Abstract
Limited prospective serosurveillance data in children regarding severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection have been reported. We prospectively investigated SARS‐CoV‐2 seropositivity in children during a 16‐month period of the coronavirus disease 2019 (COVID‐19) pandemic, including the four waves of the pandemic, before SARS‐CoV‐2 adolescents' vaccination. Serum samples from children admitted to the major tertiary Greek pediatric hospital for any cause, except for COVID‐19 infection, were randomly collected from 05/2020 to 08/2021. The study period was divided into four 4‐month periods representing relevant epidemic waves. Total SARS‐CoV‐2 antibodies for nucleocapsid protein were determined using the Elecsys® Anti‐SARS‐CoV‐2 reagent. A total of 3099 children (0–16 years) were included in the study. A total of 344 (11.1%) seropositive children were detected (males: 205 [59.5%]; median age [interquartile range [IQR]]: 3 years [0.6–10]). Seropositivity rates (%) increased during the four 4‐month periods: 1.4%, 8.6%, 17.2%, and 17.6%, respectively. A correlation of seropositivity rates in children with new diagnosed SARS‐CoV‐2 cases in the community was detected. No significant differences were detected between males and females. Seropositivity was significantly higher in hospitalized than in nonhospitalized children and in non‐Greek compared to Greek children (p < 0.001). The lowest seropositivity rate before school opening (9/2021) was detected in the age groups 6–12 years (14.4%) and 12–16 years (16.1%). However, compared with the other age groups, the lowest median antibody titers were observed in children 0–1 year (median [IQR]: 13.9 cut‐off index: [4.5–53.9] [p < 0.001]). Although the seropositivity of children was related to the community epidemic waves, the exposure was limited. Low seropositivity rates in school‐age children support the need for SARS‐CoV‐2 immunization.
Keywords: antibody, children, immunity, SARS‐CoV‐2, seroepidemiology
Highlights
SARS‐CoV‐2 seropositivity rates (%) in children residing in Athens increased from 05/2020 to 08/2021 during the four 4‐month pandemic waves: 1.4%, 8.6%, 17.2%, and 17.6%, respectively.
A correlation of seropositivity rates in children with new diagnosed SARS‐CoV‐2 cases in the community was detected.
The lowest seropositivity rate before school opening (9/2021) was detected in the age groups 6–12 years (14.4%) and 12–16 years (16.1%).
Limited exposure and low seropositivity rates in school‐age children support the need for SARS‐CoV‐2 immunization.
1. INTRODUCTION
Since the first reports of coronavirus disease 2019 (COVID‐19) and the identification of the novel coronavirus that causes severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the infection has spread at an unprecedented pace and caused more than 200 million confirmed cases in adults and children worldwide. 1 , 2
Although most pediatric COVID‐19 cases present as asymptomatic, mild, or moderate, there are rare cases of severe clinical presentation of the postinfection multisystem inflammatory syndrome, when children require hospitalization to prevent life‐threatening complications of the infection. 3 , 4 , 5
It is difficult to estimate the exact incidence rate of SARS‐CoV‐2 infection in children, mainly due to the increased number of asymptomatic cases. 6 Children are commonly infected after close contact with an infected family member within the same household, even though viral transmission rates have also been reported in several other activities, including schools. 7
Serological studies have been critical in tracking the evolution of the COVID‐19 pandemic by deciphering the true extent of transmission in different populations. 8 Estimating the true rate of SARS‐CoV‐2 infection allows public health specialists to predict the likely future course of the epidemic in specific locations or populations and to better design interventions to control the epidemic. 8 , 9
The aim of this study was to prospectively evaluate the SARS‐CoV‐2 seropositivity rates of children 0–16 years old during a 16‐month period of the pandemic in Athens, Greece before adolescents' immunization. In the study, the role of epidemiological parameters, including sex, age, origin, and hospitalization status, was also evaluated.
2. MATERIALS AND METHODS
2.1. Study design and participants
This was a prospective cohort study involving children who presented in the emergency department or were admitted for any reason to the ‘'Aghia Sophia’’ Children's Hospital, a 750‐bed tertiary pediatric hospital, which is the largest hospital for children in Greece.
To evaluate the seropositivity of children in the Athens metropolitan area, serum samples were prospectively collected from 05/2020 to 08/2021. Each month, approximately 200 serum samples were randomly collected from the Department of Clinical Biochemistry of “Aghia Sophia” Children's Hospital. Serum samples were residual sera that were ordered from paediatricians for any medical reason from hospitalized children or children from the emergency department. Children with proven COVID‐19 infection either with SARS‐CoV‐2 PCR or with the rapid test were excluded from the study. Epidemiological parameters, which included age, sex, origin, and hospitalization status, were recorded. If a child was admitted to the hospital more than once within the study period and the results of his antibody test results were positive, only those of the first positive result were included in the analysis. Further laboratory testing of the serum samples was performed anonymously using an identification code.
The study period was divided into four 4‐month different subperiods representing different phases of the pandemic: 01/05/2020–31/08/2020, 01/09/2020–31/12/2020, 01/01/2021–30/04/2021, and 01/05/2021–31/08/2021. Children from different age groups were enrolled in this study and were categorized as infants (0–1 year), toddlers (1–4 years), pre‐school (4–6 years), primary school (6–12 years), and adolescents (12–16 years).
Data on the new cases of COVID‐19 in Greece were found on the site of the Greek National Public Health Organization (GNPHO). 10
2.2. Ethical approval
The study protocol was in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards and was approved by the scientific and bioethics committee of “Aghia Sophia” Children's Hospital (No. 25609).
2.3. Antibody detection assay
Serums for SARS‐CoV‐2 antibody detection were tested in the Infectious Diseases Laboratory of the Choremeion Research facility, First Department of Pediatrics, Medical School, National and Kapodistrian University of Athens, “Aghia Sophia” Children's Hospital. Serum samples were tested using Elecsys® Anti‐SARS‐CoV‐2 (Roche Diagnostics) reagent on a Cobas e 411 immunoassay analyzer for the semiquantitative detection of total antibodies (IgA, IgM, and IgG) against SARS‐CoV‐2 nucleocapsid protein according to the manufacturer's instructions. Elecsys® Anti‐SARS‐CoV‐2 is an electrochemiluminescence immunoassay (ECLIA), which is based on a double antigen sandwich enzyme‐linked immunosorbent assay methodology. ECLIA represents a highly effective technique, with an estimated sensitivity of 99.5% (14 days from the onset of symptoms) and a specificity of 99.8%. Values of ≥1 cut‐off index (COI) are positive.
2.4. Statistical analysis
The assumption of normality was checked through kurtosis and skewness, Kolmogorov–Smirnov, and Shapiro–Wilk tests. Absolute and relative frequencies (%) were used to describe the qualitative variables, while median and interquartile range (IQR) were used for quantitative variables. Differences between qualitative variables were evaluated with the χ 2 test. To check the proportions, a binomial test was conducted (for dichotomous variables) or one sample χ2 test (for nondichotomous variables). Correlations were assessed via the Spearman correlation coefficient. The statistical significance level was set at a p‐value of <0.05. Statistical analysis was performed with the SPSS version 26.0 (IBM Corp., Released 2019. IBM SPSS Statistics for Windows, Version 26.0. IBM Corp).
3. RESULTS
A total of 3099 pediatric serum samples were tested from 1/5/2020 to 31/8/2021. More specifically, in the first subperiod were tested 793 (25.6%) samples, in the second 775 (25%), in the third 775 (25%), and in the fourth 756 (24.4%) (p: 0.75). Demographic characteristics of the study population and seropositive children in each study subperiod are presented in Table 1. The median age (IQR) of the study population was 5 years (0.9–11 years). Among the 3099 children, 1712 (55.2%) were male, 2221 (71.7%) were hospitalized, and 2260 (72.9%) were of Greek origin.
Table 1.
Distribution of the demographic characteristics of the 3099 children who included in the study in the four different study periods: 1st: 05/2020–08/2020, 2nd: 09/2020–12/2020, 3rd: 01/2021–04/2021, 4th: 05/2021–08/2021
| Study period | |||||||
|---|---|---|---|---|---|---|---|
| Total | 1st | 2nd | 3rd | 4th | |||
| Variable | Categories | n (%) | n (%) | n (%) | n (%) | n (%) | p value |
| Children tested | 3099 | 793 (25.6) | 775 (25) | 775 (25) | 756 (24.4) | 0.75 | |
| Seropositive | 344 (11.1) | 11 (1.4) | 67 (8.6) | 133 (17.2) | 133 (17.6) | <0.001 | |
| Gender | Female | 139/1387 (10.0) | 5/364 (1.4) | 31/351 (8.8) | 57/357 (16) | 46/315 (14.6) | <0.001 |
| Male | 205/1712 (12.0) | 6/429 (1.4) | 36/424 (8.5) | 76/418 (18.2) | 87/441 (19.7) | <0.001 | |
| p value | 0.09 | >0.999 | 0.898 | 0.445 | 0.08 | ||
| Age group (years) | 0–1 | 112/837 (13.4) | 5/213 (2.4) | 23/211 (10.9) | 52/253 (20.6) | 32/160 (20.0) | <0.001 |
| 1–4 | 73/642 (11.4) | 1/145 (0.7) | 11/151 (7.3) | 25/156 (16.0) | 36/190 (19.0) | <0.001 | |
| 4–6 | 37/237 (15.6) | 5/66 (7.6) | 7/51 (13.7) | 14/67 (20.9) | 11/53 (20.8) | 0.119 | |
| 6–12 | 70/882 (8.0) | 0/240 (0.0) | 13/234 (5.6) | 24/179 (13.4) | 33/229 (14.4) | <0.001 | |
| 12–16 | 52/501 (10.4) | 0/129 (0.0) | 13/128 (10.2) | 18/120 (15.0) | 21/124 (16.9) | 0.001 | |
| p value | 0.001 | <0.001 | 0.115 | 0.285 | 0.577 | ||
| Department | Hospitalized | 274/2221 (12.3) | 7/541 (1.3) | 59/540 (10.9) | 120/622 (19.3) | 88/533 (17.0) | <0.001 |
| Non‐hospitalized | 70/878 (8.0) | 4/252 (1.6) | 8/235 (3.4) | 13/153 (8.5) | 45/238 (19.0) | <0.001 | |
| p value | <0.001 | 0.75 | <0.001 | 0.001 | 0.411 | ||
| Origin | Greek | 209/2260 (9.3) | 6/580 (1.0) | 32/553 (5.8) | 95/594 (16.0) | 76/533 (14.3) | <0.001 |
| Non‐Greek | 135/839 (16.1) | 5/213 (2.4) | 35/222 (15. 8) | 38/181 (21.0) | 57/223 (25.6) | <0.001 | |
| p value | <0.001 | 0.176 | <0.001 | 0.143 | <0.001 | ||
Note: Among them, 344 (11.1%) were detected seropositive for SARS‐CoV‐2.
A total of 344 (11.1%) seropositive children were detected during the study period, with median age of 3 years (IQR: 0.6–10 years). The median age of seropositive children in the four study subperiods were: 1st: 2 years (IQR: 0.2–5), 2nd: 3 years (IQR: 0.4–11.3), 3rd: 2 years (IQR: 0.4–10.7), and 4th: 4 years (IQR: 1.1–10) (p: 0.167).
Seropositivity in different pediatric departments of the hospital was 13.9% (227/1634) in general pediatric departments, 5.1% (5/98) in pediatric intensive care unit, 8.6% (21/244) in neonatal intensive care unit, 9.7% (14/144) in surgical departments (including surgery, cardiothoracic surgery, neurosurgery, orthopedics, urology, ophthalmology, plastic surgery, and otorhinolaryngology departments), 4.9% (2/41) in the cardiology department, and 6.7% (4/60) in the neurology department.
The seropositivity of SARS‐CoV‐2 (%) during each month of the study is presented in Figure 1, 2 and the relevant monthly new cases of COVID‐19 cases in Greece, as reported by the GNPHO in Figure 1, 2. Seropositivity varied significantly among the four subperiods. Seropositivity increased from 1.4% in the 1st subperiod to 8.6% in the 2nd, 17.2% in the 3rd and 17.6% in the 4th subperiod (p < 0.001). The lowest seropositivity was detected in 08/2020 (0%) and the highest were detected in 06/2021 (21.8%) and 07/2021 (21.2%). There was a correlation of seropositivity rates in children with newly diagnosed SARS‐CoV‐2 cases in Greece with Spearman r: 0.75 (95% confidence interval: 0.3913–0.9109) (p: 0.001).
Figure 1.
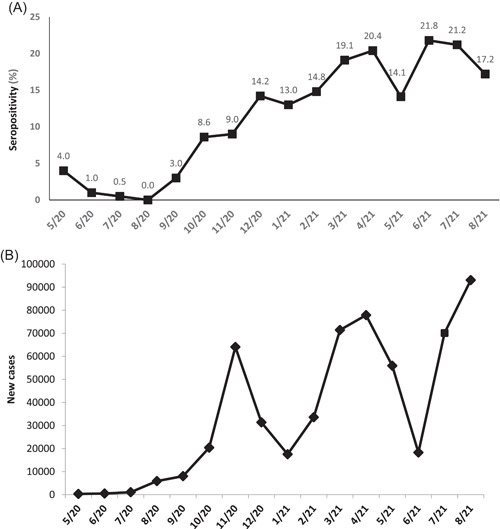
(A) Monthly seropositivity rates (%) for SARS‐CoV‐2 antibodies from 5/2020 to 8/2021 in 3099 children of Athens metropolitan area. (B) New COVID‐19 cases in Greece 5/2020–8/2021, including all age groups (data from Greek National Public Health Organization) 10
Figure 2.
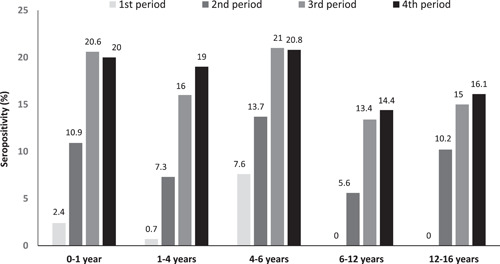
Changes in SARS‐CoV‐2 seropositivity (%) in 3099 children of different age groups from 5/2020 to 8/2021 presented in four different 4‐month periods: 1st: 5/2020–8/2020, 2nd: 9/2020–12/2020, 3rd: 1/2021–4/2021, 4th: 5/2021–8/2021 in Athens metropolitan area
The SARS‐CoV‐2 seropositivity (%) detected in the four different subperiods per age group are presented in Table 1 and Figure 1, 2. In all age groups, there was a statistically significant increase in seropositivity during the four study subperiods (p < 0.001) except for the age group of 4–6 years (p: 0.119). In all four study subperiods, the age group 4–6 years had the highest seropositivity rate, followed by the 0–1 year and the 1–4 years group. The lowest seropositivity rate at the last period was detected in the age groups 6–12 years (14.4%) and 12–16 years (16.1%).
No significant differences were detected between seropositive males and females during the study period (p: 0.09) (Table 1). Seropositivity was significantly higher in hospitalized children than in non‐hospitalized children (p: 0.0004) (Table 1). Children of non‐Greek origin had statistically significant higher seropositivity rates compared to children of Greek origin in all subperiods (p < 0.001) (Table 1).
Antibody titers per age group are presented in Figure 3. The median (IQR) antibody titer per age group was 13.9 COI (4.5–53.9) in the 0–1‐year‐old, 59.6 COI (13.3–137.9) in the 1–4 years, 63 COI (12.6–170.1) in the 4–6 years, 39 COI (9.5–133.1) in the 6–12 years, and 33.1 COI (8.1–81.2) in the 12–16 years (p: 0.001).
Figure 3.
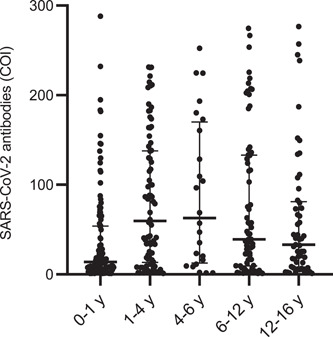
SARS‐CoV‐2 antibodies levels in 344 seropositive children of different age groups from 5/2020 to 8/2021 in the Athens metropolitan area. Bars represent median and line interquartile range (IQR) values (COI, cut‐off index; y, years)
4. DISCUSSION
In the present study, we prospectively investigated SARS‐CoV‐2 seropositivity in a large pediatric population during a 16‐month period of the COVID‐19 pandemic and the possible association with epidemiological and demographic characteristics to evaluate the need for immunization.
We detected increasing seropositivity in sequential epidemic waves and an association with the new cases in the general population indicating the increased circulation and transmission of the virus in the community. The initial low seropositivity in the first and second epidemic waves are justified due to the implementation of strict physical distancing measures, school closure, and the limited exposure of children to close contacts outside of their household. 11 In contrast, the subsequent increase coincides with the release of restriction measures, as well as the circulation of more transmissible variants of SARS‐CoV‐2. 12 , 13
In our area, children <17 years old represent almost 17% of the total population. Even taking into account the highest seropositivity rate of children during the study period (21.8%), it means that only an additional 3.7% of the total population has SARS‐CoV‐2 immunity. At the same period (end of August 2021), the GNPHO reported that people <17 years represent 12.1% of total cases. 10 For this discrepancy between pediatric confirmed cases and seropositivity, we could hypothesize that antibodies are waning over time or there is a delay between cases and antibody detection in the population. Another hypothesis could be that children who have asymptomatic or mild symptomatic infection do not display detectable antibody responses.
No differences in seropositivity rates between sexes were detected, which is consistent with findings regarding confirmed SARS‐CoV‐2 cases in Greece. 10 Data from the United States on a Nationwide Commercial Laboratory Seroprevalence Survey also support the comparable seroprevalence rates between females and males (20.4% and 20.8%, respectively), as well as the equal incidence of the disease in both women and males <17 years by August 2021. 10 , 14 , 15
In all study subperiods, pre‐school children (4–6 years old) had the highest seropositivity rate. This finding may possibly be overestimated due to the limited number of children of this age group represented in the study. Nevertheless, it may partially explain the transmission of preadolescent children in kindergartens and playgrounds, in which epidemic control strategies are not always effective due to absence of physical distancing and use of face masks. 16
An increased proportion of seropositive hospitalized compared to non‐hospitalized children during the study period (12.3% and 8%, respectively) was detected. This could be a reflection of persistent physical symptoms in children after a known or unknown SARS‐CoV‐2 infection (long‐COVID‐19), including dyspnea, cough, headache, joint, or chest pain that may require hospitalization for further evaluation. 17 Another hypothesis could be that COVID‐19 infection could affect post‐infectious general health status. This has been shown for other infections, such as measles, which has long‐term effects in childhood infectious disease morbidity and mortality. 18 However, this is a hypothesis that must be tested in large epidemiological studies.
In the present study, significant differences in seropositivity rates were found between children of Greek and non‐Greek origin in all different subperiods. It appears that SARS‐CoV‐2 alongside other infectious diseases incommensurately affects underrepresented racial and ethnic groups within society. 19 , 20 This trend may possibly be attributed to poverty, densely populated households, and to limited access to health facilities. 19 , 20 These factors lead not only to a disproportionate increase in the incidence of SARS‐CoV‐2 infection in these social groups but also to significant restrictions in laboratory tests and confirmation of the disease. 19 , 20 Public health measures regarding the vaccination of children should take into account the elevated seropositivity rates in such groups and modify their vaccination need with prospective seroepidemiology studies in the future.
COVID‐19 vaccines are an indisputable tool of major significance for pandemic control and vaccinations in children began in Greece in August 2021. The BNT162b2 COVID‐19 vaccine is authorized for adolescents aged 12–15 years, with estimated efficacy and immunogenicity rates approaching 100%. 21 , 22 Recently, the BNT162b2 COVID‐19 vaccine was licensed for children 5–11 years old. 23 The present study demonstrated that at the beginning of the upcoming school year, only a minority of children aged 5–12 years (14.4%) and 12–16 years (16.1%) will enter school and other social activities with detectable antibodies following natural SARS‐CoV‐2 infection. Study findings support immunization of this age group as it will add protection from complications such as myocarditis or multisystem inflammatory syndrome in children, facilitate the school and other activities' function, and protect the community from SARS‐CoV‐2 transmission. 5 , 24
Although we cannot determine the onset of SARS‐CoV‐2 infection in seropositive children only from a randomly detected antibody titer, the present study showed that children 0–1 year demonstrated significantly lower SARS‐CoV‐2 antibody titers compared to the other age groups. Transplacental transfer of maternal IgG from a SARS‐CoV‐2‐infected mother can be largely cleared from an infant's circulation by 6 months of age 25 and this may justify the significantly lower antibody titers of children 0–1 year compared to the other age groups. Further studies should gain insight into immune responses after SARS‐CoV‐2 infection and establish differences between age groups.
Limitations of the study include that it represents only the pediatric population of Athens and not country‐wide data. In addition, the distribution of age groups in the study does not necessarily represent the total pediatric population. Furthermore, seroepidemiology studies performed in hospitals may overestimate SARS‐CoV‐2 seropositivity as some of the children could be at the beginning of the infection or could be exposed because of their hospitalization. These potential limitations are common to most seroprevalence studies and do not degrade the utility of the data during the pandemic, as so far this is the only longitudinal study in our area. 9
5. CONCLUSION
As most children have mild symptoms after SARS‐CoV‐2 infection or remain asymptomatic, it is difficult to determine the exact incidence of COVID‐19 in the pediatric population. Through a prospective investigation of seropositivity in children, we showed that seropositivity of children is associated with epidemic waves in the community. Certain groups were affected disproportionately, indicating that public health decisions need to better protect these groups to reduce inequity in the impact of COVID‐19. Before the initiation of SARS‐CoV‐2 immunization for adolescents and younger children, the number of seropositive children is limited, which supports the need for immunization. Continuous surveillance of seroepidemiological data in children can guide and optimize public health policies on the implementation of preventive measures for transmission or immunization in schools and communities.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Alexandra Margeli and Ioannis Papassotiriou provided the samples. Filippos Filippatos, Elizabeth‐Barbara Tatsi and Charilaos Dellis collected samples and demographic data and carried out the experiments. Vasiliki Efthymiou performed the statistical analysis. Athanasios Michos and Vasiliki Syriopoulou designed and supervised the study. Elizabeth‐Barbara Tatsi helped in project supervision. Filippos Filippatos, Elizabeth‐Barbara Tatsi and Athanasios Michos wrote the initial manuscript. All authors reviewed and approved the final manuscript.
Filippatos F, Tatsi E‐B, Dellis C, et al. Seroepidemiology of SARS‐CoV‐2 in pediatric population during a 16‐month period prior to vaccination. J Med Virol. 2022;94:2174‐2180. 10.1002/jmv.27608
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Center for Systems Science and Engineering . COVID‐19 Map, Johns Hopkins Coronavirus Resource Center. 2021. Accesed September 15, 2021. https://coronavirus.jhu.edu/map.html/
- 3. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 4. Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID‐19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020;174(10):e202430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hobbs CV, Drobeniuc J, Kittle T, et al. Estimated SARS‐CoV‐2 seroprevalence among persons aged <18 years—Mississippi, May–September 2020. Morb Mortal Wkly Rep. 2021;70(9):312‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Posfay‐Barbe KM, Wagner N, Gauthey M, et al. COVID‐19 in children and the dynamics of infection in families. Pediatrics. 2020;146(2):e20201576. [DOI] [PubMed] [Google Scholar]
- 8. Cheng MP, Yansouni CP, Basta NE, et al. Serodiagnostics for severe acute respiratory syndrome‐related Coronavirus 2: a narrative review. Ann Intern Med. 2020;173(6):450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kritsotakis EI. On the importance of population‐based serological surveys of SARS‐CoV‐2 without overlooking their inherent uncertainties. Public Health Pract. 2020;1:100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greek National Public Health Organization . 2021. Accesed September 2, 2021. https://eody.gov.gr/epidimiologika-statistika-dedomena/ektheseis-covid-19/
- 11. Lee B, Raszka WV. COVID‐19 transmission and children: the child is not to blame. Pediatrics. 2020;146(2):e2020004879. [DOI] [PubMed] [Google Scholar]
- 12. Somekh I, Sharabi A, Dory Y, Simões E, Somekh E. Intrafamilial spread and altered symptomatology of SARS‐CoV‐2, during predominant circulation of lineage B.1.1.7 variant in Israel. Pediatr Infect Dis J. 2021;40(8):E310‐E311. [DOI] [PubMed] [Google Scholar]
- 13. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Factors associated with household transmission of SARS‐CoV‐2. JAMA Netw Open. 2021;4(8):e2122240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . CDC COVID Data Tracker. 2020. Accesed September 3, 2021. https://covid.cdc.gov/covid-data-tracker/#demographics/.
- 15. Centers for Disease Control and Prevention . CDC COVID Data Tracker. 2020. Accesed September 3, 2021. https://covid.cdc.gov/covid-data-tracker/#national-lab/
- 16. Centers for Disease Control and Prevention . Science Brief: Transmission of SARS‐CoV‐2 in K‐12 Schools and Early Care and Education Programs‐Updated. 2020. Accesed September 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html/ [PubMed]
- 17. Radtke T, Ulyte A, Puhan MA, Kriemler S. Long‐term symptoms after SARS‐CoV‐2 infection in children and adolescents. JAMA. 2021;326(9):869‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mina MJ, Metcalf CJ, De Swart RL, Osterhaus AD, Grenfell BT. Long‐term measles‐induced immunomodulation increases overall childhood infectious disease mortality. Science. 2015;348(6235):694‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID‐19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):707‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with Covid‐19 in New York city. JAMA Netw Open. 2020;3(12):e2026881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Food and Drug Administration . Pfizer‐BioNTech COVID‐19 Vaccine EUA Letter of Authorization reissued 05‐10‐2021. 2021. Accesed August 30, 2021. https://www.fda.gov/media/144412/download/
- 22. Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid‐19 vaccine in adolescents. N Engl J Med. 2021;385(3):239‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. FDA Authorizes Pfizer‐BioNTech COVID‐19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. Accesed November 9, 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age.
- 24. Centers for Disease Control and Prevention . CDC COVID Data Tracker. 2020. Accesed August 31, 2021. https://covid.cdc.gov/covid-data-tracker/#pediatric-data/
- 25. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715‐725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


