Abstract
Objectives
Lung ultrasound (LUS) holds the promise of an accurate, radiation‐free, and affordable diagnostic and monitoring tool in coronavirus disease 2019 (COVID‐19) pneumonia. We sought to evaluate the usefulness of LUS in the diagnosis of patients with respiratory distress and suspicion of interstitial severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pneumonia, in comparison to other imaging modalities.
Methods
This was a multicenter, retrospective study. LUS was performed, on Emergency Department (ED) arrival of patients presenting for possible COVID‐19 evaluation, by trained emergency physicians, before undergoing conventional radiologic examination or while waiting for the report. Scans were performed using longitudinal transducer orientation of the lung regions. CXR was interpreted by radiologists staffing ED radiology. Subjects were divided into two group based on molecular test results. LUS findings were compared to COVID test results, nonlaboratory data, and other imaging for each patient. Categorical variables were expressed as percentages and continuous variables as median ± standard error.
Results
A total of 479 patients were enrolled, 87% diagnosed with SARS‐CoV‐2 by molecular testing. COVID positive and COVID negative patients differed with respect to sex, presence of fever, and white blood cells count. Most common findings on lung point of care ultrasound (POCUS) for COVID‐positive patients were B‐lines, irregular pleural lines, and small consolidation. Normal chest X‐ray was found in 17.89% of cases.
Conclusions
This 479 patient cohort, with COVID‐19, found LUS to be noninferior to chest X‐ray (CXR) for diagnostic accuracy. In this study, COVID‐positive patients are most likely to show B lines and sub‐pleural consolidations on LUS examination.
Keywords: COVID‐19, lung ultrasound, point‐of‐care, SARS‐CoV‐2
Abbreviations
- ANCOVA
analysis of covariance
- CHF
chronic heart failure
- COPD
chronic obstructive pulmonary disease
- COVID‐19
coronavirus disease 2019
- CT
computed tomography
- CXR
chest X‐ray
- EAU
Emergency Acceptance Unit
- ED
Emergency Department
- ICU
intensive care unit
- LUS
lung ultrasound
- PCR
polymerase chain reaction
- POCUS
point of care ultrasound
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Coronavirus disease 2019 (COVID‐19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a newly emergent coronavirus that was first recognized in Wuhan, Hubei province, China, in December 2019. 1 Since its advent, great effort has been put to clarify the most efficient method of triaging and prioritizing patients for testing and isolation. 2 Although most patients experience mild disease, 14% of patients have lung involvement. 3 , 4 As a result, lung imaging is pivotal in every recommendation for guiding the diagnosis and management of COVID‐19 patients. 1 , 5
Preliminary reports have been published on the use of lung ultrasound (LUS) in the evaluation of patients with SARS‐CoV‐2 infection. 6 , 7 , 8 , 9 LUS is helpful in determining early lung involvement during the pauci‐symptomatic phase of the disease, being able to monitor patient condition longitudinally and play a role in decision‐making. 10 In particular, LUS in symptomatic patients with known or suspected COVID‐19 can help support the diagnosis of pneumonitis (but not specifically diagnose COVID‐19). In addition, LUS enables ruling out concerning ultrasound features that may require an additional evaluation, monitoring patients with a change in their clinical status and may avoid unnecessary additional imaging for patients whose pretest probability of an alternative or superimposed diagnosis is low. 5
Lack of a unified approach to LUS impedes its use by clinicians for prognostic judgments regarding their patients. This is caused, at least in part, by a paucity of data linking outcomes and LUS in COVID‐19. 11 Moreover, LUS lacks specificity, as underlying cardiopulmonary disease may confound the ultrasonographic presentation, 12 although there is promising accuracy particularly in pandemic surge. 13
The aim of this retrospective multicentric study was to demonstrate the usefulness of LUS in the early diagnosis of lung disease in COVID‐positive patients and compare results to chest X‐ray results.
Materials and Methods
Study Design
This is a retrospective multicenter observational study in patients admitted to the Emergency Department (ED). The study was approved by the respective local Ethics Committees of Ancona (Italy), Braga (Portugal), and Madrid (Spain). This study was compliant with the Helsinki and Oviedo declarations. To ensure the confidentiality and the data security, as well as to avoid data manipulation and loss of data, the following precautionary measures were taken:
attribution of a numeric code to each patient participating in the study to maintain their anonymity;
creation of two data collection databases to which only the study participants had access—one database contained the personal data of the patients with the numeric identification code while the second database contained the identification codes and data useful for the study;
The principal investigator for each center was responsible for the collection, storage, and transmission of study data.
The study protocol was designed and conducted to ensure adherence to the principles and procedures of good clinical practice and to comply with the laws of the respective countries of the centers that enrolled the patients as described in the following documents and accepted, with its signature, by the investigators of the study. 14 , 15 , 16 , 17 , 18 , 19
The primary objective was to evaluate the diagnostic accuracy of LUS, compared to CXR in supporting the diagnosis of interstitial viral pneumonia in COVID‐19 patients admitted to the ED 20 and its correlation with patient prognosis (age between 0 and 100 years) presenting to four different ED in Italy, Portugal, and Spain.
The secondary objective of the study was to evaluate whether any clinical features or clinical characteristics, that is, age, preexisting diseases like chronic obstructive pulmonary disease (COPD), chronic heart failure, known interstitial diseases (asbestosis, silicosis, etc.), lung cancer, recent chemotherapy, primary, or acquired immunodeficiency, may influence the accuracy of LUS diagnosis.
LUS was performed, on admission, by emergency physicians, trained in LUS, in addition to the physical examination before participants underwent conventional radiologic examination or while they were waiting for the report.
Institutions used similar methodologies for LUS scanning. 13 , 21
Radiologists interpreting CXR were those on shift for XR readings during the participants’ stay in the ED. No time or services other than the ultrasound examination were required of patients, because bedside ultrasound is routinely used in addition to the patient's physical examination compared and/or integrated into the usual diagnostic process.
Measures and Equipment
Ultrasound examinations were performed using high‐frequency linear transducers. A LA522 linear array transducer with a bandwidth of 9‐3 MHz and a CA430 convex array transducer with a bandwidth of 8‐1 MHz were used with the Esaote MyLab 25, an L12‐3 linear array transducer with a bandwidth of 12‐3 MHz with the Philips CX5, and a linear array transducer with a bandwidth of 11‐3 MHz with the GE Logiq, with lung preset.
Scans were performed using longitudinal transducer orientation of the lung regions.
X‐rays were performed using both portable and fixed standard radiology equipment (Mecall Eidos RF 439) and digitally transmitted via a picture archiving and communication system.
LUS examinations were performed using one of three machines—an Esaote MyLab 25 (Esaote, Genova, Italy), Philips CX50 Philips, Amsterdam, Netherlands), or GE Healthcare Logiq E (GE Healthcare, Little Chalfont, UK).
Participants
Inclusion Criteria
The population of interest for this study is made up of all the patients (between 0 and 100 years of age) admitted to the ED and/or to the Emergency Acceptance Units of the participating centers presenting with symptoms attributable to SARS‐CoV‐2 infection, that is, fever, cough, pharyngodynia, headache, arthromyalgia, asthenia, malaise, dyspnea, hemoptysis, diarrhea, ageusia, anosmia, and other suspicion symptoms, who have undergone CXR and complete LUS examination integrated with physical examination. Other inclusion criteria:
Any age and sex
Patients SARS CoV‐2 positive
Availability of the CXR report taken during admission
Availability of data relating to LUS integrated with physical examination
Diagnosis of interstitial pneumonia
Exclusion Criteria
None.
Data Collection
Laboratory and instrumental data from patients enrolled in our study were collected retrospectively in patients diagnosed with SARS‐CoV‐2 infection undergone LUS and CXR.
CXR report considered in the study will be the exam carried out during the patient's stay in the emergency room and reported by the specialist in imaging diagnostics.
LUS report considered in this study is the exam performed routinely, in addition to the physical examination, during the usual time spent from the patient in the emergency room.
The comparison between LUS accuracy and conventional radiology was performed in the context of the different EDs participating in the study. LUS was performed without recourse to imaging specialists because clinical ultrasound almost is a usual approach in the emergency room and useful to address the diagnostic‐therapeutic procedures of different internal, traumatic, and nontraumatic diseases.
Therefore, LUS does not bring any additional cost to the normal diagnostic path but instead allows to highlight any lesions that conventional CXR may sometimes not identify, with advantages of better diagnostic definition.
The results of this study could represent a basis for modifying the current management of patients with severe pneumonia by identifying a better diagnostic‐therapeutic path.
Statistical Analysis
All subjects were divided into two group by the dichotomous result of the molecular test. Categorical variables were shown as percentages and continuous variables were presented as the median ± standard error. Differences between positive and negative subjects on molecular testing were analyzed using t‐tests and χ 2 tests, where appropriate. Correlations between clinical variables and the molecular test results were explored by means of multiple regressions or χ 2 tests. Finally, predictors of the molecular test were verified by analysis of covariance (ANCOVA) models. For all tests, a P value <.05 was considered to indicate statistical significance.
Results
Table 1 contains patient demographic, laboratory results, historical, and physical examination findings.
Table 1.
Clinical Characteristics of Sample Overall and by Molecular Test
| Positive on Molecular Test | Negative on Molecular Test | P | |
|---|---|---|---|
| Subjects | 82.67% | 17.33% | |
| Age (y) | 66.45 ± 0.95 | 68.84 ± 2.17 | ns |
| Female | 41.16% | 59.04% | .002 |
| Fever | 70.29% | 47.50% | <.001 |
| Dyspnea | 63.23% | 49.38% | .024 |
| Cough | 24.92% | 14.63% | .049 |
| Asthenia | 22.66% | 30.00% | ns |
| Gastroenterological symptoms | 7.45% | 1.25% | .041 |
| Hemoptysis | 3.15% | 0.00% | ns |
| Arthromyalgia | 8.55% | 8.64% | ns |
| Chest pain | 8.14% | 9.88% | ns |
| Ageusia | 7.84% | 1.27% | .035 |
| Anosmia | 6.37% | 1.27% | ns |
| Bradycardia | 9.06% | 12.66% | ns |
| Faringodinia | 1.19% | 0.00% | ns |
| Cefalea | 1.19% | 0.00% | ns |
| Body temperature | 37.19°± 0.22 | 36.61°± 0.11 | ns |
| Systolic blood pressure (mmHg) | 126.53 ± 1.55 | 130.75 ± 2.64 | ns |
| Diastolic blood pressure (mmHg) | 73.80 ± 0.85 | 73.81 ± 1.64 | ns |
| Heart rate (bpm) | 88.00 ± 1.12 | 91.68 ± 1.99 | ns |
| Respiratory rate (m−1) | 17.89 ± 0.33 | 17.01 ± 0.59 | ns |
| White blood cells | 7.73 ± 0.27 | 11.17 ± 1.53 | <.001 |
| C‐reactive protein | 84.83 ± 6.95 | 70.88 ± 9.66 | ns |
| History of diabetes | 21.07% | 28.92% | ns |
| History of chronic obstructive pulmonary disease | 22.85% | 33.73% | .040 |
| History of heart failure | 26.71% | 27.71% | ns |
| History of kidney failure | 19.58% | 14.46% | ns |
| History of neoplastic disease | 12.68% | 21.25% | ns |
| History of dementia | 23.72% | 25.30% | ns |
| Total admission | 75.70% | 74.68% | ns |
| Admission not in ICU | 74.22% | 73.41% | ns |
| Need of intubation | 10.07% | 0.00% | <.001 |
| Need ICU during hospitalization | 14.95% | 3.45% | .019 |
| Mortality | 13.13% | 12.07% | ns |
Mean ± standard error. Differences in significance were analyzed using t‐test or χ 2, as appropriate. ICU, intensive care unit.
Most common findings on lung POCUS were B‐lines (Figure 1), irregular pleural line (Figure 2), and small sub‐pleural consolidations (Figure 3A,B), which were reported in 80.17%, 59.29%, and 55.32%, respectively. Normal chest X‐ray was found in 17.89%, whereas ground glass (Figure 4), interstitial pattern (Figure 5), and small consolidation (Figures 6 and 7) were reported in 31.68%, 59.70%, and 18.63%, respectively.
Figure 1.
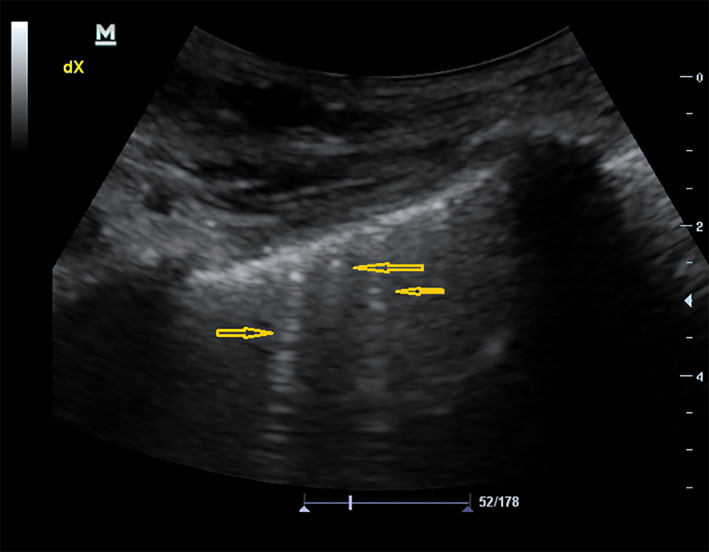
Multifocal B‐lines.
Figure 2.
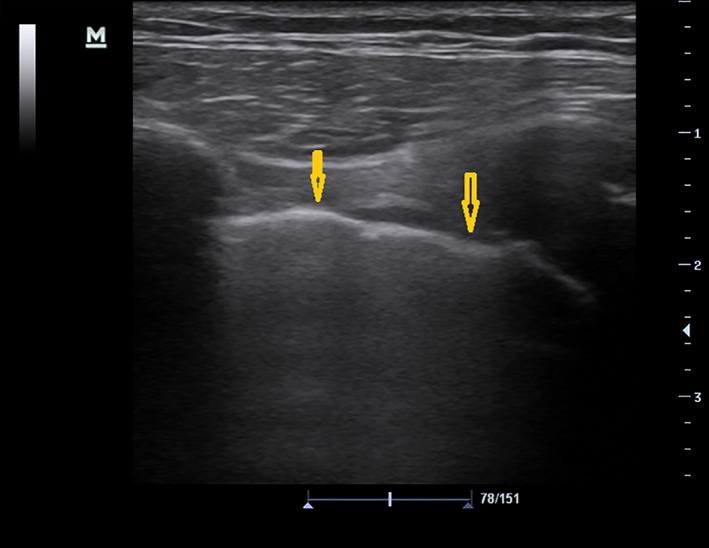
Thickening irregular line.
Figure 3.
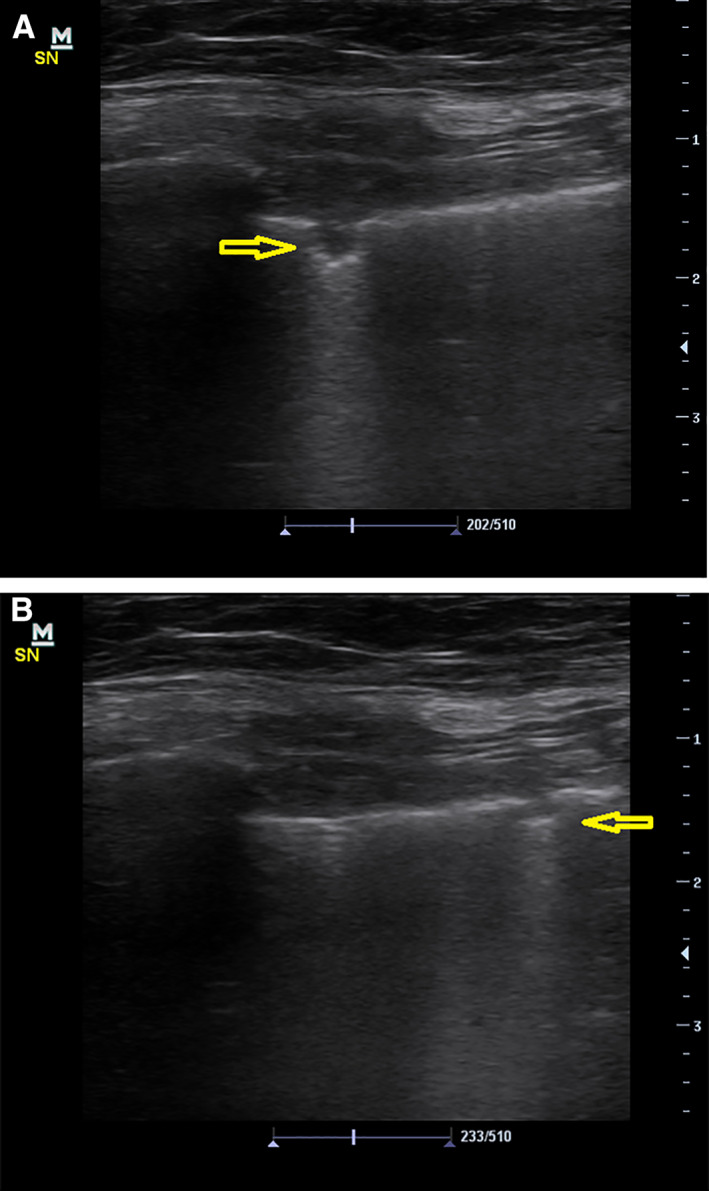
A, Small sub‐pleural consolidation. B, Small sub‐pleural consolidation.
Figure 4.
Ground glass.
Figure 5.
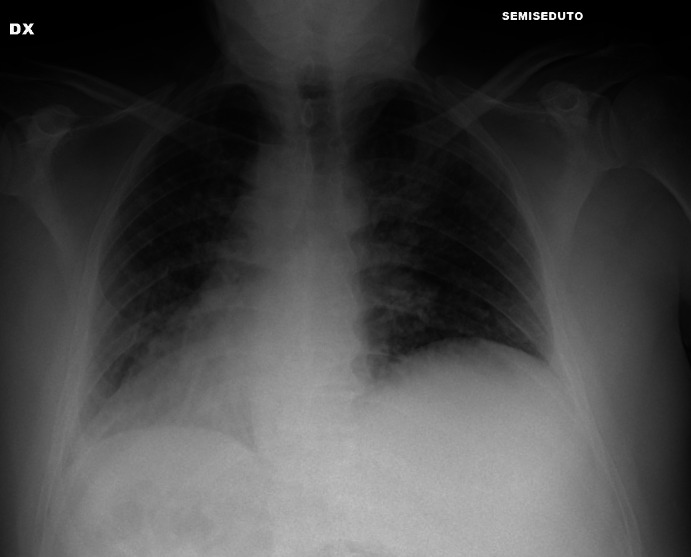
Interstitial pattern.
Figure 6.
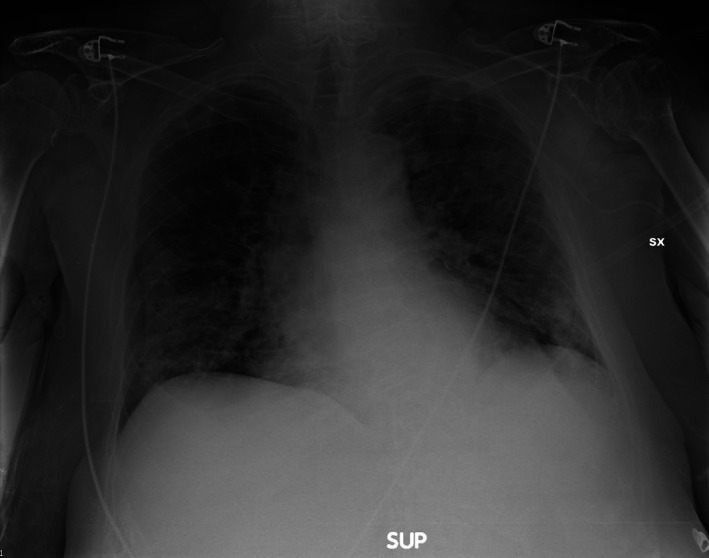
Small consolidation and interstitial pattern.
Figure 7.
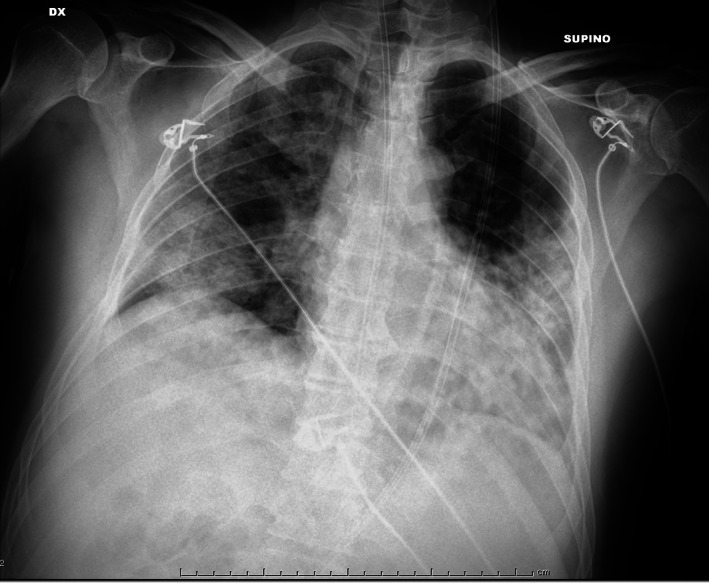
Ground glass and small consolidations.
As indicated in Table 2, the two groups of subjects differed on POCUS examination with respect to pleural effusion (P < .001) and B‐lines (P < .001). In the case of CXRs, COVID + patients presented more frequently ground glass (P = .001) and small consolidation areas (P = .018), whereas interstitial pattern (P = .009) and pleural effusion (P < .001) were mostly found in the COVID patients. Including age and sex in the multiple regression models only fever (P < .001), dyspnea (P = .045), white blood cells (P < .001), and history of COPD or neoplastic diseases (P = .033) were associated with the molecular testing results, whose variability (R 2) was explained up to 13%.
Table 2.
Ultrasound (LUS) and X‐ray Lung’s Findings Overall and by Molecular Test
| Overall | Subjects Positive on Molecular Testing | Subjects Negative on Molecular Testing | P | |
|---|---|---|---|---|
| LUS pleural effusion: absent; unilateral; bilateral | 88.26%; 4.40%; 7.34% | 91.38%; 3.39; 5.23% | 75.91%; 8.43%; 15.66% | <.001 |
| LUS B‐Lines: absent; more than 3; many and confluent | 19.83%; 36.33%; 43.84% | 16.45%; 37.22%; 43.33% | 36.14%; 31.33%; 32.53% | <.001 |
| LUS irregular pleural Line | 59.29% | 61.01% | 51.81% | ns |
| LUS consolidation: absent; small; big/lobar | 44.68%; 37.16%; 18.16% | 42.28%; 38.99%; 18.73% | 55.42%; 28.92%; 15.66% | ns |
| X‐ray normal findings | 17.89% | 18.95% | 13.25% | ns |
| X‐ray ground glass | 31.68% | 35.00% | 16.87% | .001 |
| X‐ray interstitial pattern | 59.70% | 56.84% | 72.29% | .009 |
| X‐ray small consolidation areas: absent; unilobar; multilobar | 81.37%; 11.13%; 7.50% | 79.11%; 12.01%; 8.88% | 91.57; 7.23%; 1.20% | .018 |
| X‐ray pleural effusion (absent, unilateral, bilateral) | 95.20%; 3.29%; 1.51% | 97.72%; 1.75%; 0.53% | 83.14%; 10.84%; 6.02 | <.001 |
Differences in significance were analyzed using χ 2, or analysis of variance, as appropriate.
Then, we tested, which findings on lung POCUS were predictors of whether the patient has COVID or not, after adjusting for age, sex, fever, dyspnea, white cell count, and clinical history. In ANCOVA models, including only POCUS findings, B‐lines and small consolidation, were associated with molecular test results (P = .001 and P < .001, respectively), whereas irregular pleural lines or effusions were not.
We also tested the association between chest X‐ray findings and the result of molecular testing after adjusting for the same confounders. In ANCOVA models, including only X‐ray results, ground glass aspect, small consolidations, and effusions remained significant (always P < .001), whereas normal findings and interstitial pattern did not. Finally, we tested, which predictors were associated with molecular test results in ANCOVA models, including both POCUS and X‐ray findings. After adjusting for age, sex, fever, dyspnea, white cell count, and clinical history, only consolidations were significant at POCUS (P < .001), whereas ground glass aspects, small consolidations, and effusions keep their significance at X‐ray findings (P < .001, P < .001 and P = .004, respectively). Adjusted multiple regression explained (R 2) up to 31% of the molecular test variability when both POCUS and X‐ray predictors were included in the model.
Regarding the prognostic role of POCUS, we tested, which findings could predict a need for hospital admission, orotracheal intubation, and mortality after adjusting for the same confounders. LUS pleural effusion (P = .011), small consolidation (P = .019), male sex (P = .027), and clinical history (P = .044) remained associated with the admission rate, whereas any X‐ray outcomes were not. Concerning the need for orotracheal intubation, both LUS and X‐ray findings were found to have significant role, in particular, LUS small consolidation (P = .004), X‐ray ground glass (P = .032), and X‐ray interstitial pattern (P = .007) were associated with the need for invasive mechanical ventilation. Predictors of mortality were only LUS pleural effusion (P = .045) and X‐ray small consolidation (P = .043).
Discussion
Our study results show the utility of LUS in the diagnosis of COVID‐19 infections for patients presenting to the ED. Throughout the pandemic, and for many years prior, most patients with respiratory complaints were evaluated with CXR upon ED presentation. While some centers practiced routine use of chest computed tomography (CT) in possible COVID‐19 infected patients, such a practice is unsustainable in all, but the wealthiest healthcare systems and even in these, ignores the impact of cumulative medical radiation exposure. 22 , 23 The last two years have seen repeated waves of COVID‐19 infections around the globe and even immunization has not spared countries from reexperiencing surges of infected patients requiring hospitalization and subsequent death tolls. Two of the most challenging aspects of COVID‐19 diagnosis and treatment for clinicians are the inability to easily and rapidly evaluate suspected viral victims not only for the likelihood of infection but also requirement for admission. Both of these answers can still be illusive despite the introduction of numerous polymerase chain reaction (PCR) tests and the possibility of chest CT for imaging and application of various prediction tools. PCR test return times have varied greatly during the pandemic and can unexpectedly increase during surges. CT, including all of the limitations listed previously is often unfit for rapid evaluation and triage, which may be required with high patient volumes.
POCUS presented an ideal tool for rapid evaluation, triage, 24 and easy disinfection in the case of hand held devices. However, test performance parameters have to be at least as good as CXR for a large‐scale shift in clinical practice. 25 , 26 Ours was one of the larger patient cohorts comparing LUS and CXR, and does so across multiple medical centers and countries. 27 Our patient sample also represents a range of at‐risk conditions for COVID‐19, including congestive heart failure, COPD, and diabetes. Like many studies focusing on LUS findings in COVID‐19, we also noted frequent B‐lines, irregular pleural lines, and small sub‐pleural consolidations.
In a cohort of 89 intensive care unit (ICU) patients, Alharthy et al noted that 78.6% of patients had bilateral irregular pleural lines on LUS, compared to our incidence of 59%. 28 Further, the authors detected B‐lines in 100% of cases and 61.7% had variable consolidations, while we found an incidence of 80% and 55%, respectively. These differences are likely explained by the study populations, an ICU one by Alharthy and broader one presenting to the ED with some not requiring admission or ICU care. 28
Surprisingly, upon ANCOVA analysis, only B‐lines and small consolidations were associated with COVID‐19 molecular testing results. This stands in contrast to a study by Volpicelli and colleagues who classified irregular pleural lines as equating to high probability for COVID‐19 infection. 29 Kameda et al, similarly note in their comprehensive review of LUS for COVID‐19 that irregular or thickened pleura is a common finding. 30 This is consistent with multiple other studies, which have been suggested to be specific for COVID‐19 pneumonitis. However, in our analysis, pleural irregularity did not correlate with positive COVID‐19 test results. A study by Arntfied et al, used artificial intelligence to identify COVID‐19 infection in ICU patients based on LUS, comparing accuracy to human expert sonologists. 20 The algorithm was far superior at differentiating COVID‐19 from cardiac pulmonary edema as well as non‐COVID‐related acute respiratory distress syndrome. Heat maps, a method for evaluating what portion of an image is most important to an algorithm in obtaining the correct prediction, showed that the general pleural line area was of greatest importance. While irregular pleural lines are common in patients with COVID‐19 pneumonia, it is not a pathognomonic finding.
Among our cohort, the most common sonographic finding was the presence of B‐lines. ANCOVA analysis confirmed an association between B‐lines and positive COVID‐19 testing. This may leave clinicians in a difficult position because B‐lines should be present in cases of pulmonary edema such as from congestive heart failure exacerbations, unrelated to COVID‐19 infection. In fact, some authors suggested, early in the course of the pandemic that pleural line thickening and sub‐pleural consolidations may allow differentiation between pulmonary edema unrelated to COVID‐19. Thus, it is significant in our study group that analysis showed association between the presence of sub‐pleural consolidations and positive COVID‐19 test results. This leaves clinicians with a readily available sonographic differentiator between pulmonary edema and COVID‐19 infection. Unfortunately, among our group, only 59% of positive patients had sub‐pleural consolidations identified, and this percentage may not increase significantly in even sicker patient groups such as ICU population.
Our study group analysis showed association between the presence of sub‐pleural consolidations and positive COVID‐19 test results. This finding provides clinicians with a readily available sonographic differentiator between pulmonary edema and COVID‐19 infection. However, among our COVID‐19 positive patient group, only 59% had sub‐pleural consolidations identified, and this percentage may not increase significantly in even sicker patient groups such as ICU population. Thus, the absence of sub‐pleural consolidations alone cannot be used to rule out COVID‐19 infection with pulmonary involvement.
Interestingly, in our cohort, only 18% of COVID‐19 patients had negative CXRs. The rate of negative CXR results in prior studies has ranged widely, with some reporting greater than 50%. Thus, our population may not be representative of those studies in studies with a markedly higher negative CXR rate. Upon regression modeling, only ground glass appearance, small consolidations, and effusions had a significant association with positive COVID‐19 test results. Given the regression modeling results and high negative CXR rates in other studies, CXR likely has significant diagnostic limitations for COVID‐19 lung imaging, an early cause for the high use of chest CT clinically throughout the world. Our analysis confirmed that, as well as supporting COVID‐19 diagnosis, LUS was useful in prognostic stratification relating to the need for hospitalization, orotracheal intubation, and mortality among the patients studied. In our study, both LUS pleural effusion and LUS small consolidation were associated with the need for hospitalization, whereas only the former was associated with the mortality rate.
Our study had multiple limitations. First, this was a retrospective study; however, our cohort size is significantly larger than many prior studies and adds to the published literature. Because most of our data were accumulated during a worldwide surge, the majority of our patients were found to be COVID‐19 positive on molecular testing. While LUS techniques were similar between sites, training, and actual practice could not be controlled for among such a diverse group of medical center and researchers. This is reflective of real life, but may also limit application of our results to specific settings with more or less experience. We could not control data collection given the retrospective nature of the study, but did standardize data entry to minimize error and variability.
Conclusion
In conclusion, analysis of our large 479 patient cohort with COVID‐19 found LUS to be noninferior to CXR. In addition, the presence of B lines and small sub‐pleural consolidation on LUS and ground glass appearance, small consolidations, and pleural effusion on CXR are significantly associated with a positive molecular test result for COVID‐19.
Acknowledgments
The authors wish to thank Dr Eliana Viola for her fruitful collaboration.
The authors have no conflicts of interest relevant to this article.
References
- 1. WHO . COVID‐19 Clinical Management: Living Guidance 25 January 2021 WHO/2019‐nCoV/clinical/2021.1; 2021.
- 2. Mohamed MFH, Al‐Shokri S, Yousaf Z, et al. Frequency of abnormalities detected by point‐of‐care lung ultrasound in symptomatic COVID‐19 patients: systematic review and meta‐analysis. Am J Trop Med Hyg. 2020; 103:815–821. 10.4269/ajtmh.20-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med 2020; 382:692–694. [DOI] [PubMed] [Google Scholar]
- 4. Şan İ, Bekgöz B, Usul E, et al. Role of lung ultrasonography in the diagnosis of COVID‐19 patients admitted to the emergency department. Notf Rett Med 2021; 24:15–20. 10.1007/s10049-020-00807-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma IWY, Hussain A, Wagner M, et al. Canadian internal medicine ultrasound (CIMUS) expert consensus statement on the use of lung ultrasound for the assessment of medical inpatients with known or suspected coronavirus disease 2019. J Ultrasound Med 2020; 40:1879–1892. 10.1002/jum.15571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pivetta E, Goffi A, Tizzani M, et al. Lung ultrasonography for the diagnosis of SARS‐CoV‐2 pneumonia in the Emergency Department. Ann Emerg Med 2020; 77:385–394. 10.1016/j.annemergmed.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yi Peng Q, Ting Wang X, Na Zhang L, Chinese Critical Care Ultrasound Study Group (CCUSG) . Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med 2020; 46:849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xing C, Li Q, Du H, Kang W, Lian J, Yuan L. Lung ultrasound findings in patients with COVID‐19 pneumonia. Crit Care. 2020; 24:174. 10.1186/s13054-020-02876-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poggiali E, Dacrema A, Bastoni D, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID‐19) pneumonia? Radiology. 2020; 295:E6–E6. 10.1148/radiol.2020200847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID‐19: a simple, quantitative, reproducible method. J Ultrasound Med. 2020; 39:1413–1419. 10.1002/jum.15285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Millington SJ, Koenig S, Mayo P, Volipcelli G. Lung Ultrasound for Patients With Coronavirus Disease 2019 Pulmonary Disease. Chest. 2021; 159:205–211. 10.1016/j.chest.2020.08.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson K, Butler R, Aujayeb A. Lung ultrasound in the COVID‐19 pandemic postgraduate. Med J 2021; 97:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Volpicelli G, Gargani L, Perlini S, et al. Lung ultrasound for the early diagnosis of COVID‐19 pneumonia: an international multicenter study. Intensive Care Med 2021; 47:444–454. 10.1007/s00134-021-06373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. International Conference of Harmonization (ICH) . ICH tripartite guideline for good clinical practices E6 (R1), 10th 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1Guideline.pdf. Accessed September 14, 2015.
- 15. Commission Directive 91/507/EEC of 19 July 1991 modifying the Annex to Council Directive 75/318/EEC on the approximation of the laws of Member States relating to analytical, pharmacotoxicological and clinical standards and protocols in respect of the testing of medicinal products.
- 16. D. L.vo n.211 del 24 giugno 2003. Attuazione della direttiva 2001/20/CE relativa all'applicazione della buona pratica clinica nell'esecuzione delle sperimentazioni cliniche di medicinali per uso clinico. (GU. Serie Generale n.184 del 09‐08‐2003 – Suppl. Ordinario n. 130)
- 17. D. L.vo n.200, 6 Novembre 2007, n. 200. Attuazione della direttiva 2005/28/CE recante buona pratica clinica relativa ai medicinali in fase di sperimentazione a uso umano, nonche' requisiti per l'autorizzazione alla fabbricazione o importazione di tali medicinali. (GU Serie Generale n.261 del 09‐11‐2007 ‐ Suppl. Ordinario n. 228)
- 18. Decreto Ministeriale 21 dicembre 2007. Modalita' di inoltro della richiesta di autorizzazione all'Autorita' competente, per la comunicazione di emendamenti sostanziali e la dichiarazione di conclusione della sperimentazione clinica e per la richiesta di parere al comitato etico (GU Serie Generale n. 53 del 3‐3‐2008‐Suppl. Ordinario n. 51).
- 19. Regolamento Generale sulla Protezione dei Dati (RGPD) n. 2016/679 del 27 aprile 2016; Gazzetta ufficiale dell'Unione europea 4 maggio 2016. https://www.garanteprivacy.it/regolamentoue/formazione/ https://europa.eu/youreurope/business/dealing-with-customers/data-protection/data-protection-gdpr/index_it.htm
- 20. Arntfield R, VanBerlo B, Alaifan T, et al. Development of a convolutional neural network to differentiate among the etiology of similar appearing pathological B lines on lung ultrasound: a deep learning study. BMJ Open 2021; 11:e045120. 10.1136/bmjopen-2020-045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volpicelli G, Elbarbary M, Blaivas M, et al. International Liaison Committee on Lung Ultrasound (ILC‐LUS) for International Consensus Conference on Lung Ultrasound (ICC‐LUS). International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med. 2012; 38:577–591. [DOI] [PubMed] [Google Scholar]
- 22. Caroselli C, Blaivas M, Falzetti S. Diagnostic imaging in the newborn, child and adolescent infected with SARS‐CoV‐2: is there a realistic alternative to lung‐HRCT and chest X‐rays ? A systematic review of the literature. Ultrasound Med Biol. 2021; 47:3034–3040. 10.1016/j.ultrasmedbio.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Norbedo S, Blaivas M, Raffaldi I, Caroselli C. Lung ultrasound point‐of‐view in pediatric and adult COVID‐19 infection. J Ultrasound Med. 2021; 40:899–908. 10.1002/jum.15475 [DOI] [PubMed] [Google Scholar]
- 24. Caroselli C, Cherubini A. Should lung ultrasound be always performed in older patients with possible COVID‐19 disease? Eur Geriatr Med 2021; 13:1–3. 10.1007/s41999-021-00538-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma IWY, Hussain A, Wagner M, et al. Canadian internal medicine ultrasound (CIMUS) expert consensus statement on the use of lung ultrasound for the assessment of medical inpatients with known or suspected coronavirus disease 2019. J Ultrasound Med 2021; 40:1879–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schrift D, Barron K, Arya R, Choe C. The use of POCUS to manage ICU patients with COVID‐19. J Ultrasound Med 2021; 40:1749–1761. [DOI] [PubMed] [Google Scholar]
- 27. Cappa G, Secco G, Nganso A, Ruzga R, Perlini S. The role of lung ultrasound in low‐resource settings during the coronavirus (SARS‐CoV ‐2) Pandemic. J Ultrasound Med 2021; 9999:1–3. 10.1002/jum.15755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alharthy A, Fagihi F, Memish ZA, Karakitsos D. Lung injury in COVID‐19—an emerging hypothesis. ACS Chem Neurosci. 2020; 11:2156–2158. 10.1021/acschemneuro.0c00422 [DOI] [PubMed] [Google Scholar]
- 29. Volpicelli G, Gargani L. Sonographic signs and patterns of COVID‐19 pneumonia. Ultrasound J 2020; 12:22. 10.1186/s13089-020-00171-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kameda T, Mizuma Y, Taniguchi H, Fujita M, Taniguchi N. Point‐of‐care lung ultrasound for the assessment of pneumonia: a narrative review in the COVID‐19 era. J Med Ultrason. 2021; 48:31–43. 10.1007/s10396-020-01074-y [DOI] [PMC free article] [PubMed] [Google Scholar]


