Abstract
This study investigated the effect of melatonin on clinical outcomes in patients with coronavirus disease 2019 (COVID‐19). We searched PubMed, the Web of Science, the Cochrane Library, Ovid MEDLINE, and Clinicaltrials.gov for randomized controlled trials (RCTs) published before September 11, 2021. Only RCTs that compared the clinical efficacy of melatonin with a placebo in the treatment of patients with COVID‐19 were included. The primary outcome measure was the clinical recovery rate. We included three RCTs in this meta‐analysis. Melatonin 3 mg three times daily was administered in one RCT, and 3 or 6 mg daily before bedtime in the other two trials. Treatment duration was 14 days in two RCTs and 7 days in one trial. The clinical recovery rates were 94.2% (81/86) and 82.4% (70/85) in the melatonin and control groups, respectively. Overall, patients receiving melatonin had a higher clinical recovery rate than did the controls (odds ratio [OR]: 3.67; 95% CI: 1.21−11.12; I 2 = 0%, p = 0.02). The risk of intensive care unit admission was numerically lower in the melatonin group than in the control group (8.3% [6/72] vs. 17.6% [12/68], OR: 0.45; 95% CI: 0.16−1.25; I 2 = 0%, p = 0.13), and the risk of mortality was numerically lower in the melatonin group than in the control group (1.4% [1/72] vs. 4.4% [3/68], OR: 0.32; 95% CI: 0.03−3.18; I 2 = 0%, p = 0.33). In conclusion, melatonin may help improve the clinical outcomes of patients with COVID‐19.
Keywords: COVID‐19, melatonin, outcome, SARS‐CoV‐2
Highlight
This study investigated the effect of melatonin on clinical outcomes in patients with coronavirus disease 2019.
Patients receiving melatonin had a higher clinical recovery rate than did the controls (odds ratio: 3.67; 95% CI: 1.21−11.12; I 2 = 0%, p = 0.02).
The risk of intensive care unit admission was only insignificantly lower in the melatonin group than in the control group.
The risk of mortality was insignificantly lower in the melatonin group than in the control group.
1. INTRODUCTION
As of September 10, 2021, more than 220 million confirmed cases of coronavirus disease 2019 (COVID‐19) have been reported to the World Health Organization, 1 and more than 4 million deaths have been recorded. For patients infected by severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2), the estimated case fatality rate is 2.0%. 1 Although relevant authorities worldwide, including scientists, have worked aggressively to combat the COVID‐19 pandemic, effective treatments for SARS‐CoV‐2 infection remain limited. 2 Currently, only corticosteroid and tocilizumab could help reduce the mortality of patients with COVID‐19. 3 , 4 Remdesivir can improve the clinical outcomes of hospitalized patients with COVID‐19, but it does not provide any survival benefits. 5 Although neutralizing monoclonal antibodies (mAbs) have demonstrated potential in randomized controlled trials (RCTs), current evidence is insufficient for drawing solid conclusions regarding the efficacy of SARS‐CoV‐2 neutralizing mAbs for patients with COVID‐19. 6 Therefore, more effective therapeutic options against this disease are urgently required.
To combat SARS‐CoV‐2, several artificial‐intelligence‐based approaches have been developed for the rapid identification of candidate repurposable drugs that target it. 7 , 8 Melatonin can serve as an anti‐inflammatory, an immunomodulator, and an antioxidant agent, and its efficacy has been demonstrated in multiple experimental disease models and in clinical trials for sepsis, including that triggered by viral infections; it has thus been considered as a potential treatment for COVID‐19. 9 , 10 Furthermore, several clinical studies, including RCTs, have demonstrated the efficacy of melatonin in the treatment of patients with COVID‐19. 11 , 12 , 13 , 14 However, most of these RCTs have included only a small sample size. Hence, systematic reviews evaluating the effects of melatonin in larger patient populations are necessary to ascertain its efficacy. Accordingly, we conducted this meta‐analysis of RCTs to evaluate melatonin for its efficacy and ability to improve the clinical outcomes of patients with COVID‐19.
2. METHODS
2.1. Study search and selection
We searched PubMed, the Web of Science, the Cochrane Library, Ovid MEDLINE, and Clinicaltrials.gov for RCTs published before September 11, 2021. The following search terms were used: “melatonin,” “agomelatine,” “rozerem,” “circadin,” “slenyto,” “COVID‐19,” “SARS‐CoV‐2,” “coronavirus,” “2019‐nCoV,” and “corona‐virus.” We included RCTs that compared the clinical efficacy of melatonin with a placebo in the treatment of patients with COVID‐19. The inclusion criteria were (1) patients with COVID‐19; (2) used melatonin as intervention; (3) designed as RCT; and (4) the data regarding the clinical outcome of interest were available. We excluded case reports, case series, observational studies, and retrospective cohort studies. Two investigators (S. H. L. and L. C. L.) independently screened and reviewed each study. In case of any disagreement, a third investigator (C. C. L.) made the final decision. For each included study, we extracted the following data: publication year, study design, melatonin regimens, clinical outcomes, and inflammatory markers. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyzes guidelines, 15 and the study protocol was registered in PROSPERO (CRD42021278500).
2.2. Outcome measures
The primary outcome measure was the rate of clinical recovery and the secondary outcome measures were the need for intensive care unit (ICU) admission, mortality, and inflammation markers (C‐reactive protein [CRP]).
2.3. Data analysis
We used the Cochrane risk‐of‐bias tool 16 to assess the quality of the included RCTs and their associated risk of bias. We performed all statistical analyzes using Review Manager (version 5.3; Nordic Cochrane Center). Heterogeneity was defined when I 2 > 50%. We used a fixed‐effects model when the data were homogeneous and used a random‐effects model when the data were heterogeneous. We calculated pooled odds ratios (ORs) and mean differences along with 95% CI for outcome analyses.
3. RESULTS
3.1. Study selection
The search results yielded a total of 62 studies from the online databases (n = 12 from PubMed; n = 16 from the Web of Science; n = 10 from Ovid MEDLINE; n = 14 from the Cochrane Library, and n = 10 from Clinicaltrials.gov), of which 32 were excluded as duplicates. In addition, we determined 27 studies to be irrelevant after screening their titles and abstracts. Finally, we included three RCTs 12 , 13 , 14 in this meta‐analysis (Figure 1, Appendix 1).
Figure 1.
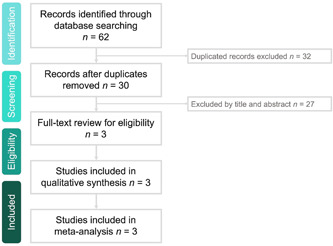
Flow diagram of study identification and eligibility assessment processes
3.2. Study characteristics
Of the three RCTs, only one 13 was a double‐blind study (Table 1). All included RCTs were single‐center studies and conducted in Iran. 12 , 13 , 14 Each of them followed a distinct melatonin regimen. In the trial by Farnoosh et al., 13 melatonin 3 mg three times daily was administered, and in the other two trials, 12 , 14 it was administered 3 or 6 mg daily before bedtime. In the trial by Mousavi et al., 14 melatonin was used for 7 days, but in the other two trials, 12 , 13 it was used for 14 days. Overall, the experimental group treated with melatonin and the control group consisted of 86 and 85 patients, respectively. In addition to melatonin given in the experimental group, the standard of care, including hydroxychloroquine, atazanavir, methylprednisolone, azithromycin, naproxen, and lopinavir/ritonavir, was applied in both experimental and control groups according to the Iranian national COVID‐19 treatment protocol. The risk of bias in each study was shown in Figure 2.
Table 1.
Characteristics of included studies
| Author, year | Study design | Study period | Study sites | Study population | Regimen of melatonin | No of patients | |
|---|---|---|---|---|---|---|---|
| Study group | Control group | ||||||
| Alizade et al. (2021) 12 | Randomized, single‐blinded trial | Between June 30, 2020 and August 5, 2020 | Tehran University of Medical Sciences in Iran | Mild to moderate COVID‐19 patients | Melatonin at 6 mg before bedtime for 14 days | 14 | 17 |
| Farnoosh et al. (2021) 13 | Randomized, double‐blind clinical trial | From April 25, 2020 to June 5, 2020 | Baqiyatallah University of Medical Sciences in Iran | Mild to moderate COVID‐19 | Melatonin at 3 mg three times daily for 14 days | 24 | 20 |
| Mousavi et al. (2021) 14 | Randomized open‐label, active‐controlled clinical trial | From April 14, 2020 to June 15, 2020 | Mazandaran University of Medical Sciences in Iran | Hospitalized patients with COVID‐19 | Melatonin at 3 mg before bedtime for 7 days | 48 | 48 |
Figure 2.
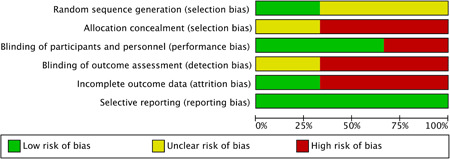
Summary of the risk‐of‐bias assessment
3.3. Primary outcome
The clinical recovery rates were 94.2% (81/86) and 82.4% (70/85) in the melatonin and control groups, respectively. Overall, patients receiving melatonin had a higher clinical recovery rate than did the controls (OR: 3.67; 95% CI: 1.21−11.12; I 2 = 0%, p = 0.02; Figure 3). The result remained unchanged when the random‐effects model was used (OR: 3.61; 95% CI: 1.17−11.16; I 2 = 0%, p = 0.03).
Figure 3.
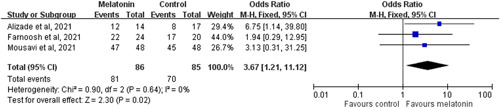
Forest plot showing the effect of melatonin compared with the control on the clinical recovery rate
3.4. Secondary outcomes
On the basis of the two RCTs 13 , 14 that reported the need for ICU admission, we found that the risk of ICU admission was numerically lower in the melatonin group than in the control group (8.3% [6/72] vs. 17.6% [12/68]). However, the difference was not statistically significant (OR: 0.45; 95% CI: 0.16−1.25; I 2 = 0%, p = 0.13). A pooled analysis of the two RCTs 13 , 14 that had available data on mortality indicated a similar trend in that the risk of mortality was numerically lower in the melatonin group than in the control group (1.4% [1/72] vs. 4.4% [3/68]). However, the difference did not reach statistically significance (OR: 0.32; 95% CI: 0.03−3.18; I 2 = 0%, p = 0.33).
Regarding inflammation markers, no significant difference was observed between the melatonin group and the control group in terms of the rate of normalization of follow‐up CRP levels (OR: 1.14; 95% CI: 0.34−3.89; I 2 = 0%, p = 0.83) or the values of follow‐up CRP (MD, −1.03; 95% CI: −3.47 to 1.42; I 2 = 0%, p = 0.41).
4. DISCUSSION
In this meta‐analysis, we reviewed three RCTs 12 , 13 , 14 to investigate the additional use of melatonin for the treatment of patients with COVID‐19. First, we observed that melatonin was associated with a higher clinical recovery rate than a placebo in the treatment of patients with COVID‐19. This result was based on the analysis of the three RCTs with low heterogeneity. Second, although the melatonin group had a numerically lower risk of ICU admission and mortality than did the control group, the differences were not statistically significant. Finally, melatonin was also associated with numerically lower levels of inflammation in terms of follow‐up CRP, although no statistically significant difference was observed between the two groups. These findings are consistent with those of recent observational studies. 11 , 17 Castillo et al. 17 presented a case series of 10 patients and demonstrated that patients with COVID‐19 receiving a high dose of melatonin (36−72 mg/day) experienced clinical improvement more rapidly, had less need for mechanical ventilation (MV), had a shorter hospital stay, and possibly experienced lower mortality than did patients not given melatonin during the same period. Ramlall et al. demonstrated that melatonin exposure after intubation was associated with a positive outcome for periods of intubation in 112 patients with COVID‐19 who required MV (adjusted hazard ratio: 0.127; 95% CI, 0.06−0.269; p < 0.05). Overall, these findings indicate that melatonin may positively impact the clinical outcome of patients with COVID‐19 and suggest a potential role for melatonin in the treatment of patients infected by SARS‐CoV‐2.
According to our review of the literature, the present study is the first meta‐analysis to investigate the usefulness of melatonin in patients with COVID‐19 and to demonstrate its potential benefits for improving clinical outcomes. Although this study cannot provide plausible mechanisms, melatonin is a well‐known hormone that exhibits anti‐inflammatory and antioxidative activity. 18 In addition, studies have demonstrated its ability to protect against acute lung injury or acute respiratory distress syndrome caused by viral and other pathogens. 8 , 19 , 20 Melatonin is also effective in reducing vessel permeability and anxiety. 19 , 20 , 21 It can be used for sedation and improving sleep quality in critically ill patients. 21 All these characteristics might result in improved clinical outcomes for patients with COVID‐19.
This study has several limitations. The number of study patients was small; hence, some differences between this study and control groups might have reached statistical significance had the numbers been adequate. All three RCTs 12 , 13 , 14 were single‐center studies and were conducted in Iran, which could limit their generalizability. Additional large‐scale RCTs are required to validate our findings; currently, several ongoing RCTs are investigating the usefulness of melatonin as a treatment for patients with COVID‐19 in both the ICU and in outpatient departments. 22 , 23 , 24 , 25 Although there were some differences in study design between the three included RCTs, 12 , 13 , 14 all the outcome analyses in this study demonstrated low heterogeneity, which might minimize the confounding effect of heterogeneity. Melatonin is used to treat circadian rhythm sleep‐wake disorders and insomnia; however, in this study, we did not evaluate its effect on the sleep quality of patients with COVID‐19. Because only one RCT 14 reported results regarding this factor and it confirmed that oral melatonin could substantially improve the sleep quality of hospitalized patients with COVID‐19. 14 The safety of melatonin treatment was not evaluated in this study due to the lack of available data for analysis. Although the safety of melatonin has been demonstrated in clinical practice, 26 , 27 , 28 further study is still required to assess patient tolerance of melatonin in the treatment of COVID‐19. Finally, because the definition of the rate of clinical recovery was not comprehensively described in the original studies, we were unable to make an imprecise statement regarding this outcome.
In conclusion, this meta‐analysis revealed that melatonin may help improve the clinical outcomes of patients with COVID‐19 and suggested its potential role. However, further large‐scale research is required to confirm our findings.
AUTHOR CONTRIBUTIONS
Conception: Shao‐Huan Lan, Chih‐Cheng Lai. Study design: Shao‐Huan Lan, Hong‐Zin Lee, Chien‐Ming Chao. Analysis and interpretation: Shao‐Huan Lan, Hong‐Zin Lee, Chien‐Ming Chao, Shen‐Peng Chang, Li‐Chin Lu. Drafted or written: Chih‐Cheng Lai. Substantially revised or critically review: Chih‐Cheng Lai.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
APPENDIX 1. SEARCH STRATEGY
| PubMed search strategy—last searched on September 11, 2021 | Results | |
|---|---|---|
| 1 | Search: ((((agomelatine[Title/Abstract]) OR (Rozerem[Title/Abstract])) OR (Circadin[Title/Abstract])) OR (Slenyto[Title/Abstract])) OR (melaton*[Title/Abstract]) | 27154 |
| 2 | Search: ((((Covid‐19[Title/Abstract]) OR (SARS‐CoV‐2[Title/Abstract])) OR (coronaviru[Title/Abstract])) OR (2019‐nCoV[Title/Abstract])) OR (corona‐virus[Title/Abstract]) | 178993 |
| 3 | Search: random*[Title/Abstract] | 1254316 |
| 4 | Search: ((((((agomelatine[Title/Abstract]) OR (Rozerem[Title/Abstract])) OR (Circadin[Title/Abstract])) OR (Slenyto[Title/Abstract])) OR (melaton*[Title/Abstract])) AND (((((Covid‐19[Title/Abstract]) OR (SARS‐CoV‐2[Title/Abstract])) OR (coronavirus[Title/Abstract])) OR (2019‐nCoV[Title/Abstract])) OR (corona‐virus[Title/Abstract]))) AND (random*[Title/Abstract]) | 12 |
| Web of Science search strategy—last searched on September 11, 2021 | Results | |
|---|---|---|
| 1 | melaton* (Topic) or Agomelatine (Topic) or Rozerem (Topic) or Circadin (Topic) or Slenyto (Topic) | 55952 |
| 2 | Covid‐19 (TOPIC) or SARS‐CoV‐2 (TOPIC) or coronavirus (TOPIC) or 2019‐nCoV (TOPIC) or corona‐virus (TOPIC) | 304144 |
| 3 | Random* (Topic) | 3539064 |
| 4 | #1 AND #2 AND #3 | 16 |
| Ovid Medline search strategy—last searched on September 11, 2021 | Results | |
|---|---|---|
| 1 | (melaton* or Agomelatine or Rozerem or Circadin or Slenyto).ab. | 24793 |
| 2 | (Covid‐19 or SARS‐CoV‐2 or coronavirus or 2019‐nCoV or corona‐virus).ab. | 108783 |
| 3 | Random*.ab. | 1191078 |
| 4 | 1 and 2 and 3 | 10 |
| Cochrane Library search strategy—last searched on September 11, 2021 | ||
|---|---|---|
| 1 | (melaton*):ti,ab,kw OR (Agomelatine):ti,ab,kw OR (Rozerem):ti,ab,kw OR (Circadin):ti,ab,kw OR (Slenyto):ti,ab,kw | 3382 |
| 2 | (Covid 19):ti,ab,kw OR (SARS CoV 2):ti,ab,kw OR (coronavirus):ti,ab,kw OR (2019 nCoV):ti,ab,kw OR (corona virus):ti,ab,kw | 7295 |
| 3 | (Random*):ti,ab,kw | 1100367 |
| 4 | #1 AND #2 AND #3 | 14 |
| Clinicaltrials.gov search strategy—last searched on September 11, 2021 | ||
|---|---|---|
| 1 | Melatonin | |
| 2 | COVID‐19 | |
| 3 | 1 AND 2 | 10 |
Lan S‐H, Lee H‐Z, Chao C‐M, Chang S‐P, Lu L‐C, Lai C‐C. Efficacy of melatonin in the treatment of patients with COVID‐19: a systematic review and meta‐analysis of randomized controlled trials. J Med Virol. 2022;94:2102‐2107. 10.1002/jmv.27595
Shao‐Huan Lan, Hong‐Zin Lee, and Chien‐Ming Chao contributed equally to this study.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. WHO. Accessed September 10, 2021. https://covid19.who.int/
- 2. Lai CC, Chao CM, Hsueh PR. Clinical efficacy of antiviral agents against coronavirus disease 2019: a systematic review of randomized controlled trials. J Microbiol Immunol Infect. 2021;54(5):767‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shankar‐Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL‐6 antagonists and mortality among patients hospitalized for COVID‐19: a meta‐analysis. JAMA. 2021;326(6):499‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324(13):1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai CC, Chen CH, Wang CY, Chen KH, Wang YH, Hsueh PR. Clinical efficacy and safety of remdesivir in patients with COVID‐19: a systematic review and network meta‐analysis of randomized controlled trials. J Antimicrob Chemother. 2021;76(8):1962‐1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kreuzberger N, Hirsch C, Chai KL, et al. SARS‐CoV‐2‐neutralising monoclonal antibodies for treatment of COVID‐19. Cochrane Database Syst Rev. 2021;9(9):Cd013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network‐based drug repurposing for novel coronavirus 2019‐nCoV/SARS‐CoV‐2. Cell Discov. 2020;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Artigas L, Coma M, Matos‐Filipe P, et al. In‐silico drug repurposing study predicts the combination of pirfenidone and melatonin as a promising candidate therapy to reduce SARS‐CoV‐2 infection progression and respiratory distress caused by cytokine storm. PLoS One. 2020;15(10):e0240149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Acuña‐Castroviejo D, Escames G, Figueira JC, de la Oliva P, Borobia AM, Acuña‐Fernández C. Clinical trial to test the efficacy of melatonin in COVID‐19. J Pineal Res. 2020;69(3):e12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reiter RJ, Abreu‐Gonzalez P, Marik PE, Dominguez‐Rodriguez A. Therapeutic algorithm for use of melatonin in patients with COVID‐19. Front Med. 2020;7:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramlall V, Zucker J, Tatonetti N. Melatonin is significantly associated with survival of intubated COVID‐19 patients. medRxiv. 2020. 10.1101/2020.10.15.20213546 [DOI] [Google Scholar]
- 12. Alizadeh Z, Keyhanian N, Ghaderkhani S, Dashti‐Khavidaki S, Shokouhi Shoormasti R, Pourpak Z. A pilot study on controlling coronavirus disease 2019 (COVID‐19) inflammation using melatonin supplement. Iran J Allergy Asthma Immunol. 2021;20(4):494‐499. [PubMed] [Google Scholar]
- 13. Farnoosh G, Akbariqomi M, Badri T, et al. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID‐19: a randomized, double‐blind clinical trial. Arch Med Res. 2021; S0188‐4409(21):00141‐00147. 10.1016/j.arcmed.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mousavi SA, Heydari K, Mehravaran H, et al. Melatonin effects on sleep quality and outcomes of COVID‐19 patients: an open‐label, randomized, controlled trial. J Med Virol. 2021;94(1):263‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103‐112. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castillo RR, Quizon GRA, Juco MJM, et al. Melatonin as adjuvant treatment for coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC‐19 PRO): a case series. Melatonin Res. 2020;3(3):297‐310. [Google Scholar]
- 18. Zhang R, Wang X, Ni L, et al. COVID‐19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cross KM, Landis DM, Sehgal L, Payne JD. Melatonin for the early treatment of COVID‐19: a narrative review of current evidence and possible efficacy. Endocr Pract. 2021;27(8):850‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Srinivasan V, Mohamed M, Kato H. Melatonin in bacterial and viral infections with focus on sepsis: a review. Recent Pat Endocr Metab Immune Drug Discov. 2012;6(1):30‐39. [DOI] [PubMed] [Google Scholar]
- 21. Lewandowska K, Małkiewicz MA, Siemiński M, Cubała WJ, Winklewski PJ, Mędrzycka‐Dąbrowska WA. The role of melatonin and melatonin receptor agonist in the prevention of sleep disturbances and delirium in intensive care unit ‐ a clinical review. Sleep Med. 2020;69:127‐134. [DOI] [PubMed] [Google Scholar]
- 22. Ameri A, Asadi MF, Kamali M, et al. Evaluation of the effect of melatonin in patients with COVID‐19‐induced pneumonia admitted to the intensive care unit: a structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. García IG, Rodriguez‐Rubio M, Mariblanca AR, et al. A randomized multicenter clinical trial to evaluate the efficacy of melatonin in the prophylaxis of SARS‐CoV‐2 infection in high‐risk contacts (MeCOVID trial): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodríguez‐Rubio M, Figueira JC, Acuña‐Castroviejo D, Borobia AM, Escames G, de la Oliva P. A phase II, single‐center, double‐blind, randomized placebo‐controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID‐19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ziaei A, Davoodian P, Dadvand H, et al. Evaluation of the efficacy and safety of melatonin in moderately ill patients with COVID‐19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferlazzo N, Andolina G, Cannata A, et al. Is melatonin the cornucopia of the 21st century? Antioxidants. 2020;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non‐pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281. 10.1186/s13643-019-1163-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramos E, López‐Muñoz F, Gil‐Martín E, et al. The coronavirus disease 2019 (COVID‐19): key emphasis on melatonin safety and therapeutic efficacy. Antioxidants. 2021;10(7):1152. 10.3390/antiox10071152 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


