Abstract
Background
Pain is a clinical feature of COVID‐19, however, data about persistent pain after hospital discharge, especially among ICU survivors is scarce. The aim of this study was to explore the incidence and characteristics of new‐onset pain and its impact on Health‐Related Quality of Life (HRQoL), and to quantify the presence of mood disorders in critically ill COVID‐19 survivors.
Methods
This is a preliminary report of PAIN‐COVID trial (NCT04394169) presenting a descriptive analysis in critically ill COVID‐19 survivors, following in person interview 1 month after hospital discharge. Pain was assessed using the Brief Pain Inventory, the Douleur Neuropathique 4 questionnaire and the Pain Catastrophizing Scale. HRQoL was evaluated with the EQ 5D/5L, and mood disorders with the Hospital Anxiety and Depression Scale (HADS).
Results
From 27 May to 19 July 2020, 203 patients were consecutively screened for eligibility, and 65 were included in this analysis. Of these, 50.8% patients reported new‐onset pain; 38.5% clinically significant pain (numerical rating score ≥3 for average pain intensity); 16.9% neuropathic pain; 4.6% pain catastrophizing thoughts, 44.6% pain in ≥2 body sites and 7.7% widespread pain. Patients with new‐onset pain had a worse EQ‐VAS and EQ index value (p < 0.001). Pain intensity was negatively correlated to both the former (Spearman ρ: −0.546, p < 0.001) and the latter (Spearman ρ: −0.387, p = 0.001). HADS anxiety and depression values equal or above eight were obtained in 10.8% and 7.7% of patients, respectively.
Conclusion
New‐onset pain in critically ill COVID‐19 survivors is frequent, and it is associated with a lower HRQoL.
Trial registration No.: NCT04394169. Registered 19 May 2020. https://clinicaltrials.gov/ct2/show/NCT04394169.
Significance
A substantial proportion of severe COVID‐19 survivors may develop clinically significant persistent pain, post‐intensive care syndrome and chronic ICU‐related pain. Given the number of infections worldwide and the unprecedented size of the population of critical illness survivors, providing information about the incidence of new‐onset pain, its characteristics, and its influence on the patients’ quality of life might help establish and improve pain management strategies.
1. INTRODUCTION
Long‐term physical or mental health status problems are common in critically ill patients after discharge from ICU. In 2012, a new medical term,‘post‐intensive care syndrome’ (PICS), was adopted by the Society of Critical Care Medicine to refer to this entity (Elliott et al., 2014). Currently, survivors 1 year after ICU admission outnumber the deceased by three to one (Brinkman et al., 2013). However, this success has come at the cost of increasing disability and decreasing survivors’ quality of life (QoL), which also impacts negatively on caregivers (Cameron et al., 2016; Herridge et al., 2003, 2016; Torres et al., 2017). Critical illness survivors display a high prevalence of moderate to extreme chronic pain, with the latter entailing a significant limitation in work and social activity, and becoming a major cause of disability in Europe (Barbaglia et al., 2017; Baumbach et al., 2016; Mäkinen et al., 2020). Components of the PICS such as depression, anxiety, post‐traumatic stress disorder (PTSD) and cognitive impairment are also prevalent in chronic pain. These problems show a bi‐directional relationship and have been associated with a worse prognosis (Fishbain et al., 2017; Linton & Bergbom, 2011).
COVID‐19 has led to a large number of hospital admissions. As of 5 October 2021 more than 237 million cases of COVID‐19 have been reported worldwide (WHO Coronavirus Disease [COVID‐19] Dashboard, n.d.), with up to 5% of patients requiring re‐admission to the ICU (Ferrando et al., 2020; Hozhabri et al., 2020). Despite this large number of individuals that could be potentially affected by PICS and chronic ICU‐related pain, there are no prospective studies to date reporting data on critically ill COVID‐19 patients’ pain, its characteristics, and its relationship with self‐perceived QoL. Moreover, pain is a common symptom after acute COVID‐19, and chronic pain could be another consequence of this disease (Carfì et al., 2020; Kemp et al., 2020; Meyer‐Frießem et al., 2021). Although scientific evidence is insufficient, prevention and early pain management could improve medium or long‐term outcomes, so it is imperative to call for proper identification and management of this condition (Katz et al., 2015; Mills et al., 2019).
The hypothesis was that survivors of critical COVID‐19 would often present with pain and display a decrease in self‐perceived QoL, as well as an increased incidence of anxiety and depression disorders (Inoue et al., 2019). The aim of the study is to investigate the prevalence and characteristics of new‐onset pain in COVID‐19 ICU survivors 1 month after hospital discharge. Additional objectives include the assessment of the relationship between new‐onset pain and pain intensity with QoL via the Health‐related Quality of Life (HRQoL) questionnaire and the quantification of the presence of mood disorders.
2. METHODS
2.1. Study design
This is a preliminary descriptive report of an ongoing single‐centre clinical trial aiming to assess the efficacy of a combined intervention program to mitigate chronic pain in COVID‐19 ICU survivors (NCT04394169). The study was approved by the Ethics Committee of the Hospital Clinic of Barcelona (approval number HCB/2020/0549) on 14 May 2020. The study protocol has been submitted for publication (Ojeda et al., 2021). Participants were enrolled 1 month after hospital discharge after giving their written informed consent. All study procedures were performed following the ethical standards of the Declaration of Helsinki. This study followed the ‘Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)’ statement guidelines for observational cohort studies (von Elm et al., 2007).
2.2. Study population and data collection
Adult survivors from critically severe COVID‐19 infection—confirmed by polymerase chain reaction‐based tests on respiratory tract samples—with at least one of the following inclusion criteria were eligible for participation: (i) an Acute Physiology and Chronic Health Evaluation (APACHE) II score at ICU admission >14; (ii) an ICU length of stay over 10 days; (iii) ICU‐acquired Weakness; (iv) delirium during ICU admission. These criteria were in accordance with those previously recommended for critical illness monitoring and rehabilitation program (Busico et al., 2019); delirium was added as it has been associated with long‐term cognitive and functional deficits in multiple studies (Rengel et al., 2019; Sampson et al., 2020). The exclusion criteria were the following: (i) central nervous system degenerative disease; (ii) terminal illness; (iii) insufficient understanding of the Spanish language; (iv) difficulty in completing follow‐up due to a living distance >50 km from the Hospital Clínic of Barcelona; (v) not providing informed consent for the study (for details, see Methods S1).
The site's clinical database allowed identification of all the patients who were discharged from the intensive care units, and those who met the inclusion criteria were recruited.
One month after their discharge from hospital, these patients were contacted via a phone call to check whether they would be interested in participating in this trial and were invited to an introductory interview in which they received information about the study.
After enrolment, a baseline in person interview was conducted by researchers (AC, TC, OC‐T, JA, MA) 1 month after hospital discharge. Demographic and socioeconomic data along with medical history—including mental health and chronic pain disorders—were collected. The presence of previous chronic pain was evaluated using the definition proposed by Baumbach et al. (2018): ‘more than occasional pain (e.g. short headache/toothache) in the last 4 weeks before ICU admission’ (see Methods S1). Information regarding their recent ICU stay also was included. Finally, questionnaires were self‐administered to assess pain, QoL and mood disorders (see Methods S1 for demographic and patient characteristics variables definitions).
2.3. Study definitions and outcomes
2.3.1. New‐onset pain and its characteristics
Pain was evaluated via the Brief Pain Inventory short form questionnaire (BPI‐SF) (Cs & Km, 1994) (see Appendix S1). The definition of new onset‐pain was an affirmative answer to the first question of the BPI‐SF questionnaire: ‘Throughout our lives, most of us have had pain from time to time (such as minor headaches, sprains and toothaches). Have you had pain other than these everyday kinds of pain?’. If the answer was positive, the Investigator confirmed the presence of new‐onset pain after verifying that the pain was not present before admission to ICU or prior to Covid‐19 infection. The patients were instructed not to consider acute covid symptoms when asked whether they had pain prior to ICU admission.
Pain location, intensity and interference in daily life activities were registered using the specific BPI‐SF questionnaire. Following the Initiative on Methods, Measurements, and Pain Assessment in Clinical Trials (IMMPACT) recommendations, clinically meaningful pain was defined by a numerical rating score (NRS) ≥3 for average pain intensity (Gewandter et al., 2015). Widespread pain was also assessed (see Methods S1.) Every time new‐onset pain was detected, neuropathic pain (NP) and pain catastrophizing were screened and assessed with the Douleur Neuropathique 4 interview questionnaire (DN4‐interview) (see Appendix S2) and the Pain Catastrophizing Scale (PCS) (see Appendix S3), respectively (Bouhassira et al., 2005; Darnall et al., 2017). A score ≥30 in the PCS was considered as a clinically relevant level of catastrophizing (Darnall et al., 2017). Once a positive NP screen was obtained, the affected anatomical area was confirmed by the Investigator.
2.3.2. HRQoL
HRQoL, as assessed by the EQ 5D/5L (Herdman et al., 2011) (see Appendix S4), determines the QoL according to a descriptive system, a visual analogic scale (VAS) and an index value. The descriptive system evaluates five dimensions: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression, each scored according to a scale of 1 (no problems) to 5 (extreme problems). Responses are coded as single‐digit numbers expressing the severity level selected for each dimension. The digits for the five dimensions can be combined in a 5‐digit code that describes the respondent's health state. The VAS measures the patient's perception of their overall current health, from 0—the worst imaginable health—to 100—the best imaginable health. An index value can also be obtained by applying a formula that attaches values (weights) to each of the levels in each dimension. The index is calculated by deducting the appropriate weights from 1, the value for full health (i.e. state 11111) (EuroQol Research Foundation, 2019). The index was obtained by using the EQ 5D/5L Index Value Calculator (Developed by the EuroQol Group Version 2.0.) (Van Hout et al., 2012).
2.3.3. Relationship between new‐onset pain and health‐related quality of life
The EQ‐VAS and index values between individuals presenting with pain and those without pain were compared. The relationship between pain intensity and HRQoL was also assessed.
2.3.4. Anxiety and depression
The presence of anxiety and/or depression was assessed by the Hospital Anxiety and Depression Scale (HADS) (Bjelland et al., 2002) (see Appendix S5). According to Bjelland's review, values equal or greater than a cut‐off point of 8 were considered abnormal anxiety or depression values (Bjelland et al., 2002). Additionally, the relationship between anxiety, depression, HRQoL, and new‐onset pain was also assessed.
2.4. Statistical analysis
No sample size calculation was carried out for this preliminary analysis. Notably, during this descriptive project analysis, no outcomes for the ongoing clinical trial were assessed. Continuous variables were expressed as median (interquartile range), and categorical variables were presented as numbers (percentages). Missing data were minimal (below 0.5%) and missing observations were not imputed. Comparisons between groups were conducted with either the Fisher or the Mann–Whitney test. Correlations between ordinal variables were analysed with Spearman's Rho without accounting for any order interactions. A threshold of 0.05 was used for statistical significance. All reported tests are two‐sided. The R software was used for all the analysis (R Foundation for Statistical Computing).
3. RESULTS
From 27 May to 19 July 2020, 203 critically ill COVID‐19 ICU survivors were consecutively screened; 65 patients were finally included in the study and they completed the questionnaires. These cases belong to the first wave of the COVID‐19 pandemic in Spain. Patients´ flowchart is shown in Figure 1. A summary of demographics and characteristics for patients without new‐onset pain, patients with new‐onset pain, and for both groups are presented in Table 1.
FIGURE 1.
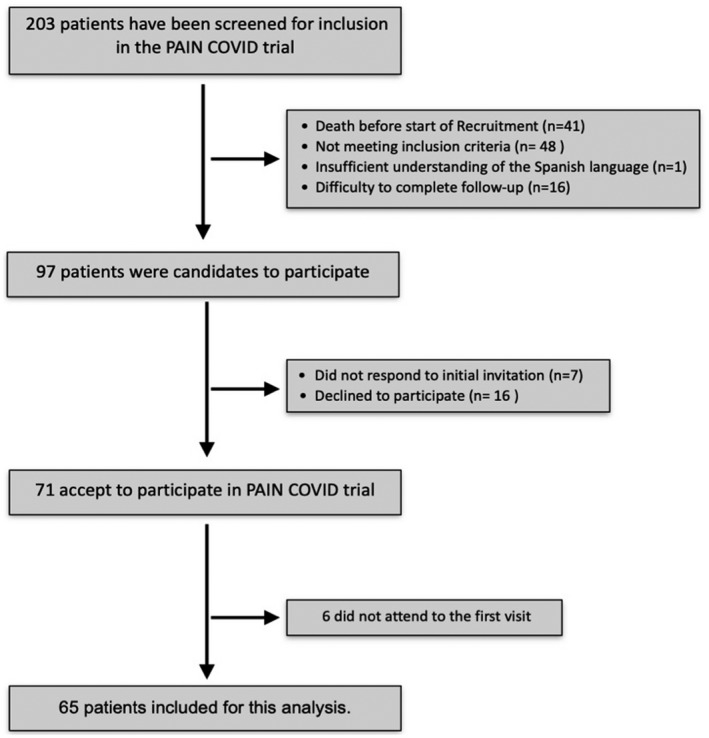
Patient inclusion flowchart. Covid‐19 ICU Survivors meeting PAIN‐COVID trial inclusion/exclusion criteria were contacted before 1 month after hospital discharge. A total of 65 patients were included for the analysis. Difficulty in completing follow‐up: home distance over 50 km from the Hospital Clinic of Barcelona
TABLE 1.
Summary of demographics and characteristics of patients without new onset pain, with new onset pain, and for both groups (all patients)
| Characteristics | All patients (n = 65) | No pain (n = 32) | New onset pain (n = 33) | p value |
|---|---|---|---|---|
| Age (years) | 65 (57–70) | 68 (61–76) | 60 (55–69) | 0.005 |
| Male sex | 48 (73.8) | 25 (78.1) | 23 (69.7) | 0.574 |
| Current tobacco smoking status | 25 (38.5) | 11 (34.4) | 14 (42.4) | 0.612 |
| Body mass index (kg/m2) | 27 (25–31.4) | 26.5 (24.9–30.2) | 28.3 (25–32) | 0.434 |
| History of psychiatric disease | 10 (15.4) | 4 (12.5) | 6 (18.2) | 0.733 |
| History of chronic pain | 8 (12.3) | 2 (6.2) | 6 (18.2) | 0.258 |
| Opioid tolerance patient | 4 (6.2) | 0 | 4 (12.1) | 0.114 |
| Barthel Index | 100 (95–100) | 100 (95–100) | 100 (95–100) | 0.46 |
| Charlson Comorbidity Index Score | 3 (2–4) | 3 (2–4) | 2 (1–3) | 0.033 |
| S.O.F.A | 5 (3–7) | 4 (3–6) | 6 (3–8) | 0.043 |
| A.P.A.C.H.E II | 12 (9–15) | 12 (10–15.2) | 12 (8–15) | 0.594 |
| Mechanical ventilation | 50 (76.9) | 21 (65.6) | 29 (87.9) | 0.042 |
| Mechanical ventilation (days) | 15 (9–22) | 13 (8–17) | 16.5 (10–23.2) | 0.161 |
| Tracheostomy | 34 (52.3) | 13 (40.6) | 21 (63.6) | 0.084 |
| Use of neuromuscular blocking agents | 31 (47.7) | 10 (31.2) | 21 (63.6) | 0.013 |
| Non‐invasive ventilation | 20 (30.8) | 12 (37.5) | 8 (24.2) | 0.29 |
| Hypoxemia >24 h | 60 (92.3) | 30 (93.8) | 30 (90.9) | 1 |
| Sepsis | 42 (64.6) | 18 (56.2) | 24 (72.7) | 0.2 |
| Vasopressor therapy | 49 (75.4) | 20 (62.5) | 29 (87.9) | 0.023 |
| Total corticoids dose (mg) | 1968 (1267–2700) | 881.5 (334–1250) | 850 (625–1300) | 0.52 |
| Sedation (total days) | 15 (9 −20) | 14 (9 −18) | 17 (10–20.5) | 0.213 |
| Acute renal injury | 28 (43.1) | 10 (31.2) | 18 (54.5) | 0.08 |
| Replacement renal therapy | 5 (7.7) | 3 (9.4) | 2 (6.1) | 0.672 |
| D‐Dimerum maximum (ng/ml) | 6400 (3400–10,000) | 7350 (3700–10,000) | 6100 (3300–10,000) | 0.59 |
| Ferrityn maximum (ng/ml) | 1968 (1267–2700) | 1943 (1149.8–2475) | 2200 (1267–2900) | 0.276 |
| C‐reactive protein maximum (mg/dl) | 20.3 (12–27) | 17.1 (11.3–25.2) | 22.0 (17–29) | 0.094 |
| Days with increased C‐reactive protein | 8 (5–18) | 6.5 (3.8–14) | 8 (5–18) | 0.275 |
| LOS ICU (days) | 25 (15–33) | 19.5 (11.8–30.5) | 28 (16–34) | 0.203 |
| LOS Hospital (days) | 36 (25–47) | 33 (23–45.2) | 40 (30–50) | 0.141 |
| Delirium | 25 (38.5) | 12 (38.7) | 13 (39.4) | 1 |
| ICU acquired weakness | 41 (63.1) | 17 (53.1) | 24 (72.7) | 0.127 |
| Days after discharge from the ICU | 72.5 (63.75–82.75) | 67 (62.5–81.5) | 78 (65–84) | 0.189 |
Data are in number and proportions (%) for categorical variables and in median and interquartile range (IQR) for continuous variables. Non‐invasive ventilation: patients without intubation all along the process. p value refers to the statistical comparison between ‘No pain’ and ‘New onset pain’ patients. Bold values are statistically significant values.
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; LOS, length of stay; S.O.F.A, Sequential Organ Failure Assessment Score.
Comparison between ‘No pain’ and ‘New onset pain’ patients identified as factors significantly associated with the development of new onset pain among severe COVID‐19 survivors: a younger age, a lower Charlson Comobirdity Index Score, a higher SOFA, mechanical ventilation, use of neuromuscular blocking agents and the use of vasopressor therapy (Table 1).
3.1. New‐onset pain and characteristics
Thirty‐three (50.8%) patients presented new‐onset pain; 25 (38.5%) reported clinically significant pain (NRS ≥3 for average pain intensity); 18 (27.6%) had a BPI‐SF intensity score ≥3 and a BPI‐SF interference score ≥3; 10 (15.4%) patients reported NP, and 3 (4.6%) patients had a clinically relevant level of catastrophizing.
Among those experiencing new‐onset pain, median BPI average pain item was 4 (IQR 3–5), median BPI‐SF score was 3 (IQR 2–4) for intensity and 3 (IQR 1–5) for interference. Figure 2 shows the BPI average pain item and its self‐perceived impact on daily life activities.
FIGURE 2.
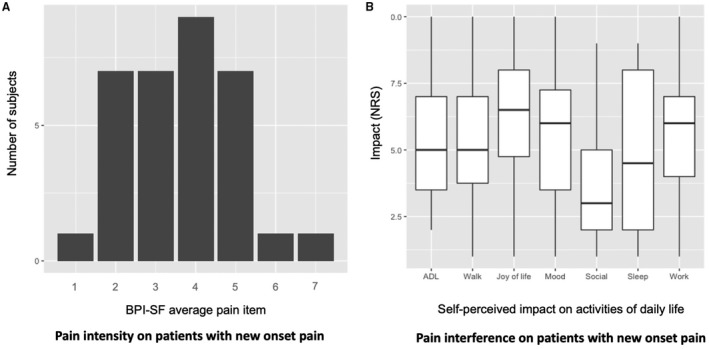
Pain intensity and pain interference in daily life activities on those patients with new onset pain. (a) BPI average pain intensity item (0–10) (b) BPI Self‐perceived impact on activities of daily life in patients with new‐onset pain. The box plots indicate the median interquartile range. ADL, activities of daily life; BPI‐SF, Brief pain inventory Short Form; NRS, numeric rating scale
3.1.1. Pain location
Twenty‐nine (44.6%) patients reported pain in two or more body locations, and 5 (7.7%) reported widespread pain. Upper extremities were the most affected body location (18 [27.7%] patients), followed by lower extremities (12 [18.5%] patients); 10 (15.4%) patients reported shoulder pain. Figure 3 shows a body map with the percentage of pain reported in each anatomical area.
FIGURE 3.
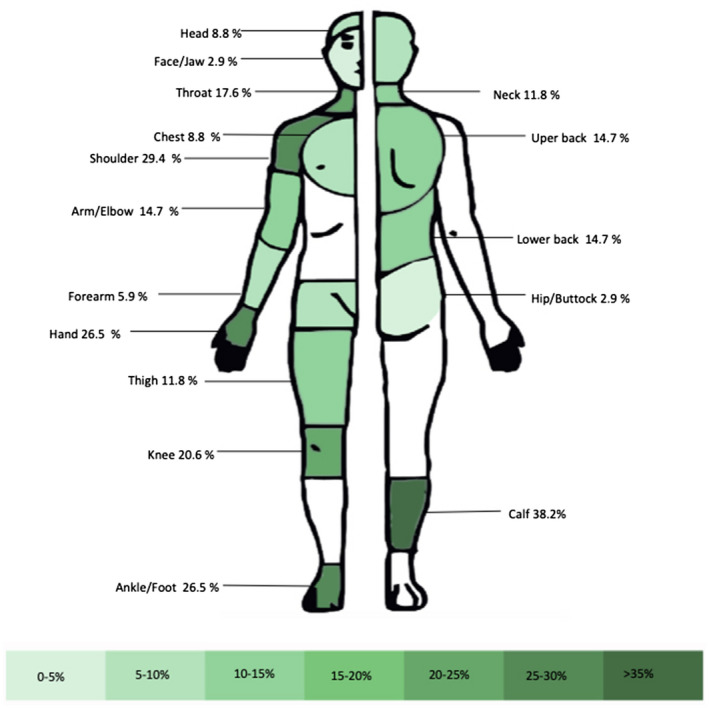
Body map of pain locations completed by patients in the BPI‐SF. BPI‐SF, Brief Pain Inventory Short Form
3.1.2. Chronic pain patients
Of the 8 (12.3%) patients who reported previous chronic pain, 6 (9.2%) had new‐onset pain, and 4 (6.2%) experienced worsening of their previous chronic pain. The main features of these patients are shown in Table 2.
TABLE 2.
Chronic pain patients characteristics
| Patient ID | 3 | 13 | 14 | 23 | 30 | 41 | 46 | 51 |
|---|---|---|---|---|---|---|---|---|
| Age | 68 | 47 | 60 | 60 | 54 | 60 | 70 | 71 |
| Gender | Male | Female | Male | Male | Female | Female | Female | Male |
| Previous pain diagnosis | Politopic osteoarthritis: hands, back and knees | Abdominal pain: Polycystic disease | Cervical pain | Postherpetic neuralgia | Quervain tendinitis; heel pain; low back pain; migraine | Right knee osteoarthritis | Migraine |
Ankylosing Spondylitis Axial pain Knee and hip osteoarthritis |
| Psychiatric pathology | None | None | None | None | Major depressive disorder | Major depressive disorder | None | None |
| EQ pain/discomfort dimension | 2 (slight problems) | 3 (moderate problems) | 2 (slight problems) | 2 (slight problems) | 3 (moderate problems) | 3 (moderate problems) | 4 (severe problems) | 2 (slight problems) |
| BPI‐SF: first question | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| New onset pain confirmed by researcher | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| BPI average pain item | — | 5 | 4 | — | 5 | 2 | 5 | 4 |
| BPI intensity score | — | 7 | 3.75 | — | 6 | 1.5 | 4 | 3.5 |
| BPI interference score | — | 7.14 | 6.29 | — | 7 | 2.75 | 3.29 | 1.29 |
| PCS ≥30 | — | Yes | Yes | — | No | No | No | No |
| DN4 Interview | — | Yes | No | — | No | No | No | No |
| HADS anxiety ≥8 | Yes | Yes | Yes | No | No | No | No | No |
| HADS depression ≥8 | Yes | Yes | No | No | No | No | No | No |
| Chronic pain worsening | No | No | Yes | No | Yes | Yes | Yes | No |
| Observations | New pain: myofasial neck and right shoulder girdle pain. Foot and left leg neuropathic pain | New pain: mechanical low back pain radiating to lower extremities | New pain: knees, and hands arthralgia, generalized myalgia | New pain: left thoracic pain | New pain: mechanical neck pain | New pain: right feet (decubitus ulcer) |
BPI‐SF questionnaire first question: ‘Throughout our lives, most of us have had pain from time to time (such as minor headaches, sprains, and toothaches). Have you had pain other than these everyday kinds of pain?’. BPI‐SF intensity score: mean of the 4 intensity questions. BPI‐SF interference score: mean of the 7 interference questions.
Abbreviations: BPI‐SF, Brief Pain Inventory Short Form; DN4, Douleur Neuropathique 4 Questionnaire; HADS, Hospital Anxiety and Depression Scale; PCS, Pain Catastrophizing Scale.
3.1.3. Neuropathic pain
Ten of 33 (30%) patients with new‐onset pain had a positive screen for NP, the affected areas being: Both feet (n = 3), leg and foot (n = 2), foot (n = 1), thigh (n = 1), shoulder girdle (n = 1) and upper limb (n = 1). Table 3 summarizes the characteristics of these patients. It should be noted that 8 of 10 patients with NP presented ICU‐acquired weakness.
TABLE 3.
Neurophatic pain patients characteristics
| Patient ID | 6 | 13 | 17 | 26 | 31 | 38 | 43 | 47 | 54 | 69 |
|---|---|---|---|---|---|---|---|---|---|---|
| Neurophatic pain localization and characteristics | Numbness of the upper right limb | Left foot allodynia | Dysesthesia in both feet | Hyperesthesia left forearm and hand. Right hand paresthesias | Dysesthesias in the right thigh | Right shoulder girdle pain | Left leg and foot hyperesthesia | Bilateral tingling in both feet | Bilateral tingling in both feet | Left leg and foot hyperesthesia |
| Age | 49 | 47 | 77 | 49 | 72 | 59 | 69 | 55 | 25 | 54 |
| Gender | Male | Female | Female | Male | Male | Male | Male | Male | Male | Female |
| Psychiatric pathology | Depression | |||||||||
| Chronic pain | No | Yes | No | No | No | No | No | No | No | No |
| ICU acquired weakness | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| EQ 5D Index | 0.838 | 0.378 | 0.484 | 0.649 | 0.818 | 0.576 | 0.818 | 0.527 | 0.813 | 0.549 |
| EQ VAS | 90 | 50 | 50 | 85 | 40 | 65 | 80 | 50 | 70 | 80 |
| BPI average pain item | 4 | 5 | 7 | 2 | 5 | 3 | 2 | 5 | 3 | 6 |
| BPI intensity score | 4.25 | 7 | 4.25 | 2 | 4.75 | 3.5 | 2 | 2.5 | 2 | 5.75 |
| BPI interference score | 6.14 | 7.14 | 6.57 | 1 | 3.86 | 2.43 | 1.57 | 3 | 1.29 | 3 |
| PCS >30 | No | Yes | No | No | No | No | No | No | No | No |
| HADS anxiety ≥8 | No | Yes | No | No | No | No | No | No | No | No |
| HADS depression ≥8 | No | Yes | Yes | No | No | No | No | No | No | No |
BPI‐SF intensity score: mean of the 4 intensity questions. BPI‐SF interference score: mean of the 7 interference questions.
Abbreviations: BPI‐SF, Brief Pain Inventory Short Form; HADS, Hospital Anxiety and Depression Scale; PCS, Pain Catastrophizing Scale.
3.2. HRQoL
Only 9 (5.8%) patients reported self‐perceived full health status. Median EQ Index Value was 0.8 (IQR 0.57–0.87), and median EQ VAS was 70 (IQR 60–80). (See Table S1 for EQ 5D/5L frequencies and proportions reported for each dimension and level.)
3.3. New‐onset pain and HRQoL
As expected, individuals displaying new‐onset pain had a significantly worse EQ VAS (65 [50–75] vs. 80 [69–86], p < 0.001). In addition, these individuals showed a worse EQ index value (0.65 [0.55–0.80] vs. 0.87 [0.80–1], p < 0.001). Pain intensity was negatively correlated to EQ VAS measurements (Spearman ρ: −0.41, p < 0.001) and EQ index value (Spearman ρ: −0.55, p < 0.001), as shown in Figure 4.
FIGURE 4.
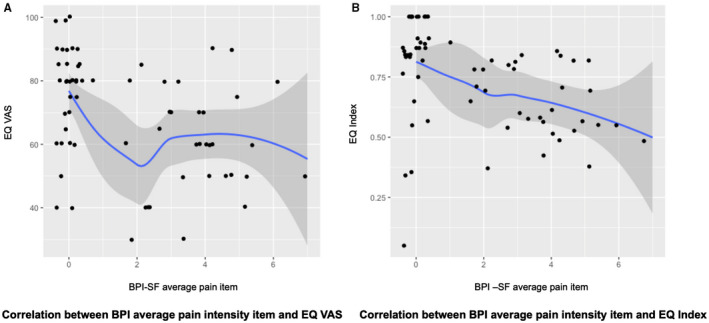
New‐onset pain and Health Related Quality of Life. (a) Correlation between BPI average pain intensity item and EQ VAS. (b) Correlation between BPI average pain intensity item and EQ Index. BPI‐SF, Brief Pain Inventory Short Form; VAS, visual analogue scale
3.4. Anxiety and depression disorders
In 7 (10.8%) patients a HADS anxiety value ≥ of 8 was reported, and 5 (7.7%) patients displayed a HADS depression value ≥8. Anxiety was related to a worse HRQoL in the EQ index value (0.49 [0.36–0.63] vs. 0.82 [0.60–0.87], p = 0.006), but not in the EQ VAS (60 [45–75] vs. 72 [60–80], p = 0.19). Regarding depression, it was related to worse HRQoL both in the EQ index value (50 [40–50] vs. 75 [60–81], p = 0.007) and in the EQ VAS (0.42 [0.38–0.48] vs. 0.82 [0.60–0.87], p = 0.02).
New‐onset pain occurred in 3 of the 7 patients with a HADS anxiety value ≥8 (p = 0.71), and in 4 of the 5 patients with a HADS depression value ≥8 (p = 0.36).
4. DISCUSSION
To the best of our knowledge, this is the first study that prospectively assesses the incidence of new‐onset pain, its characteristics, and its influence on the QoL in very severe COVID‐19 survivors.
Murat et al. exanimated the characteristics of pain in COVID‐19 (Murat et al., 2020). However, prospective studies evaluating the incidence and characteristics of persistent pain after infection are non‐existent (Meyer‐Frießem et al., 2021).
Pain research after COVID‐19 is starting, so it is necessary to establish an appropriate terminology. Soares et al. proposed a classification (Pain not related to COVID‐19; Pain directly related to COVID‐19; and Pain aggravated by COVID‐19) (Soares et al., 2021), that could not be adapted to the current study because it could not be assumed that new‐onset pain was related to COVID‐19 in all patients since it is also frequent in other critical illness survivors (Baumbach et al., 2016; Koster‐Brouwer et al., 2020).
Most studies assessing pain in ICU survivors, and after COVID‐19, use a subdomain of the QoL scales (Kemp et al., 2019; Taboada et al., 2020). Consensus statement on physical rehabilitation in ICU survivors recommend to assess pain using the VAS (Major et al., 2016). However, given the multidimensional nature of pain, appropriate management will only be achieved after detailed assessment with specific instruments (Bendinger & Plunkett, 2016).
Half of the critical COVID‐19 survivors reported new pain in the first month after hospital discharge, and that was frequently associated with a significantly worse QoL.
Studies evaluating new‐onset pain in ICU survivors reported an incidence between 18% and 44% (Battle et al., 2013; Baumbach et al., 2016; Koster‐Brouwer et al., 2020). In COVID‐19 patients, a prevalence of new‐onset pain of around 19% had been informed (Caronna et al., 2020; Soares et al., 2021). Comparison of the current results with the previously reported ones is difficult, due to the impossibility to assess chronic pain according to widely used criteria, also because of the short period of time allocated to pain assessment (Treede et al., 2015), and due the characteristics of the study population (severe critical illness survivors).
Despite the study limitations and given the large number of patients that can be affected with this problem worldwide, the authors believe it is essential to report these findings, that also include the identification of factors associated with new onset pain (Table 1).
4.1. Characteristics of pain
4.1.1. Severity
38.5% and 75.8% of patients reported clinically significant pain, and new‐onset pain respectively. This is a critical finding, because pain intensity is a risk factor to develop chronic pain (Fletcher et al., 2015; Mills et al., 2019).
4.1.2. Pain location
As it has been observed in non COVID‐19 ICU survivors’ studies, most subjects with new‐onset pain (87.9%) reported pain in multiple body areas. Similarly, shoulder pain was frequently reported (30%) (Battle et al., 2013). The shoulder is susceptible to muscle atrophy resulting from neuromuscular relaxation, corticosteroid therapy, sedation and mechanical ventilation (Koster‐Brouwer et al., 2020), implantation of invasive devices in the area (Battle et al., 2013) and/or manoeuvres used to move the patients, often depending on the pressure exerted on the joint (Yachou et al., 2020). Brachial plexus injury during prone positioning for mechanical ventilation has been identified as another potential cause (Guérin et al., 2020). Therefore, a protocol for proning procedures and the identification of pain and functional limitation after patient extubation is desirable (Quick & Brown, 2020). Widespread pain was observed in 15.2% of patients reporting new‐onset pain. A chronic fibromyalgia‐like symptoms after severe acute respiratory syndrome has been described (Moldofsky & Patcai, 2011), with neuroinflammation being a possible pathophysiological factor. Thus, further studies are needed to demonstrate generalized hyperalgesia (suggesting central sensitization) using quantitative sensory testing (Kemp et al., 2020).
4.1.3. Neurophatic pain
Thirty percent of patients suffering from new‐onset pain had a positive screening for NP. Chronic NP pain has been reported in non‐COVID‐19 ICU survivors (Baumbach et al., 2017; Koster‐Brouwer et al., 2020). Despite the fact that the DN4 questionnaire is only validated for chronic pain patients (Hansson et al., 2019), NP in the early stages is linked with chronic NP development, (Beloeil et al., 2017; Martinez et al., 2012; Searle et al., 2009) and this, in turn, with QoL impairment (Colloca et al., 2017). Thus, early management of this condition is believed to be essential.
Small nerve fiber pathology might present as NP with different patterns (Devigili et al., 2008) and is common in ICU survivors {Citation} (Baumbach et al., 2017; Latronico et al., 2013). Thirty‐three percent of patients with NP presented with allodynia or hyperesthesia in one foot and leg, suggesting peroneal nerve injury. Prolonged immobility, muscle atrophy, and contracture can cause peroneal nerve compression (Rubinos & Ruland, 2016). To avoid compression neuropathies, proper patient positioning in bed is crucial (Grigoriadis et al., 2009). Critical illness polyneuropathy has been previously described (Kemp et al., 2020), and the results of this study showed that 80% of patients with NP had ICU‐acquired weakness. Moreover, in our study, 70% of patients with NP reported pain in the lower extremities, suggesting that polyneuropathy may explain a significant proportion of new‐onset NP in this population. The presence of unilateral NP in the foot, may suggest the appearance of a mononeuropathy or early stages of a polyneuropathy.
Human coronaviruses are neurotropic, and their association with neuroinflammation and various neurological disorders is well documented (Yachou et al., 2020). Moreover, the COVID‐19 virus can trigger a cytokine storm in specific populations, with immediate—but also probably lasting—effects on the nervous system (Wu et al., 2020). Although there are no conclusive data about the persistence of neuroinflammation in COVID‐19 survivors, NP could be a manifestation of this process.
4.1.4. Chronic pain patients
Seventy‐five percent of patients with chronic pain had new‐onset pain. This finding is in line with previous reports, with chronic pain in another location being an important risk factor for developing new chronic pain. (Mills et al., 2019). However, the use in this study of a chronic pain definition based on the work of Baumbach et al. (2016), which has broader criteria than the IASP definition (Treede et al., 2015), might lead to an overestimation of previous chronic pain incidence.
4.2. Anxiety and depression
A lower prevalence of anxiety and depression was found in this population when compared to previously documented prevalence in non‐COVID‐19 ICU survivors at 2 and 3 months after hospital discharge (Davydow et al., 2009; Hatch et al., 2018). Alternatively, patients could develop a post‐traumatic growth, defined as positive changes that a person can experience due to struggling with trauma (Tedeschi & Calhoun, 1996). The Polyanna Syndrome, described as being ‘blind optimistic’ to a bad situation (Sirgy, 2002), could also explain the unexpected low incidence of depression given the survival to the pandemic.
4.3. HRQoL
The EQ VAS evidenced that they had a better perception of their health status than expected, which could be related to the feeling of being a survivor of the pandemic. Additionally, there is controversy about the impact of ICU‐delirium on QoL after hospital discharge (Abelha et al., 2013; Luz et al., 2020). In this study, ICU‐delirium was included as inclusion criteria, although not a mandatory one and it might have led to the underestimation of HRQoL scores. However, in our results, patients with ICU‐delirium did not show a worse EQ index value compared to patients without ICU‐delirium (0.78 [0.54–0.86] vs. 0.8 [0.58–0.88], p = 0.55).
4.4. Correlation of clinically significant pain and HRQoL
Clinically significant pain was correlated with worse HRQoL, and these findings have been described for many pathologies, usually related to an injury as a trigger of pain (Langerud et al., 2018). This association sheds light on the importance of improving the characterization of physical and mental trajectories after ICU admission to provide the best long‐term patient care.
4.5. Limitations
The main limitation of this study is the small size of the cohort from a single centre and the lack of a control group of non‐COVID‐19 ICU survivors. In addition, this preliminary analysis of the PAIN‐COVID cohort trial is purely descriptive and, as sample size was not calculated, some of the results could be unpowered.
Second, PAIN‐COVID trial excludes patients with neurodegenerative diseases (Carniglia et al., 2017), and includes patients with ICU‐acquired weakness and delirium, which are related to persistent pain, and could have led to an over‐estimation of new‐onset pain.
Moreover, data were analysed 1 month after hospital discharge and therefore some components of PICS, such as PTSD and new‐onset chronic pain, were left out of the analysis. Also, the chronic pain definition used when collecting basal patient characteristics may limit the comparison of this study findings with other studies’, and eventually undiagnosed persistent cognitive impairment after ICU‐delirium may lead to error when completing the questionnaires.
5. CONCLUSIONS
In this prospective, observational study, new‐onset pain was found to be frequent in critically ill COVID‐19 survivors 1 month after hospital discharge. Pain was associated with daily life activity limitations and with a lower HRQoL. Characteristics of this new‐onset pain were also described to help determine the best strategies for managing this healthcare problem in future studies.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the study conception and design. The study was designed by Dr Ojeda. Preparation, patient recruitment and data collection were performed by Dr Calvo, Dr Cuñat, Dr Comino‐Trinidad, Dr Aliaga and Dr Arias. The first draft of the manuscript was written by Dr Ojeda and Dr Calvo, who share the first authorship of this manuscript; all the authors commented on previous versions of the manuscript. Dr Mellado‐Artigas performed the data analysis. Dr Dürsteler, Dr Martinez‐Pallí and Dr Ferrando reviewed the different contributions of all the authors and contributed to the structure of this manuscript. All the authors read and approved the final manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff of the Pain Unit and the Surgical Intensive Care Unit.
Ojeda, A. , Calvo, A. , Cuñat, T. , Mellado‐Artigas, R. , Comino‐Trinidad, O. , Aliaga, J. , Arias, M. , Ferrando, C. , Martinez‐Pallí, G. , & Dürsteler, C. (2022). Characteristics and influence on quality of life of new‐onset pain in critical COVID‐19 survivors. European Journal of Pain, 26, 680–694. 10.1002/ejp.1897
Antonio Ojeda and Andrea Calvo first authorship shared.
REFERENCES
- Abelha, F. J. , Luís, C. , Veiga, D. , Parente, D. , Fernandes, V. , Santos, P. , Botelho, M. , Santos, A. , & Santos, C. (2013). Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Critical Care (London, England), 17(5), R257. 10.1186/cc13084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaglia, G. , Adroher, N. D. , Vilagut, G. , Bruffaerts, R. , Bunting, B. , Caldas de Almeida, J. M. , Florescu, S. , de Girolamo, G. , de Graaf, R. , Haro, J. M. , Hinkov, H. , Kovess‐Masfety, V. , Matschinger, H. , & Alonso, J. (2017). Health conditions and role limitation in three European Regions: A public‐health perspective. Gaceta Sanitaria, 31(1), 2–10. 10.1016/j.gaceta.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Battle, C. E. , Lovett, S. , & Hutchings, H. (2013). Chronic pain in survivors of critical illness: A retrospective analysis of incidence and risk factors. Critical Care, 17(3), R101. 10.1186/cc12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach, P. , Götz, T. , Günther, A. , Weiss, T. , & Meissner, W. (2016). Prevalence and characteristics of chronic intensive care‐related pain: The role of severe sepsis and septic shock. Critical Care Medicine, 44(6), 1129–1137. 10.1097/CCM.0000000000001635 [DOI] [PubMed] [Google Scholar]
- Baumbach, P. , Götz, T. , Günther, A. , Weiss, T. , & Meissner, W. (2017). Somatosensory functions in survivors of critical illness. Critical Care Medicine, 45(6), e567–e574. 10.1097/CCM.0000000000002309 [DOI] [PubMed] [Google Scholar]
- Baumbach, P. , Götz, T. , Günther, A. , Weiss, T. , & Meissner, W. (2018). Chronic intensive care‐related pain: Exploratory analysis on predictors and influence on health‐related quality of life. European Journal of Pain (London, England), 22(2), 402–413. 10.1002/ejp.1129 [DOI] [PubMed] [Google Scholar]
- Beloeil, H. , Sion, B. , Rousseau, C. , Albaladejo, P. , Raux, M. , Aubrun, F. , Martinez, V. ; SFAR Research Network . (2017). Early postoperative neuropathic pain assessed by the DN4 score predicts an increased risk of persistent postsurgical neuropathic pain. European Journal of Anaesthesiology, 34(10), 652–657. 10.1097/EJA.0000000000000634 [DOI] [PubMed] [Google Scholar]
- Bendinger, T. , & Plunkett, N. (2016). Measurement in pain medicine. BJA Education, 16(9), 310–315. 10.1093/bjaed/mkw014 [DOI] [Google Scholar]
- Bjelland, I. , Dahl, A. A. , Haug, T. T. , & Neckelmann, D. (2002). The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research, 52(2), 69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- Bouhassira, D. , Attal, N. , Alchaar, H. , Boureau, F. , Brochet, B. , Bruxelle, J. , Cunin, G. , Fermanian, J. , Ginies, P. , Grun‐Overdyking, A. , Jafari‐Schluep, H. , Lantéri‐Minet, M. , Laurent, B. , Mick, G. , Serrie, A. , Valade, D. , & Vicaut, E. (2005). Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain, 114(1–2), 29–36. 10.1016/j.pain.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Brinkman, S. , de Jonge, E. , Abu‐Hanna, A. , Arbous, M. S. , de Lange, D. W. , & de Keizer, N. F. (2013). Mortality after hospital discharge in ICU patients*. Critical Care Medicine, 41(5), 1229–1236. 10.1097/CCM.0b013e31827ca4e1 [DOI] [PubMed] [Google Scholar]
- Busico, M. , das Neves, A. , Carini, F. , Pedace, M. , Villalba, D. , Foster, C. , García Urrutia, J. , Garbarini, M. , Jereb, S. , Sacha, V. , & Estenssoro, E. (2019). Follow‐up program after intensive care unit discharge. Medicina Intensiva (English Edition), 43(4), 243–254. 10.1016/j.medine.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Cameron, J. I. , Chu, L. M. , Matte, A. , Tomlinson, G. , Chan, L. , Thomas, C. , Friedrich, J. O. , Mehta, S. , Lamontagne, F. , Levasseur, M. , Ferguson, N. D. , Adhikari, N. K. J. , Rudkowski, J. C. , Meggison, H. , Skrobik, Y. , Flannery, J. , Bayley, M. , Batt, J. , dos Santos, C. , … Herridge, M. S. (2016). One‐year outcomes in caregivers of critically ill patients. New England Journal of Medicine, 374(19), 1831–1841. 10.1056/NEJMoa1511160 [DOI] [PubMed] [Google Scholar]
- Carfì, A. , Bernabei, R. , Landi, F. ; Gemelli Against COVID‐19 Post‐Acute Care Study Group . (2020). Persistent symptoms in patients after acute COVID‐19. JAMA, 324(6), 603–605. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniglia, L. , Ramírez, D. , Durand, D. , Saba, J. , Turati, J. , Caruso, C. , Scimonelli, T. N. , & Lasaga, M. (2017). Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediators of Inflammation, 2017, 1–23. 10.1155/2017/5048616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caronna, E. , Ballvé, A. , Llauradó, A. , Gallardo, V. J. , Ariton, D. M. , Lallana, S. , López Maza, S. , Olivé Gadea, M. , Quibus, L. , Restrepo, J. L. , Rodrigo‐Gisbert, M. , Vilaseca, A. , Hernandez Gonzalez, M. , Martinez Gallo, M. , Alpuente, A. , Torres‐Ferrus, M. , Pujol Borrell, R. , Alvarez‐Sabin, J. , & Pozo‐Rosich, P. (2020). Headache: A striking prodromal and persistent symptom, predictive of COVID‐19 clinical evolution. Cephalalgia, 40(13), 1410–1421. 10.1177/0333102420965157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca, L. , Ludman, T. , Bouhassira, D. , Baron, R. , Dickenson, A. H. , Yarnitsky, D. , Freeman, R. , Truini, A. , Attal, N. , Finnerup, N. B. , Eccleston, C. , Kalso, E. , Bennett, D. L. , Dworkin, R. H. , & Raja, S. N. (2017). Neuropathic pain. Nature Reviews Disease Primers, 3, 17002. 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cs, C. , & Km, R. (1994). Pain assessment: Global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore, 23(2), 129–138. http://europepmc.org/article/MED/8080219 [PubMed] [Google Scholar]
- Darnall, B. D. , Sturgeon, J. A. , Cook, K. F. , Taub, C. J. , Roy, A. , Burns, J. W. , Sullivan, M. , & Mackey, S. C. (2017). Development and validation of a daily pain catastrophizing scale. The Journal of Pain, 18(9), 1139–1149. 10.1016/j.jpain.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydow, D. S. , Gifford, J. M. , Desai, S. V. , Bienvenu, O. J. , & Needham, D. M. (2009). Depression in general intensive care unit survivors: A systematic review. Intensive Care Medicine, 35(5), 796–809. 10.1007/s00134-009-1396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devigili, G. , Tugnoli, V. , Penza, P. , Camozzi, F. , Lombardi, R. , Melli, G. , Broglio, L. , Granieri, E. , & Lauria, G. (2008). The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain, 131(Pt 7), 1912–1925. 10.1093/brain/awn093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, D. , Davidson, J. E. , Harvey, M. A. , Bemis‐Dougherty, A. , Hopkins, R. O. , Iwashyna, T. J. , Wagner, J. , Weinert, C. , Wunsch, H. , Bienvenu, O. J. , Black, G. , Brady, S. , Brodsky, M. B. , Deutschman, C. , Doepp, D. , Flatley, C. , Fosnight, S. , Gittler, M. , Gomez, B. T. , … Needham, D. M. (2014). Exploring the scope of post‐intensive care syndrome therapy and care: Engagement of non‐critical care providers and survivors in a second stakeholders meeting. Critical Care Medicine, 42(12), 2518–2526. 10.1097/CCM.0000000000000525 [DOI] [PubMed] [Google Scholar]
- EuroQol Research Foundation . (2019). EQ‐5D‐5L user guide. https://euroqol.org/publications/user‐guides/
- Ferrando, C. , Mellado‐Artigas, R. , Gea, A. , Arruti, E. , Aldecoa, C. , Bordell, A. , Adalia, R. , Zattera, L. , Ramasco, F. , Monedero, P. , Maseda, E. , Martínez, A. , Tamayo, G. , Mercadal, J. , Muñoz, G. , Jacas, A. , Ángeles, G. , Castro, P. , Hernández‐Tejero, M. , … Hernández‐Sanz, M. L. (2020). Características, evolución clínica y factores asociados a la mortalidad en UCI de los pacientes críticos infectados por SARS‐CoV‐2 en España: Estudio prospectivo, de cohorte y multi‐céntrico. Revista Española De Anestesiología Y Reanimación, S0034935620301870. 10.1016/j.redar.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbain, D. A. , Pulikal, A. , Lewis, J. E. , & Gao, J. (2017). Chronic pain types differ in their reported prevalence of post ‐traumatic stress disorder (PTSD) and there is consistent evidence that chronic pain is associated with PTSD: An evidence‐based structured systematic review. Pain Medicine, 18(4), 711–735. 10.1093/pm/pnw065 [DOI] [PubMed] [Google Scholar]
- Fletcher, D. , Stamer, U. M. , Pogatzki‐Zahn, E. , Zaslansky, R. , Tanase, N. V. , Perruchoud, C. , Kranke, P. , Komann, M. , Lehman, T. , Meissner, W. ; euCPSP group for the Clinical Trial Network group of the European Society of Anaesthesiology . (2015). Chronic postsurgical pain in Europe: An observational study. European Journal of Anaesthesiology, 32(10), 725–734. 10.1097/EJA.0000000000000319 [DOI] [PubMed] [Google Scholar]
- Gewandter, J. S. , Dworkin, R. H. , Turk, D. C. , Farrar, J. T. , Fillingim, R. B. , Gilron, I. , Markman, J. D. , Oaklander, A. L. , Polydefkis, M. J. , Raja, S. N. , Robinson, J. P. , Woolf, C. J. , Ziegler, D. , Ashburn, M. A. , Burke, L. B. , Cowan, P. , George, S. Z. , Goli, V. , Graff, O. X. , … Walco, G. A. (2015). Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. Pain, 156(7), 1184–1197. 10.1097/j.pain.0000000000000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis, K. , Efstathiou, I. , Petrianos, I. , Zikos, P. , Ioannou, A. , Armaganidis, A. , & Vasileiadis, G. (2009). Pressure exerted in the peroneal nerve depending on the different positions in the intensive care unit bed. The Internet Journal of Rehabilitation, 1(2), 1–5. [Google Scholar]
- Guérin, C. , Albert, R. K. , Beitler, J. , Gattinoni, L. , Jaber, S. , Marini, J. J. , Munshi, L. , Papazian, L. , Pesenti, A. , Vieillard‐Baron, A. , & Mancebo, J. (2020). Prone position in ARDS patients: Why, when, how and for whom. Intensive Care Medicine, 46(12), 2385–2396. 10.1007/s00134-020-06306-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, P. , Baron, R. , & Stubhaug, A. (2019). Acute neuropathic pain: Equivalent or different to chronic neuropathic pain? A call for gathering of scientifically based information on acute neuropathic pain. Pain, 160(11), 2413–2414. 10.1097/j.pain.0000000000001650 [DOI] [PubMed] [Google Scholar]
- Hatch, R. , Young, D. , Barber, V. , Griffiths, J. , Harrison, D. A. , & Watkinson, P. (2018). Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: A UK‐wide prospective cohort study. Critical Care, 22. 10.1186/s13054-018-2223-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman, M. , Gudex, C. , Lloyd, A. , Janssen, M. , Kind, P. , Parkin, D. , Bonsel, G. , & Badia, X. (2011). Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Quality of Life Research, 20(10), 1727–1736. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge, M. S. , Cheung, A. M. , Tansey, C. M. , Matte‐Martyn, A. , Diaz‐Granados, N. , Al‐Saidi, F. , Cooper, A. B. , Guest, C. B. , Mazer, C. D. , Mehta, S. , Stewart, T. E. , Barr, A. , Cook, D. , & Slutsky, A. S. (2003). One‐year outcomes in survivors of the acute respiratory distress syndrome. New England Journal of Medicine, 348(8), 683–693. 10.1056/NEJMoa022450 [DOI] [PubMed] [Google Scholar]
- Herridge, M. S. , Chu, L. M. , Matte, A. , Tomlinson, G. , Chan, L. , Thomas, C. , Friedrich, J. O. , Mehta, S. , Lamontagne, F. , Levasseur, M. , Ferguson, N. D. , Adhikari, N. K. J. , Rudkowski, J. C. , Meggison, H. , Skrobik, Y. , Flannery, J. , Bayley, M. , Batt, J. , Santos, C. D. , … Cameron, J. I. (2016). The RECOVER program: Disability risk groups and 1‐year outcome after 7 or more days of mechanical ventilation. American Journal of Respiratory and Critical Care Medicine, 194(7), 831–844. 10.1164/rccm.201512-2343OC [DOI] [PubMed] [Google Scholar]
- Hozhabri, H. , Piceci Sparascio, F. , Sohrabi, H. , Mousavifar, L. , Roy, R. , Scribano, D. , De Luca, A. , Ambrosi, C. , & Sarshar, M. (2020). The global emergency of novel coronavirus (SARS‐CoV‐2): An update of the current status and forecasting. International Journal of Environmental Research and Public Health, 17(16), 5648. 10.3390/ijerph17165648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, S. , Hatakeyama, J. , Kondo, Y. , Hifumi, T. , Sakuramoto, H. , Kawasaki, T. , Taito, S. , Nakamura, K. , Unoki, T. , Kawai, Y. , Kenmotsu, Y. , Saito, M. , Yamakawa, K. , & Nishida, O. (2019). Post‐intensive care syndrome: Its pathophysiology, prevention, and future directions. Acute Medicine & Surgery, 6(3), 233–246. 10.1002/ams2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, J. , Weinrib, A. , Fashler, S. , Katznelson, R. , Shah, B. , Ladak, S. , Jiang, J. , Li, Q. , McMillan, K. , Santa Mina, D. , Wendtlandt, K. , McRae, K. , Tamir, D. , Lyn, S. , de Perrot, M. , Rao, V. , Grant, D. , Roche‐Nagle, G. , Cleary, S. , … Clarke, H. (2015). The Toronto General Hospital Transitional Pain Service: Development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. Journal of Pain Research, 8, 695–702. 10.2147/JPR.S91924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, H. I. , Corner, E. , & Colvin, L. A. (2020). Chronic pain after COVID‐19: Implications for rehabilitation. British Journal of Anaesthesia, 125(4), 436–440. 10.1016/j.bja.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, H. I. , Laycock, H. , Costello, A. , & Brett, S. J. (2019). Chronic pain in critical care survivors: A narrative review. British Journal of Anaesthesia, 123(2), e372–e384. 10.1016/j.bja.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster‐Brouwer, M. E. , Rijsdijk, M. , van Os, W. K. M. , Soliman, I. W. , Slooter, A. J. C. , de Lange, D. W. , van Dijk, D. , Bonten, M. J. M. , & Cremer, O. L. (2020). Occurrence and risk factors of chronic pain after critical illness. Critical Care Medicine, 48(5), 680–687. 10.1097/CCM.0000000000004259 [DOI] [PubMed] [Google Scholar]
- Langerud, A. K. , Rustøen, T. , Småstuen, M. C. , Kongsgaard, U. , & Stubhaug, A. (2018). Health‐related quality of life in intensive care survivors: Associations with social support, comorbidity, and pain interference. PLoS One, 13(6), e0199656. 10.1371/journal.pone.0199656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico, N. , Filosto, M. , Fagoni, N. , Gheza, L. , Guarneri, B. , Todeschini, A. , Lombardi, R. , Padovani, A. , & Lauria, G. (2013). Small nerve fiber pathology in critical illness. PLoS One, 8(9), e75696. 10.1371/journal.pone.0075696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton, S. J. , & Bergbom, S. (2011). Understanding the link between depression and pain. Scandinavian Journal of Pain, 2(2), 47–54. 10.1016/j.sjpain.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Luz, L. F. D. S. , dos Santos, M. C. , Ramos, T. A. , de Almeida, C. B. , Rover, M. C. , Dal’Pizzol, C. P. , Pohren, C. L. D. S. , Martins, A. V. D. S. , & Boniatti, M. M. (2020). Delirium and quality of life in critically ill patients: A prospective cohort study. Revista Brasileira De Terapia Intensiva, 32(3), 426–432. 10.5935/0103-507X.20200072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major, M. E. , Kwakman, R. , Kho, M. E. , Connolly, B. , McWilliams, D. , Denehy, L. , Hanekom, S. , Patman, S. , Gosselink, R. , Jones, C. , Nollet, F. , Needham, D. M. , Engelbert, R. H. H. , & van der Schaaf, M. (2016). Surviving critical illness: What is next? An expert consensus statement on physical rehabilitation after hospital discharge. Critical Care, 20, 10.1186/s13054-016-1508-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen, O. J. , Bäcklund, M. E. , Liisanantti, J. , Peltomaa, M. , Karlsson, S. , & Kalliomäki, M.‐L. (2020). Persistent pain in intensive care survivors: A systematic review. British Journal of Anaesthesia, 125(2), 149–158. 10.1016/j.bja.2020.04.084 [DOI] [PubMed] [Google Scholar]
- Martinez, V. , Ammar, S. B. , Judet, T. , Bouhassira, D. , Chauvin, M. , & Fletcher, D. (2012). Risk factors predictive of chronic postsurgical neuropathic pain: The value of the iliac crest bone harvest model. Pain, 153(7), 1478–1483. 10.1016/j.pain.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Meyer‐Frießem, C. H. , Gierthmühlen, J. , Baron, R. , Sommer, C. , Üçeyler, N. , & Enax‐Krumova, E. K. (2021). Pain during and after COVID‐19 in Germany and worldwide: A narrative review of current knowledge. Pain Reports, 6(1), e893. 10.1097/PR9.0000000000000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, S. E. E. , Nicolson, K. P. , & Smith, B. H. (2019). Chronic pain: A review of its epidemiology and associated factors in population‐based studies. British Journal of Anaesthesia, 123(2), e273–e283. 10.1016/j.bja.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldofsky, H. , & Patcai, J. (2011). Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post‐SARS syndrome; a case‐controlled study. BMC Neurology, 11(1), 37. 10.1186/1471-2377-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat, S. , Dogruoz Karatekin, B. , Icagasioglu, A. , Ulasoglu, C. , İçten, S. , & Incealtin, O. (2020). Clinical presentations of pain in patients with COVID‐19 infection. Irish Journal of Medical Science. 10.1007/s11845-020-02433-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda, A. , Calvo, A. , Cuñat, T. , Artigas, R. M. , Comino‐Trinidad, O. , Aliaga, J. , Arias, M. , Ahuir, M. , Ferrando, C. , & Dürsteler, C. (2021). Rationale and study design of an early care, therapeutic education, and psychological intervention program for the management of post‐intensive care syndrome and chronic pain after COVID‐19 infection (PAIN‐COVID): Study protocol for a randomized controlled trial. Trials, 22(1), 486. 10.1186/s13063-021-05463-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick, T. , & Brown, H. (2020). A commentary on Prone Position Plexopathy during the COVID‐19 pandemic. https://www.boa.ac.uk/resources/knowledge‐hub/a‐commentary‐on‐prone‐position‐plexopathy‐during‐the‐covid‐19‐pandemic.html [Google Scholar]
- Rengel, K. F. , Hayhurst, C. J. , Pandharipande, P. P. , & Hughes, C. G. (2019). Long‐term cognitive and functional impairments after critical illness. Anesthesia & Analgesia, 128(4), 772–780. 10.1213/ANE.0000000000004066 [DOI] [PubMed] [Google Scholar]
- Rubinos, C. , & Ruland, S. (2016). Neurologic complications in the intensive care unit. Current Neurology and Neuroscience Reports, 16(6), 57. 10.1007/s11910-016-0651-8 [DOI] [PubMed] [Google Scholar]
- Sampson, E. L. , West, E. , & Fischer, T. (2020). Pain and delirium: Mechanisms, assessment, and management. European Geriatric Medicine, 11(1), 45–52. 10.1007/s41999-019-00281-2 [DOI] [PubMed] [Google Scholar]
- Searle, R. D. , Simpson, M. P. , Simpson, K. H. , Milton, R. , & Bennett, M. I. (2009). Can chronic neuropathic pain following thoracic surgery be predicted during the postoperative period? Interactive Cardiovascular and Thoracic Surgery, 9(6), 999–1002. 10.1510/icvts.2009.216887 [DOI] [PubMed] [Google Scholar]
- Sirgy, M. J. (2002). The psychology of quality of life. Springer Netherlands. 10.1007/978-94-015-9904-7 [DOI] [Google Scholar]
- Soares, F. H. C. , Kubota, G. T. , Fernandes, A. M. , Hojo, B. , Couras, C. , Costa, B. V. , Lapa, J. D. D. S. , Braga, L. M. , Almeida, M. M. D. , Cunha, P. H. M. D. , Pereira, V. H. H. , Morais, A. D. S. D. , Teixeira, M. J. , Ciampi de Andrade, D. ; Pain in the Pandemic Initiative Collaborators . (2021). Prevalence and characteristics of new‐onset pain in COVID‐19 survivours, a controlled study. European Journal of Pain, ejp.1755. 10.1002/ejp.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboada, M. , Moreno, E. , Cariñena, A. , Rey, T. , Pita‐Romero, R. , Leal, S. , Sanduende, Y. , Rodríguez, A. , Nieto, C. , Vilas, E. , Ochoa, M. , Cid, M. , & Seoane‐Pillado, T. (2020). Quality of life, functional status, and persistent symptoms after intensive care of COVID‐19 patients. British Journal of Anaesthesia. 10.1016/j.bja.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, R. G. , & Calhoun, L. G. (1996). The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. Journal of Traumatic Stress, 9(3), 455–471. 10.1007/BF02103658 [DOI] [PubMed] [Google Scholar]
- Torres, J. , Carvalho, D. , Molinos, E. , Vales, C. , Ferreira, A. , Dias, C. C. , Araújo, R. , & Gomes, E. (2017). The impact of the patient post‐intensive care syndrome components upon caregiver burden. Medicina Intensiva, 41(8), 454–460. 10.1016/j.medin.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Treede, R.‐D. , Rief, W. , Barke, A. , Aziz, Q. , Bennett, M. I. , Benoliel, R. , Cohen, M. , Evers, S. , Finnerup, N. B. , First, M. B. , Giamberardino, M. A. , Kaasa, S. , Kosek, E. , Lavand'homme, P. , Nicholas, M. , Perrot, S. , Scholz, J. , Schug, S. , Smith, B. H. , … Wang, S.‐J. (2015). A classification of chronic pain for ICD‐11. Pain, 156(6), 1003–1007. 10.1097/j.pain.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hout, B. , Janssen, M. F. , Feng, Y.‐S. , Kohlmann, T. , Busschbach, J. , Golicki, D. , Lloyd, A. , Scalone, L. , Kind, P. , & Pickard, A. S. (2012). Interim Scoring for the EQ‐5D‐5L: Mapping the EQ‐5D‐5L to EQ‐5D‐3L value sets. Value in Health, 15(5), 708–715. 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- von Elm, E. , Altman, D. G. , Egger, M. , Pocock, S. J. , Gøtzsche, P. C. , & Vandenbroucke, J. P. (2007). Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. British Medical Journal, 335(7624), 806–808. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Coronavirus Disease (COVID‐19) Dashboard . (n.d.). https://covid19.who.int
- Wu, Y. , Xu, X. , Chen, Z. , Duan, J. , Hashimoto, K. , Yang, L. , Liu, C. , & Yang, C. (2020). Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain, Behavior, and Immunity, 87, 18–22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachou, Y. , El Idrissi, A. , Belapasov, V. , & Ait Benali, S. (2020). Neuroinvasion, neurotropic, and neuroinflammatory events of SARS‐CoV‐2: Understanding the neurological manifestations in COVID‐19 patients. Neurological Sciences, 41(10), 2657–2669. 10.1007/s10072-020-04575-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


