration of MIP and on the 5th‐day posttreatment. CT scan was performed on an average 5.8th day after the onset of symptoms as per the method described in our earlier publication 15 and on the 5th day after randomization. Briefly, images were taken in single inspiration breath‐hold, and two radiologists separately analyzed CT images. The semiquantitative method was used to calculate the percentage of lung involvement and was classified as mild (<25%), moderate (25%–50%), severe (51%–75%), and diffuse (>75%).
2.6. Outcomes
We identified all‐cause mortality during hospitalization in both groups as the primary outcome, while secondary outcomes considered in the present study included differences in mean oxygen requirement, oxygen saturation, and change in immune cum inflammatory markers. Short‐term safety of MIP was evaluated on the basis of liver function test, kidney function test, hemoglobin level, death, and premature discontinuation of treatment (in MIP group).
2.7. Statistical analysis
In the present interventional study, we did not conduct a prior sample size calculation. The MIP cohort (n = 105) was a sample of convenience and included all patients treated with MIP. The BST control sample size was estimated by matching at 1:2 (cases to controls) by propensity score analysis. Continuous variables were expressed as mean ± SE; interquartile range while categorical variables were expressed as count/percentage with odds ratio. Comparison for continuous variables was estimated by Mann–Whitney U test, and categorical data were compared by χ 2 test. Time‐dependent events in the MIP group were compared by a two‐sided log‐rank test (hazard ratio [HR] and 95% confidence interval) on Kaplan–Meier survival analysis across days of hospitalization. High‐sensitive C‐reactive protein (Hs‐CRP) concentration across different days of treatment was compared by repeated‐measures mixed‐model, and p < 0.05 was considered statistically significant, and statistics were performed with IBM SPSS Statistics, version 20 and statographics 18. 22
3. RESULTS
3.1. Comparison of baseline characteristics in two groups
A total of 105 patients were included in the MIP group. Similarly, from the retrospective historical control group, out of 1894 patients admitted from March 2020, 210 BST control patients were identified to serve as a comparator group for the MIP group. Henceforth clinical, biochemical, and radiological characteristics between these groups were compared (Tables 1 and 2).
Table 1.
Baseline demographic and clinical characteristics of severely ill COVID‐19 patients of BST arm (n = 210) and MIP arm (n = 105)
| Characteristics | BST (n = 210) | MIP (n = 105) | p |
|---|---|---|---|
| Age (mean years: IQR) | 52.23 ± 19.78 (28–67) | 56.89 ± 25.22 (32–87) | 0.53 |
| Male | 187 (89.04%) | 92 (87.61%) | 0.93 |
| Cough | 195 (92.85%) | 91 (87.02%) | 0.91 |
| Productive sputum | 78 (37.14%) | 34 (32.38%) | 0.45 |
| Sore throat | 133 (63.33%) | 67 (63.80%) | 0.94 |
| Fever | 87 (41.42%) | 43 (40.95%) | 0.93 |
| Anorexia | 41 (19.52%) | 19 (18.09%) | 0.56 |
| Rhinitis | 37 (17.61%) | 21 (20%) | 0.32 |
| Insomnia | 30 (14.28%) | 15 (14.28%) | 0.87 |
| Hymoptypsis | 21 (10%) | 8 (7.61%) | 0.13 |
| Dysgusia | 31 (14.76%) | 15 (14.28%) | 0.82 |
| Nausia | 13 (6.19%) | 6 (5.71%) | 0.56 |
| Diarhoea | 32 (15.28%) | 17 (16.19%) | 0.67 |
| Myalgia | 132 (62.85%) | 62 (59.04%) | 0.42 |
| Fatigue | 51 (24.28%) | 24 (22.85%) | 0.31 |
| Headache | 47 (22.38%) | 17 (22.85%) | 0.90 |
| Oropharyngeal congestion | 103 (49.04%) | 45 (42.85%) | 0.12 |
| Comorbidities | |||
| Chronic obstructive pulmonary disease | 47 (22.38%) | 24 (22.85%) | 0.45 |
| Asthma | 29 (13.80%) | 12 (11.42%) | 0.69 |
| Diabetes mellitus | 81 (38.57%) | 48 (45.71%) | 0.21 |
| Cardiomyopathy | 84 (40.0%) | 52 (49.52%) | 0.15 |
| Chronic kidney disease | 51 (24.28%) | 19 (18.09%) | 0.37 |
| Cancer | 5 (2.37%) | 3 (2.85%) | 0.74 |
| Chronic liver disease | 61 (29.04%) | 29 (27.61%) | 0.89 |
| Cardio vascular disease | 41 (19.52%) | 16 (15.23%) | 0.27 |
| Thyroid disorder | 58 (27.61%) | 34 (32.38%) | 0.51 |
| Setriod use | 29 (13.80%) | 14 (13.33%) | 0.94 |
| Smoker | 91 (43.33%) | 37 (35.28%) | 0.15 |
| Anemia | 103 (49.05%) | 56 (53.33%) | 0.76 |
| Therapy | |||
| Hydoxychloroquine and azithromycin | 210 (100%) | 105 (100%) | 1.00 |
| Ivermectin | 210 (100%) | 105 (100%) | 1.00 |
| Doxycycline | 39 (18.57%) | 15 (14.28%) | 0.86 |
| Prednisolone | 35 (16.66%) | 16 (15.23%) | 0.85 |
| Dexamethasone | 73 (34.78%) | 34 (32.38%) | 0.90 |
| Remdesivir | 21 (10.0%) | 12 (11.42%) | 0.83 |
| Favipiravir | 31 (14.76%) | 15 (14.28%) | 0.95 |
Abbreviations: BST, best standard treatment; COVID‐19, coronavirus disease of 2019; IQR, interquartile range; MIP, Mycobacterium indicus pranii.
Table 2.
Baseline biochemical characteristics of critically ill COVID‐19 patients of BST arm (n = 210) and MIP arm (n = 105)
| Parameters | BST arm (n = 210) | MIP arm (n = 105) | p | ||||
|---|---|---|---|---|---|---|---|
| 95% CI (lower bound–upper bound) | Mean ± SE | IQR | 95% CI (lower bound–upper bound) | Mean ± SE | IQR | ||
| Temperature | (35.90–41.01) | 38.92 ± 0.89 | 3.30 | (36.12–40.85) | 37.19 ± 0.91 | 2.93 | 0.64 |
| SPO2% | (82–85) | 81.97 ± 2.12 | 8.0 | (76.12–84.94) | 80.53 ± 2.05 | 13 | 0.19 |
| White blood cell count (103/L) | (6597–8569) | 7596 ± 480 | 4589 | (7833.44–10819.89) | 7326.66 ± 696.21 | 4500 | 0.45 |
| Lymphocyte(106/L) | 1456–1987 | 1562 ± 189 | 602 | 1467.33–1733.56 | 1596.12 ± 256.89 | 415 | 0.89 |
| Platelet count(109/L) | 141–194 | 156 ± 20.56 | 63 | 123.56–193.77 | 189.99 ± 17.78 | 71 | 0.12 |
| Blood sugar(mg/dl) | 91.27–155.45 | 96.12 ± 17.45 | 32 | 56.89–104.56 | 85.78 ± 13.98 | 51 | 0.56 |
| Bilirubin(mg/dl) | 1.79–3.82 | 1.92 ± 0.43 | 0.74 | 1.22–3.78 | 1.89 ± 0.34 | 0.79 | 0.12 |
| Heart rate (beats/min) | (82–96) | 91.34 ± 5.34 | 09 | (83.13–97.66) | 90.40 ± 3.38 | 25 | 0.65 |
| Respiration rate (breaths/min) | (16–38) | 31.10 ± 2.10 | 10 | (21.28–29.91) | 35.60 ± 2.01 | 11 | 0.19 |
| Creatinine (mg/dl) | (0.86–1.87) | 1.21 ± 0.12 | 0.45 | (1.00–1.54) | 1.27 ± 0.12 | 0.70 | 0.43 |
| Hemoglobin (g/dl) | (11–15) | 12.53 ± 0.31 | 3.71 | (10.50–13.13) | 11.82 ± 0.61 | 2.60 | 0.61 |
| SGOT (IU/L) | (38–59) | 42.12 ± 5.32 | 14.56 | (38.85–51.79) | 45.32 ± 3.01 | 21.0 | 0.32 |
| SGPT (IU/L) | (59–78) | 63.67 ± 7.33 | 39.67 | (54.23–77.49) | 65.86 ± 5.42 | 15.0 | 0.67 |
| ALP (IU/L) | (167–149) | 134.39 ± 7.82 | 7.97 | (119.04–178.01) | 148.53 ± 13.74 | 67.0 | 0.68 |
Abbreviations: ALP, alkaline phosphatase; BST, best standard treatment; CI, confidence interval; COVID‐19, coronavirus disease of 2019; IQR, interquartile range; MIP, Mycobacterium indicus pranii; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic‐pyruvic transaminase.
3.2. Relationship between hospital stay inflammatory/cytokine response in severely ill COVID‐19 patients
To identify role of inflammatory/cytokine response in severely ill COVID‐19 patients, we attempted to evaluate correlation between inflammatory/cytokine responses and number of days for hospital stay in severely ill COVID‐19 patients. From results of present study, significantly positive correlation was observed between hospital stay and plasma levels of procalcitonin (y = 2.145x + 213.2; r² = 0.810; p < 0.05), ferritin (y = 1.792x + 178.1; r² = 0.763; p < 0.05), D‐dimer (y = 1.313x + 178.5; r² = 0.678; p < 0.05), and Hs‐CRP (y = 1.897x + 278.2; r² = 0.410; p < 0.05). Furthermore plasma ferritin levels were found to have positive correlation between levels of Hs‐CRP (y = 0.980x + 567.8; r² = 0.810; p < 0.05) and lactate dehydrogenase (LDH) (y = 1.567x + 167.9; r² = 0.734; p < 0.05), similarly LDH was found to be positively correlated with Hs‐CRP levels (y = 1.634x + 167.8; r² = 0.762; p < 0.05). On similar line, ILs (IL‐1, IL‐2, and IL‐6) and tumor necrosis factor (TNF)‐α were correlated with hospital stay of severely ill COVID‐19 patients (Figure 1). From these results, we found positive correlation between hospital stay and levels of IL‐1 (y = 0.109x + 65.20; r² = 0.365; p < 0.05), IL‐6 (y = 1.077x + 129.8; r² = 0.670; p < 0.05), and IL‐2 (y = 0.034x + 20.52; r² = 0.365; p < 0.01), while in present study we could not find any significant correlation between TNF‐α, IL‐4, IL‐10, and interferon (INF)‐γ (y = 0.003x + 1.653; r² = 0.023; p = 0.879) levels and hospitalization (Figure 1).
Figure 1.
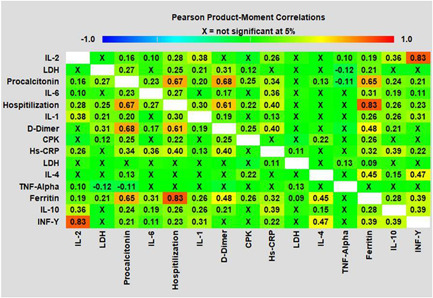
Associations between increased serum inflammatory factors, interleukins (IL‐1; IL‐2, IL‐4, IL‐6, IL‐10, and INF‐ γ) and TNF‐α levels with hospital stay in coronavirus disease of 2019 patients (Pearson's product‐moment correlations) correlation analyses were conducted between variables and were considered significant at p < 0.05; p < 0.01; p < 0.0001. Hs‐CRP, high‐sensitive C‐reactive protein
3.3. Proinflammatory and immune response in patients under two different arms of treatment
On the day of randomization when ILs and TNF‐α responses of patients were compared in two treatment arms, we could not find any significant difference in pretreatment values of IL‐1, IL‐2, IL‐6, and TNF‐α. On the 5th day of treatment, a significant reduction was observed in values of IL‐1 in the MIP group (p = 0.03), while a nonsignificant reduction in values of IL‐1 was observed in the BST group. Similarly, in both groups, IL‐2 was significantly reduced in MIP (0.05) and BST (0.05) group, although levels of IL‐6 and TNF‐α were reduced in both treatment arms, reduction in posttreatment values remained non significantly reduced up to 5th day of treatment (Figure 2A–D). When these parameters were compared among two treatment groups on the 5th day, significantly lower levels (IL‐1: p = 0.04; IL‐2: p = 0.04; IL‐6: p = 0.03; TNF‐α: p = 0.01) were found in the MIP group compared to the BST group. Similarly, in the present study on the 5th day of randomization, we observed a significant reduction in levels of IL‐4 in both treatment arms, while no significant difference was observed between these treatment arms when values were compared on 5th day (Figure 3A). On similar lines when levels of INF‐γ were compared in the MIP group levels showed significant reduction while as in the BST group levels remained more or less nonsignificantly reduced. In addition, when levels of INF‐γ were compared on 5th day between these groups, we could find significantly lowered levels in the MIP group compared to the BST group (Figure 3C). Contrarily to these findings, we could not observe any significant reduction in levels of IL‐10 in both the treatment arms (Figure 3B)
Figure 2.
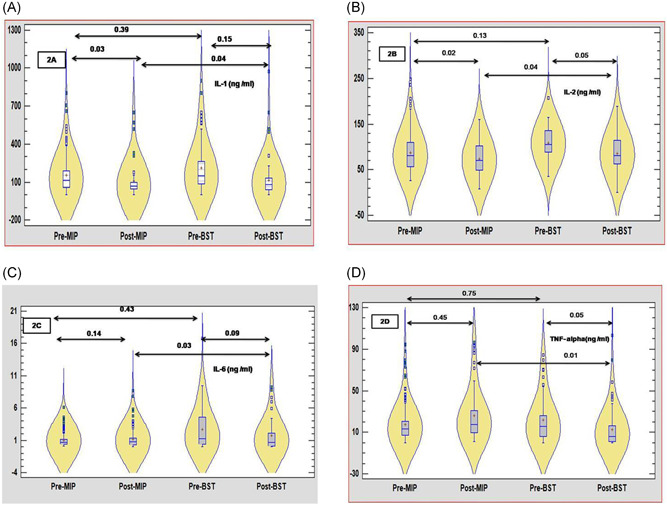
(A–D) The violin plots of interleukin‐1 (IL‐1), IL‐6, and TNF‐α level in patients with coronavirus disease of 2019 (COVID‐19) in severely ill COVID‐19 patients under best standard treatment (BST) arm and Mycobacterium indicus pranii (MIP) arm. The red dot in the violin is the median value, and the blue rectangle is the 25th and 75th percentiles. (A) On 5th day of treatment, a significant reduction was observed in values of IL‐1 in the MIP group (p = 0.03) while a nonsignificant reduction in values of IL‐1 was observed in the BST group. (B) Similarly in both groups IL‐2 was significantly reduced in MIP (0.05) and BST (0.05) groups. (C and D) Nonsignificant reduction in values of IL‐6 and TNF‐α was observed
Figure 3.

(A–C) The box plots of interleukin‐4 (IL‐4), IL‐10, and INF‐γ level in patients with coronavirus disease of 2019 (COVID‐19) in severely ill COVID‐19 patients under best standard treatment (BST) arm and Mycobacterium indicus pranii (MIP) arm
In a similar fashion on the day of randomization, when proinflammatory/inflammatory markers were compared between BST and MIP groups, we could not observe any significant difference between the two treatment arms. When these proinflammatory/inflammatory markers were compared on the 5th‐day posttreatment, levels of procalcitonin (p = 0.02), ferritin (p = 0.09), Hs‐CRP (p = 0.05), and levels of D‐dimer (p < 0.001) were found to be decreased significantly in the MIP group compared to levels of these parameters on the day of randomization. Although significant reduction was observed in posttreatment values of LDH (p = 0.05) in the BST group, the gradient of reduction was below the gradient of reduction observed in the MIP group. Posttreatment values compared in BST and MIP revealed an early reduction of proinflammatory/inflammatory markers in the MIP group compared to the BST group (Figure 4A–F ). In the present study when posttreatment values of BST and MIP group were compared, a significant reduction in levels of ferritin (0.02) and Hs‐CRP (0.05) was observed in the MIP group compared to BST group, while as significantly lower levels of D‐dimer were observed in BST arm compared to MIP arm (Figure 4B,D,F). Furthermore, on the 5th‐day post randomization, no significant difference was observed in levels of procalcitonin, ferritin, Hs‐CRP, and D‐dimer in the BST group compared to values on the day of randomization. Results of this study revealed the superiority of MIP therapy in the amelioration of inflammatory response in severely ill COVID‐19 patients compared to BST therapy (Table 3).
Figure 4.
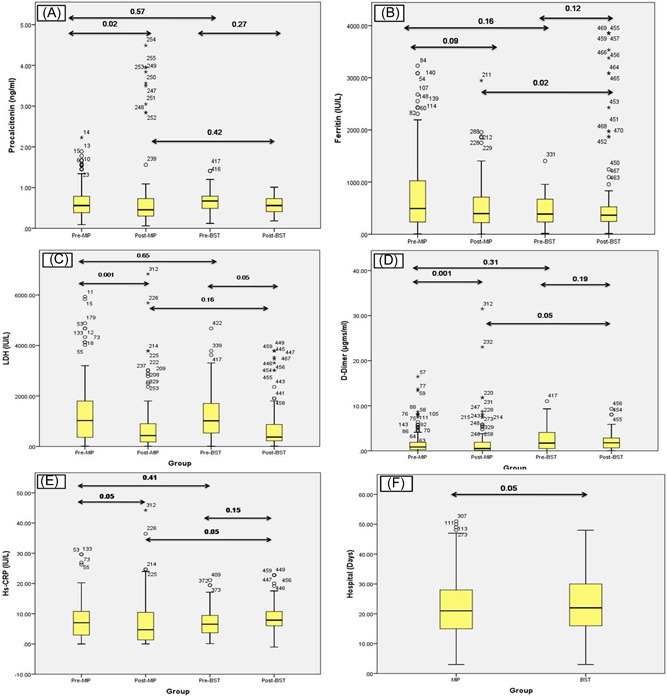
(A–F) Dynamic changes of high‐sensitive C‐reactive protein (Hs‐CRP), D‐dimer, LDH, ferritin, procalcitonin. During hospitalization, the horizontal lines represent the median value in each group. On 5th‐day posttreatment, levels of procalcitonin (p = 0.02), ferritin (p = 0.09), Hs‐CRP (p = 0.05), and levels of D‐dimer (p < 0.001) were found to be decreased significantly in Mycobacterium indicus pranii (MIP) group. Significant reduction was observed in posttreatment values of LDH (p = 0.05) in best standard treatment (BST) group, but the gradient of reduction was below the gradient of reduction observed in the MIP group
Table 3.
Comparison of hemato‐biochemical parameters of MIP and BST in severely ill COVID‐19 patients
| Parameters | Group | Before treatment | After treatment | Wilcoxon signed rank | |||
|---|---|---|---|---|---|---|---|
| Means (SD) | Means (SD) | Positive ranks | Negative ranks | Mean difference (Z) | p | ||
| Temperature (C) | BST (133) | 38.53 ± 2.34 | 37.56 ± 2.19 | 34 | 99 | −1.93 | 0.321 |
| MIP (86) | 39.05 ± 1.31 | 36.96 ± 3.14 | 49 | 37 | −2.12 | ≤0.05 | |
| Respiration (breaths/min) | BST (133) | 34.91 ± 2.76 | 28.45 ± 5.34 | 56 | 77 | −2.78 | 0.07 |
| MIP (86) | 35.03 ± 4.21 | 28.76 ± 4.11 | 54 | 32 | −5.47 | ≤0.001 | |
| Hemoglobin (g/dl) | BST (133) | 11.34 ± 1.67 | 12.47 ± 1.45 | 93 | 40 | +2.11 | 0.13 |
| MIP (86) | 11.90 ± 2.11 | 11.45 ± 2.31 | 49 | 37 | +0.21 | 0.52 | |
| WBC (103/L) | BST (133) | 7456.±371 | 5193 ± 456 | 45 | 88 | −2167 | ≤ 0.05 |
| MIP (86) | 7298 ± 561 | 5943 ± 513 | 35 | 37 | −2234 | ≤0.05 | |
| SGOT (IU/L) | BST (133) | 44.78 ± 5.34 | 37.12 ± 4.61 | 49 | 84 | −5.67 | 0.21 |
| MIP (86) | 43.14 ± 4.23 | 34.45 ± 6.21 | 42 | 44 | −3.45 | 0.06 | |
| SGPT (IU/L) | BST (133) | 72.45 ± 12.05 | 52.56 ± 10.21 | 77 | 56 | −13.13 | 0.19 |
| MIP (86) | 68.89 ± 15.45 | 55.22 ± 12.45 | 28 | 58 | −8.89 | 0.26 | |
| ALP (IU/L) | BST (133) | 123.56 ± 14.90 | 104.12 ± 16.45 | 58 | 75 | −22.90 | 0.09 |
| MIP (86) | 112.45 ± 10.23 | 95.67 ± 8.47 | 28 | 58 | −20.78 | 0.11 | |
| Creatinine (mg/dl) | BST (133) | 1.09 ± 0.32 | 0.92 ± 0.31 | 48 | 85 | −0.67 | 0.42 |
| MIP (86) | 1.21 ± 0.24 | 0.93 ± 0.23 | 21 | 65 | −0.43 | 0.51 | |
Abbreviations: ALP, alkaline phosphatase; BST, best standard treatment; COVID‐19, coronavirus disease of 2019; MIP, Mycobacterium indicus pranii; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic‐pyruvic transaminase; WBC, white blood cell.
3.4. Primary and secondary outcomes in patients under two different arms of treatment
The present study revealed 77 death out of 210 patients in the BST group with HR = 1.406 (95% CI: 0.734–0.892; p = 0.021), while out of 105 patients treated with MIP, 14 patients died with HR = 0.234 (95% CI: 0.264–2.31) (p = 0.001) (Figure 5). In the MIP group remaining 91 surviving patients showed improvement in clinical conditions as indicated by body temperature reduction, improved SPO2, modulation of inflammatory/immune response, improvement in chest imaging (Figure 6), and reduction in the need for mechanical ventilation compared to 133 surviving patients in BST group. In the MIP group, the mean need for oxygen reduced from 11.69 L/min; SD: 3.40; (range: 5–16) at Day 0 to 3.61 L/min; SD: 2.63 (range: 0–8) at Day 5 (14 patients were weaned off ventilation on 3rd and 4th day of treatment) with a mean difference of −6.182 L/min and p = 0.001 (Wilcoxon signed‐rank test). While as in the BST group mean the need for oxygen reduced from 11.34 L/min; SD: 3.34; (range: 5–16) at Day 0 to 8.54 L/min; SD: 2.12(range: 3–12) at Day 5 (16 patients were weaned off ventilation on 5th day of treatment) with a mean difference of 3.87 and p = 0.05. Similarly, oxygen saturation (SPO2) levels increased (80.07 ± 8.30 on Day 0 to 91.38 ± 5.70 mean difference was +10.45 and p = 0.001in MIP group and 82.45 ± 14.45 on Day 0 to 88.98 ± 15.56 mean difference was +5.45 and p = 0.51 in BST group) on 5th day of treatment (Table 4). From Table 4, it can be appreciated that on the 5th day of treatment need for oxygen was significantly reduced in almost all patients in the MIP group, with 14 patients being weaned off from ventilation, 21 patients required low oxygen flow, and other 56 patients required moderate oxygen flow (Table 4). Chest CT scan findings on the day before treatment with MIP showed lesions, which are characteristic for COVID‐19 (Figure 6). When comparing CT scan findings on the 5th day of treatment, 77 patients (in the MIP group and 75 patients in the BST group) showed improvement in pulmonary lesions and resolution of lesions towards normality. The resolution of pulmonary lesions observed in CT scan findings in the MIP group were completely in agreement with levels of oxygen saturation observed in these patients, where almost 100% of patients showed a dramatic increase in oxygen saturation on the 5th‐day posttreatment. An important finding of the present study was a significant reduction (p = 0.01) in hospitalization stay in MIP group compared to a hospital stay in BST group as in present study patients were discharged from hospital following twin criteria which include: (i) negative results on PCR for SARS‐CoV‐2 on two test conducted on 3 days apart, (ii) absence of fever for 3 days before discharge from hospital (Figure 4F).
Figure 5.
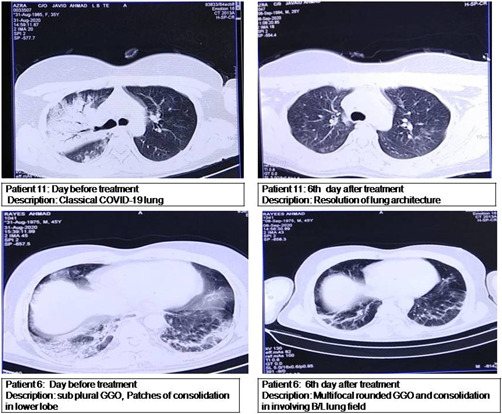
Radiological findings in severe coronavirus disease of 2019 (COVID‐19) patients treated with Mycobacterium indicus pranii
Figure 6.
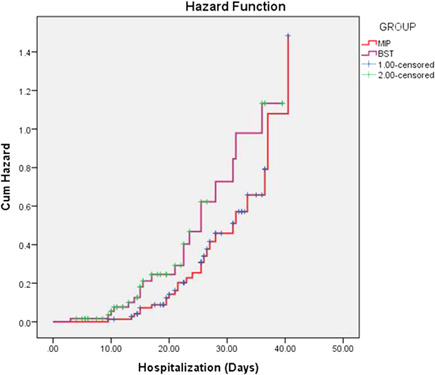
Kaplan–Meier estimates of the time from intervention (administration of Mycobacterium indicus pranii [MIP] and best standard treatment [BST]) to death or to improvement. The ordinal scale ranging from Category 1 (death) to Category 0 (discharged with full return to baseline physical function)
Table 4.
Comparison of primary and secondary outcomes in BST arm and convalescent plasma arm
| Characteristics | Pre‐BST (n = 133) | Post‐BST(n = 133) | p | Pre‐MIP (n = 91) | Post‐MIP (n = 91) | p |
|---|---|---|---|---|---|---|
| Need for high flow mechanical ventilation | 92.85% | 83.51% | ≤0.05 | 90.69% | 50% | ≤0.01 |
| Hospitalization (days) | 25.31 ± 12.83 | 18.37 ± 13.77 | ≤0.01 | |||
| All‐cause mortality | 36.66% | 13.33% | ≤0.01 | |||
| SPO2 (%) (with posttreatment comparison on 5th day) | 81.02 ± 12.82 | 86.22 ± 13.22 | 0.02 | 80.07 ± 8.30 | 91.38 ± 5.70 | ≤0.01 |
| Negative conversion of SARS‐CoV‐2 on basis of RT‐PCR on 7th day | 79 (59.39%) | 67 (73.62%) | ≤0.05 | |||
| Negative conversion of SARS‐CoV‐2 on basis of RT‐PCR on 14th day | 87 (65.41%) | 83 (91.20%) | ≤0.01 | |||
| Retrogression to moderate diseases | 75 (56.39%) | 71 (78.02%) | ≤0.05 | |||
Abbreviations: BST, best standard treatment; MIP, Mycobacterium indicus pranii; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
From Tables 4 and 5, it can be seen that MIP therapy is quite superior compared to BST therapy in amelioration of clinical symptoms and offers therapeutic benefits in severely ill COVID‐19 patients as indicated by primary and secondary outcomes of diseases. Based on the findings, it can be well postulated that MIP offers therapeutic benefits by modulation of immune‐inflammatory pathways, henceforth ameliorating/preventing cytokine‐induced tissue damage as indicated by normalization of lung architecture.
Table 5.
Comparison of secondary outcome in BST arm and convalescent plasma arm
| Characteristics | BST arm (n = 133) | MIP arm (n = 91) | p |
|---|---|---|---|
| Resolution of cough on 5th day; n (%) | 85 (63.90%) | 67 (73.62%) | 0.43 |
| Resolution of fever on 5th day; n (%) | 95 (71.42%) | 66 (72.52%) | 0.82 |
| Resolution of myalgia on 5th day; n (%) | 58 (43.60%) | 87 (95.60%) | ≤0.01 |
| Resolution of sore throat on 5th day; n (%) | 62 (46.61%) | 71 (78.02%) | ≤0.01 |
| Resolution of shortness of breath on 5th day; n (%) | 39 (29.32%) | 73 (80.21%) | ≤0.01 |
| Days of respiratory support postenrollment | 65 (48.87%) | 56 (61.53%) | ≤0.05 |
| Need for invasive mechanical ventilation on 5th day postenrollment; n (%) | 71 (53.38%) | 35 (38.46%) | ≤0.01 |
Abbreviations: BST, best standard treatment; MIP, Mycobacterium indicus pranii.
3.5. Adverse events in MIP group
On the 5th day of treatment, total leukocyte count (TLC) levels in 35 patients, hemoglobin in 49, neutrophil 35, and lymphocyte in 45 were decreased compared to Day 0 values. Similarly, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic‐pyruvic transaminase (SGPT), and creatinine levels were increased in 42 (4 values missing), 28 (2 values missing), and 21 patients, respectively. It is important to note all these changes were nonsignificant, except creatinine levels were significantly reduced on the 5th day. None of the patients in the MIP group exhibited any major complication during hospitalization that can be attributed to the use of MIP in severely ill COVID‐19 patients.
These findings are of immense utility as the majority of countries are facing a shortage of mechanical ventilators, which results in a difficult task for the clinician in prioritizing patients for intensive care unit (ICU), and subsequently, this shortage results in increased mortality in hospitalized patients. The significant effect of MIP was on estimates of the probability of survival or reduction in the need for mechanical ventilation.
4. DISCUSSION
With the spread of COVID‐19 across the globe, researchers from different clinical settings claimed a wide range of drugs as effective therapeutic remedies against SARS‐CoV‐2, but in the majority of the case, these claims were refuted by placebo‐controlled double‐blind clinical trials. 22 Presently there is no effective treatment available against COVID‐19, although a wide spectrum of drugs has been repurposed, and the safety and efficacy of these drugs are yet to be evaluated. 23 Until the date of drafting, this manuscript there are only 3–4 studies that have reported a reduction in intensive care stay of severely ill COVID‐19 patients. 24 Present study reports that inclusion of MIP in therapeutic protocol results in a significant reduction in the number of patients that need mechanical ventilation and MIP results in decreased mortality in severely ill COVID‐19 patients. Association was stronger when the need for ventilation or death was considered together as a composite parameter. Results of the present study are in agreement with earlier studies using Tocilizumab recombinant humanized monoclonal antibody directed against IL‐6 receptor 25 and anakinra recombinant human IL‐1 receptor antagonist. 26 Both studies were based on the hypothesis of hyper inflammation ad immune dysregulation implicated in the severe progression of COVID‐19. Immunotherapeutic against COVID‐19 mostly work by suppression of inflammatory cytokines, inhibition of kinases, and complement cascade. 27 However, there is still a lack of completely assured drugs against COVID‐19.
Inefficacy of a wide spectrum of antiviral drugs against SARS‐CoV‐2 has questioned their use in COVID‐19, and concurrently there are a number of large cohort studies that report hyper‐inflammation and immune dysfunction named as cytokine storm as pathogenic hotspots in severe progression in COVID‐19. It is widely accepted that cytokine storm significantly increases ICU stay, the need for mechanical ventilation, and increased mortality in COVID‐19. 28 , 29 Abnormal immune‐inflammatory response causes hyper‐activation of CD4 and CD8 lymphocytes, which subsequently causes tissue necrosis, and fibrosis in visceral organs. 30 Autopsy of pulmonary tissue and CT scan findings have revealed bilateral diffuse alveolar damage and fibroblastic multiplication in alveolar tissues. 31 In spite of ample evidence offered by a plethora of studies, immune modulators have been nevertheless very rarely introduced in the therapeutic protocol of COVID‐19 patients. During our literature survey, we found only a limited number of immunologically active drugs in a limited number of western countries included in the regular treatment with encouraging results in severely ill COVID‐19 patients. 32 , 33
Based on these findings and earlier use of MIP, it cannot be denied the beneficial role of MIP in the regulation of immune pathways and amelioration of hyperactive inflammatory state in SARS‐CoV‐2. Therefore, we decided to include MIP at a dose rate of 0.1 ml intramuscular three times a day at three different sites for 3 consecutive days with standard therapy used in our institute. In the present case, a series of critically ill COVID‐19 patients treated with MIP were compared with critically ill COVID‐19 patients treated without MIP. In this real‐life setting, we found a significant reduction in the need for mechanical oxygenation or death in severely infected COVID‐19 patients treated with standard therapy with the inclusion of MIP compared to patients treated with standard therapy without MIP. The association with the use of MIP was found stronger with respect to normalization of lung architecture (CT scan), a significant increase in oxygen saturation, reduction/weaning off mechanical ventilation. Furthermore, concomitant improvement in biological and clinical parameters offers indirect evidence of the beneficial/effective role of MIP in COVID‐19 patients. Our results are in concurrence with earlier studies on a small cohort of COVID‐19 patients who reported a decrease in overall death rates and early clearance of viral load in patients treated with different immune modulators. 34 Classical findings in severe COVID‐19 patients are multiple organ damage caused by cytokine storm induced by SARS‐CoV‐2, and currently, in Indian conditions, no immune‐modulator is widely included in the COVID‐19 population because of a wide spectrum of causes, which range from efficacy, safety, availability, and economic reasons. 35 , 36 However, corticosteroids used in earlier patient populations have given controversial findings, which have led many clinicians to discontinue their use. 37 Earlier studies have reported the immunotherapeutic as well as the immunoprophylactic effect of MIP. These findings are attributed to balancing inflammatory pathways, early protective Th1 immune response, downregulation of Th2 response, and immune modulation. 32 , 38 Earlier studies have reported that MIP when used as an adjuvant in combination with other standard drugs, the combinational therapeutic protocol, results in apoptosis of hyperactivated immune cells henceforth keeps an effective check on hyper inflammation. 39 Henceforth, it can be proposed that MIP prevents cytokine storm‐induced lung damage in COVID‐19. In addition to this, MIP has been found to result in accelerated viral clearance by activation of the MyD88 pathway in Th1‐mediated innate immune response. 40 Furthermore, immune‐modulatory action of MIP was reported by a recent clinical trial conducted on COVID‐19 patients. The study reported a significant reduction in C‐reactive protein (an inflammatory marker) in those patients who were treated with MIP. 41 Furthermore, a recently genomic‐wide association study has attributed the immune‐modulatory role of MIP to high antigenic potential. 42 MIP, as an adjuvant to standard therapy against tuberculosis, revealed immune modulation and regulation of inflammatory process in later part treatment is perhaps useful in the restoration of normal tissue architecture. 43 Furthermore, there are several reports on the aerosol route of delivery of MIP to induce local lung immune response. 44 Taken together, these findings, in addition to the results from the present study, suggest that MIP modulates immune response by regulating/subduing cytokine storm and simultaneously cause activation of those immune pathways, which result in enhanced viral clearance.
In clinical settings, when patients are under a wide range of therapeutic preparations simultaneously, it is very difficult to assign side effects to a particular drug. Assessment of side effects associated with the use of MIP in the present study was an increase in alkaline phosphatase levels, SGOT, SGPT, and deceases in TLC and hemoglobin in some patients. However, the use of MIP in earlier studies has not reported any adverse effects associated with the use of the drug. Similarly, in the present study, we could not find any adverse effect during the hospitalization of COVID‐19 patients. However, to have a clear view of safety, patients undergoing treatment with MIP should be followed for a longer duration.
In conclusion, the use of MIP use for 3 days at a dose of 0.1 ml three times resulted in a significant reduction in the stay of patients in ICU and mortality in severe COVID‐19 patients. Taken together, MIP gives an opportunity for use in COVID‐19 patients in developing countries like India. The beneficial role it offers ranges from its reported immunomodulatory role, balancing inflammatory pathway, economically cheap, and easily available.
Nevertheless, the limitation of the present study was our results are based on a small cohort population, and we cannot rule out sampling and time‐varying confounders that might have affected results in our study; our study was an open‐label study that might have affected various clinical decisions, the study being nonrandom so the effect of unmeasured confounding cannot be ruled out. Therefore, it is suggested that clinical trials with a large sample size and of longer duration should be conducted to understand the beneficial role of MIP in COVID‐19 populations.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
Institutional ethical committee approved study (CDSCO U/P No: EC/NEW/INST/2020/7452/16
AUTHOR CONTRIBUTIONS
Naveed Nazir Shah, Khurshid Ahmad Dar, Syed Quibtiya, Aaliya Mohi Ud Din Azad, and Mehvish Mushtaq contributed significantly to the conception and design, and/or collection of data. Showkat Ul Nabi, Sofi Imtiyaz Ali, Muzafar Ahmad Rather, Wajid Mohammad Sheikh, and Showkeen Muzamil Bashir helped in data analysis and interpretation, assisted in writing the manuscript, and revised the manuscript decisively for imperative intellectual content. Naveed Nazir Shah is accountable for the quality of the data and the reliability of the data collection and analysis.
ACKNOWLEDGMENT
The authors are obliged to all the staff members of Chest Disease Hospital Srinagar, India, for their kind support in the supervision of all COVID‐19 patients.
Shah NN, Dar KA, Quibtiya S, et al. Repurposing of Mycobacterium indicus pranii for the severe form of COVID‐19 patients in India: A cohort study. J Med Virol. 2022;94:1906‐1919. 10.1002/jmv.27547
DATA AVAILABILITY STATEMENT
The raw data used to support the findings of the present study are accessible from the corresponding author upon request.
REFERENCES
- 1. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. 10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Symptoms page. Accessed February 22, 2021. https://www.cdc.gov/coronavirus/2019-ncov/CDC
- 3. Ingraham NE, Lotfi‐Emran S, Thielen BK, et al. Immunomodulation in COVID‐19. Lancet Respir Med. 2020;8(6):544‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol. 2014;80(6):509‐514. [DOI] [PubMed] [Google Scholar]
- 5. Veronin MA, Lang A, Reinert JP. Remdesivir and coronavirus disease 2019 (COVID‐19): essential questions and answers for pharmacists and pharmacy technicians. J Pharm Technol. 2021;202(37):62‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borbone N, Piccialli G, Roviello GN, Oliviero G. Nucleoside analogs and nucleoside precursors as drugs in the fight against SARS‐CoV‐2 and other coronaviruses. Molecules. 2021;26(4):986. 10.3390/molecules26040986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vicidomini C, Roviello V, Roviello GN. Molecular basis of the therapeutical potential of clove (Syzygium aromaticum L.) and clues to its anti‐COVID‐19 Utility. Molecules. 2021;26:1880. 10.3390/molecules26071880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strizova Z, Smetanova J, Bartunkova J, Milota T. Principles and challenges in anti‐COVID‐19 vaccine development. Int Arch Allergy Immunol. 2021;182:339‐349. 10.1159/000514225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. CSIR fights COVID‐19 . Accessed May 8, 2020. https://urdip.res.in/COVID.19/vertical3.jsp
- 10. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaudhry H, Zhou J, Zhong Y, et al. Role of cytokines as a double‐edged sword in sepsis. In Vivo. 2013;27:669‐684. [PMC free article] [PubMed] [Google Scholar]
- 12. Saini V, Raghuvanshi S, Talwar GP, et al. Polyphasic taxonomic analysis establishes Mycobacterium indicus pranii as a distinct species. PLoS One. 2009;4(7):e6263. 10.1371/journal.pone.0006263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma SK, Katoch K, Sarin R, et al. Efficacy and safety of Mycobacterium indicus pranii as an adjunct therapy in Category II pulmonary tuberculosis in a randomized trial. Sci Rep. 2017;7(1):3354. 10.1038/s41598-017-03514-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta S, Singh S, Chouhan K. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatology Venereol Leprol. 2014;80:509‐514. [DOI] [PubMed] [Google Scholar]
- 15. Sharma P, Kar HK, Kaur H, et al. Induction of lepromin positivity and immunoprophylaxis in household contacts of multibacillary leprosy patients: a pilot study with a candidate vaccine, Mycobacterium w. Int J Lepr Other Mycobact Dis. 2000;68:136‐142. [PubMed] [Google Scholar]
- 16. Jaiswal SR, Malhotra P, Mitra DK, Mitra DK, Chakrabarti S. Focusing on a unique innate memory cell population of natural killer cells in the fight against COVID‐19: harnessing the ubiquity of cytomegalovirus exposure. Mediterr J Hematol Infect Dis. 2020;12(1):e2020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson B, Bekker LG, Ress S, Kaplan G. Recombinant interleukin 2 adjunctive therapy in multidrug‐resistant tuberculosis. Novartis Found Symp. 1998;217:99‐106. [DOI] [PubMed] [Google Scholar]
- 18. Gupta A, Ahmad FJ, Ahmad F, et al. Efficacy of Mycobacterium indicus pranii immunotherapy as an adjunct to chemotherapy for tuberculosis and underlying immune responses in the lung. PLoS One. 2012;7(7):39215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh IG, Mukherjee R, Talwar GP, Kaufmann SH. In vitro characterization of T cells in Mycobacterium w‐vaccinated mice. Infect Immun. 1992;60:257‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Health Commission of the People's Republic of China . Chinese management guideline for COVID‐19 (version 6.0). February 19, 2020. Accessed April 6, 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b.817.pdf
- 22. Winters R, Winters A, Amedee RG. Statistics: a brief overview. Ochsner J. 2010;10(3):213‐216. [PMC free article] [PubMed] [Google Scholar]
- 23. Parry AH, Wani AH, Yaseen M, et al. Spectrum of chest computed 348 tomographic (CT) findings in coronavirus disease‐19 (COVID‐19) patients in India. Eur J Radiol. 2020;129:139‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomized, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395:1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID‐19. N Engl J Med. 2020;382:2327‐2336. 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thorlund K, Dron L, Park J, et al. A real‐time dashboard of clinical trials for COVID‐19. Lancet Digit Health. 2020;2(6):e286‐e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bayat M, Asemani Y, Mohammadi MR, Sanaei M, Namvarpour M, Reyhaneh E. An overview of some potential immunotherapeutic options against COVID‐19. Int Immunopharmacol. 2021;95:107516. 10.1016/j.intimp.2021.107516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID‐19: interleukin‐6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID‐19: a cohort study. Lancet Rheumatol. 2020;2(7):e393‐e400. 10.1016/S2665-9913(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pipeline Review . Tocilizumab improves significantly clinical outcomes of patients with moderate or severe COVID‐19 pneumonia. April 28, 2020. Accessed June 4, 2020. https://pipelinereview.com/index
- 32. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395:473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019‐nCoV pneumonia. Lancet. 2020;195:683‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seguin A, Glacier L, Boutboul D, Lemiale V, Azoulay E. Pulmonary involvement in patients with hemophagocytic lymphohistiocytosis. Chest. 2016;149:1294‐1301. [DOI] [PubMed] [Google Scholar]
- 36. Cavalli G, De Luca G, Campochiaro C, et al. Interleukin‐1 blockade with high‐dose anakinra in patients with COVID‐19, acute respiratory distress syndrome, and hyper inflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325‐e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klopfenstein T, Zayet S, Lohse A, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID‐19 patients. Med Mal Infect. 2020;50:397‐400. 10.1016/j.medmal.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pandey RK, Bhatt KH, Dahiya Y. Mycobacterium indicus pranii supernatant induces apoptotic cell death in mouse peritoneal macrophages in vitro . PLoS One. 2011;6:e17093. 10.1371/journal.pone.0017093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bezemer GFG, Garssen J. TLR9 and COVID‐19: a multidisciplinary theory of a multifaceted therapeutic target. Front Pharmacol. 2021;11:601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sehgal IS, Guleria R, Singh S, Siddiqui MS, Agarwal R. A randomized trial of Mycobacterium w in critically ill patients with COVID‐19: ARMY‐1. ERJ Open Res. 2021;7(2):00059‐02021. 10.1183/23120541.00059-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharma P, Mukherjee R, Talwar GP, et al. Immunoprophylactic effects of the anti‐leprosy MIP vaccine in household contacts of leprosy patients: clinical field trials with a follow up of 8‐10 years. Lepr Rev. 2005;76(2):127‐143. [PubMed] [Google Scholar]
- 43. Perrin FM, Lipman MC, McHugh TD, Gillespie SH. Biomarkers of treatment response in clinical trials of novel antituberculosis agents. Lancet Infect Dis. 2007;7(7):481‐490. [DOI] [PubMed] [Google Scholar]
- 44. Jeyanathan M, Mu J, McCormick S, et al. Murine airway luminal antituberculosis memory CD8 T cells by mucosal immunization are maintained via antigen‐driven in situ proliferation, independent of peripheral T cell recruitment. Am J Respir Crit Care Med. 2010;181(8):862‐872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used to support the findings of the present study are accessible from the corresponding author upon request.


