Dear Editor,
Since the first detection of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) in 2019, the continuous interest in gaining insights into its epidemiology and evolution is widespread. Despite showing a proofreading system involving (but not limited to) the nonstructural protein 14 exoribonuclease activity, 1 which characterizes an evolutionary pattern with a lower global mutation rate when compared with other RNA viruses, the SARS‐CoV‐2 has been also undergoing biologically relevant Spike's protein amino acid substitutions in different Variants of Interest (VOI) and Variants of Concern (VOC) as a result of convergent and directional evolution, which means that multiple and specific sites share identical mutations. 2
Regardless of retrospective circulation in Europe (doi:10.1038/d41586-021-03610-3), the SARS‐CoV‐2 Omicron VOC was first detected in South Africa through the excellent work conducted by Dr. de Oliveira's research team, together with the Department of Health and scientists from the Network for Genomic Surveillance in South Africa (http://www.sun.ac.za/english/Lists/news/DispForm.aspx?ID=8785). With 35 non‐synonymous reported mutations found in the Spike's protein amino acid, of which 15 on the receptor‐binding domain (RBD) and 10 of those on the receptor‐biding Motif (RBM), the concern about the potential increased spread and virulence of the Omicron variant is justified.
Noteworthily, with the exception of S371L substitution, in a span of 12 months (October 2020–2021), all other mutations in Spike's RBD and RBM had been already described and predicted to promote different phenotypic characteristics (Table 1). Further importance is given to the latter region (RBM) as it mediates the recognition to the human angiotensin‐converting enzyme 2 receptors (ACE2) being, therefore, an important neutralizing antibody target 12 , 13 (Figure 1). It is also worth mentioning that the low frequency of some substitutions in many virus strains is different from the emergence of the combination of these substitutions in a particular strain.
Table 1.
Amino acid substitutions present in SARS‐CoV‐2 Spike (receptor‐binding domain) Omicron variant
| Substitutions | Span of time from the first date of publication (in months) | Publication month, year | References |
|---|---|---|---|
| S373P | 0 | Oct, 2020 | Long et al. 3 |
| G496Sa | 5 | Mar, 2021 | Teng et al. 4 |
| G339D | 6 | Apr, 2021 | Smaoui and Yahyaoui 5 |
| S375F | 6 | Apr, 2021 | Chen et al. 6 |
| G446S,a T478K,a Q493K,a and Y505Ha | 8 | Jun, 2021 | Verma and Subbarao 7 |
| K417N | 8 | Jun, 2021 | Barton et al. 8 |
| N440K,a S477N,a and N501Y a | 9 | July, 2021 | Gan et al. 9 |
| Q498Ra | 10 | Aug, 2021 | Zahradník et al. 10 |
| E484Aa | 12 | Oct, 2021 | Laurini et al. 11 |
Receptor‐binding motif; In bold: shared substitutions in SARS‐CoV‐2 VOI and/or VOC.
Additional information: ECDC: https://www.ecdc.europa.eu/en/covid-19/variants-concern
Figure 1.
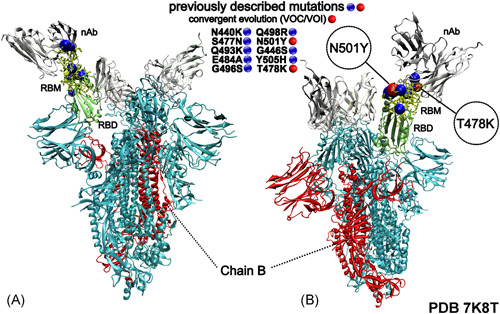
Three‐dimensional structural representation of the SARS‐CoV‐2 Spike protein exhibiting the mutations present in Omicron variant receptor‐binding motif (RBM). Blue and red spheres represent the RBM's substitutions. nAb, vaccine‐induced neutralizing‐antibody (silver); RBM, receptor‐binding motif (yellow) and RBD, receptor‐binding domain (green). The image was created with the Visual Molecular Dynamics (VMD) v.1.9.3 (http://www.ks.uiuc.edu/Research/vmd/)
We also found the additional Omicron Spike amino acid substitutions L141F, R346K, and V367F, also previously described. 3 The substitution L141F had not been cataloged by the World Health Organization (WHO) as present in this VOC. Furthermore, the substitution V367F is also not labeled by the WHO (i.e., “n/a”) and, according to the European Centre for Disease Prevention and Control (ECDC) (https://www.ecdc.europa.eu/en/covid-19/variants-concern), it was first detected in the United Kingdom in December 2020. Moreover, the substitution R346K, classified by WHO as Mu variant, was first detected in Colombia in January 2021.
Additionally, we draw attention to the following additional mutations found in the Omicron variant such as the substitution G142D (Spike's N‐terminal domain, under convergent evolution and present in Kappa and Delta variants), and P681H (Spike's C‐terminal domain 2, furin cleavage site, present in Alpha, Gamma, Lambda, Mu and Theta variants), 2 both corresponding to important regions targeted by neutralizing antibodies. 14 , 15 The substitution Q498R appears to be epistatic to N501Y. 10 This in a general context somehow let us know: how little we are sequencing, how fast the virus evolves, and how far we are behind in bringing it to the end. Nevertheless, such SARS‐CoV‐2 evolutionary signatures show us a way forward.
Taking into account that SARS‐CoV‐2 variants carry homoplasy traits from independent evolution, 16 where the viral effective population size has earned identical site‐specific mutations in the Spike protein (i); assuming that unidentified SARS‐CoV‐2 variants and sub‐lineages carrying new as well as several previously described mutations are very likely to be circulating worldwide (ii) and considering a high vaccine coverage with two/booster‐dose schedules (iii): should we expect a high number of severe infections from different SARS‐CoV‐2 variants?
Someway, it brings evidence that the currently available immunizers are effective against distinct SARS‐CoV‐2 variants and a series of further sub‐lineages. Even considering a diminished vaccine efficacy, they can still be protective, as supported by a series of well‐conducted studies through different vaccine strategies. 17 , 18 , 19
To better understand the evolution of the SARS‐CoV‐2 Omicron variant, on December 11 we carried out some additional analysis by collecting whole‐genome sequences (n = 146) from Hong Kong, Botswana, South Africa, Canada, Australia, Italy, Belgium, Israel, Austria, England, and Germany, which were downloaded from the Global Initiative on Sharing Avian Influenza Data‐EpiCoV (GISAID‐EpiCoV). Sequences from SARS‐CoV‐2 Alpha (n = 1719), Beta (n = 5870), Gamma (n = 5), and Delta (n = 10 133) variants isolated from Africa were also included in the analysis. Data sets were filtered out (0% of degenerated bases) via the biopython‐based software Sequence Cleaner (https://biopython.org/wiki/Sequence_Cleaner), aligned with the SARS‐CoV‐2 reference coding‐sequence (NC_045512.2) through MAFFT v.7 (https://mafft.cbrc.jp/alignment/software/closelyrelatedviralgenomes.html) and edited by the UGENE v.38.1 platform. 20
Subsequently, the sequences were submitted to the Datamonkey web‐server for the Genetic Algorithm for Recombination Detection (GARD), Single Breakpoint (SPB), and Pairwise Homoplasy Index (PHI, SplitsTree v.4.17.0, using default settings, available at: http://www.splitstree.org/) algorithms. In silico evidence of recombination was found when only the sequences from the SARS‐CoV‐2 Beta, Delta, and Omicron VOC were aligned together, indicating that the variants cocirculation can enhance recombination events (Supporting Information). Remarkably, Beta, Delta, and Omicron are the most predominant variants circulating in Africa (https://www.bmj.com/content/375/bmj.n3013).
Finally, giving special emphasis to areas with high COVID‐19 immunization coverage plus booster‐dose schedules presenting a low ratio of serious diseases, hospitalization, and death from different SARS‐CoV‐2 variants (https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf); and through viral molecular evolution approaches, our hypothesis is that the currently available immunizers from different technologies leveraged for vaccine production, taking into consideration the different levels of vaccines effectiveness, are highly probable to be effective against SARS‐CoV‐2 Omicron variant, which further increases the urgency of vaccination programs.
In view of the SARS‐CoV‐2 evolutionary signatures, it is also believed that such a proposition can be also addressed to other unidentified genetic lineages from the Greek alphabet which will prospectively label future SARS‐CoV‐2 variants. By providing useful insights into a linear vaccine perspective, we believe that these insights add important knowledge to the state of the art of the COVID‐19 disease pandemic in general and to vaccine efficacy considerations in particular.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
Robert Andreata‐Santos, Luiz Mário Ramos Janini and Ricardo Durães‐Carvalho are supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil, grant 2021/05661‐1 (RA‐S), grant 2020/08943‐5 (LMRJ), and grants 2019/01255‐9 and 2021/03684‐4 (Young Investigator Program) (RD‐C). The authors also thank the National Laboratory for Scientific Computing (LNCC/MCTI, Brazil) for providing HPC resources of the Santos Dumont supercomputer (ID #45691).
DATA AVAILABILITY STATEMENT
Sequences downloaded are available as Supporting Information file.
REFERENCES
- 1. Abdelrahman Z, Li M, Wang X. Comparative review of SARS‐CoV‐2, SARS‐CoV, MERS‐CoV, and influenza A respiratory viruses. Front Immunol. 2020;11:552909. 10.3389/fimmu.2020.552909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nunes DR, Braconi CT, Ludwig‐Begall LF, Arns CW, Duraes‐Carvalho R. Deep phylogenetic‐based clustering analysis uncovers new and shared mutations in SARS‐CoV‐2 variants as a result of directional and convergent evolution. medRxiv. 2021;12:434. 10.1101/2021.10.14.21264474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. Molecular architecture of early dissemination and massive second wave of the SARS‐CoV‐2 virus in a major metropolitan area. mBio. 2020;11(6):e02707‐e02720. 10.1128/mBio.02707-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu F, Xiang R, Deng X, et al. Systemic effects of missense mutations on SARS‐CoV‐2 spike glycoprotein stability and receptor‐binding affinity. Brief Bioinform. 2021;22(2):1239‐1253. 10.1093/bib/bbaa233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smaoui MR, Yahyaoui H. Unraveling the stability landscape of mutations in the SARS‐CoV‐2 receptor‐binding domain. Sci Rep. 2021;11(1):9166. 10.1038/s41598-021-88696-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teng S, Sobitan A, Rhoades R, Liu D, Tang Q. Prediction and mitigation of mutation threats to COVID‐19 vaccines and antibody therapies. Chem Sci. 2021;12(20):6929‐6948. 10.1039/d1sc01203g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verma J, Subbarao N. In silico study on the effect of SARS‐CoV‐2 RBD hotspot mutants' interaction with ACE2 to understand the binding affinity and stability. Virology. 2021;561:107‐116. 10.1016/j.virol.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J, Gao K, Wang R, Wei GW. Effects of common mutations in the SARS‐CoV‐2 Spike RBD and its ligand, the human ACE2 receptor on binding affinity and kinetics. eLife. 2021;10:e70658‐e76948. 10.7554/eLife.70658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verma J, Subbarao N. Structural modeling of the SARS‐CoV‐2 spike/human ACE2 complex interface can identify high‐affinity variants associated with increased transmissibility. J Mol Biol. 2021;433(15):167051‐167116. 10.1016/j.jmb.2021.167051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barton MI, Macgowan SA, Kutuzov MA, Dushek O, Barton GJ, van der Merwe PA. SARS‐CoV‐2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat Microbiol. 2021;6(9):1188‐1198. 10.1038/s41564-021-00954-4 [DOI] [PubMed] [Google Scholar]
- 11. Gan HH, Twaddle A, Marchand B, Gunsalus KC. Molecular rationale for SARS‐CoV‐2 spike circulating mutations able to escape bamlanivimab and etesevimab monoclonal antibodies. Sci Rep. 2021;11(1):20274. 10.1038/s41598-021-99827-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zahradnik J, Marciano S, Shemesh M, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laurini E, Marson D, Aulic S, Fermeglia A, Pricl S. Receptor‐binding domain‐specific human neutralizing monoclonal antibodies against SARS‐CoV and SARS‐CoV‐2. Signal Transduc Target Ther. 2020;5(1):212. 10.1038/s41392-020-00318-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cerutti G, Guo Y, Zhou T, et al. Potent SARS‐CoV‐2 neutralizing antibodies directed against spike N‐terminal domain target a single supersite. Cell Host Microbe. 2021;29(5):819‐833. 10.1016/j.chom.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson BA, Xie X, Bailey AL, et al. Loss of furin cleavage site attenuates SARS‐CoV‐2 pathogenesis. Nature. 2021;591(7849):293‐299. 10.1038/s41586-021-03237-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Dorp L, Acman M, Richard D, et al. Emergence of genomic diversity and recurrent mutations in SARS‐CoV‐2. Infect Genet Evol. 2020;83:104351. 10.1016/j.meegid.2020.104351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pegu A, O'Connell SE, Schmidt SD, et al. Durability of mRNA‐1273 vaccine‐induced antibodies against SARS‐CoV‐2 variants. Science. 2021;373(6561):1372‐1377. 10.1126/science.abj4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X‐N, Huang Y, Wang W, et al. Effectiveness of inactivated SARS‐CoV‐2 vaccines against the Delta variant infection in Guangzhou: a test‐negative case‐control real‐world study. Emerg Microbes Infect. 2021;10(1):1751‐1759. 10.1080/22221751.2021.1969291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernal JL, Gower C, Andrews N. Effectiveness of Covid‐19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(25):e92. 10.1056/NEJMc2113090 [DOI] [PubMed] [Google Scholar]
- 20. Okonechnikov K, Golosova O, Fursov M. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 2012;28(8):1166‐1167. 10.1093/bioinformatics/bts091 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
Sequences downloaded are available as Supporting Information file.


