Abstract
The benefits of baricitinib in coronavirus disease‐2019 are inadequately defined. We performed a systematic review and meta‐analysis of studies of baricitinib to determine its clinical efficacy and adverse events in patients with COVID‐19. Databases were searched from their inception to September 5, 2021. The primary outcome was the coefficient of mortality. We also compared secondary indicators and adverse events between baricitinib treatment and placebo or other treatments. Twelve studies of 3564 patients were included and assessed qualitatively (modified Jadad and Newcastle–Ottawa Scale scores). Baricitinib effectively improved the mortality rate (relative risk of mortality = 0.56; 95% confidence interval: 0.46–0.69; p < 0.001; I 2 = 2%), and this result was unchanged by subgroup analysis. Baricitinib improved intensive care unit admission, the requirement for invasive mechanical ventilation, and improved the oxygenation index. Data from these studies also showed that baricitinib slightly reduced the risk of adverse events. Regarding the choice of the drug dosage of baricitinib, the high‐dose group appeared to have additional benefits for clinical efficacy. Our study shows that baricitinib may be a promising, safe, and effective anti‐severe acute respiratory syndrome‐coronavirus‐2 drug candidate, with the advantages of low cost, easy production, and convenient storage.
Keywords: baricitinib, COVID‐19, efficacy, JAK‐inhibitor, safety
1. INTRODUCTION
The severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2/coronavirus disease‐2019 [COVID‐19]) pandemic has emerged as an extraordinary public health challenge. According to the World Health Organization's latest weekly epidemiological update on COVID‐19 (September 7, 2021), the cumulative number of cases reported globally since 2019 currently exceeds 220 million. Additionally, the number of deaths caused by this infection has surpassed 4.5 million. The number of new cases has been increasing globally over the past two months, even as vaccines have been administered in multiple countries, with more than 4.4 million cases reported in the past week (30 August–5 September 2021). 1 Vaccines are still lacking in many low‐ and middle‐income countries. Therefore, effective treatments need to be found before a vaccine is widely developed and used in countries worldwide. The leading cause of death of COVID‐19 is acute respiratory distress syndrome, while a cytokine storm is thought to be the leading cause of multiple organ failure and acute respiratory distress syndrome. 2 Upon viral infection, sustained excessive secretion of proinflammatory cytokines leads to dysregulation of the innate immune system, and this cytokine storm attracts large numbers of inflammatory cells to infiltrate the lungs, ultimately causing immune damage. 3 Secretion of cytokines, such as interleukin (IL)−1β, IL‐6, tumor necrosis factor‐α, and IL‐1,8, is significantly increased in patients with COVID‐19. 4
Signal transducer and activator of transcription (STAT) signaling is a major cellular signaling pathway involved in the inflammatory response. 5 Accordingly, effective inhibition of cytokine storms is crucial for preventing severe COVID‐19 complications and reducing mortality. Baricitinib appears to be a safe and efficacious drug for treating COVID‐19 infections. Baricitinib is a Janus kinase (JAK1/JAK2) inhibitor developed to treat patients suffering from rheumatoid arthritis. 6 JAK‐STAT signaling is critical to multiple cellular processes, including survival, differentiation, and proliferation. 7 The JAK‐STAT pathways control the magnitude and duration of cytokine signaling to Type I and Type II cytokine receptors. Several inflammatory factors are involved in the pathogenesis of malaria through JAK‐STAT pathways, including IL‐6 and granulocyte‐macrophage colony‐stimulating factors. JAK1 and JAK2 inhibitors target these signaling pathways, which suppress the activation of inflammatory cells and reduce acute inflammatory responses.
On November 19, 2020, baricitinib received emergency authorization from the US Food and Drug Administration for use in combination with Remdesivir to treat hospitalized or suspected patients with COVID‐19. This emergency Food and Drug Administration authorization was based on a clinical trial conducted by the National Institute of Allergy and Infectious Diseases (ACTT‐2). 8 This trial showed that the combination of baricitinib and Remdesivir reduced mortality over a 28‐day treatment period compared with Remdesivir alone. Recently, new data from a randomized, controlled trial (RCT) showed that the 28‐day all‐cause mortality rate was 8% (n = 62) for baricitinib and 13% (n = 100) for placebo (hazard ratio [HR] = 0.57; 95% confidence interval [CI]: 0.41–0.78; p = 0.0018), with a 38.2% relative reduction in mortality. 9 This result supports the use of baricitinib in patients with COVID‐19.
The clinical use of baricitinib in patients with COVID‐19 remains questionable. Although previous meta‐analyses of JAK inhibitors have been published, 10 , 11 , 12 none of them examined the clinical efficacy and adverse events of baricitinib, and only one RCT 8 was included in these studies. Therefore, the clinical efficacy and adverse events of baricitinib still need to be investigated.
2. METHODS
This is a systematic review and meta‐analysis study of clinical trials and observational studies. Append research in this systematic review and meta‐analysis were chosen as most likely attaining the coming criteria: follow the PICO framework (P, Populations—hospitalized coronavirus disease 2019 patients; I, Interventions—treatment with baricitinib; C, Comparator/Control—a group of patients who only receive standard of care therapy or any other medications as control/placebo and did not receive treatment with baricitinib; O, Outcomes—mortality, intensive care unit admission, the requirement for invasive mechanical ventilation, the oxygenation index, choice of the drug dosage, and the risk of adverse events), The Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) criteria 13 were followed.
2.1. Inclusion and exclusion criteria
Studies that met all the following criteria were included: (1) the studies were in the English language, and baricitinib was used alone or with other therapies in patients with COVID‐19; (2) the efficacy and safety of baricitinib were investigated in adults with COVID‐19; (3) clinical outcomes of interest (all‐cause mortality, disease severity, intensive care unit [ICU] admission, invasive mechanical ventilation, and adverse events) were reported; (4) case‐control, cohort, and randomized or non‐randomized clinical trial research were contained; (5) all studies besides correspondence or review articles, case‐series or case report studies, studies reported other than in English language, research focusing on children below 18 years old were excluded.
2.2. Search strategy and study quality assessment
PubMed, Embase, Clinical Trial, and Web of Science were searched from inception to September 5, 2021 by three investigators (ZL, JN, and YX). We used the keywords “Baricitinib” AND “SARS‐CoV‐2,” OR “coronavirus disease 2019” OR “Covid‐19.” The search terms are detailed in the Appendix. These investigators independently screened titles and abstracts generated by the search. After selection, full electronic articles were then carefully evaluated for data extraction.
2.3. Data extraction and quality assessment
Three authors (ZL, JN, and YX) extracted data independently using predefined standardized forms. Each full article that met the inclusion criteria was carefully reviewed, and the following baseline information was extracted: first author, publication year, study type, number of total participants, number of participants receiving baricitinib, number of participants receiving other drugs, and the modified Jadad 14 or Newcastle–Ottawa Scale (NOS) score. 15 The outcome measures were mortality, intensive care unit admission, the requirement for invasive mechanical ventilation, the oxygenation index, choice of the drug dosage, and the risk of adverse events.
Three authors assessed the quality of each study involved in this review independently. Randomized studies and clinical trials included in the final analyses were scored by one investigator (ZL) to formally assess the risk of bias using the modified Jadad score. Non‐randomized studies included in the final analyses were scored by one investigator (JN) using the Newcastle–Ottawa Scale (NOS). There was adjudication by one investigator (YX) when there was disagreement.
The modified Jadad scale was used to evaluate the quality of clinical trials, including the randomization (0 or 2), blinding (0 or 2), description of withdrawals and dropouts (0 or 1), inclusion/exc1usion criteria (0 or 1), adverse effects (0 or 1), and statistical analysis (0 or 1) of each study. The studies were scored from 0 to 8, and 1–3 signified low‐quality while 4–8 signified high‐quality. 16 NOS was used to evaluate the quality of observational studies. The representativeness of the exposed cohort (0 or 1), selection of the nonexposed cohort (0 or 1), ascertainment of exposure (0 or 1), none of the subjects had the disease they were studying at the start of the study (0 or 1), comparability (0 or 1), noncomparability (0 or 1), method (0 or 1), follow‐up time (0 or 1), and adequacy of follow‐up of cohorts (0 or 1) were reported for the NOS score. Research is graded as good quality if it scores ≥7. 17
2.4. Data analysis
Forest plots were generated using Review Manager V.5.3 software (Cochrane Collaboration). To calculate the risk ratio (RR) and its 95% 95% CI for the mortality, ICU admission, mechanical ventilation, and adverse events outcomes, we utilize Mantel–Haenszel's formula. We used the Inverse Variance method to obtain the mean difference (MD) and its standard deviations (SDs) for the oxygenation index outcome. The I 2 statistic was exerted to assess the heterogeneity with a value of <25% considered a low degree of heterogeneity, 26%–50% moderate degree of heterogeneity, and >50% considered a high degree of heterogeneity. Because of the small number of studies, we did not test publication bias as any test would have low power to distinguish chance from real asymmetry. 18
3. RESULTS
3.1. Design and quality assessment of included studies
The PRISMA flow diagram is shown in Figure 1. We initially identified 512 articles. We then identified 46 highly relevant articles by searching titles and abstracts and eliminating repetitions. After examining the content further, 12 studies comprising 3564 patients 8 , 9 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 remained. Out of 12 pieces of research, two were double‐blind, randomized clinical trials, four were non‐randomized clinical trials, and six were prospective cohort research and retrospective cohort research Figure 2. The quality assessment is shown in Figure 3A,B.
Figure 1.
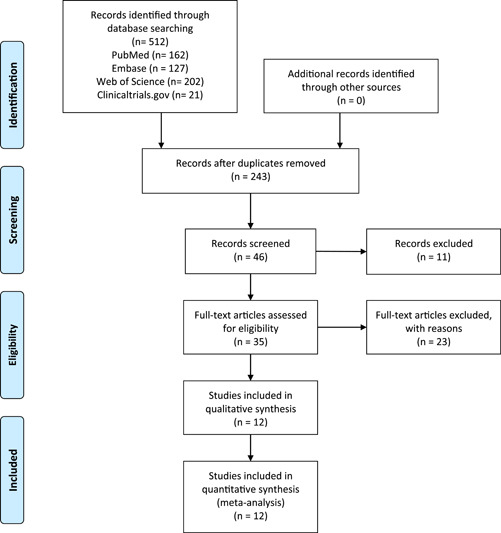
PRISMA flow diagram
Figure 2.
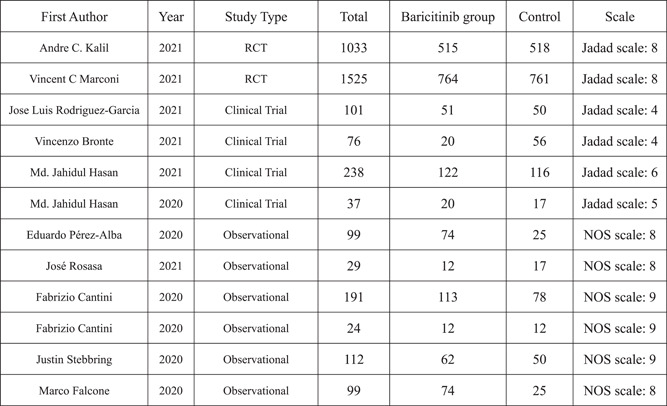
Included studies
Figure 3.
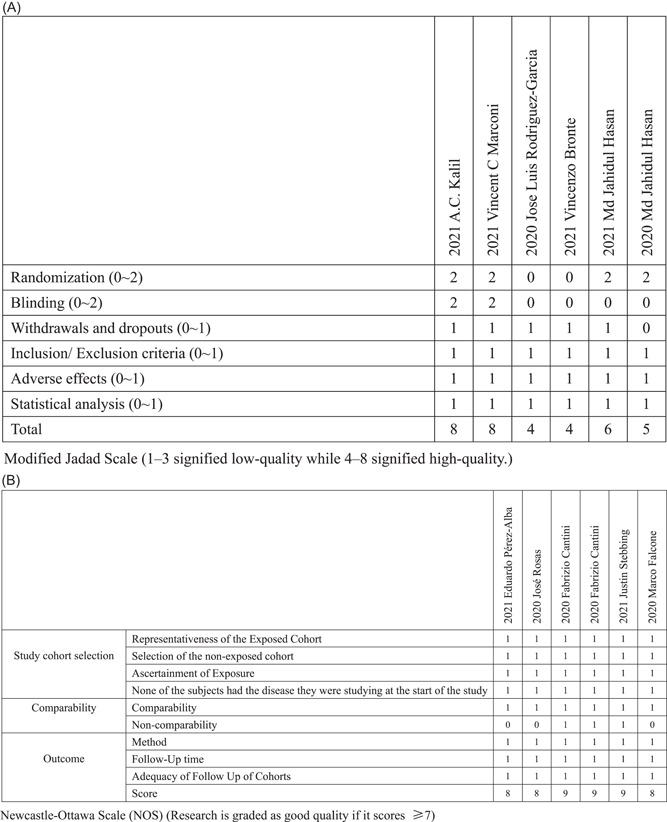
Bias risk assessment
3.1.1. Baseline characteristics of the included studies
The studies included data on the first author, publication year, study type, number of total participants, number of participants receiving baricitinib, and number of participants receiving other drugs (Figure 2). See Figure 3, for the modified Jadad scale and NOS scale.
3.2. Meta‐analysis results for clinical efficacy
3.2.1. Mortality outcomes
The outcome of mortality in the baricitinib group versus the control groups is shown in Figure 4A. Nine studies 8 , 9 , 19 , 20 , 23 , 24 , 25 , 27 , 28 including 3827 patients reported the mortality rate. Pooled results showed that baricitinib had a lower mortality rate compared with that in the control groups (relative risk [RR] = 0.56; 95% CI: 0.46–0.69; p < 0.001; I 2 = 2%; Figure 4A). Three studies of 2632 patients 8 , 9 , 27 reported the effect of baricitinib versus placebo in patients (RR = 0.63; 95% CI: 0.49–0.81; p < 0.001; I 2 = 0%; Figure 4B). Three studies of 582 patients 20 , 25 , 28 reported the effect of baricitinib versus hydroxychloroquine in patients (RR = 0.29; 95% CI: 0.14–0.60; p < 0.001; I 2 = 49%; Figure 4B).
Figure 4.
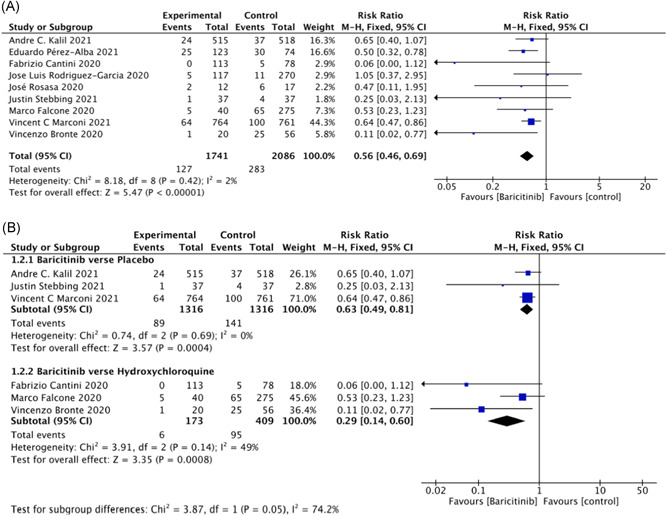
Mortality outcomes
3.2.2. ICU admission outcomes
Five studies 19 , 24 , 25 , 26 , 27 reported ICU admission and included 672 patients. Baricitinib significantly reduced the requirement of ICU support in patients with COVID‐19 (RR = 0.12; 95% CI: 0.04–0.31; p < 0.001; I 2 = 15%; Figure 5A).
Figure 5.
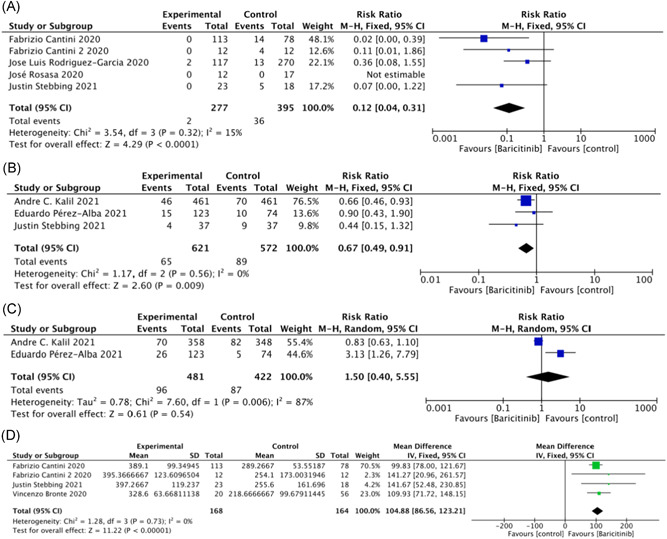
Secondary indicators
3.2.3. Invasive mechanical ventilation outcomes
Three studies 8 , 23 , 27 reported invasive mechanical ventilation and included 1193 patients. Baricitinib significantly reduced the requirement of invasive mechanical ventilation in patients with COVID‐19 (RR = 0.67; 95% CI: 0.49 – 0.91; p < 0.01; I 2 = 0%; Figure 5B).
3.2.4. Noninvasive mechanical ventilation outcomes
Pooled data from two studies 8 , 23 of 903 patients showed that the requirement of noninvasive mechanical ventilation was similar between the baricitinib group and the control groups (RR = 1.50; 95% CI: 0.40–5.55; p = 0.54; I 2 = 87%; Figure 5C).
3.2.5. Oxygenation index outcomes
Four studies 20 , 25 , 26 , 27 including 332 patients investigated the effect of baricitinib on the oxygenation index (OI); MD = 104.88 (95% CI: 86.56–123.21; p < 0.001; I 2 = 0%; Figure 5D). Baricitinib significantly increased the oxygenation index at discharge compared with admission.
3.3. Meta‐analysis results of adverse events
When we examined adverse events caused by baricitinib treatment in patients with COVID‐19, we found some common adverse events, such as infections, embolism events, liver dysfunction, renal and urinary disorders, and mental disorders. Therefore, we performed subgroup analysis of the different adverse events.
Six studies 8 , 9 , 19 , 23 , 27 including 3132 patients investigated the effect of baricitinib on infections. In the baricitinib group, the risk of infections was marginally reduced compared with that in the control groups (RR = 0.80; 95% CI: 0.66–0.96; p < 0.05; I 2 = 45%; Figure 6). In two RCTs 8 , 9 with 2558 participants, baricitinib decreased the risk of serious adverse events compared with controls (RR = 0.81; 95% CI: 0.68–0.96; p < 0.05; I 2 = 0%; Figure 6).
Figure 6.
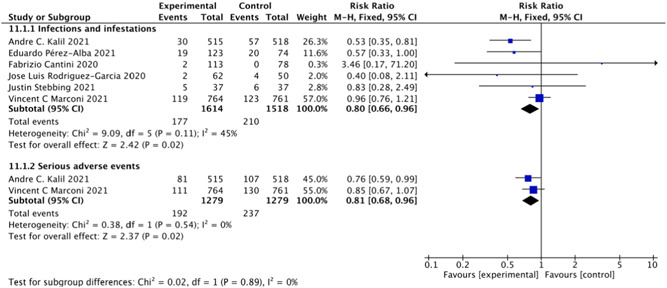
Infections and serious adverse events
Four studies 8 , 9 , 19 , 23 including 2867 patients investigated the effect of baricitinib on embolism events. The baricitinib group had the same risk of embolism events as the control groups (RR = 1.23; 95% CI: 0.80–1.90; p = 0.34; I 2 = 0%; Figure 7). We also included two studies 8 , 9 with 2558 patients and four studies 8 , 9 , 19 , 23 with 2867 patients that examined the effect of baricitinib on the rates of deep venous thrombosis (RR = 1.67; 95% CI: 0.74–3.80; p = 0.22; I 2 = 0%; Figure 7) and pulmonary embolism (RR = 1.83; 95% CI: 0.90–3.71; p = 0.10; I 2 = 0%; Figure 7). The pooled results showed no significant differences between the baricitinib group and the control groups in these outcomes.
Figure 7.
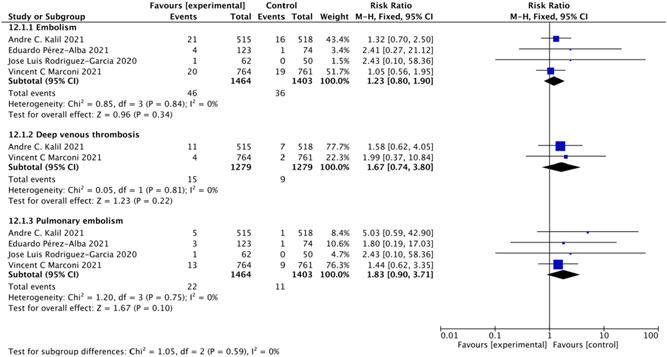
Embolism events
We included four studies 8 , 25 , 26 , 27 with 1322 patients that investigated the effect of baricitinib on liver dysfunction. The baricitinib group appeared to be similar to the control groups in causing liver dysfunction (RR = 1.11; 95% CI: 0.60–2.04; p = 0.73; I 2 = 66%; Figure 8). Two studies 8 , 19 including 1154 patients examined the effect of baricitinib on mental disorders. A meta‐analysis did not show any significant difference in the rate of mental disorders between the baricitinib group and control groups (RR = 0.93, 95% CI: 0.30–2.87; p = 0.12; I 2 = 0%; Figure 8). Finally, the baricitinib group appeared to be similar to the control groups regarding the rate of renal and urinary disorders in three studies 8 , 19 , 23 including 1342 patients (RR = 1.04; 95% CI: 0.75–1.46; p = 0.80; I 2 = 0%; Figure 8).
Figure 8.
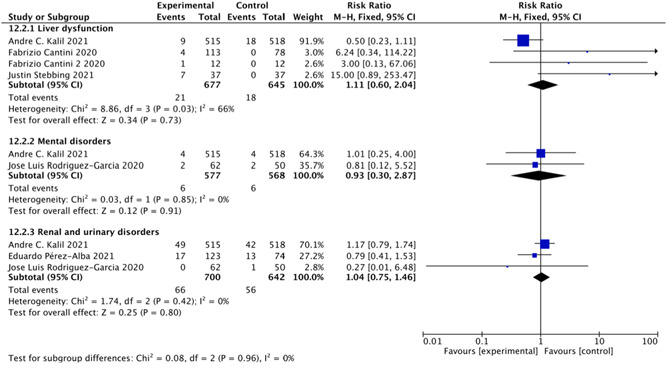
Secondary adverse events
These findings suggested that the safety of baricitinib treatment was almost identical to that in controls. Additionally, baricitinib treatment resulted in a lower risk of infections and serious adverse events than in controls.
3.4. Additional baricitinib loading dose
Previous studies 21 , 22 suggested that an additional baricitinib loading dose improved the clinical outcome of COVID‐19. An 8‐mg daily oral dose of baricitinib resulted in early stabilization of respiratory function, a decline in the requirement of critical care support, and reduced rehospitalization and mortality rates compared with the usual 4‐mg daily oral dose of baricitinib in severe COVID‐19 pneumonia. Hasan et al. 21 , 22 did two studies, one of which was blood oxygen saturation level was stabilized (≥94% on room air) earlier in the high dose (HD) group compared to the usual dose (UD) group (5 [IQR: 4–5]/8 [IQR: 6–9], p < 0.05). Patients in the HD group required intensive care unit (ICU) and intubation supports more in the UD group than that in patients of the HD group (17.2%/9%, p < 0.05; 11.2%/4.1%, p > 0.05; N = 116/122, respectively). The 30‐day mortality and 60‐day rehospitalization rate were higher in the UD group than the HD group (6%/3.3%, p < 0.01; 11.9%/7.6%, p > 0.05; N = 116/122, respectively). And the other was the requirement of intensive care unit and mechanical ventilation support was higher in the control group than in the case group (29.4% [N = 17]/10% [N = 20], p < 0.05; 11.8% [N = 17]/5% [N = 20], p > 0.05], respectively).
4. DISCUSSION
To the best of our knowledge, this is the first systematic review and meta‐analysis of the efficacy and safety of baricitinib as a potential therapeutic candidate for SARS‐CoV‐2. We found that baricitinib use was associated with a significant reduction in mortality in patients with COVID‐19. Baricitinib reduced the risk of death in patients compared with patients with placebo and hydroxychloroquine, which further validated our findings. Additionally, the risk of ICU admission, the requirement for invasive mechanical ventilation, and the discharge oxygenation index were significantly improved after using baricitinib in patients with COVID‐19. Moreover, benefits were observed with baricitinib treatment 4 mg daily, but a more robust benefit was observed with baricitinib 8 mg daily. We also found no clinically meaningful differences in safety between the baricitinib group and the control groups. Baricitinib also had a reduced risk of new infections and serious adverse events compared with that in controls.
In contrast to three previously published meta‐analyses 10 , 11 , 12 that summarized published studies of JAK inhibitors, in our study, we specifically focused on the clinical efficacy and adverse events of baricitinib. We included two recent multicenter, double‐blind, randomized, placebo‐controlled trials, and several recent high‐quality, observational studies of baricitinib to improve the validity of our conclusions. We also performed a detailed analysis of the rates of adverse events and serious adverse events and the effect of different baricitinib doses. This more detailed analysis has more clinical significance compared with other related studies.
Previous studies 10 , 11 , 12 have suggested that new‐onset infections and thrombotic events are the main adverse events with baricitinib treatment. However, our meta‐analysis showed that the incidence of new‐onset infections was reduced by baricitinib treatment compared with controls. This may be due to the accelerated recovery of patients using baricitinib, 29 , 30 or to the fact that the included studies used baricitinib for a short period of time, and we look forward to more studies in the future. Similar results were also shown in two RCTs, COV‐BARRIER and ACTT‐2, which included 2558 patients, especially regarding the incidence of serious adverse events. However, our study showed no significant difference in other common adverse reactions between baricitinib treatment and controls.
Baricitinib (C16H17N7O2S), an adenosine triphosphate competitive kinase inhibitor that selectively, strongly, and reversibly inhibits JAK1 and JAK2 enzymes, was predicted to be a potential therapeutic agent against SARS‐CoV‐2 using artificial intelligence algorithms. 31 Baricitinib inhibits the intracellular signaling pathway of cytokines that are elevated in severe COVID‐19, including IL‐2, IL‐6, IL‐10, interferon‐γ, and granulocyte‐macrophage colony‐stimulating factor. 32 Baricitinib acts against SARS‐CoV‐2 by impairing AP2‐associated protein kinase 1 and preventing SARS‐CoV‐2 cellular entry and infectivity. 33 Baricitinib also improves the lymphocyte count in patients with COVID‐19. 34 Furthermore, baricitinib has few interactions with other drugs, and excretion rates are largely unchanged, making it useful for older adults with underlying disease. 35
JAK inhibitors (JAKinibs) are biological agents that inhibit Type I/II cytokine receptors. They are currently used to treat a number of diseases, and second‐generation selective Jakinibs are being designed and studied. 36 Baricitinib, fedratinib, and ruxolitinib are highly potent selective JAK inhibitors approved for indications such as rheumatoid arthritis (RA) and some serious skin diseases, 37 , 38 all three of which are powerful anti‐inflammatory agents. Current studies show that baricitinib is the most suitable of the three for the treatment of COVID‐19 in terms of its clinical efficacy and few side effects. Due to the low plasma protein binding rate of baricitinib, with only 50% plasma protein (plasma protein binding of ruxolitinib: 97%/fedratinib: 95%), 39 and the least interaction with cytochrome P450 enzymes and drug transporters, it has great potential in combination with other drugs to treat COVID‐19. For example, Khan and Durairaj 40 advocate the combination of baricitinib with methotrexate (MTX) in the treatment of COVID‐19. MTX and chloroquine were the most common drugs in the control group. MTX inhibits the production of potentially toxic metabolic compounds (transmethylation products) that accumulate in chronically inflamed tissues. 41 and reduces intracellular glutathione, thus altering cellular redox states and decreasing macrophage recruitment and function. 42 MTX has been shown to have significant effects on neutrophil chemotaxis, leading to accumulation of adenosine in cells and extracellular, decreasing the secretion of TNF‐β, IFN, and IL‐6. 43 Another study conducted by Stebbing et al. 39 mentioned the combination of baricitinib with direct‐acting lopinavir/ritonavir, remdesivir, or other antivirals. With a molecular weight of 371.42 Da, 44 baricitinib is available in tablet form, which is convenient for transport and storage. Otherwise, low‐dose consumption of baricitinib (2–10 mg orally once a day) in comparison to fedratinib (400 mg orally once a day) makes it have better medication compliance. Baricitinib also has the advantages of oral administration, a simple route of administration, and a reasonable price, making it suitable for use in low‐ and middle‐income countries. Both chloroquine and the derivative molecule hydroxychloroquine have in vitro activity against SARS‐CoV and SARS‐CoV‐2. 45 Hydroxychloroquine is thought to impair the terminal glycosylation of the angiotensin‐converting enzyme 2 (ACE2) receptor, which is the binding site for the envelope spike glycoprotein and has been shown to inhibit endolysosome function. In addition, hydroxychloroquine may have greater in vitro activity against SARS‐CoV‐2 than chloroquine. 46
There are many limitations to our study as follows. First, although two high‐quality, multicenter RCTs were included, we also included some high‐quality observational studies. More multicenter, double‐blind, randomized trials are required to validate our results. Second, the method of assessing the severity of patients at baseline was not uniform across studies. Therefore, more studies using uniform evaluation methods for severity are still required. Third, the effect of baricitinib treatment may be affected by other drugs taken simultaneously, and the results do not only reflect the clinical effect of baricitinib. Fourth, most of the current studies on the safety of baricitinib were short‐term. Therefore, the incidence of more long‐term adverse reactions still needs to be further investigated. Finally, there have been few studies on the optimal dose of baricitinib for SARS‐CoV‐2. This optimal dose needs to be further explored in future studies.
5. CONCLUSIONS
Our study systematic review and meta‐analysis that baricitinib may represent a promising, safe, and effective anti‐SARS‐CoV‐2 drug candidate, with the advantages of a low cost, easy production, and convenient storage. In the future, investigation of the clinical effects and safety of baricitinib in patients with SARS‐CoV‐2 and investigation of different levels of severity and novel variant strains will likely lead to the widely available precise use of baricitinib.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Luqian Zhou contributed to determining the outline and content of the systematic review and meta‐analysis. Zhiwei Lin and Jianyi Niu contributed to retrieving literature. Zhiwei Lin, Jianyi Niu, Yifan Xu, Lijie Qin, and Jiabin Ding contributed to writing a draft of this manuscript. All authors contributed to revising the draft critically for important intellectual content, providing final confirmation of the revised version, and being responsible for all aspects of the work. The authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by the Basic Research Program of Guangzhou (202102010224), Clinical Transformation Program of the First Affiliated Hospital of Guangzhou Medical University (ZH201802, ZH201914), High‐Level University Program of Guangzhou Medical University (2017(160), and Opening Project of State Key Laboratory of Respiratory Disease (SKLRD‐0P‐202115).
1.
PubMed
Search: ((COVID‐19) OR (((((((((((((((((((((((((((((((((((COVID‐19) OR (COVID‐19 Virus Disease)) OR (COVID‐19 Virus Disease)) OR (COVID‐19 Virus Diseases)) OR (Disease, COVID‐19 Virus)) OR (Virus Disease, COVID‐19)) OR (COVID‐19 Virus Infection)) OR (COVID‐19 Virus Infection)) OR (COVID‐19 Virus Infections)) OR (Infection, COVID‐19 Virus)) OR (Virus Infection, COVID‐19)) OR (2019‐nCoV Infection)) OR (2019 nCoV Infection)) OR (2019‐nCoV Infections)) OR (Infection, 2019‐nCoV)) OR (Coronavirus Disease‐19)) OR (Coronavirus Disease 19)) OR (2019 Novel Coronavirus Disease)) OR (2019 Novel Coronavirus Infection)) OR (2019‐nCoV Disease)) OR (2019 nCoV Disease)) OR (2019‐nCoV Diseases)) OR (Disease, 2019‐nCoV)) OR (COVID19)) OR (Coronavirus Disease 2019)) OR (Disease 2019, Coronavirus)) OR (SARS Coronavirus 2 Infection)) OR (SARS‐CoV‐2 Infection)) OR (Infection, SARS‐CoV‐2)) OR (SARS CoV 2 Infection)) OR (SARS‐CoV‐2 Infections)) OR (COVID‐19 Pandemic)) OR (COVID‐19 Pandemic)) OR (COVID‐19 Pandemics)) OR (Pandemic, COVID‐19))) AND ((baricitinib) OR (((LY3009104) OR (Olumiant)) OR (INCB028050))).
Embase
Search: (‘covid‐19’:ab,ti OR ‘covid‐19’:ab,ti OR ‘covid‐19 virus disease’:ab,ti OR ‘covid‐19 virus disease’:ab,ti OR ‘covid‐19 virus diseases’:ab,ti OR ‘disease, covid‐19 virus’:ab,ti OR ‘virus disease, covid‐19’:ab,ti OR ‘covid‐19 virus infection’:ab,ti OR ‘covid‐19 virus infection’:ab,ti OR ‘covid‐19 virus infections’:ab,ti OR ‘infection, covid‐19 virus’:ab,ti OR ‘virus infection, covid‐19’:ab,ti OR ‘2019‐ncov infection’:ab,ti OR ‘2019 ncov infection’:ab,ti OR ‘2019‐ncov infections’:ab,ti OR ‘infection, 2019‐ncov’:ab,ti OR ‘coronavirus disease‐19’:ab,ti OR ‘coronavirus disease 19’:ab,ti OR ‘2019 novel coronavirus disease’:ab,ti OR ‘2019 novel coronavirus infection’:ab,ti OR ‘2019‐ncov disease’:ab,ti OR ‘2019 ncov disease’:ab,ti OR ‘2019‐ncov diseases’:ab,ti OR ‘disease, 2019‐ncov’:ab,ti OR ‘covid19’:ab,ti OR ‘coronavirus disease 2019’:ab,ti OR ‘disease 2019, coronavirus’:ab,ti OR ‘sars coronavirus 2 infection’:ab,ti OR ‘sars‐cov‐2 infection’:ab,ti OR ‘infection, sars‐cov‐2’:ab,ti OR ‘sars cov 2 infection’:ab,ti OR ‘sars‐cov‐2 infections’:ab,ti OR ‘covid‐19 pandemic’:ab,ti OR ‘covid 19 pandemic’:ab,ti OR ‘covid‐19 pandemics’:ab,ti OR ‘pandemic, covid‐19’:ab,ti) AND ('baricitinib’:ab,ti OR ‘ly3009104’:ab,ti OR ‘olumiant’:ab,ti OR ‘incb028050’:ab,ti).
Clinical Trial.gov
Search: Baricitinib AND COVID‐19
Web of Science
Search: ((((((((((((((((((((((((((((((((((((TS = (COVID‐19)) OR TS = (COVID‐19)) OR TS = (COVID‐19 Virus Disease)) OR TS = (COVID‐19 Virus Disease)) OR TS = (COVID‐19 Virus Diseases)) OR TS = (Disease, COVID‐19 Virus)) OR TS = (Virus Disease, COVID‐19)) OR TS = (COVID‐19 Virus Infection)) OR TS = (COVID‐19 Virus Infection)) OR TS = (COVID‐19 Virus Infections)) OR TS = (Infection, COVID‐19 Virus)) OR TS = (Virus Infection, COVID‐19)) OR TS = (2019‐nCoV Infection)) OR TS = (2019 nCoV Infection)) OR TS = (2019‐nCoV Infections)) OR TS = (Infection, 2019‐nCoV)) OR TS = (Coronavirus Disease‐19)) OR TS = (Coronavirus Disease 19)) OR TS = (2019 Novel Coronavirus Disease)) OR TS = (2019 Novel Coronavirus Infection)) OR TS = (2019‐nCoV Disease)) OR TS = (2019 nCoV Disease)) OR TS = (2019‐nCoV Diseases)) OR TS = (Disease, 2019‐nCoV)) OR TS = (COVID‐19)) OR TS = (Coronavirus Disease 2019)) OR TS = (Disease 2019, Coronavirus)) OR TS = (SARS Coronavirus 2 Infection)) OR TS = (SARS‐CoV‐2 Infection)) OR TS = (Infection, SARS‐CoV‐2)) OR TS = (SARS CoV 2 Infection)) OR TS = (SARS‐CoV‐2 Infections)) OR TS = (COVID‐19 Pandemic)) OR TS = (COVID‐19 Pandemic)) OR TS = (COVID‐19 Pandemics)) OR TS = (Pandemic, COVID‐19)) AND (((TS = (baricitinib)) OR TS = (ly3009120)) OR TS = (olefiant)) OR TS = (incb028060).
Lin Z, Niu J, Xu Y, Qin L, Ding J, Zhou L. Clinical efficacy and adverse events of baricitinib treatment for coronavirus disease‐2019 (COVID‐19): A systematic review and meta‐analysis. J Med Virol. 2022;94:1523‐1534. 10.1002/jmv.27482
Zhiwei Lin, Jianyi Niu, and Yifan Xu contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Weekly epidemiological update on COVID‐19 − 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19
- 2. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID‐19. J Infect. 2020;80(6):607‐613. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choudhary S, Sharma K, Silakari O. The interplay between inflammatory pathways and COVID‐19: A critical review on pathogenesis and therapeutic options. Microb Pathog. 2021;150:104673. 10.1016/j.micpath.2020.104673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valizadeh H, Abdolmohammadi‐Vahid S, Danshina S, et al. Nano‐curcumin therapy, a promising method in modulating inflammatory cytokines in COVID‐19 patients. Int Immunopharmacol. 2020;89(Pt B):107088. 10.1016/j.intimp.2020.107088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goker Bagca B, Biray Avci C. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID‐19. Cytokine Growth Factor Rev. 2020;54:51‐62. 10.1016/j.cytogfr.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van De Laar CJ, Oude Voshaar MAH, Fakhouri WKH, et al. Cost‐effectiveness of a JAK1/JAK2 inhibitor vs a biologic disease‐modifying antirheumatic drug (bDMARD) in a treat‐to‐target strategy for rheumatoid arthritis. Clinicoecon Outcomes Res. 2020;15(12):213‐222. 10.2147/CEOR.S231558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singer JW, Al‐Fayoumi S, Taylor J, Velichko S, O'Mahony A. Comparative phenotypic profiling of the JAK2 inhibitors ruxolitinib, fedratinib, momelotinib, and pacritinib reveals distinct mechanistic signatures. PLoS One. 2019;14(9):e0222944. 10.1371/journal.pone.0222944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid‐19. N Engl J Med. 2021;384(9):795‐807. 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID‐19 (COV‐BARRIER): a randomised, double‐blind, parallel‐group, placebo‐controlled phase 3 trial. Lancet Respir Med. 2021. 10.1016/S2213-2600(21)00331-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen CY, Chen WC, Hsu CK, Chao CM, Lai CC. Clinical efficacy and safety of Janus kinase inhibitors for COVID‐19: a systematic review and meta‐analysis of randomized controlled trials. Int Immunopharmacol. 2021;99:108027. 10.1016/j.intimp.2021.108027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wijaya I, Andhika R, Huang I, et al. The use of Janus Kinase inhibitors in hospitalized patients with COVID‐19: Systematic review and meta‐analysis. Clin Epidemiol Glob Health. 2021;11:100755. 10.1016/j.cegh.2021.100755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen CX, Wang JJ, Li H, Yuan LT, Gale RP, Liang Y. JAK‐inhibitors for coronavirus disease‐2019 (COVID‐19): a meta‐analysis. Leukemia. 2021;35(9):2616‐2620. 10.1038/s41375-021-01266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 14. Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156‐175. 10.2522/ptj.20070147 [DOI] [PubMed] [Google Scholar]
- 15. Lo CK, Mertz D, Loeb M. Newcastle‐Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cogn Disord. 2001;12(3):232‐236. 10.1159/000051263 [DOI] [PubMed] [Google Scholar]
- 17. Margulis AV, Pladevall M, Riera‐Guardia N, et al. Quality assessment of observational studies in a drug‐safety systematic review, comparison of two tools: the Newcastle‐Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359‐368. 10.2147/CLEP.S66677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. CMAJ. 2007;176(8):1091‐1096. 10.1503/cmaj.060410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez‐Garcia JL, Sanchez‐Nievas G, Arevalo‐Serrano J, Garcia‐Gomez C, Jimenez‐Vizuete JM, Martinez‐Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS‐CoV‐2 pneumonia: an observational cohort study. Rheumatology. 2021;60(1):399‐407. 10.1093/rheumatology/keaa587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID‐19. J Clin Invest. 2020;130(12):6409‐6416. 10.1172/JCI141772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hasan MJ, Rabbani R, Anam AM, et al. Impact of high dose of baricitinib in severe COVID‐19 pneumonia: a prospective cohort study in Bangladesh. BMC Infect Dis. 2021;21(1):427. 10.1186/s12879-021-06119-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasan MJ, Rabbani R, Anam AM, Huq SMR. Additional baricitinib loading dose improves clinical outcome in COVID‐19. Open Med. 2020;16(1):41–46. 10.1515/med-2021-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pérez‐Alba E, Nuzzolo‐Shihadeh L, Aguirre‐García GM, et al. Baricitinib plus dexamethasone compared to dexamethasone for the treatment of severe COVID‐19 pneumonia: a retrospective analysis. J Microbiol Immunol Infect. 2021. 10.1016/j.jmii.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosas J, Liaño FP, Cantó ML, et al. Experience with the use of baricitinib and tocilizumab monotherapy or combined, in patients with interstitial pneumonia secondary to coronavirus COVID‐19: a real‐world study. Reumatol Clin. 2020;S1699‐258X(20):30271–0. 10.1016/j.reuma.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of Baricitinib in COVID‐19 moderate pneumonia; multicentre study. J Infect. 2020;81(4):647‐679. 10.1016/j.jinf.2020.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID‐19: a pilot study on safety and clinical impact. J Infect. 2020;81:318‐356. 10.1016/j.jinf.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stebbing J, Sánchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS‐CoV‐2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7(1):eabe4724. 10.1126/sciadv.abe4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Falcone M, Tiseo G, Barbieri G, et al. Role of low‐molecular‐weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7(12):ofaa563. 10.1093/ofid/ofaa563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert‐Bennett E. Role of hospital surfaces in the transmission of emerging health care‐associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38(5 suppl 1):S25‐S33. 10.1016/j.ajic.2010.04.196 [DOI] [PubMed] [Google Scholar]
- 30. Wang L, Baser O, Wells P, et al. Predictors of hospital length of stay among patients with low‐risk pulmonary embolism. J Health Econ Outcomes Res. 2019;6(2):84‐94. 10.36469/9744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stebbing J, Krishnan V, de Bono S, et al. Mechanism of baricitinib supports artificial intelligence‐predicted testing in COVID‐19 patients. EMBO Mol Med. 2020;12(8):e12697. 10.15252/emmm.202012697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang X, Zhang Y, Qiao W, Zhang J, Qi Z. Baricitinib, a drug with potential effect to prevent SARS‐COV‐2 from entering target cells and control cytokine storm induced by COVID‐19. Int Immunopharmacol. 2020;86:106749. 10.1016/j.intimp.2020.106749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019‐nCoV acute respiratory disease. Lancet. 2020;395(10223):e30‐e31. 10.1016/S0140-6736(20)30304-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim JS, Lee JY, Yang JW, et al. Immunopathogenesis and treatment of cytokine storm in COVID‐19. Theranostics. 2021;11(1):316‐329. 10.7150/thno.49713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalil AC, Stebbing J. Baricitinib: the first immunomodulatory treatment to reduce COVID‐19 mortality in a placebo‐controlled trial. Lancet Respir Med. 2021;81(4):1349–1351. 10.1016/S2213-2600(21)00358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz DM, Bonelli M, Gadina M, O'Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12(1):25‐36. 10.1038/nrrheum.2015.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374(13):1243‐1252. 10.1056/NEJMoa1507247 [DOI] [PubMed] [Google Scholar]
- 38. Guttman‐Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate‐to‐severe atopic dermatitis: a phase 2 parallel, double‐blinded, randomized placebo‐controlled multiple‐dose study. J Am Acad Dermatol. 2019;80(4):913‐921. 10.1016/j.jaad.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 39. Stebbing J, Phelan A, Griffin I, et al. COVID‐19: combining antiviral and anti‐inflammatory treatments. Lancet Infect Dis. 2020;20(4):400‐402. 10.1016/S1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khan S, Durairaj S. JAK Inhibition with methotrexate as treatment for COVID‐19 is a double‐edged sword. Int Arch Allergy Immunol. 2020;181(7):563‐564. 10.1159/000508750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nesher G, Osborn TG, Moore TL. Effect of treatment with methotrexate, hydroxychloroquine, and prednisone on lymphocyte polyamine levels in rheumatoid arthritis: correlation with the clinical response and rheumatoid factor synthesis. Clin Exp Rheumatol. 1997;15(4):343‐347. [PubMed] [Google Scholar]
- 42. Phillips DC, Woollard KJ, Griffiths HR. The anti‐inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br J Pharmacol. 2003;138(3):501‐511. 10.1038/sj.bjp.0705054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cronstein BN. Going with the flow: methotrexate, adenosine, and blood flow. Ann Rheum Dis. 2006;65(4):421‐422. 10.1136/ard.2005.049601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shi JG, Chen X, Lee F, et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol. 2014;54(12):1354‐1361. 10.1002/jcph.354 [DOI] [PubMed] [Google Scholar]
- 45. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. 10.1186/1743-422X-2-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71(15):732‐739. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


