Abstract
CoronaVac was the first vaccine approved in Brazil for use in healthcare workers (HCWs). However, there is limited information about it, with little long‐term evidence on post‐vaccination antibody persistence. This study evaluated the antibody response to SARS‐CoV‐2 in 1237 HCWs after the first (1D), second dose (2D), and 6 months postvaccination (6mA2D) with CoronaVac. The seropositivity was 88% at 1D, increasing to 99.8% at 2D, but decreasing to 97.9% at 6mA2D, which was also observed at the analyzed antibody levels. Interestingly, the levels in females were higher than males, and we found a positive correlation with previous SARS‐CoV‐2 infection. Participants with comorbidities had lower levels suggesting the need to monitor for a potential booster dose. Our findings suggest that CoronaVac induced a robust antibody response that wanes significantly over time. Further longitudinal studies are needed to identify whether the antibodies will decline or plateau at a lower level.
Keywords: CoronaVac, healthcare workers, IgG antibody, SARS‐CoV‐2, serology
Highlights
Our findings suggest that CoronaVac induced a robust antibody response that wanes significantly over time.
In our study, two doses of CoronaVac were capable of induction an antibody response in people ≥51 years old.
The seropositivity and the levels of antibodies were higher in females when compared to males.
We found a positive correlation with previous SARS‐CoV‐2 infection, previously infected participants had a significantly higher antibody response than previously uninfected participants.
Our findings suggest that patients with chronic diseases may need a booster shot of CoronaVac vaccine.
Individuals with immune‐mediated diseases developed a significant humoral response following the administration of two doses of CoronaVac, albeit with lower antibody titers.
1. INTRODUCTION
In late December 2019, clusters of patients with pneumonia of unknown sources were reported in Wuhan, China. The etiological agent was quickly identified as a new coronavirus, subsequently named as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) and identified as a cause of the Coronavirus Disease 2019 (COVID‐19). 1 , 2 Being highly transmissible, SARS‐CoV‐2 has spread fast all over the world, leading to World Health Organization (WHO) declare a pandemic, in early March of 2020. 3
The SARS‐CoV‐2 coronavirus pandemic has been an unprecedented level of global collaboration that has led to a rapid development of vaccines. Various candidate vaccines are being developed and tested, including nucleic acid vaccines, inactivated virus vaccines, live attenuated vaccines, protein or peptide subunit vaccines, and viral‐vectored vaccines. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13
In Brazil, the CoronaVac was approved for emergency use and vaccination started on January 2021, in health care workers (HCWs). HCWs were prioritized in the vaccination programs because they are directly exposed to infected patients and can receive a high viral load. 14 , 15 CoronaVac was developed by Chinese biopharmaceutical company Sinovac Life Sciences and uses the inactivated whole SARS‐CoV‐2 virus. In the vaccination schedule using a CoronaVac, a second dose of vaccine should occur 2–4 weeks after the first dose. 16
The early protective efficacy of a vaccine is primarily conferred by the induction of antigen‐specific antibodies. Long‐term protection requires the persistence of vaccine antibodies above protective thresholds and/or the maintenance of immune memory cells capable of reactivation after subsequent viral exposure. 17 A rapid decay of anti‐SARS‐CoV‐2 antibodies in convalescent COVID‐19 patients has been reported, raising the question of whether COVID‐19 vaccines will elicit long‐lasting immune protection. 18 , 19 , 20 , 21 , 22 , 23
With the continued potential for more transmissible SARS‐CoV‐2 variants, data on antibody dynamics of vaccine‐induced immune responses are essential to understand the protection and durability of vaccine and clarify the need for further booster doses. Limited information is available about CoronaVac, with little long‐term evidence on the postvaccination antibody persistence. Therefore, this study aimed to evaluate the antibody response to CoronaVac up to 6 months after the second dose, in a cohort of HCWs, and correlate the findings with age, gender, previous SARS‐CoV‐2 infection, and pre‐existing diseases.
2. METHODS
2.1. Ethical aspects
The study was approved by the Ethics Committee of the Hospital Geral Dr. César Cals, through CAAE 39691420.7.0000.5049.
2.2. Healthcare worker (HCW) cohort
For the purpose of this study, 1237 HCWs of both genders, aged 18 years and above, that have received two doses of the vaccine CoronaVac between February to April 2021 were included after an informed consent. An additional evaluation was performed between July and September 2021, after 6 months they received the second dose, when 805 HCWs that were included on the previous phases remained. HCWs who did not take the vaccine or who were unable to collect blood samples within the period determined by the study were excluded. This study involved 29 institutions that provide health care services in Ceará State, Brazil. Twenty‐five institutions are components of public Brazilian health system and 4 are private organizations. Services that provide primary care until reference hospitals of high‐complexity procedures were included in the study.
2.3. Study design
All participants answered a questionnaire with information on demographic and clinical data. The history of COVID‐19 was considered before the first dose administration or after the second dose. HCWs who had COVID‐19 between the first and second dose of CoronaVac were excluded of the study. Therefore, The HCWs with COVID‐19 history, included in the study, received the same CoronaVac schedule than HCWs who did not have COVID‐19. The second dose were administered 28 days after the first dose in all participants. Sequential serum samples were collected from HCWs at three different times: 28 days after the first vaccine dose (1D), 30 days (2D) and 6 months after the second dose of CoronaVac (6mA2D). The participants’ blood was collected at the workplace. The samples were sent to the Serology Laboratory of the COVID‐19 Diagnosis Support Unit of Fundação Oswaldo Cruz, Ceará, Brazil, for serological analysis.
2.4. Laboratory analysis
All samples were tested for immunoglobulin G (IgG) anti‐ SARS‐CoV‐2 using the SARS‐CoV‐2 IgG II Quant Assay (Abbott). The chemiluminescent immunoassay measures antibodies against the receptor‐binding domain (RBD) of the S1‐subunit of the SARS‐CoV‐2 S protein and presents 100% positive agreement with the plaque reduction neutralization test. This antibody test has a sensitivity and specificity of 99.4% and 99.6%, respectively. 24 All steps of the assay were realized according to the manufacturer's instructions. The chemiluminescence signal was detected by the ARCHITECT i2000SR equipment (Abbott). The analyzer calculates antibody concentration in arbitrary concentration units (AU/ml). Results ≥50 AU/ml (cutoff value) are reported as positive and results <50 AU/ml are reported as negative.
2.5. Statistical analysis
GraphPad Prism (version 5.01) was used for statistical analyses and generation of scatter plots. The variables of data were considered non‐normally distributed and the statistical analyze was performed using medians and interquartile range (IQR) for continuous variables, and median (minimum and maximum) or frequency values for qualitative variables. For qualitative variables was used Fisher's test. For the anti‐S IgG levels was used Mann–Whitney test to analyze two groups within the same evaluation time, and Kruskal–Wallis test with subsequent Dunn's multiple comparison testing for group comparisons between the different evaluation times. In all analyses, p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Cohort description
The cohort consisted of 1237 HCWs that received two doses of the CoronaVac vaccine. The cohort had a greater representation from female individuals, with 960 (77.6%) female and 277 (22.4%) male. The age distribution of this cohort was as follows: 18–30 years old, 266 (21.5%); 31–50 years old, 808 (65.3%), 51 years and older, 163 (13.2%) (Table 1). HCWs consisted in 55.3% healthcare assistants, 22.4% administrative staff, 14.0% assistant staff, 7.7% medical doctors and 0.5% health trainees.
Table 1.
HCWs included in the study by age and gender
| HCWs no. (%) | |||||
|---|---|---|---|---|---|
| Gender | Female | Male | Total | ||
| Age (years) | No. (%) | Median (min–max) | No. (%) | Median (min–max) | No. (%) |
| 18–30 | 195 (20.3) | 26 (19–30) | 71 (25.6) | 27 (20–30) | 266 (21.5) |
| 31–50 | 630 (65.6) | 39 (31–50) | 178 (64.3) | 38 (31–50) | 808 (65.3) |
| ≥51 | 135 (14.1) | 56 (51–70) | 28 (10.1) | 54 (51–64) | 163 (13.2) |
| Total | 960 (77.6) | 277 (22.4) | 1237 (100) | ||
Abbreviations: HCW, healthcare worker; min, minimum; max, maximum.
Although all HCWs completed their allocated two‐dose vaccination schedule, serum samples were obtained from 805 professionals after 6 months following the second dose, 638 females (79.3%) and 167 (20.7%) males.
3.2. Antibody dynamics after vaccination according to age and gender
The antibody response was studied at three time points (Table 2). In 1D, IgG was detectable in 1089 (88%) of HCWs. The seropositivity was 92.5% in 18–30 age group, 88.1% in the 31–50 age group and 80.4% in the ≥51 years group. The lowest seropositivity in HCWs with more than 51 years was statistically significant when compared with the youngest group (odds ratio [OR]: 3.00, 95% confidence interval [CI]: 1.65–5.46; p = 0.0002). Seropositivity was higher among females (90.5%) than males (79.4%) (OR: 2.47, 95% CI: 1.72–3.55; p < 0.0001) and was found to be highest in both women and men between 18 and 30 years (95.9% and 83.1%, respectively) (OR: 4.75, 95% CI: 1.85–12.19; p = 0.0011). Among HCWs between 31 and 50 years, IgG were found in 90.3% of females and 79.2% of males (OR: 2.54, 95% CI: 1.61–.98; p = 0.0001), and among those more than 51 years, 82.2% in females and 71.4% in males, but no statistically significant difference was found between the genders in this age group (p = 0.1982). In 2D, IgG was detected in 1235 (99.8%) HCWs. Two HCWs (0.2%) remained seronegative, one female (58 years old, with hypertension, diabetes, and autoimmune disease (Sjogren's syndrome), without previous RT‐PCR test for SARS‐CoV‐2) and one male (56 years, without comorbidities, with previous negative RT‐PCR). In 6mA2D, IgG positivity was found in 789 of 805 (97.9%) HCWs. 16 (2%) participants became seronegative, 11 females (nine females between 31 and 50 years; two females ≥51 years) and five males (two males between 18 and 30 years; three males between 31 and 50 years). The female seronegative in 2D was found seropositive in 6mA2D, but the male seronegative following two doses remained seronegative.
Table 2.
Anti‐S IgG positivity in HCWs vaccinated with CoronaVac, according to age and gender
| Characteristics of HCWs |
No. positive/No. tested (%) Time points |
||||
|---|---|---|---|---|---|
| Age (years) | Gender | 1D | p‐value | 2D | 6mA2D |
| 18–30 | Female | 187/195 (95.9) | 0.0011 | 195/195 (100) | 98/98 (100) |
| Male | 59/71 (83.1) | 71/71 (100) | 34/36 (94.4) | ||
| Total | 246/266 (92.5) | 266/266 (100) | 132/134 (98.5) | ||
| 31–50 | Female | 571/630 (90.3) | 0.0001 | 630/630 (100) | 435/444 (98.0) |
| Male | 141/178 (79.2) | 178/178 (100) | 111/114 (97.4) | ||
| Total | 712/808 (88.1) | 808/808 (100) | 546/558 (97.8) | ||
| ≥51 | Female | 111/135 (82.2) | 0.1982 | 134/135 (99.2) | 94/96 (97.9) |
| Male | 20/28 (71.4) | 27/28 (96.4) | 17/17 (100) | ||
| Total | 131/163 (80.4) | 161/163 (98.8) | 111/113 (98.2) | ||
| Total | Female | 869/960 (90.5) | <0.0001 | 959/960 (99.9) | 627/638 (98.3) |
| Male | 220/277 (79.4) | 276/277 (99.6) | 162/167 (97.0) | ||
| 1089/1237 (88.0) | 1235 (99.8) | 789/805 (97.9) | |||
Abbreviations: HCW, healthcare worker; IgG, immunoglobulin G; 1D: after 28 days of the first dose of vaccine; 2D: after 30 days of the second dose; 6mA2D: after 6 months of the second dose. Statistical analysis was performed using the Fisher's test.
Elevated anti‐S IgG levels in the HCWs vaccinated were found, with median IgG levels of 723.4 AU/ml (IQR, 109.6–1873) in 1D. Notably, these levels increased significantly to 1208 AU/ml (IQR, 706.1–2236) (p < 0.001) in 2D, although, the levels decreased significantly to 470.1 AU/ml (IQR, 191.3–1140), in 6mA2D (p < 0.0001) (Figure 1).
Figure 1.
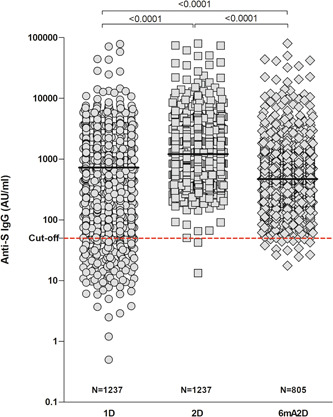
Anti‐S IgG levels in healthcare workers vaccinated with Coronavac. The evaluation was performed after 28 days of the first dose (1D), 28 days of the second dose (2D), and 6 months after the second dose of CoronaVac (6mA2D). Data were expressed by the concentration AU/ml for median values and the comparisons were performed with the Kruskal–Wallis test and Dunn's multiple testing correction
The evaluation of IgG titers between males and females showed levels increased in females in 1D and 2D (Figure 2A). In 1D, the median IgG level was 761.6 AU/ml (IQR, 136.1–2021) in females and 626.6 AU/ml (IQR, 62.30–1499) in males, increasing to 1252 AU/ml (IQR, 741.7–2388) in females and 959.6 AU/ml (IQR, 627.1–1782) in males, in 2D. However, the median decreased to 487.3 AU/ml (IQR, 198.1–1180) in females and 404.6 in males (IQR, 150.8–1029), in 6mA2D. There was statistically difference between genders in terms of antibody levels in 1D and 2D (p < 0.001) but no difference was found in 6mA2D (p = 0.037).
Figure 2.
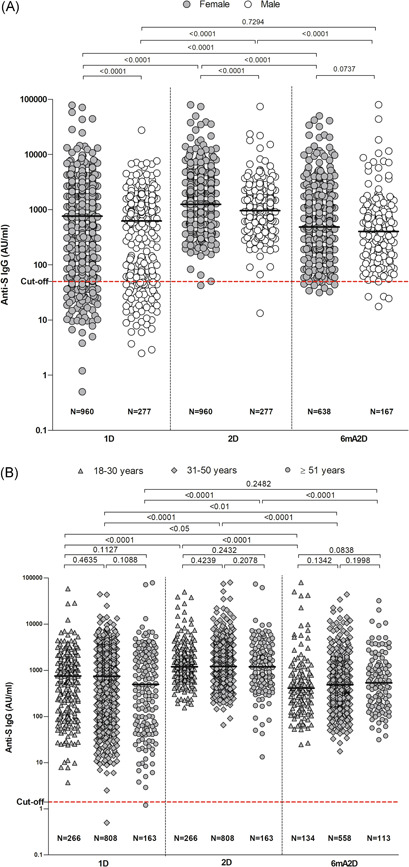
Anti‐S IgG levels by female and male individuals (A), and by age group (B). The evaluation was performed after 28 days of the first dose (1D), 28 days of the second dose (2D), and 6 months after the second dose with CoronaVac (6mA2D). Data were expressed by the concentration AU/ml for median values. The comparisons between groups of different time points were performed with the Kruskall–Wallis test and Dunn's multiple testing correction, and the comparisons between groups of the same time point were performed with test t and Mann–Whitney; p‐values < 0.0001 are reported as exact numbers
Antibody levels were compared between the age groups, but no statistically significant difference was found (Figure 2B). In 1D, the median lgG level was 755.3 AU/ml (IQR: 140.8–1.612) in the age group 18–30 years, 742.3 AU/ml (IQR: 111–1907) in the age group 31–50 years and 495.9 AU/ml (IQR: 62.4–2225) in the age group ≥51 years. In 2D, the median IgG level increased to 1197 AU/ml (IQR 745.6–2086), 1213 AU/ml (IQR, 716.1–2273), and 1191 AU/ml (IQR: 554.7–2422), respectively. In 6mA2D, the IgG titers decreased to 411.5 AU/ml (IQR: 174.2–876.6) in the age group 18–30 years, 489.5 AU/ml (IQR: 194.4–1145) in the age group 31–50 years, and to 537.4 AU/ml (IQR: 172.4–1325) in the age group >51 years.
3.3. Antibody dynamics after vaccination according to previous infection
The antibody response to CoronaVac was evaluated in relation to a previous SARS‐CoV‐2 infection, confirmed by RT‐PCR. While answering the questionnaire, 596 (48%) participants reported symptoms of COVID‐19 before vaccination. The main symptoms reported were loss of taste or smell (96%), fever or chills (48%) congestion or runny nose (40%), headache (39%), sore throat (35%), muscle or body aches (33%) cough (32%), fatigue (31%), and diarrhea (20%). Five hundred and thirty (42.8%) HCWs reported they were tested by RT‐PCR for SARS‐CoV‐2, before the first dose of vaccine. Among these, 259 (48.9%) had test results that were positive, 268 (50.6%) had results that were negative and 3 (0.5%) had results that were indeterminate.
Postvaccination antibody response positively correlated with prior COVID‐19 (before the first dose of vaccine). In 1D, IgG was detected in 96.5% of HCWs who had previous COVID‐19% and 87.3% of HCWs who did not have previous COVID‐19 (OR: 4.03; 95% CI: 1.89–8.59; p < 0.0001). In 2D, IgG was detected in all 259 (100%) HWCs with a history of COVID‐19 and in 267 (99.6%) of HCWs without history of COVID‐19. All participants with prior COVID‐19 infection remained seropositive in 6mA2D, but 3.2% of those participants without prior COVID‐19 became seronegative.
Antibody titers were found five times higher in those who had COVID‐19 (median IgG level: 1512 AU/ml; IQR: 754.1–2545) than those who did not had (median IgG level: 303.6 AU/ml; IQR: 80.73–1131) in 1D (p < 0.0001) and 1.4 times higher in those who had the disease (median IgG level: 1460 AU/ml; IQR: 864.4–2564) than those who did not have (median IgG level: 1066 AU/ml; IQR: 631.6–2080), in 2D (p = 0.0004) (Figure 3). In 6mA2D, the median IgG levels were 587.5 AU/ml (IQR: 292.5–1184) in the group with previous disease and 307.7 AU/ml (IQR: 153.2–978.4) in the group without previous disease (p < 0.0001).
Figure 3.
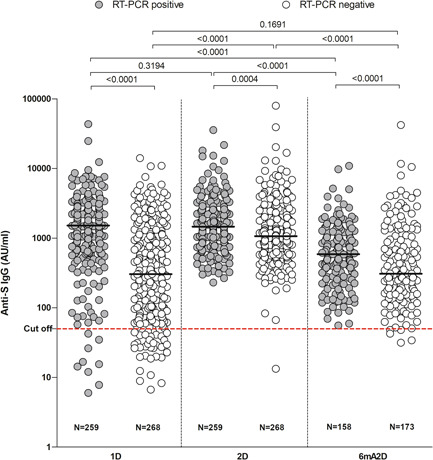
Anti‐S IgG levels in healthcare workers vaccinated with Coronavac, according to previous COVID‐19 infection. The quantitative assessment of IgG was measured after 28 days of the first dose (1D), 28 days of the second dose (2D), and 6 months after the second dose with CoronaVac (6mA2D). The groups were divided in positive RT‐PCR and negative RT‐PCR before vaccination. Data were expressed by the concentration AU/ml for median values. The comparisons between groups of different time points were performed with the Kruskal–Wallis test and Dunn's multiple testing correction, and the comparisons between groups of the same time point were performed with test t and Mann–Whitney; p‐values < 0.0001 are reported as exact numbers
The IgG levels after the second dose were compared with the first dose. In the HCWs without previous COVID, IgG levels increased 3.5 times (p < 0.0001). However, in the group of HCWs with previous COVID‐19, an increase of anti‐S IgG was not observed after the second dose of vaccine (p = 0.3194).
A total of 187/805 (23.2%) reported symptoms of COVID‐19 in the period of 2–6 months after the second dose. Among them, 88/805 (10.9%) reported a RT‐PCR test, 72/88 (81.8%) tested negative and 16/88 (18.1%) tested positive. The group tested positive was analyzed separately and exhibited the highest levels of IgG in 6mA2D (median IgG level: 2804.0 AU/ml; IQR: 664.8–11,090).
3.4. Antibody response to vaccination in participants with pre‐existing diseases
In this study, 239 (19%) participants informed that they have comorbidities. Hypertension (44%), diabetes (20%), obesity (15%), asthma (12%), dyslipidemia (4%), and cardiopathy (2%) were the main comorbidities reported.
Seropositivity for Anti‐S IgG was similar in HCWs with comorbidities compared to those without comorbidities. However, the comparison of antibody titles after vaccination between the groups showed IgG levels higher in the group without comorbidities. In 1D, the median title of IgG was 790.8 AU/ml, (IQR: 128.9–1916) in participants without comorbidities and 480.2 AU/ml (IQR: 83.68–1720) in participants with comorbidities (p = 0.0111). In 2D, the median titles were 1.233 AU/ml (IQR: 727.9–2266) and 1.055 AU/ml (IQR: 598.6–2096), respectively (p = 0.0321). In 6mA2D, the concentrations were 492.1 AU/ml (IQR: 203.8–1188) in the group without comorbidities and 323.9 AU/ml (IQR: 149.2–1003) in the group with comorbidities (p = 0.0090) (Figure 4A).
Figure 4.
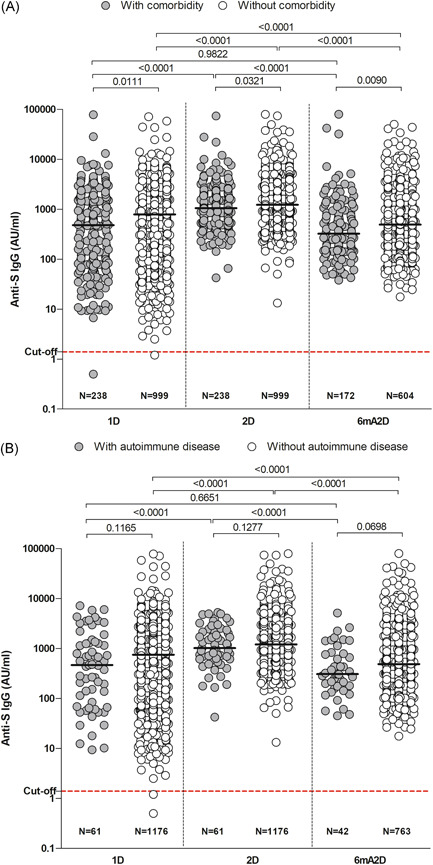
Anti‐S IgG in healthcare workers according to comorbidity (A) and autoimmune disease (B). The evaluation was performed after 28 days of the first dose (1D), 28 days of the second dose (2D), and 6 months after the second dose with CoronaVac (6mA2D). Data were expressed by the concentration AU/ml for median values. The comparisons between groups of different time points were performed with the Kruskal–Wallis test and Dunn's multiple testing correction, and the comparisons between groups of the same time point were performed with test t and Mann–Whitney; p‐values < 0.0001 are reported as exact numbers
A small number of HCWs (5%) had an autoimmune disease. No differences were found in IgG positivity between HCWs with immune‐mediate diseases and those who did not. In the same way, no significant difference in antibody titers were found between the groups (Figure 4B).
4. DISCUSSION
This study demonstrated that the first dose of CoronaVac already elicits a good immune response in HCWs. The antibody status of HCWs in this phase might reflect community‐acquired immunity, resulting from SARS‐CoV‐2 exposure in daily medical practice. The second dose of vaccine induced a powerful boost in the humoral immunity. However, 6 months after the second dose, the antibody response decreased significantly. The decline of antibodies is expected as not all vaccine‐induced plasmablasts commit or are maintained as long‐lived memory plasma cells. 25 Thus, the success of vaccine is dependent on the generation and maintenance of immunological memory. 26
According to the findings, the rate of antibody positivity and the amount of antibody titers were significantly higher in females. The difference in antibody responses is one of the most well‐conserved sex differences in immunology contributing to susceptibility to infectious diseases and responses to vaccines in males and females. 27 Sex‐based immunological differences can explain the male‐biased COVID‐19 mortality 28 and the more robust female immune response to COVID‐19 vaccines reported in the literature. 29 , 30 , 31 , 32
Patient age has been identified as a risk factor for severe illness and death in COVID‐19. Vaccine responses are diminished in the older adults due to immune senescence. In this study, although there was no significant difference in antibody titers between the age groups, the participants ≥51 years exhibited the lowest IgG anti‐S titers, mainly after the first dose of vaccine. However, after the second dose of vaccine, the immunogenicity reached a level close to the others age group. Bayram et al. 29 and Bueno et al. 33 also reported relatively low seropositivity in individuals aged 60 years and older, after the first dose of CoronaVac, but similar to that found in younger individuals after the second dose. Thus, in the present study two doses of CoronaVac were capable of induction an antibody response in people ≥51 years old. In addition, the results show that age has a less significant effect at later time point, 6 months after the second dose of vaccine. Similar finding was reported by Naaber et al. 25 evaluating the effect of age in COVID‐19‐mRNA vaccinated individuals. Therefore, the findings indicate the benefit of the second dose for older people and its effect to level up the short‐term vaccination response. Although, the long‐term evidence on the postvaccination antibody persistence in older populations remains to be studied.
After the vaccination, previously infected participants had a significantly higher antibody levels than previously uninfected participants. Post‐vaccination antibody levels positively correlated with previous infection were described by other studies. 29 , 34 , 35 , 36 , 37 Interestingly, an increase of antibodies in previously infected participants was not observed after the second dose of vaccine. HCWs with a history of COVID‐19 seems to reach a peak of antibodies already in the first dose, so that a second dose does not increase the response. This phenomenon can be explained by the fact that in a conventional multidose vaccine schedule, the first dose generates a primary immune response, and the second dose generates the boosted anamnestic response. In patients who had COVID‐19, their prior infections will serve as a priming dose of antigen; the first vaccination dose then, in effect, becomes the booster shot. 38 Additionally, several studies make note that the second dose in this situation provides virtually no additional boost to antibody levels. 29 , 38 , 39 , 40 , 41 , 42 , 43 , 44 These findings may suggest that people who had COVID‐19 can benefit from one just dose of CoronaVac.
People with comorbidities are well known as high‐risk groups for COVID‐19. Many COVID‐19 associated comorbidities affect the function of the immune system, which in turn directly impacts the response to COVID‐19. Furthermore, the drugs prescribed for these comorbidities also influence the progression of COVID‐19. 45 Antibody titers were significantly lower in participants with comorbidities. Geisen et al. 46 and Bayram et al. 29 also reported low levels of antibodies against SARS‐CoV‐2 spike protein following two‐dose of vaccination with CoronaVac in patients with chronic diseases. These data suggest that patients with chronic diseases may need a booster shot of CoronaVac vaccine.
Immune‐mediate diseases are at risk of both severe COVID‐19 and for insufficient response to vaccinations due to immunosuppression. In this study, no significant difference was found in antibody responses to CoronaVac in this group. The HCWs with immune‐mediated diseases developed a significant humoral response following the administration of two doses of CoronaVac, albeit with lower antibody titers. Similar observation can be found in other inactivated vaccine studies. 47 , 48 Whether these individuals should get a booster dose warrants further studies.
This study has limitations since we the seroprevalence of SARS‐CoV‐2 antibodies in HCWs before vaccination was not evaluated. Therefore, the seroconversion rate was not defined. In addition, no specific risk group such as individuals with age over 70 years was included. Furthermore, the male population was underrepresented, hence conclusions on gender differences in vaccine response need further studies. Finally, the neutralizing activity of antibodies and T cell responses were not assessed.
5. CONCLUSION
Taken together, the study reports a robust evidence of antibody response to CoronaVac after two doses, which were declined at 6 months postvaccination. Further longitudinal studies are needed to identify whether the antibodies will continue to decline or plateau at a lower level and determine what level is protective, clarifying the necessity of booster doses.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Marcela H. G. Fonseca conceived of the presented idea and wrote the first draft. Tamiris de F. G. de Souza processed the data, performed the analysis, drafted the manuscript, and designed the figures. Fernanda M. de Carvalho Araújo revised and gave intellectual inputs in the manuscript. Luiz O. M.de Andrade supervised, edited, and revised the manuscript. Marcela H. G. Fonseca took the lead in writing the manuscript. All authors discussed the results and commented on the manuscript.
ACKNOWLEDGMENTS
We thank the 1237 volunteers and the 29 health care institutions for participating in this study. We also thank the researcher Dr. Paulo Goberlânio de Barros Silva for the statistical analysis. The project is funded by Fundação Oswaldo Cruz and Ministério da Saúde, Brazil.
Fonseca MHG, de Souza TdFG, de Carvalho Araújo FM, de Andrade LOM. Dynamics of antibody response to CoronaVac vaccine. J Med Virol. 2022;94:2139‐2148. 10.1002/jmv.27604
DATA AVAILABILITY STATEMENT
Data are available on request due to privacy or ethical restrictions. The data that support the findings of this study are available from the corresponding author.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel Coronavirus from patients with pneumonia in china. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS‐CoV‐2 and COVID‐19. Nat Rev Microbiol. 2021;19(3):141‐154. 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed. 2020;91(1):157‐160. 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poland GA, Ovsyannikova IG, Kennedy RB. SARS‐CoV‐2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595‐1606. 10.1016/S0140-6736(20)32137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham BS. Rapid COVID‐19 vaccine development. Science. 2020;368(6494):945‐946. 10.1126/science.abb8923 [DOI] [PubMed] [Google Scholar]
- 6. Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576‐577. 10.1038/d41586-020-01221-y [DOI] [PubMed] [Google Scholar]
- 7. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396(10249):467‐478. 10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2—preliminary Report. N Engl J Med. 2020;383(20):1920‐1931. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586(7830):516‐527. 10.1038/s41586-020-2798-3 [DOI] [PubMed] [Google Scholar]
- 10. Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID‐19 vaccine R&D. Science. 2020;368(6494):948‐950. 10.1126/science.abc5312 [DOI] [PubMed] [Google Scholar]
- 11. Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS‐CoV‐2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951‐960. 10.1001/jama.2020.15543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18‐59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181‐192. 10.1016/S1473-3099(20)30843-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet. 2020;395(10240):1845‐1854. 10.1016/S0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gómez‐Ochoa SA, Franco OH, Rojas LZ, et al. COVID‐19 in health‐care workers: a living systematic review and meta‐analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021;190(1):161‐175. 10.1093/aje/kwaa191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Lancet. COVID‐19: protecting health‐care workers. Lancet. 2020;395(10228):922. 10.1016/S0140-6736(20)30644-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization Interim Recommendations for Use of the Inactivated COVID‐19 Vaccine, CoronaVac, Developed by Sinovac: Interim Guidance. WHO; 2021. https://apps.who.int/iris/handle/10665/341454 [Google Scholar]
- 17. Siegrist CA. 2 ‐ Vaccine immunology. Plotkin's vaccines. 7th end. Elsevier; 2018:16‐34.e7. 10.1016/B978-0-323-35761-6.00002-X [DOI] [Google Scholar]
- 18. Lu L, Zhang H, Zhan M, et al. Antibody response and therapy in COVID‐19 patients: what can be learned for vaccine development? Sci China Life Sci. 2020;63(12):1833‐1849. 10.1007/s11427-020-1859-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Zuiani A, Fischinger S, et al. Quick COVID‐19 healers sustain anti‐SARS‐CoV‐2 antibody production. Cell. 2020;183(6):1496‐1507.e16. 10.1016/j.cell.2020.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma H, Zhao D, Zeng W, et al. Decline of SARS‐CoV‐2‐specific IgG, IgM and IgA in convalescent COVID‐19 patients within 100 days after hospital discharge. Sci China Life Sci. 2021;64(3):482‐485. 10.1007/s11427-020-1805-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poland GA, Ovsyannikova IG, Kennedy RB. SARS‐CoV‐2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595‐1606. 10.1016/S0140-6736(20)32137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasan A, Al‐Ozairi E, Al‐Baqsumi Z, Ahmad R, Al‐Mulla F. Cellular and humoral immune responses in Covid‐19 and immunotherapeutic approaches. Immunotargets Ther. 2021;10:63‐85. 10.2147/ITT.S280706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021;184(4):861‐880. 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abbott Core Laboratory . SARS‐CoV‐2 Immunoassays: advancing diagnostics of COVID‐19. Available at: https://corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2-
- 25. Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. 10.1016/j.lanepe.2021.100208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palm AE, Henry C. Remembrance of things past: long‐term b cell memory after infection and vaccination. Front Immunol. 2019;10:1787. 10.3389/fimmu.2019.01787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626‐638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 28. Jin JM, Bai P, He W, et al. Gender differences in patients with COVID‐19: focus on severity and mortality. Front Public Health. 2020;8:152. 10.3389/fpubh.2020.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bayram A, Demirbakan H, Günel Karadeniz P, Erdoğan M, Koçer I. Quantitation of antibodies against SARS‐CoV‐2 spike protein after two doses of CoronaVac in healthcare workers. J Med Virol. 2021;93(9):5560‐5567. 10.1002/jmv.27098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sauré D, O'Ryan M, Torres JP, Zuniga M, Santelices E, Basso LJ. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID‐19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis. 2021;22:56‐63. 10.1016/S1473-3099(21)00479-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shrotri M, Krutikov M, Palmer T, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV‐19 and BNT162b2 against SARS‐CoV‐2 infection in residents of long‐term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;21(11):1529‐1538. 10.1016/S1473-3099(21)00289-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demonbreun AR, Sancilio A, Velez ME, et al. COVID‐19 mRNA vaccination generates greater immunoglobulin g levels in women compared to men. J Infect Dis. 2021;224(5):793‐797. 10.1093/infdis/jiab314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bueno SM, Abarca K, González PA, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in a subgroup of healthy adults in Chile. Clin Infect Dis. 2021:ciab823. 10.1093/cid/ciab823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS‐CoV‐2 antibody response following vaccination with BNT162b2 and mRNA‐1273. JAMA. 2021;326(15):1533‐1535. 10.1001/jama.2021.15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manisty C, Otter AD, Treibel TA, et al. Antibody response to first BNT162b2 dose in previously SARS‐CoV‐2‐infected individuals. Lancet. 2021;397(10279):1057‐1058. 10.1016/S0140-6736(21)00501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS‐CoV‐2. JAMA. 2021;325(14):1467‐1469. 10.1001/jama.2021.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med. 2021;384(14):1372‐1374. 10.1056/NEJMc2101667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Purushotham JN, van Doremalen N, Munster VJ. SARS‐CoV‐2 vaccines: anamnestic response in previously infected recipients. Cell Res. 2021;31:827‐828. 10.1038/s41422-021-00516-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saadat S, Tehrani ZR, Logue J, et al. Single dose vaccination in healthcare workers previously infected with SARS‐CoV‐2. MedRxiv. 2021. 10.1101/2021.01.30.21250843 [DOI] [Google Scholar]
- 40. Ciccone EJ, Zhu DR, Ajeen R, et al. SARS‐CoV‐2 seropositivity after infection and antibody response to mRNA‐based vaccination. MedRxiv. 2021. 10.1101/2021.02.09.21251319 [DOI] [Google Scholar]
- 41. Mazzoni A, Di Lauria N, Maggi L, et al. First‐dose mRNA vaccination is sufficient to reactivate immunological memory to SARS‐CoV‐2 in subjects who have recovered from COVID‐19. J Clin Invest. 2021;131(12):e149150. 10.1172/JCI149150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Callegaro A, Borleri D, Farina C, et al. Antibody response to SARS‐CoV‐2 vaccination is extremely vivacious in subjects with previous SARS‐CoV‐2 infection. J Med Virol. 2021;93(7):4612‐4615. 10.1002/jmv.26982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med. 2021;384(14):1372‐1374. 10.1056/NEJMc2101667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ebinger JE, Fert‐Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nat Med. 2021;27(6):981‐984. 10.1038/s41591-021-01325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Callender LA, Curran M, Bates SM, Mairesse M, Weigandt J, Betts CJ. The impact of pre‐existing comorbidities and therapeutic interventions on COVID‐19. Front Immunol. 2020;11:1991. 10.3389/fimmu.2020.01991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti‐SARS‐CoV‐2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80(10):1306‐1311. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Medeiros‐Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27(10):1744‐1751. 10.1038/s41591-021-01469-5 [DOI] [PubMed] [Google Scholar]
- 48. Seyahi E, Bakhdiyarli G, Oztas M, et al. Antibody response to inactivated COVID‐19 vaccine (CoronaVac) in immune‐mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int. 2021;41(8):1429‐1440. 10.1007/s00296-021-04910-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request due to privacy or ethical restrictions. The data that support the findings of this study are available from the corresponding author.


